Abstract
Objectives
We studied the association and agreement between questionnaire data and biomarkers of marine food among Greenland Inuit.
Design
Cross sectional study.
Methods
The study population comprised 2,224 Inuit, age 18+ (43% men); data collected 2005–2008 in Greenland. Using a food frequency questionnaire (FFQ), we calculated consumption of seal, whale, and fish (g/day) and as meals/month, intake of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), total N3, and mercury. We measured erythrocyte membrane fatty acids (FA) and whole blood mercury (Hg). Associations were assessed by Pearson correlation and agreement between the 2 methods was assessed by Bland–Altman plots depicting mean difference between the methods. Using multiple linear regressions, the associations were studied between whole blood mercury, erythrocyte FA and frequency or gram per day of seal, whale, and fish.
Results
Partial correlations ranged from r=0.16, p<0.0001 (DHA) to r=0.56, p<0.0001 (mercury). The best fitted lines were found for mercury and DHA. Mean difference was negative for mercury but positive for all the FA biomarkers. In a multiple logistic regression analysis, the best association was found between whole blood mercury and seal consumption, both as frequency in meals and actual intake gram per day: β=1.07 µg (95% CI: 1.06; 1.08) and β=1.04 µg (95% CI: 1.03; 1.04), respectively.
Conclusion
Mercury showed the best correlation and agreement between calculated and measured values. Calculated actual intake in gram per day and frequency of meals showed similar associations with whole blood mercury and erythrocyte membrane FAs.
Keywords: food frequency questionnaire, biomarkers, fatty acids, mercury, agreement, Inuit
The lifestyle of Inuit in Greenland is undergoing a transition from a fisher-hunter society, with a physically active lifestyle and a diet based on the food available from the natural environment, to a westernized society, with imported foods high in saturated fats and refined sugars combined with a sedentary lifestyle. Parallel to this a rapid increase in the prevalence of lifestyle diseases such as type 2 diabetes and obesity has been observed (1). To follow future dietary changes in the population and to investigate how foods and nutrients affect disease outcomes, methods are needed to estimate and rank food intake.
Dietary assessment by questionnaire
A frequently used method to estimate food intake in epidemiological studies is the food frequency questionnaire (FFQ). FFQ is non-invasive for the respondent and can be conducted either as an interviewer-guided questionnaire or as a self-administered questionnaire. The purpose of the FFQ is to obtain retrospective information about average dietary intake during the preceding months. Many studies include estimation of the typical portion size for each food item to estimate consumed amounts, although a large within-person variation exists in portion size for most food items (2). This increased variation can decrease the validity of the questionnaire.
Fatty acids as biomarkers of dietary intake
As an alternative to questionnaire data, biomarkers can provide a surrogate measurement of past dietary intake. Biomarkers are especially useful when food composition tables are inaccurate or have many missing values. The traditional Arctic diet differs from most other regions in the world by a high intake of fish and marine mammals such as seal, whale, and walrus. Hence, the diet of Inuit is very high in marine fatty acids (FA), especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The FAs from the diet will distribute in the adipose tissue, in plasma and in the phospholipids of erythrocytes and these tissue concentrations of FA can be used as biomarkers of past fat intake. The erythrocyte membrane has a dynamic turnover of FAs from the diet (3). Long-chain FAs are particularly useful as biomarkers since cell membrane composition of FAs has been shown to be more sensitive to dietary N6 and N3 FAs than monounsaturated fat or saturated fat (4). A controlled cross-over intervention trial, studying the short-term effect of dietary FA composition on erythrocyte membrane FAs found that EPA and DHA were more reliable as biomarkers of dietary FA intake than was saturated fat (5). Intake of N3 and N6 polyunsaturated FAs (PUFA) assessed by an FFQ have been compared with PUFA measured in erythrocyte membranes, and erythrocyte membranes were found to provide good biomarkers of PUFA intake measured in American White women (6). Humans can synthesize EPA and DHA from alpha-linoleic acid (ALA), but it has been found that the endogenous synthesis is very limited (7). This makes EPA and DHA possible biomarkers for marine food intake. Fatty acids measured in erythrocyte membranes were more strongly correlated to actual intake measured by FFQ data compared with plasma FAs (8).
