Abstract
BACKGROUND AND PURPOSE
FTY720 (Fingolimod) is a recently approved orally administered drug for the treatment of multiple sclerosis. Phase II and III clinical trials have demonstrated that this drug modestly increases BP. We previously showed that inhibition of sphingosine kinase increases vascular tone and BP in hypertensive, but not normotensive rats. Since FTY720 is reported to have inhibitory effects on sphingosine kinase, we investigated whether FTY720 increases vascular tone and BP only in hypertensive rats via this mechanism.
EXPERIMENTAL APPROACH
The contractile and BP modulating effects of FTY720 were studied in vivo and ex vivo (wire myography) in age-matched normotensive Wistar Kyoto (WKY) rats and spontaneously hypertensive rats (SHRs).
KEY RESULTS
Oral administration of FTY720 induced an increase in mean arterial pressure in SHR, whereas a decrease in BP was observed in WKY rats, as measured 24 h after administration. Similar to the sphingosine kinase inhibitor dimethylsphingosine (DMS), FTY720 induced large contractions in isolated carotid arteries from SHR, but not in those from WKY. In contrast, the phosphorylated form of FTY720 did not induce contractions in isolated carotid arteries from SHR. FTY720-induced contractions were inhibited by endothelium denudation, COX and thromboxane synthase inhibitors, and by thromboxane receptor antagonism, indicating that (like DMS-induced contractions) they were endothelium-dependent and mediated by thromboxane A2.
CONCLUSIONS AND IMPLICATIONS
These data demonstrate that FTY720 increases vascular tone and BP only in hypertensive rats, most likely as a result of its inhibitory effect on sphingosine kinase.
Keywords: FTY720, Fingolimod, hypertension, sphingosine kinase, endothelium, vasoconstriction, EDCF, sphingolipids
Introduction
The immunosuppressant drug FTY720 (fingolimod) is a recently approved therapeutic addition to the treatment options of relapsing multiple sclerosis (Brinkmann, 2009). FTY720 is a sphingosine analogue that is phosphorylated in vivo to (S)-FTY720-P by sphingosine kinase (mainly type 2) (Billich et al., 2003; Albert et al., 2005), the enzyme responsible for the production of the endogenous bioactive sphingolipid sphingosine-1-phosphate (S1P). (S)-FTY720-P is a high-affinity ligand at four of the five S1P receptors (S1P1,3,4,5) and leads to degradation of S1P1 receptors on T-lymphocytes, which subsequently results in a reduced lymphocyte egress and thus a T-lymphocyte-specific immunosuppression (Adachi and Chiba, 2008; Thangada et al., 2010; Verzijl et al., 2010). In phase II and III clinical studies, a modest rise in BP (3–5 mmHg) was detected in patients treated with FTY720 (Cohen et al., 2010; Comi et al., 2010; Kappos et al., 2010). Although the active metabolite (S)-FTY720-P has vasoactive properties (both vasodilatation and vasoconstriction have been reported) (Tölle et al., 2005; Salomone et al., 2008), until now the exact mechanism by which FTY720 induces this rise in BP in vivo remains elusive.
We have previously shown that hypertension, both experimental as well as human essential hypertension, is associated with profound alterations in vascular sphingolipid biology (Spijkers et al., 2011a). These alterations could be restored by selective anti-hypertensive treatment in the spontaneously hypertensive rat (SHR) (Spijkers et al., 2011b). Sphingolipids, such as ceramide and S1P, are bioactive lipids that play an important role in cellular signalling. They do not only play a crucial role in cell growth (Hannun and Obeid, 2008; Pyne and Pyne, 2010), but they are also involved in vascular function (Nixon et al., 2007; Alewijnse and Peters, 2008; Spijkers et al., 2010; Schuchardt et al., 2011). S1P, for instance, via activation of endothelial S1P receptors, is known to activate eNOS and thereby to induce vasodilatation (Dantas et al., 2003; Mulders et al., 2009). In contrast, we have shown that ceramide, a precursor of S1P, is a potent stimulator of endothelium-mediated thromboxane A2 (TXA2) synthesis in isolated carotid arteries of SHRs, thereby inducing endothelium-dependent vasoconstriction (Spijkers et al., 2011a). Interestingly, the latter phenomenon is only present in vessels from hypertensive rats, because of an increased endothelial expression of the enzymes involved in TXA2 synthesis. This mechanism is also thought to be involved in the large endothelium-dependent vasoconstrictions induced by pharmacological inhibition of sphingosine kinase, by means of dimethylsphingosine (DMS), in isolated carotid arteries from SHR, but not in those from normotensive rats. Moreover, inhibition of sphingosine kinase in SHR in vivo results in a marked rise in BP, while it has no effect, or even lowers BP in normotensive Wistar Kyoto (WKY) rats (Spijkers et al., 2011a).
