Abstract
BACKGROUND AND PURPOSE
IL-6 plays crucial roles in cardiac hypertrophy, cardiac fibrosis and heart failure. Activation of β-adrenoceptors induced IL-6 production in neonatal mouse cardiac fibroblasts (NMCFs) through a Gs/adenylate cyclase/cAMP/p38 MAPK pathway but independent of PKA. However, how cAMP activates p38 MAPK is still not defined. In this study, we have assessed the role of the exchange protein directly activated by cAMP (Epac) and PKCδ in p38 MAPK activation and IL-6 production by stimulated by the β-adrenoceptor agonist isoprenaline in NMCFs.
EXPERIMENTAL APPROACH
The IL-6 concentration in cell culture supernatants was measured by ELISA. The levels of phosphorylated and total p38 MAPK and PKCδ were determined by Western blot analysis. The translocation of PKCδ was determined by immunoblotting the soluble and particulate fractions. Expression of Epac1 or PKCδ was knocked down by the corresponding, adenovirus-mediated, small hairpin RNA (shRNA).
RESULTS
In NMCFs, activation of β-adrenoceptors enhanced PKCδ phosphorylation and translocation. Furthermore, knock-down of the PKCδ isoform using an adenovirus-mediated shRNA markedly down-regulated IL-6 induction by NMCFs stimulated with isoprenaline. Moreover, knock-down of Epac1 confirmed that Epac1 was upstream of PKCδ in IL-6 production. Additionally, both Epac1 and PKCδ mediated the p38 MAPK activation induced by isoprenaline.
CONCLUSIONS AND IMPLICATIONS
β-Adrenoceptor agonists activate a cAMP/Epac/PKCδ/p38 MAPK pathway to produce IL-6 in NMCFs. This study identifies Epac as the link between cAMP and p38 MAPK signalling pathways and demonstrates that PKCδ can function as a novel downstream effector of this β-adrenoceptor/cAMP/Epac pathway.
Keywords: β-adrenoceptors, interleukin-6, cardiac fibroblasts, Epac, PKCδ
Introduction
Inflammation is a key component in the cardiac remodelling process in response to cardiovascular diseases such as hypertension, myocardial infarction and heart failure. IL-6 is a multifunctional cytokine and has a broad range of activities modulating not only innate immune responses but also cell growth and differentiation. There is clinical evidence for a role of IL-6 in the pathophysiology of heart failure, in terms of events such as cardiac hypertrophy, cardiac fibrosis and apoptosis. It is correlated with the severity of heart failure and is a better predictor for the severity, prognosis and mortality of heart failure (Tsutamoto et al., 1998; Haugen et al., 2008). Administration of anti-IL-6 receptor antibody to inhibit IL-6 signalling suppressed left ventricular remodelling after myocardial infarction in mice(Kobara et al., 2010). Of note, the increase in plasma levels of IL-6 in heart failure patients was mainly associated with sympathetic activation, and treatment with β-adrenoceptor antagonists showed an independent and significant negative relation with plasma levels of IL-6 levels (Tsutamoto et al., 1998; Mabuchi et al., 2000; receptor nomenclature follows Alexander et al., 2011). Moreover, elevated plasma levels of IL-6 were reported to mediate adverse myocardial remodelling (Melendez et al., 2010) and chronic β-adrenoceptor stimulation increased myocardial IL-6 production in rat myocardium (Murray et al., 2000). Furthermore, our group has previously reported that cardiac fibroblasts, rather than cardiomyocytes, serve as the predominant source of IL-6 in response to β-adrenoceptor stimulation in mouse myocardium (Yin et al., 2003). We also demonstrated a non-canonical pathway – Gs/cAMP/p38 MAPK – mediated the IL-6 production induced by isoprenaline (Yin et al., 2006). However, the details of how cAMP activated p38 MAPK in IL-6 production induced by β-adrenoceptor agonists remained unknown.
Protein kinase C (PKC) is a family of enzymes with three classes: classical, novel and atypical. Classical (α, β and γ) and novel PKCs (δ, ε, θ, η) rely on phospholipids for activation, whereas atypical PKCs (ζ, ι and λ) do not require these lipids. Mouse cardiac fibroblasts express various PKC isoforms, including α, β, ε, η, δ and ζ (Braun et al., 2003). A recent study suggests the participation of PKCδ in transactivation of ERK1/2 induced by angiotensin II in cardiac fibroblasts (Olson et al., 2008). Several PKC isoforms have been shown to be required for regulation of IL-6 expression (Smyth et al., 2006; Hao et al., 2010), but which specific PKC isoforms are involved in β-adrenoceptor-induced IL-6 secretion is still to be determined.
Classically, increased cAMP has been linked with activation of protein kinase A (PKA) (Taylor et al., 1990). However, recent studies have disclosed PKA-independent effects of cAMP, which suggests a possible involvement of other cAMP effectors, such as the exchange protein directly activated by cAMP (Epac), acting as cAMP-activated guanine nucleotide exchange factors for Rap and Ras (Bos, 2003; Gloerich and Bos, 2010). The two variants of Epac, Epac1 and Epac2, are activated by physiologically relevant concentrations of cAMP. Epac1 is highly expressed in the heart and has affinity for cAMP comparable to that of the PKA holoenzyme (Dao et al., 2006; Metrich et al., 2008). There is increasing evidence that Epac regulates the assembly of gap junctions (Duquesnes et al., 2010), Ca2+ movement and the contractile machinery (Pereira et al., 2007; Cazorla et al., 2009), and plays critical roles in cardiac remodelling (Morel et al., 2005; Metrich et al., 2008). However, the role of Epac in the regulation of cytokines in the heart during pathological remodelling is still unclear.