Whole blood mercury as biomarker
The traditional diet in Greenland also exposes the Inuit to lead, mercury and selenium, and all can be used as biomarkers of intake of traditional food. Due to pollution, mercury is found in high concentrations throughout the Arctic and intake of mercury has been shown to be very high in Greenland, exceeding the limits set by FAO/WHO (9). It accumulates in animal tissue in the form of methyl-mercury (MeHg). Human exposure to MeHg in Greenland is mainly from marine mammals because mercury accumulates in the top predators of the food chain. Seal, which is a top predator mammal, has high concentrations of mercury, particular in liver tissue (10). Elimination of mercury from the body occurs primarily from urine and faces with an absorbed dose half-life of 1–2 months (11). As a biomarker, MeHg can be used because almost all MeHg is absorbed in the intestine, and it has been shown that during a long-term exposure a good relation between intake and blood values was found (12). Fontaine et al. (13) studied the mercury concentration in blood among Inuit residing in Nunavik, Québec, and found the most important dietary source of whole blood mercury was marine mammal meat. Furthermore, a positive association between calculated consumption of marine mammals and FAs measured in erythrocyte membranes and whole blood mercury was found in previous studies of fish intake (14,15). Nevertheless, biomarkers are not the gold standard, and biomarker data are also associated with uncertainties. Blood samples are sensitive to storage and mode of handling, for instance. In this study long-chain N3 FA of the erythrocyte membrane will be used as indication of the proportion of FA consumed. The same holds for mercury as a biomarker: it reflects the proportion of marine meat intake (12,16). Thus, we aimed to study the association and agreement between questionnaire data and biomarkers of marine food and to assess whether estimation of portion size has an effect on the association to biomarkers.
Materials and methods
Recruitment
Data for this cross-sectional study were collected from 2005 to 2008. In total, 7 towns and 8 villages all over Greenland were included. The study was approved by the ethical review committee for Greenland. Participants were informed by letter prior to data collection and oral and written information was given at the beginning of data collection. All participants gave written informed consent.
Participants
Participants were selected as a stratified random sample of adults (18+) with residence in Greenland. Greenland was divided into 12 regions. From each region we chose a number of villages and towns to be included in the study. In towns a random sample of 11–22% were drawn from the central person register (CPR). In villages, all adult inhabitants were invited to participate. The final sample selected from the CPR consisted of 3,652 persons, both Inuit and Danes. From that sample 2,600 Inuit chose to participate in this study with valid data on questionnaire and biomarkers. The full description of data collection and study sample has been described in details in a previous publication (17). Ethnicity was determined at enrolment based on the primary language of the participant and self-identification.
Questionnaire
Questionnaires were translated from Danish into Greenlandic and translated back into Danish in order to validate the translation. Interviews were conducted in Greenlandic or Danish according to the wish of the participant. We recorded the sex and smoking status of all participants as: non-smokers, current smokers, or former smokers. In a self-administered questionnaire data on alcohol consumption were obtained. From these data we classified participants as daily drinkers, weekly alcohol consumers, monthly consumers, and abstainers.
Semi-quantitative food frequency questionnaire
Dietary data were collected by a food frequency questionnaire. The questionnaire included 25 traditional and 43 imported energy contributing food items. We obtained data about frequency of consumption, estimated portion sizes and seasonal variation. Portion sizes were estimated from 4 different serving sizes illustrated by photos. Consumed amounts were calculated for each food item as portions per day multiplied by portion size. For traditional foods we additionally recorded seasonal length and took that into consideration when calculating the yearly consumption. Missing information on portion size was substituted by gender-specific medians. Missing information on seasonal length was substituted by community specific medians. From published food tables we calculated intake of energy and macronutrients (18–20). Individuals who reported energy intake lower than 3,350 kJ/day (men) or 2,100 kJ/day (women) or higher than 17,000 kJ/day (men) and 15,000 kJ/day (women) were excluded from the analyses.