While FTY720 is phosphorylated mainly by sphingosine kinase type 2, it has been reported to be a potent inhibitor of sphingosine kinase type 1 (Vessey et al., 2007; Tonelli et al., 2010). Therefore, we hypothesized that FTY720, similar to DMS, increases vascular tone and BP in hypertensive but not normotensive rats. Here we show that FTY720 indeed elevates BP only in hypertensive rats and induces vasoconstriction in isolated carotid arteries from SHR via TXA2 production.
Methods
Animals
Six-month-old male SHRs and WKY rats were purchased from Charles River (Maastricht, the Netherlands) and handled according to a protocol approved by the Animal Ethical Committee of the University of Amsterdam, the Netherlands. The animals were housed with access to food and water ad libitum under a 12 h light/dark cycle. For myography experiments, rats were anaesthetized by i.p. injection of 75 mg·kg−1 pentobarbital (O.B.G., Utrecht, the Netherlands). Heparin (750 IU, Leo Pharma B.V., Weesp, the Netherlands) was injected i.p. to prevent blood coagulation and thrombocyte-derived S1P release.
Tail-cuff BP measurements
FTY720 (0.3 mg·kg−1) was given orally by gastric gavage. For awake BP measurements, 24 h after FTY720 administration, the CODA™ monitor (Kent Scientific Corporation, Torrington, CT, USA) was used. In brief, rats were fixed in a transparent animal holder and placed on a heating pad. The rat was left untouched and fixated for a couple of minutes before placing the tail-cuffs. Then, tail-cuffs were placed loosely fitting over the tail slightly below the tail base. An average of eight repeated tail-cuff cycles was performed per rat per condition. During the experiment, care was taken to ensure minimal stress to the animals.
Immunohistochemistry and quantification
Carotid artery segments from untreated SHR and WKY rats were collected directly after dissection and rapidly submerged in OCT Compound (TissueTek, Sakura, Alphen aan de Rijn, the Netherlands) and frozen in liquid nitrogen with subsequent storage at −80°C. Frozen sections (5 µm) were cut on a Leica CM3050S cryostat and dried by cold pressurized air and fixed in 100% acetone during 1 min. Then, slides were washed shortly in 0.1% PBS/BSA (w v−1) and incubated with blocking buffer (2% PBS/BSA) for 30 min at room temperature. After a short wash, slides were incubated with the primary antibody against sphingosine kinase 1 (Cayman Chemical Co., Ann Arbor, MI, USA, #10006822; 1/50 dilution) dissolved in 0.1% PBS/BSA overnight at 4°C. Following a triple wash in 0.1% PBS/BSA for 5 min, the appropriate A546-labelled secondary antibody (Invitrogen, Carlsbad, CA, USA, #A-11010; 1/400 dilution) was applied for 1 h at room temperature. After a triple wash, the antibody against von Willebrand Factor (GeneTex, Irvin, CA, USA, #GTX74830; 1/200 dilution) was applied for 1 h at room temperature as a marker of the endothelium. After another triple wash, the final A488-labelled secondary fluorescent antibody (Invitrogen, #A-11029, 1/400 dilution) was applied. Finally, after a triple wash, DAPI containing mounting medium (Santa Cruz Biotechnology, Santa Cruz, CA, USA, #sc-24941) was applied and vessels were imaged using a Nikon Eclipse TE2000-U fluorescence microscope (Plan Fluor ELWD 20× objective, Nikon DXM1200F digital camera) with NIS Elements AR 2.30 software. The region of interest was determined for each segment by detection of the endothelial marker von Willebrand Factor, without any information on the protein being quantified to ensure unbiased recording. Then, the appropriate filter setting was chosen to record the mean fluorescence intensity using the NIS Elements software on the raw unprocessed images. For both endothelium and smooth muscle cell determinations, an intensity threshold was selected to exclude background fluorescence. All settings and exposure times were applied to all slides equally for quantification of the appropriate protein.
Arterial preparation and isometric force recording
The left common carotid artery was carefully excised in a range just distal from the bifurcation until the level of the aortic arch and immediately placed in Krebs-Henseleit buffer (118.0 mM NaCl, 4.7 mM KCl, 25.0 mM NaHCO3, 1.2 mM MgSO4, 1.8 mM CaCl2, 1.1 mM KH2PO4 and 5.6 mM glucose) at room temperature, aerated with 5% CO2/95% O2, pH 7.4. Four segments of carotid artery were carefully prepared and two stainless steel wires with a diameter of 40 µm (Goodfellow, Huntingdon, UK) were inserted into the lumen of each vessel segment. In selected cases the endothelium was removed mechanically (by rolling a luminally inserted PE-50 tube several times) before the tissue was mounted. The segments were then transferred into organ baths of a four-channel wire myograph (610M, Danish Myo Technology, Aarhus, Denmark) and subjected to a normalization procedure according to Mulvany and Halpern (1977). In brief, the individual circumference was adjusted to 90% of the value that the particular vessel would have had at a transmural pressure of 100 mmHg. Afterwards, the arteries were equilibrated for 30 min and the buffer was replaced after each period of 15 min. The preparations were contracted twice for 10 min with a depolarizing high K+ Krebs-Henseleit solution (100 mM NaCl was replaced by 100 mM KCl) at intervals of 15 min. Subsequently the vessels were precontracted with the α1-adrenoceptor agonist phenylephrine (0.3 µM). After a steady level of contraction force, >60% of previous KCl-induced depolarization contraction, had been attained, one concentration (10 µM) of the endothelium-dependent vasodilator methacholine was added to assess the endothelial integrity. For endothelium-denuded vessels, a relaxation <5% indicated successful denudation, and these segments were included accordingly. After washing, again 100 mM KCl was added to the vessel segments. After washing and a 30 min pre-incubation of inhibitors or their vehicle (in all cases dimethylsulfoxide), DMS (10 µM), FTY720 (10 µM) or FTY720-P (10 µM) were added and the vascular responses were recorded for an additional 50 min. Isometric force of contraction was measured continuously and all data are presented in mN·mm−1 segment length (except for raw tracings).
Data analysis and statistics
The isometric tension measurements in carotid artery segments and BP/heart rate measurements are presented as mean ± SEM with n being the number of individual rats. Peak contraction values during the myography experiments were determined and expressed as relative tension (mN·mm−1) and presented in histograms. Statistics were performed by Student's t-test (Figures 2–4) and one-way anova including Dunnett's multiple comparisons test (95% confidence interval) with FTY720 values as control (Figure 5). All statistical analyses were performed using Prism (GraphPad Prism Software, San Diego, CA, USA). Values of P < 0.05 were considered to be statistically significant.
Figure 2.
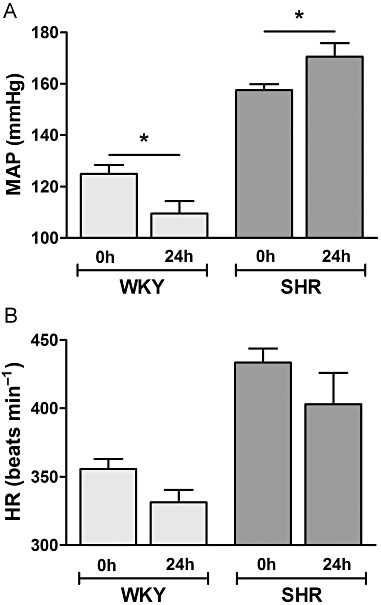
BP response to oral FTY720 challenge in SHR and WKY rats. (A) Tail-cuff mean arterial pressure (MAP) measurement in WKY and SHR before (0 h) and after 24 h after oral (0.3 mg·kg−1 FTY720) challenge and (B) corresponding heart rate (HR). Data expressed as mean ± SEM, n = 4, *P < 0.05.
Figure 4.
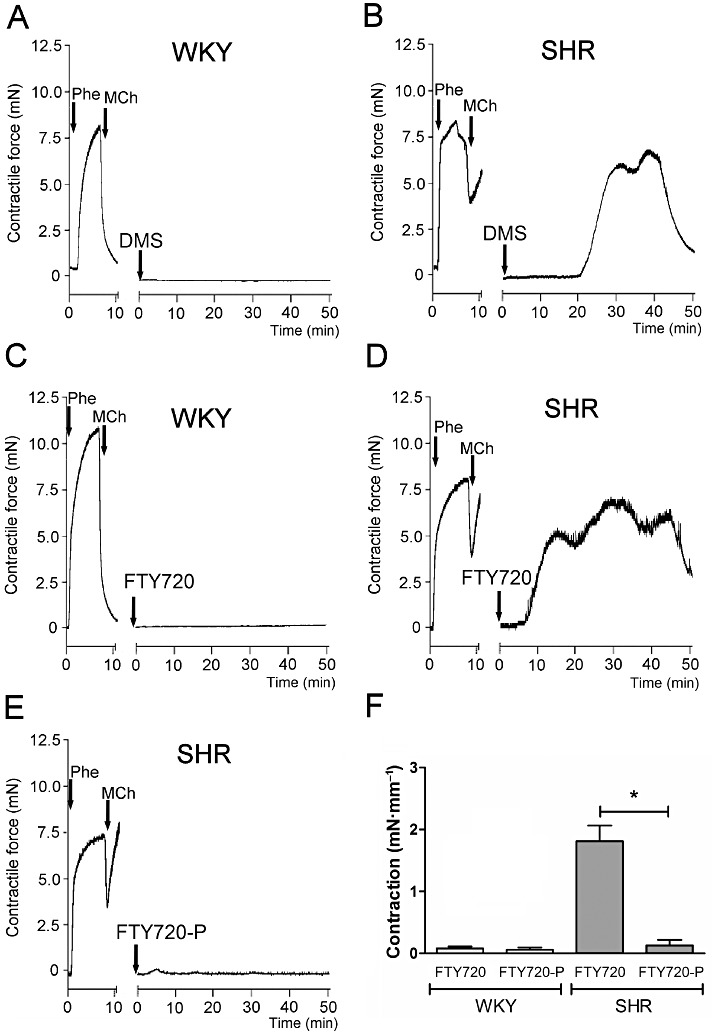
Contractile effect of DMS, FTY720 and FTY720-P on carotid arteries of SHR and WKY. Typical tracings showing the contractile effects of DMS (A + B), FTY720 (FTY, C + D) and FTY720-P (FTY-P, E) in isolated carotid arteries from WKY rats (A + C) and SHR (B, D + E). Please note the profound endothelial dysfunction in arteries from SHR as evidenced by a decreased relaxant response to methacholine (MCh; 10 µM) in phenylephrine (Phe; 0.3 µM) precontracted artery segments. Quantified data of FTY720-induced effects (F) are expressed as mean ± SEM in mN·mm−1 segment length, n = 3–6, *P < 0.05.