The intake of FAs from FFQ was calculated. For total N3 FA, we used the total N3 FAs value in the nutrient databases, which include the sum of all detectable N3 FAs. We also calculated EPA, DHA, and finally the mercury intake from various nutrient databases (10,20). Estimation was done for the total dietary intake. We estimated the consumption of traditional food (g/day) and the total energy intake (kJ/day). We calculated the consumption of seal, whale and fish (g/day). Traditional food included marine mammals, fish, game birds and terrestrial animals and polar bear. Seal included meat and blubber from seal, inner organs, and dried or salted meat products. Whale meat included beluga, narwhal, and other whales, dried whale meat, whale blubber, muktuk, and walrus. Fish included salmon or trout, cod, Greenland halibut, capelin, mussels, shrimp, crab, and a category called “other fish”.
Markers of dietary marine food intake
Blood samples with valid measurement of erythrocyte membrane FAs and whole blood mercury were obtained from 2,600 participants and the study sample was further reduced to 2,224 individuals when participants with unrealistic energy intake were excluded from analyses. The measurement of FAs in erythrocyte membranes was performed at the Lipid Research Centre, Centre Hospitalier Université Laval. A total of 40 FAs including N3 and N6 FAs and trans-FAs were measured. The composition of phospholipids of erythrocyte membranes was measured after total lipid extraction with chloroform/methanyl mixture, phospholipid separation by thin layer chromatography and methylation of FAs, followed by capillary GLC using a DB-23 column in a HP-Packard GC chromatograph. Whole blood mercury was analysed at Centre de Toxicologie, Institut National de Santé Public, Québec, Canada by inductively coupled mass spectrometry (ICP-MS). Detection limit: Mercury 0.5 mmol/L.
Data analyses and statistical methods
Means were reported with standard deviation. By Pearson correlation adjusted for age we assessed the linear association between biomarkers and FFQ. For the further analyses all dietary variables were log transformed before analyses and transformed back by 10x before reporting. We assessed the overall agreement between the FFQ and biomarkers by the Bland–Altman method of agreement (21). Both FFQ variables and biomarker variables were log transformed to achieve normal distribution. For each Bland–Altman plot the limits of agreement (LOA) were calculated as mean(difference)±1.96 * standard deviation(difference) with the assumption of normal distribution. To study the association between frequency of marine food intake versus biomarkers and estimated quantity versus biomarkers we made regression models with erythrocyte membrane EPA, DHA, total N3 FAs, and whole blood mercury as dependent outcomes. All 4 biomarkers were tested in relation to frequency of seal-, whale-, and fish consumption (meals/month) and in relation to estimated amount of seal, whale and fish consumed (g/day). The analyses were adjusted for age, total energy intake (kJ/day), smoking (non-smoker vs. former smoker or current smoker), and alcohol intake (abstainers vs. drinking). Bland–Altman plots were produced in STATA ver. 11. All other statistical analyses were performed in SPSS v. 19.0.
Results
With a participation rate of 68%, the total study sample consisted of 2,600 Inuit included in the final data set. In the final sample valid clinical information on biomarkers and FFQ data were available for 2,224 participants, 43% were men. The mean age for the final study population was 45 SD:15 years. This sample had realistic energy intake (kJ/day) calculated from the FFQ and were all of self-identified Inuit ethnicity. In Table I, baseline characteristics are shown for the total study population. The prevalence of smoking was high. Among the 86% active or former smokers 65% were still active smokers. The majority of the population consumed alcohol; however, 1% only drank on a daily basis whereas 20% drank on a weekly basis.
Table I.
Baseline characteristics of the Greenland study population (n=2,224). Means (SD) and proportions
| Unit | Total mean (SD) | |
|---|---|---|
| Men | % | 43 |
| Age | Years | 44.7 (14.7) |
| Former or current smokers | % | 86.3 |
| Drinking alcohol | % | 90.3 |
| Measured in blood | ||
| Erythrocyte membrane eicosapentanoeic acid | Mean, % of total FA | 2.4 (1.6) |
| Erythrocyte membrane docosahexaenoic acid | Mean, % of total FA | 4.3 (2.2) |
| Erythrocyte membrane total N3 FAs | Mean, % of total FA | 10.0 (4.2) |
| Whole blood mercury | Mean, µg/L | 21.1 (24.7) |
| Calculated intakes from FFQ | ||
| Energy | kJ/d | 8734.1 (3113.3) |
| Traditional food | g/d | 168.5 (144.2) |
| Seal | g/day | 46.9 (64.3) |
| Whale | g/day | 27.6 (50.4) |
| Fish | g/day | 61.2 (67.4) |
| Frequency of seal consumption | meals/month | 10.5 (11.7) |
| Frequency of whale consumption | meals/month | 4.2 (7.2) |
| Frequency of fish consumption | meals/month | 15.4 (13.7) |
| Eicosapentanoeic acid | % of total fat | 2.4 (2.4) |
| Docosahexaenoic acid | % of total fat | 1.8 (1.8) |
| Total N3 FAs | % of total fat | 7.6 (6.1) |
| Intake of mercury | µg/day | 38.9 (45.2) |
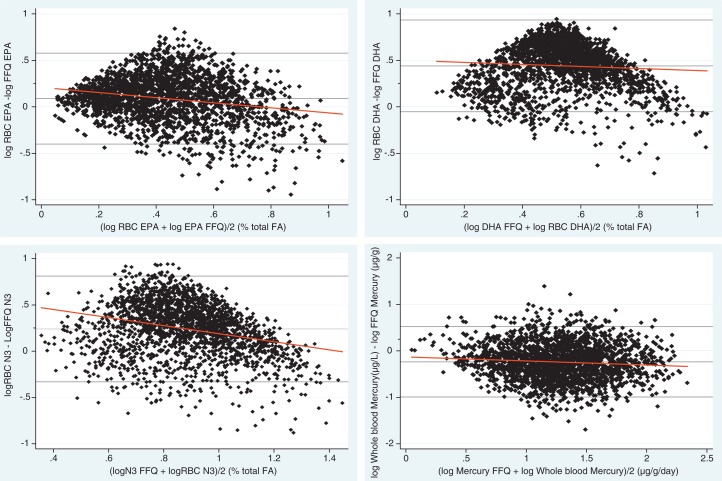
Unadjusted and adjusted Pearson correlations are presented in Table II between calculated and measured intake of whole blood mercury µg/L, erythrocyte membrane total N3 FAs, EPA, and DHA as percentages of total fat. The correlations only decreased slightly after age-adjustment. Partial correlation coefficients ranged from r = 0.16 (DHA) to 0.56 (Mercury). Table II also presents the back-transformed values of the mean difference of biomarkers and FFQ values and the back-transformed limits of agreement. The Bland–Altman plot indicated that the FFQ values for mercury were higher compared with the biomarkers. The opposite was found for total N3 FA, EPA and DHA, where the mean difference was above 0.
Table II.
Correlation coefficients of calculated mercury intake and whole blood mercury, and dietary FAs intake and erythrocyte membrane FAs. Values for Bland–Altman mean difference between biomarker and FFQ value with limits of agreement were transformed back by 10x. Adult Inuit in Greenland 2005–2008 (n=2,224)
| Nutrient/biomarker | Crude Pearson correlation | p-Value | Partial correlation* | p-Value | Geometric mean difference biomarker – calculated FFQ | Bland–Altman limits of agreement (LOA) |
|---|---|---|---|---|---|---|
| Total N3 FAs % of total fat | 0.22 | <0.0001 | 0.20 | 0.0001 | 0.74 | −0.53; 5.48 |
| EPA % of total fat | 0.40 | <0.0001 | 0.38 | 0.0001 | 0.23 | −0.60; 2.79 |
| DHA % of total fat | 0.18 | <0.0001 | 0.16 | 0.0001 | 1.76 | −0.12; 7.62 |
| Mercury in µg | 0.57 | <0.0001 | 0.56 | 0.0001 | −0.43 | −0.90; 2.29 |
Age adjusted correlation.
The Bland–Altman plots in Fig. 1 illustrate the agreement between log-transformed calculated intake and log-transformed measured biomarker. The best agreement was observed between calculated and measured mercury. The Bland–Altman plots showed a large variability between the calculated and measured values. The difference between the 2 methods were larger at higher intake of FAs.
Fig. 1.
Bland–Altman plots for EPA, DHA, total N3 fatty acids, and mercury with best fitted line. Differences in biomarkers and calculated nutrients plotted against the mean of the 2 methods for adult Inuit in Greenland (n=2,224).