Figure 5.
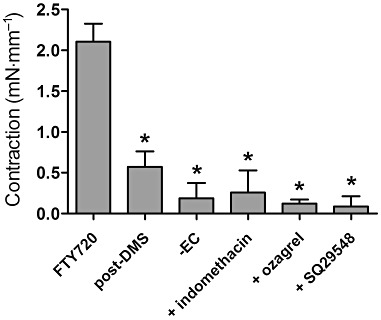
Mechanism of FTY720-induced contractions in isolated carotid arteries from SHR. FTY720-induced contractions after incubation with DMS (10 µM), in the absence of endothelium (-EC), in the presence of the COX inhibitor indomethacin (10 µM), the thromboxane synthase inhibitor ozagrel (10 µM) and the TP receptor antagonist SQ29548 (10 µM). Data are expressed as mean ± SEM in mN·mm−1 segment length, n = 4–6, *P < 0.05 compared with vehicle (dimethylsulfoxide).
Chemicals
Acetyl-β-methylcholine (methacholine), phenylephrine, indomethacin and ozagrel were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA); DMS from Biomol International L.P. (Plymouth, PA, USA) and SQ29548 from Alexis Biochemical (San Diego, CA, USA). FTY720 and FTY720-P were synthesized according to previously described methods (Albert et al., 2005). All other chemicals were from Sigma and of analytical grade. The stated drug and molecular target nomenclature conforms to BJP's latest Guide to Receptors and Channels (Alexander et al., 2011).
Results
Oral challenge with FTY720 elevates BP in vivo in SHR, but not WKY
Figure 1 shows the structural similarities of FTY720 and the sphingosine kinase inhibitor DMS, both derivatives of sphingosine, the natural substrate for sphingosine kinases. To investigate whether FTY720, like DMS, raises BP preferentially in hypertensive animals (Spijkers et al., 2011a), SHR and WKY rats were given oral FTY720 (once 0.3 mg·kg−1 in saline) and BP/heart rate was recorded using tail-cuff non-invasive BP measurements before and 24 h after FTY720 challenge. After 24 h of the oral dose of FTY720, BP was lowered in the WKY, whereas BP in SHR was elevated (n = 4–7, P < 0.05) (Figure 2A). Heart rate, however, was equally reduced in WKY and SHR (Figure 2B).
Figure 1.

Chemical structures of the sphingosine derivates dimethylsphingosine and FTY720.
Sphingosine kinase 1 expression is elevated in SHR carotid artery segments compared with those of WKY
As the main target of DMS and unphosphorylated FTY720 is sphingosine kinase, the protein expression profile of sphingosine kinase 1 in carotid arteries of WKY and SHR was assessed. As indicated by immunohistochemistry, in both the endothelium and smooth muscle layer of isolated SHR carotid artery segments, the expression of sphingosine kinase 1 was higher than that in WKY carotid artery segments (Figure 3).
Figure 3.
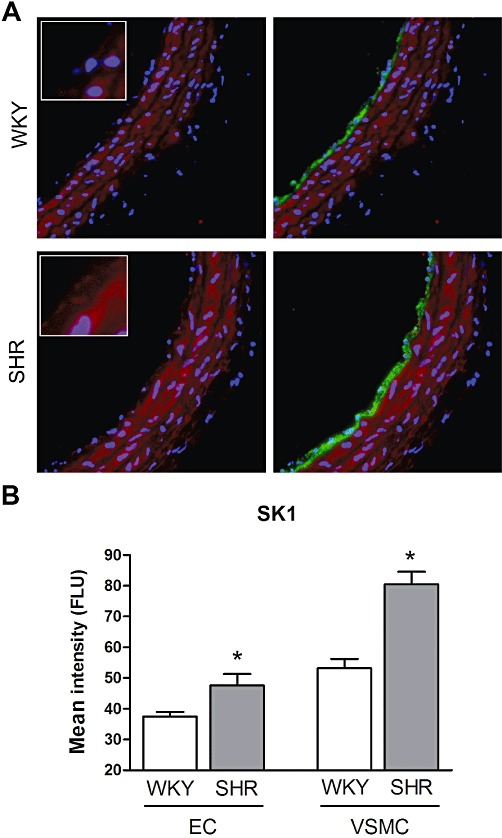
Quantitative immunohistochemistry on sphingosine kinase 1 expression. (A) Typical example the of the expression profile of sphingosine kinase 1 (red) and cell nuclei (blue; left) and additionally with vWF endothelium marker (green; right) in WKY and SHR carotid artery. (B) Staining intensity quantified as endothelium (EC)-specific expression and vascular smooth muscle cell (VSMC)-specific expression. Data expressed as mean fluorescent light units (FLU) ± SEM, n = 5–6, *P < 0.05.