Univariate analyses showed that alcohol consumption (abstainers vs. drinkers) and total energy intake (kJ/day) were associated with both whole blood mercury and FAs. Smoking (non-smokers vs. former smokers/current smokers) was only related to whole blood mercury. In Table III and Table IV linear regression models for the association with whole blood mercury, erythrocyte membrane total N3 FA, EPA, and DHA with seal, whale and fish consumption are shown. Analyses were adjusted for age, total energy intake, alcohol consumption and smoking status. Small but highly significant associations were found between estimated intake of seal, whale and fish and each of the biomarkers. Only fish intake was not associated with whole blood mercury (Table III). A similar pattern was found between frequency of seal, whale and fish meals and the 4 biomarkers, however, no association was found between fish meals and whole blood mercury or between whale meals and DHA.
Table III.
Linear multiple regression analyses for the association with whole blood mercury and erythrocyte membrane N3 FA, EPA, DHA estimated quantity of seal, whale, and fish among adult Inuit in Greenland 2005–2008 (n=2,224)
| Model* | β | 95% CI | p-Value |
|---|---|---|---|
| Variables entered in prediction-model for whole blood mercury (µg/L) | |||
| Seal (10 g/day) | 1.069 | (1.064; 1.076) | <0.0001 |
| Whale (10 g/day) | 1.028 | (1.021; 1.035) | <0.0001 |
| Fish (10 g/day) | 1.005 | (1.000; 1.009) | 0.09 |
| Variables entered in prediction-model for erythrocyte membrane total N3 FA (% of total fat) | |||
| Seal (10 g/day) | 1.016 | (1.012; 1.019) | <0.0001 |
| Whale (10 g/day) | 1.005 | (1.002;1.009) | 0.01 |
| Fish (10 g/day) | 1.007 | (1.005; 1.009) | <0.0001 |
| Variables entered in prediction-model for erythrocyte membrane EPA (% of total fat) | |||
| Seal (10 g/day) | 1.023 | (1.021; 1.026) | <0.0001 |
| Whale (10 g/day) | 1.007 | (1.002; 1.009) | <0.0001 |
| Fish (10 g/day) | 1.009 | (1.007; 1.012) | <0.0001 |
| Variables entered in prediction-model for erythrocyte membrane DHA (% of total fat) | |||
| Seal (10 g/day) | 1.012 | (1.007; 1.014) | <0.0001 |
| Whale (10 g/day) | 1.005 | (1.000; 1.009) | 0.01 |
| Fish (10 g/day) | 1.007 | (1.005; 1.012) | <0.0001 |
Models were adjusted for age, total energy intake (kJ/day), smoking, and alcohol consumption.
Table IV.
Linear multiple regression analyses for the association with whole blood mercury and erythrocyte membrane N3 FA, EPA, DHA frequency of seal, whale, and fish meals among adult Inuit in Greenland 2005–2008 (n=2,224)
| Model* | β | 95% CI | p-Value |
|---|---|---|---|
| Variables entered in prediction-model for whole blood mercury (µg/L) | |||
| Frequency seal 1 meal/month | 1.035 | (1.030; 1.038) | <0.0001 |
| Frequency whale 1 meal/month | 1.019 | (1.014; 1.023) | <0.0001 |
| Frequency fish 1 meal/month | 0.998 | (0.998; 1.002) | 0.3 |
| Variables entered in prediction-model for erythrocyte membrane total N3 FA (% of total fat) | |||
| Frequency seal 1 meal/month | 1.009 | (1.007; 1.009) | <0.0001 |
| Frequency whale 1 meal/month | 1.002 | (1.000; 1.005) | 0.04 |
| Frequency fish 1 meal/month | 1.002 | (1.000; 1.005) | <0.0001 |
| Variables entered in prediction-model for erythrocyte membrane EPA (% of total fat) | |||
| Frequency seal 1 meal/month | 1.012 | (1.011; 1.014) | <0.0001 |
| Frequency whale 1 meal/month | 1.002 | (1.000; 1.007) | 0.008 |
| Frequency fish 1 meal/month | 1.002 | (1.002; 1.005) | <0.0001 |
| Variables entered in prediction-model for erythrocyte membrane DHA (% of total fat) | |||
| Frequency seal 1 meal/month | 1.007 | (1.005; 1.009) | <0.0001 |
| Frequency whale 1 meal/month | 1.002 | (1.000; 1.005) | 0.1 |
| Frequency fish 1 meal/month | 1.002 | (1.000; 1.005) | 0.005 |
Models were adjusted for age, total energy intake (kJ/day), smoking, and alcohol consumption.