DMS and FTY720 induce marked contractile responses in isolated carotid artery segments of SHR but not in those of normotensive WKY rats
Isolated carotid arteries of normotensive WKY rats were completely unresponsive to DMS. In contrast, DMS (10 µM) induced profound transient contractions in artery segments of SHRs as described previously (Spijkers et al., 2011a) (a DMS tracing is shown as a reference in Figure 4A,B). Similar to DMS, FTY720 did not evoke any response in carotid artery segments from WKY rats (Figure 4C,F), but induced large transient contractions in segments from SHR (Figure 4D,F). Importantly, in contrast to its parent compound, the phosphorylated form of FTY720 (i.e. FTY720-P) did not induce any substantial response in segments from SHR nor WKY (Figure 4E and Supporting Information Figure S1, respectively). Thus, only unphosphorylated FTY720 induced transient vasoconstrictions in carotid arteries of SHR but not in those of WKY (Figure 4F).
FTY720 and DMS induce vasoconstriction in carotid artery segments of SHR via a similar mechanism
DMS-induced vasoconstriction has previously been shown to be endothelium-dependent and to be sensitive to COX and TXA2 synthase inhibition and could be antagonized by the thromboxane/prostaglandin (TP) receptor antagonist SQ29548 (Spijkers et al., 2011a).
To investigate whether FTY720 and DMS induce contractions in carotid artery segments from SHR via a similar mechanism, we either removed the endothelium or pre-incubated the vascular segments with DMS, applied inhibitors of aforementioned enzymes or the TP receptor antagonist before the addition of FTY720. These substances did not alter baseline tension. FTY720-induced vasoconstriction was diminished in DMS-pretreated segments (Figure 5 and Supporting Information Figure S2). Also endothelial denudation blunted the contractile response to FTY720, indicating that the contractions were indeed endothelium-dependent (Figure 5). Furthermore, the COX inhibitor indomethacin (10 µM), the thromboxane synthase inhibitor ozagrel (10 µM) and the TP receptor antagonist SQ29548 (10 µM) all inhibited the FTY720-induced vasoconstriction (Figure 5).
Discussion and conclusions
FTY720 (fingolimod) is a recently approved oral treatment option for multiple sclerosis (Brinkmann, 2009). In clinical trials, FTY720 as compared with placebo had a superior efficacy over a 2 year period in patients with relapsing–remitting multiple sclerosis (Kappos et al., 2010), and proved more effective than intramuscular interferon β-1a over a 12 month period (Cohen et al., 2010). Phase II and the aforementioned phase III clinical trials have shown that FTY720 is generally well tolerated and the reported adverse effects are, further to adverse effects due to immunosuppression, also of a cardiovascular nature. Besides transient effects on heart rate (bradycardia) and atrioventricular conduction, a moderate increase in BP of approximately 3–5 mmHg in 4–6% of treated patients was observed that persisted during treatment (Cohen et al., 2010; Comi et al., 2010; Kappos et al., 2010). In addition, one case of severe peripheral arterial vasospasm was reported in a patient after 7 days of FTY720 treatment (Schwarz et al., 2010).
In a previous report, we showed that hypertension is associated with marked alterations in vascular sphingolipid biology (Spijkers et al., 2011a). Shifting the ceramide/S1P ratio towards ceramide, for instance, by pharmacological inhibition of sphingosine kinase, triggers endothelium-dependent production of TXA2 in hypertensive animals, thus inducing vasoconstriction. Accordingly, intravenous infusion of DMS induces a marked increase in BP in anaesthetized SHR, whereas it has no effect, or even lowers BP in WKY rats (Spijkers et al., 2011a). Interestingly, ceramide levels in arterial tissue and plasma were significantly higher in SHR compared with those in normotensive WKY rats. The fact that humans with stage 2/3 hypertension also display elevated levels of ceramide in blood plasma indicates that human essential hypertension is also associated with alterations in sphingolipid biology. As several reports have clearly demonstrated that FTY720 has profound inhibitory effects on sphingosine kinase, we were prompted to investigate whether FTY720, like the sphingosine kinase inhibitor DMS, increases vascular tone and BP only in SHR.
In a pilot experiment we investigated whether FTY720 shows a similar divergent behaviour as DMS in vivo by infusing FTY720 i.v. into anaesthetized rats, but FTY720 caused marked effects on cardiac frequency in these rats making it difficult to measure BP responses. As mentioned before, the cardiac effects of FTY720 are well known and have been described in laboratory animals and humans (Forrest et al., 2004; Schmouder et al., 2006). However, as the bradycardia induced by FTY720 is known to be transient, in the present study we administered FTY720 orally and measured the BP response after 24 h. While in WKY rats orally administered FTY720 reduced BP, we observed an increase in BP in SHR 24 h after its application. At this time point, heart rate was still reduced in both groups to the same extent. This latter results excludes the possibility that the changes in heart rate are responsible for the divergent response in BP we observed. Hence, these experiments confirm that FTY720 induces comparable BP responses in normotensive and hypertensive animals as the sphingosine kinase inhibitor DMS. To determine whether similar mechanisms are involved, we performed ex vivo experiments in isolated carotid arteries. In these blood vessels, sphingosine kinase 1 expression is elevated in SHR compared with WKY, suggesting enhanced sensitivity to both DMS and FTY720 inhibition. The myography experiments clearly demonstrated that FTY720, like DMS, induces vasoconstriction only in arteries from hypertensive rats and not in those from normotensive WKY rats. In contrast, FTY720-P, the phosphorylated derivative of FTY720, did not constrict the artery segments of either WKY or SHR, most likely due to the fact that FTY720-P has no sphingosine kinase inhibitory effects. This finding excludes the possibility that the constriction response to FTY720 is caused by S1P receptor stimulation via FTY720-P, which is formed via sphingosine kinase 2 activity in the artery segment.
The transient vasoconstriction induced by FTY720 in isolated carotid arteries from SHR but not WKY rats closely resembled the vascular effects of DMS. In addition, the finding that the FTY720-induced contractions were substantially diminished in segments pretreated with DMS suggests that DMS and FTY720 induce vasoconstriction via a similar mechanism. The DMS-induced contractions have been shown to be endothelium-dependent and mediated via the eicosanoid TXA2 (Spijkers et al., 2011a). We demonstrated that FTY720-induced contractions were abolished by mechanical removal of the endothelium before the addition of the compound. Moreover, the contractions were potently inhibited by COX and thromboxane synthase inhibition and TP receptor antagonism. Together these results indicate that FTY720 and DMS induce constriction via a similar mechanism. In our previous study, we demonstrated that increased expression of calcium-independent PLA2, COX and thromboxane synthase (all enzymes involved in TXA2 synthesis) in carotid artery segments of SHR accounts for this phenomenon.
Whether this mechanism (i.e. sphingosine kinase inhibition) also contributes to the increase in BP induced by FTY720 in humans remains unclear. Although these data are unfortunately not available in literature, it would be interesting to see whether the observed increases in BP in multiple sclerosis patients are specifically in those patients that already have hypertension or endothelial dysfunction.
In addition to its sphingosine kinase inhibitory, FTY720, after phosphorylation to FTY720-P in vivo, may induce vasoconstriction via stimulation of S1P receptors on smooth muscle cells (Nixon et al., 2007; Salomone et al., 2008). However, vasoconstriction to FTY720-P is restricted to particular vascular beds, such as coronary and basilar arteries (Salomone et al., 2008). As we demonstrated in this study, FTY720-P does not induce vasoconstriction in carotid arteries. In other peripheral arteries, FTY720-P induces an endothelium-dependent vasodilatation via stimulation of endothelial S1P1 and/or S1P3 receptors (Dantas et al., 2003; Tölle et al., 2005). Therefore, it is unlikely that stimulation of vascular S1P receptors exclusively contributes to the increase in BP observed in patients treated with FTY720. In addition, this does not account for the divergent responses to FTY720 in normotensive and hypertensive animals. In this regard, it is important to note that the carotid artery is a conduit and not a resistance vessel. Interestingly, we did not observe contractile responses to FTY720 and DMS in mesenteric arteries from SHR or WKY; whereas FTY720 did induce vasodilatation in preconstricted mesenteric arteries (data not shown). This may be in accordance with observations that sphingosine kinase 1 expression in mesenteric arteries is rather low compared with cerebral vessels (Salomone et al., 2010). The fact that we observe clear BP increases to FTY720 in SHR, however, suggests that certain resistance vessels in vivo do contract. This may be due to direct TXA2 release in vivo in the resistance vascular bed itself, or to paracrine/endocrine effects of TXA2 released from other vascular beds.
Another important aspect to keep in mind when extrapolating the in vitro data to the in vivo situation is the metabolism of FTY720. One could argue that FTY720 in vivo is rapidly converted to FTY720-P, and that in contrast to the in vitro situation the vasculature in vivo is mainly exposed to FTY720-P. However, several pharmacokinetic studies (Kovarik et al., 2007a,b; Zollinger et al., 2011) have demonstrated that FTY720 is absorbed very slowly; peak FTY720 plasma levels are reached approximately 36 h after oral administration of a single dose. A recent study performed with radiolabelled FTY720 in humans (Zollinger et al., 2011) clearly demonstrated that FTY720 is only partially phosphorylated in vivo. Because of similar half-life values of FTY720 and FTY720-P, the ratio between the pro-drug and the metabolite remains rather constant and amounts to approximately 2. Thus, in vivo, endothelial cells are also exposed to higher concentrations of FTY720 than FTY720-P.