Discussion
This cross-sectional study compared intake of marine foods assessed by questionnaire and measured biomarkers for marine food. The study showed that simple correlation coefficients measuring the linear associations were, to some extent, in accordance with Bland–Altman plots measuring agreement. Mercury and EPA had the highest correlations between calculated values (FFQ) and measured biomarkers; concurrently, mercury alone showed the best fitted line between measured whole blood mercury and calculated mercury intake from FFQ.
This study also found that estimating portion sizes for the consumption of marine foods did not improve the association with biomarkers compared with association with frequency of consumption only. Additional estimation of a portion size for each food item can be costly, both in time and money, in dietary assessment trials. Hence, it is important to assess the benefit of the extra workload that estimation of portion sizes involves. Additional estimation of portion size in an FFQ can introduce extra variation in data due to inter-individual variation in the estimation of frequency and portion size. An earlier study tested the use of semi-quantitative FFQ versus FFQ (22). Pars (22) found a better association between frequency of consumption and whole blood mercury than estimated portion sizes and whole blood mercury. However, portion sizes improved the association to whole blood mercury among the younger participants (<36 years of age).
Seal was the single marine food with the best association with both whole blood mercury and FAs. Fontaine et al. found that marine mammal meat contributed to a 1.17 µg/L (values were reported log-transformed: β=0.07) increase in whole blood mercury for each gram per day extra consumption of marine mammal meat (13). This supports our results: our regression model of whole blood mercury showed that seal (10 g/day) increased whole blood mercury by 1.069 µg/L (p<0.0001). Fish consumption, both as quantity and frequency, had a less strong association to biomarkers; nevertheless, fish constituted a relatively large part of the traditional food intake. Our study population had a median intake of 40 g fish per day (range: 860 g) compared with 8 g seal/day (range: 300 g). A possible explanation for the lack of an association between fish intake and levels of biomarkers could be that marine mammals, such as seal, are top-predator animals and therefore the last step in the bioaccumulation of mercury. Greenlandic databases report that the average mercury content of marine fish in Greenland is 0.071 µg/g wet weigh compared with 0.355 µg/g wet weight in seal and a content of 0.583 µg/g wet weight in toothed whale meat (16,23). From these values, the explanation for the lack of association with whole blood mercury from fish intake could be due to lower average content of mercury in the various fish species versus seal or whale. Lucas et al. studied total N3 FAs in erythrocyte membrane as a biomarker for marine mammal and fish consumption among Inuit in Nunavik (24): per gram increase of marine mammal meat per day the erythrocyte membrane total N3 FA in erythrocyte membranes increased by 1.15% (measured as % of total FA content). In that study, fish also contributed to erythrocyte membrane total N3 FA by an 0.37% increase per gram fish increase/day (24).