Next to inhibition of sphingosine kinase, FTY720 has been reported to inhibit cytoplasmic PLA2 (Payne et al., 2007) and ceramide synthase (Berdyshev et al., 2009). It is unlikely, however, that these properties are involved in the contractile effects of FTY720 reported here; because of the role of arachidonic acid metabolites and ceramide in vessels from SHR as mentioned before, one would expect the opposite effect.
In conclusion, we clearly demonstrated that FTY720 induces vasoconstriction in isolated carotid arteries and raises BP in hypertensive, but not normotensive animals, most likely via its inhibitory effect on sphingosine kinase. Whether this mechanism contributes to increases in BP during FTY720 treatment in humans remains to be investigated.
Acknowledgments
This study was performed within the framework of Top Institute Pharma project T2-108.
Glossary
- DMS
dimethylsphingosine
- FTY720
2-amino-[2-(4-n-octylphenyl)ethyl]-1,3-propanediol
- FTY720-P
FTY720 phosphate
- S1P
sphingosine-1-phosphate
- SHR
spontaneously hypertensive rat
- SQ29548
[1S-[1 alpha,2 beta (5Z),3 beta,4 alpha]-7-[3-[[2-[(phenylamino) carbonyl]hydrazino]methyl]-7-oxabicyclo[2.2.1] hept-2-yl]-5-heptenoic acid
- TP
receptor, thromboxane/prostaglandin receptor
- TXA2
thromboxane A2
Conflict of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Raw tracing of an ex vivo WKY carotid artery segment incubated with FTY720-P (10 μM).Phenylephrine (Phe), methacholine (MCh).
Figure S2 Raw tracing of an ex vivo SHR carotid artery segment, pre-incubated with either DMS (10 μM) or vehicle control (DMSO) and subsequent FTY720 (10 μM). Dimethylsphingosine (DMS), dimethylsulfoxide (DMSO), phenylephrine (Phe), methacholine (MCh).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Adachi K, Chiba K. FTY720 story. Its discovery and the following accelerated development of sphingosine 1-phosphate receptor agonists as immunomodulators based on reverse pharmacology. Perspect Med Chem. 2008;1:11–23. [PMC free article] [PubMed] [Google Scholar]
- Albert R, Hinterding K, Brinkmann V, Guerini D, Müller-Hartwieg C, Knecht H, et al. Novel immunomodulator FTY720 is phosphorylated in rats and humans to form a single stereoisomer. Identification, chemical proof, and biological characterization of the biologically active species and its enantiomer. J Med Chem. 2005;48:5373–5377. doi: 10.1021/jm050242f. [DOI] [PubMed] [Google Scholar]
- Alewijnse AE, Peters SL. Sphingolipid signalling in the cardiovascular system: good, bad or both? Eur J Pharmacol. 2008;585:292–302. doi: 10.1016/j.ejphar.2008.02.089. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdyshev EV, Gorshkova I, Skobeleva A, Bittman R, Lu X, Dudek SM, et al. FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J Biol Chem. 2009;284:5467–5477. doi: 10.1074/jbc.M805186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Dévay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- Brinkmann V. FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol. 2009;158:1173–1182. doi: 10.1111/j.1476-5381.2009.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Barkhof F, Comi G, Hartung H-P, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- Comi G, O'Connor P, Montalban X, Antel J, Radue EW, Karlsson G, et al. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3-year results. Mult Scler. 2010;16:197–207. doi: 10.1177/1352458509357065. [DOI] [PubMed] [Google Scholar]
- Dantas AP, Igarashi J, Michel T. Sphingosine 1-phosphate and control of vascular tone. Am J Physiol Heart Circ Physiol. 2003;284:H2045–H2052. doi: 10.1152/ajpheart.01089.2002. [DOI] [PubMed] [Google Scholar]
- Forrest M, Sun S-Y, Hajdu R, Bergstrom J, Card D, Doherty G, et al. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J Pharmacol Exp Ther. 2004;309:758–768. doi: 10.1124/jpet.103.062828. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Kappos L, Radue E-W, O'Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- Kovarik JM, Hartmann S, Bartlett M, Riviere GJ, Neddermann D, Wang Y, et al. Oral-intravenous crossover study of fingolimod pharmacokinetics, lymphocyte responses and cardiac effects. Biopharm Drug Dispos. 2007a;28:97–104. doi: 10.1002/bdd.535. [DOI] [PubMed] [Google Scholar]
- Kovarik JM, Slade A, Voss B, Schmidli H, Riviere GJ, Picard F, et al. Ethnic sensitivity study of fingolimod in White and Asian subjects. Int J Clin Pharmacol Ther. 2007b;45:98–109. doi: 10.5414/cpp45098. [DOI] [PubMed] [Google Scholar]