The Bland–Altman plots for mercury and FAs showed generally poor agreement. There are several explanations for this finding. Bland–Altman plots were originally meant for measuring agreement between devices or tools measuring the same clinical outcome. In our analyses, we calculated intake of a nutrient or component and compared it with a measured counterpart. Intake and measured content in blood will never be the same, so our bias (mean difference) will never be 0. We chose to adapt the Bland–Altman method because we had continuous variables, and to see the initial agreement between the methods. This is the purpose of the Bland–Altman method: to visualize agreement in the initial phases of analyses. Regarding the biological explanations for our findings, the agreement between calculated and measured FAs could be influenced by metabolism of FAs. In a supplementation study, Brown et al. showed that the DHA content of erythrocyte membranes decreased more slowly back to baseline values than the EPA content did after ended supplementation (25). This shows that there are differences in the metabolism of N3 FAs. We found the weakest association between measured and calculated DHA (r2=0.16, p<0.0001). From this, it seems that EPA is a better biomarker for short-term intake (3–4 months) and DHA is a better biomarker for long-term intake (6 months or more). Our results are supported by a previous study that also found lower association between DHA compared with EPA when erythrocyte values and FFQ data were compared. However, interestingly, the study also found that plasma EPA compared with erythrocyte EPA improved the agreement with calculated FFQ intake (22). Another plausible reason for the lack of agreement between calculated and measured values is the general lack of values in the nutrient databases. Most of the nutrient values for traditional foods were based on few observations (typically<5), which increased the variation of the mean value. Furthermore, some food items were not found in the nutrient tables; accordingly, we had to find alternative food items (e.g. the value for seal as the ringed seal, which we decided was the most widespread kind, despite knowing other species were available for consumption). The FFQ also has methodological bias. The most obvious bias is the recall bias (respondent memory lapses); however, incorrect estimation of portion sizes and incorrect estimation of seasonal length and frequency of consumption also play a role in the quality of FFQ data. The questionnaire is a pre-printed list of foods, and there is always a risk that foods were eaten that was not included in the listed food items. Another source of bias can be in the generation of FFQ data where missing values of season length and portion size were replaced with median values of these variables. Overall, this may lead to a systematic underestimation of certain nutrients (26). Finally, within-food variation may occur due to storage, processing and differences in food preparation. A strength of our study was the interviewers who were bilingual and performed the interview according to the participant's preference in either Danish or Greenlandic.
In conclusion, mercury is the best biomarker of marine mammal intake among Inuit in Greenland but is not a suitable biomarker for fish intake in this population. Furthermore estimation of portion sizes did not improve association with biomarkers.
Acknowledgements
Karen Elise Jensen's Foundation provided financial support for data collection. Financial support for data analyses and the writing of the present paper was provided by the Danish Agency for Science, Technology and Innovation (Danish Council for Independent Research) and the Faculty of Health Sciences, University of Southern Denmark.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Hansen JC, Deutch B, Odland JØ. Dietary transition and contaminants in the Arctic: emphasis on Greenland. Int J Circumpolar Health. 2008;(Suppl 2):6–96. doi: 10.1080/22423982.2007.11864604. [DOI] [PubMed] [Google Scholar]
- 2.Buzzard M. Food-frequency methods. In: Willet W, editor. Nutritional epidemiology. New York: Oxford University Press; 1998. pp. 74–100. [Google Scholar]
- 3.Hunter D. Biochemical indicators of dietary intake. In: Willet W, editor. Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998. pp. 219–43. [Google Scholar]
- 4.Hulbert AJ, Turner N, Storlien LH, Else PL. Dietary fats and membranes function: implicatons for metabolism and disease. Biol Rev. 2005;80:155–69. doi: 10.1017/s1464793104006578. [DOI] [PubMed] [Google Scholar]
- 5.Poppitt SD, Kilmartin P, Butler P, Keogh GF. Assessment of erythrocyte phospholipid fatty acid composition as a biomarker for dietary MUFA, PUFA or saturated fatty acid intake in a controlled cross-over intervention trial. Lipids Health Dis. 2005;4:30. doi: 10.1186/1476-511X-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orton HD, Szabo NJ, Clare-Salzler M, Norris JM. Comparison between omega-3 and omega-6 polyunsaturated fatty acid inakes as assessed by a food frequency questionnaire and erythrocyte membrane fatty acid composition in young children. Eur J Clin Nutr. 2008;62:733–8. doi: 10.1038/sj.ejcn.1602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plourde M, Cunnane S. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl Physiol Nutr Metab. 2007;32:619–34. doi: 10.1139/H07-034. [DOI] [PubMed] [Google Scholar]
- 8.Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr. 2007;86:74–81. doi: 10.1093/ajcn/86.1.74. [DOI] [PubMed] [Google Scholar]
- 9.Johansen P, Pars T, Bjerregaard P. Lead, cadmium, mercury and selenium intake by Greenlanders from local marine food. Sci Total Environ. 2000;245:187–94. doi: 10.1016/s0048-9697(99)00443-x. [DOI] [PubMed] [Google Scholar]
- 10.Johansen P, Muir D, Asmund G, Riget F. Contaminants in the traditional Greenland diet. 2004. National Environmental Research Institute, Denmark. 74 pp -NERI Technical Report No. 492. http://technical-reports.dmu.dk. [DOI] [PubMed]
- 11.Clarkson TW. Mercury. J Am Coll Toxicol. 1989;8:1291–6. [Google Scholar]
- 12.Hansen JC. Aarhus: Aarhus University; 1988. Exposure to heavy metals in Greenlanders (Hg, Se, Cd & Pb): a review of an Arctic environmental study; pp. 1–78. [Google Scholar]
- 13.Fontaine J, Dewailly E, Benedetti JL, Pereg D, Ayotte P, Déry S. Re-evaluation of blood mercury, lead, and cadmium concentrations in the Inuit population of Nunavik (Québec): a cross-sectional study. Environ Health. 2008;7 doi: 10.1186/1476-069X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amiano P, Dorronsoro M, de Renobales M, Ruiz de Grodoa JC, Irigoien I. EPIC Group of Spain. Very-long-chain omega-3 fatty acids as markers for habitual fish intake in a population consuming mainly lean fish: the EPIC cohort of Gipuzkoa. Eur J Clin Nutr. 2001;55:827–32. doi: 10.1038/sj.ejcn.1601242. [DOI] [PubMed] [Google Scholar]
- 15.Brantsæter AL, Haugen M, Thomassen Y, Ellingsen DG, Ydersbond TA, Hagve TA. Exploration of biomarkers for total fish intake in pregnant Norwegian women. Public Health Nutr. 2009;13:54–62. doi: 10.1017/S1368980009005904. [DOI] [PubMed] [Google Scholar]
- 16.Dietz R, Riget F, Johansen P. Lead, cadmium, mercury and selenium in Greenland marine animals. Sci Total Environ. 1996;186:67–93. doi: 10.1016/0048-9697(96)05086-3. [DOI] [PubMed] [Google Scholar]
- 17.Bjerregaard P. Inuit Health in Transition – Greenland survey 2005–2008. Population sample and survey methods. (2nd revised edition) 2010:26 s. [Google Scholar]
- 18.National Food Institute. Danish food composition databank ed. 7.01 [cited 2008 February 1] Available from: http//www.foodcomp.dk.
- 19.CINE. Traditional Food Composition Nutribase; 2005 [cited 2008 February 1] Available from: http://www.mcgill.ca/cine/resources/nutrient/
- 20.Health Canada. Canada nutrient file. Canada Nutrient File v 2007b; 2007 [cited 2008 February 1] Available from: http://www.hc-sc.gc.ca/fn-an/nutrition/fiche-nutri-data/index_e.html.
- 21.Bland MJ, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;i:307–10. [PubMed] [Google Scholar]
- 22.Pars T. Det Sundhedsvidenskabelige Fakultet: Københavns Universitet; 2000. Forbruget ad traditionelle grønlandske fødevarer i Vestgrønland (Contemporary use of traditional food in West Greenland). [dissertation] [in Danish] [Google Scholar]
- 23.Dietz R, Johansen P, Riget F, Asmund G. In: AMAP Greenland 1994–1996. Danish Environmental Protection Agency, Environmental Project No. 356. Data on heavy metals in the Greenland marine environment before 1994; pp. 247–350. [Google Scholar]
- 24.Lucas M, Poust F, Blanchet C, Ferland A, Déry S, Abdous B. Is marine mammal fat or fish intake most strongly associated with omega-3 blood levels among the Nunavik Inuit? Prostaglandins Leukot Essent Fatty Acids. 2010;83:143–50. doi: 10.1016/j.plefa.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Brown AJ, Pang E, Roberts DCK. Persistent changes in the fatty acid composition of erythrocyte membranes after moderate intake of n-3 polyunsaturated fatty acids: study design implications. Am J Clin Nutr. 1991;54:668–73. doi: 10.1093/ajcn/54.4.668. [DOI] [PubMed] [Google Scholar]
- 26.Gibson RS. Measurement errors in dietary assessment. In: Gibson RS, editor. Principles of nutritional assessment. New York: Oxford University Press; 1990. pp. 85–96. [Google Scholar]