- Mulders AC, Mathy M-J, Meyer zu Heringdorf D, Braak ter M, Hajji N, Olthof DC, et al. Activation of sphingosine kinase by muscarinic receptors enhances NO-mediated and attenuates EDHF-mediated vasorelaxation. Basic Res Cardiol. 2009;104:50–59. doi: 10.1007/s00395-008-0744-x. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Nixon GF, Mathieson FA, Hunter I. The potential roles of sphingolipids in vascular smooth-muscle function. Biochem Soc Trans. 2007;35:908–909. doi: 10.1042/BST0350908. [DOI] [PubMed] [Google Scholar]
- Payne SG, Oskeritzian CA, Griffiths R, Subramanian P, Barbour SE, Chalfant CE, et al. The immunosuppressant drug FTY720 inhibits cytosolic phospholipase A2 independently of sphingosine-1-phosphate receptors. Blood. 2007;109:1077–1085. doi: 10.1182/blood-2006-03-011437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- Salomone S, Potts EM, Tyndall S, Ip PC, Chun J, Brinkmann V, et al. Analysis of sphingosine 1-phosphate receptors involved in constriction of isolated cerebral arteries with receptor null mice and pharmacological tools. Br J Pharmacol. 2008;153:140–147. doi: 10.1038/sj.bjp.0707581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomone S, Soydan G, Ip PC, Hopson KM, Waeber C. Vessel-specific role of sphingosine kinase 1 in the vasoconstriction of isolated basilar arteries. Pharmacol Res. 2010;62:465–474. doi: 10.1016/j.phrs.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmouder R, Serra D, Wang Y, Kovarik JM, DiMarco J, Hunt TL, et al. FTY720: placebo-controlled study of the effect on cardiac rate and rhythm in healthy subjects. J Clin Pharmacol. 2006;46:895–904. doi: 10.1177/0091270006289853. [DOI] [PubMed] [Google Scholar]
- Schuchardt M, Tölle M, Prüfer J, van der Giet M. Pharmacological relevance and potential of sphingosine 1-phosphate in the vascular system. Br J Pharmacol. 2011;163:1140–1162. doi: 10.1111/j.1476-5381.2011.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz A, Korporal M, Hosch W, Max R, Wildemann B. Critical vasospasm during fingolimod (FTY720) treatment in a patient with multiple sclerosis. Neurology. 2010;74:2022–2024. doi: 10.1212/WNL.0b013e3181e3972b. [DOI] [PubMed] [Google Scholar]
- Spijkers LJ, Alewijnse AE, Peters SL. Sphingolipids and the orchestration of endothelium-derived vasoactive factors: when endothelial function demands greasing. Mol Cells. 2010;29:105–111. doi: 10.1007/s10059-010-0042-y. [DOI] [PubMed] [Google Scholar]
- Spijkers LJ, van den Akker RF, Janssen BJ, Debets JJ, De Mey JG, Stroes ES, et al. Hypertension is associated with marked alterations in sphingolipid biology: a potential role for ceramide. PLoS One. 2011a;6:e21817. doi: 10.1371/journal.pone.0021817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijkers LJ, Janssen BJ, Nelissen J, Meens MJ, Wijesinghe D, Chalfant CE, et al. Antihypertensive treatment differentially affects vascular sphingolipid biology in spontaneously hypertensive rats. PLoS One. 2011b;6:e29222. doi: 10.1371/journal.pone.0029222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangada S, Khanna KM, Blaho VA, Oo ML, Im D-S, Guo C, et al. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med. 2010;207:1475–1483. doi: 10.1084/jem.20091343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tölle M, Levkau B, Keul P, Brinkmann V, Giebing G, Schönfelder G, et al. Immunomodulator FTY720 induces eNOS-dependent arterial vasodilatation via the lysophospholipid receptor S1P3. Circ Res. 2005;96:913–920. doi: 10.1161/01.RES.0000164321.91452.00. [DOI] [PubMed] [Google Scholar]
- Tonelli F, Lim KG, Loveridge C, Long J, Pitson SM, Tigyi G, et al. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell Signal. 2010;22:1536–1542. doi: 10.1016/j.cellsig.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzijl D, Peters SL, Alewijnse AE. Sphingosine-1-phosphate receptors: zooming in on ligand-induced intracellular trafficking and its functional implications. Mol Cells. 2010;29:99–104. doi: 10.1007/s10059-010-0041-z. [DOI] [PubMed] [Google Scholar]
- Vessey DA, Kelley M, Zhang J, Li L, Tao R, Karliner JS. Dimethylsphingosine and FTY720 inhibit the SK1 form but activate the SK2 form of sphingosine kinase from rat heart. J Biochem Mol Toxicol. 2007;21:273–279. doi: 10.1002/jbt.20193. [DOI] [PubMed] [Google Scholar]
- Zollinger M, Gschwind H-P, Jin Y, Sayer C, Zécri F, Hartmann S. Absorption and disposition of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in healthy volunteers: a case of xenobiotic biotransformation following endogenous metabolic pathways. Drug Metab Dispos. 2011;39:199–207. doi: 10.1124/dmd.110.035907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