We have explored the mechanisms leading to β-adrenoceptor-induced p38 MAPK activation and IL-6 production in neonatal mouse cardiac fibroblasts (NMCFs). We found that β-adrenoceptor-induced activation of p38 MAPK required Epac-dependent activation of PKCδ. This cAMP/Epac/PKCδ/p38 MAPK pathway is a novel mediator of β-adrenoceptor-induced IL-6 production induced by β-adrenoceptor agonists.
Methods
Cell culture
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of Peking University Health Science Center (LA2010-037) and adhered to the American Physiological Society's ‘Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training’. One-day-old Balb/c mice were obtained from the Medical Experimental Animal Center of Peking University. NMCFs were prepared as previously described (Yin et al., 2006). In brief, central thoracotomy was performed after neonatal mice were deeply anaesthetized with 1.0% isoflurane (Baxter Healthcare Corp., Guayama, Puerto Rico, USA). The hearts were quickly excised and immediately embedded in freezing HBSS. Cardiomyocytes were dispersed by digestion with 0.25% trypsin (Gibco, Paisley, UK) and 0.05% collagenase II (Sigma, St. Louis, MO, USA) at 37°C. Then isolated cardiac fibroblasts were cultured with 10% fetal bovine serum (Hyclone) and antibiotics (50 mg·mL−1 streptomycin, 50 U·mL−1 penicillin) at 37°C in 5% CO2 atmosphere. Cells at the first passage were used in this experiment, and immunostaining and examination of morphology demonstrated that the cultured cells were pure cardiac fibroblasts (>97%). Cells were serum-starved for 24 h in serum-free medium before treatment. All the treatments including different kinase inhibitors, PKCδ translocation inhibitor and adenovirus transfection did not affect cell viability, confirmed by light microscopy (Leica DMIRE2, Germany) and assay of LDH in the cell culture supernatant (Supporting Information Figure S1).
ELISA for IL-6
The concentration of IL-6 in the culture supernatant was measured by a commercially available ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. All samples were assayed in triplicate.
PKC activity assay
The StressXpress PKC Kinase Activity Assay Kit (Assay Designs/Stressgen, Ann Arbor, MI, USA) was used to measure PKC serine–threonine activity according to the manufacturer's instructions. This kit utilizes a specific synthetic peptide as a substrate for PKC (all isoforms) and a polyclonal antibody that recognizes the phosphorylated form of the substrate. Samples were collected at different time points and analysed in duplicate. Kinase activity (optical density per µg protein) was quantified by the fold change in absorbance over the unstimulated levels.
Preparation of soluble and particulate fractions
Cultured cells were lysed in lysis buffer containing 50 mM Tris–HCl (pH 7.5), 250 mM sucrose, 50 mM NaF, 5 mM EDTA, 10 mM EGTA, 5 mM dithiothreitol, 50 µg·mL−1 PMSF and 0.1 mM leupeptin. The lysate was centrifuged at 100 000×g for 60 min, and the supernatant was used as soluble fraction. The pellet was resuspended in lysis buffer containing 0.2% Triton X-100 and incubated for 60 min at 4°C. The pellet was centrifuged as before, and the supernatant was used as the particulate fraction. Translocation ratio was calculated as the fold amount of PKC or PKCδ in the particulate fraction over the amount in non-treated cells.
Western blot analysis
NMCFs were grown to confluence in growth media and rendered quiescent by serum starvation for 24 h. After the cell samples were lysed in 60 µL lysis buffer, the protein concentration was estimated by BCA protein assay kit (Pierce, Rockford, IL, USA). Proteins (30 µg) were loaded onto 10% SDS polyacrylamide gel and electrophoretically transferred to nitrocellulose membranes (Pall, Port Washington, NY, USA). The sheets were analysed with antibodies according to the supplier's protocol, and immunolabelled bands were visualized by use of the SuperSignal West Pico chemiluminescence kit (Perbio, Cramlington, Northumberland, UK).
Constructs of mouse Epac1 or PKCδ short-hairpin RNA
The target sequences for mouse Epac1 (GenBank accession NM_144850) or mouse PKCδ (GenBank accession NM_011103) were 2059–2077 CTA CTC AGG AAG TTC ATC A or 702–720 CTC ACC GAT TCA AGG TTT A, respectively; Scrambled sequences was TTC TCC GAA CGT GTC ACG T (Pager and Dutch, 2005). Chemically synthesized oligonucleotides were annealed and then ligated into the BglII/HindIII sites of pAdTrack-HP (Zhao et al., 2003). The recombinant shuttle vector was then co-transformed into Escherichia coli BJ5183 cells with use of a pAdEasy-1 adenoviral backbone plasmid, both of which were kindly provided by Dr. B. Vogelstein (Johns Hopkins University, Baltimore, MD, USA) (He et al., 1998). Recombinant virus was produced in human embryonic kidney (HEK) 293A cells and purified by the ViraBind Adenovirus Purification Kit (Cell Biolabs, San Diego, CA, USA). Viral titer was determined by testing multiplicity of infection in HEK 293A cells and was used to infect cells at 50 plaque-forming units per cell. Under these conditions, approximately 98% of cells were infected. The knock-down efficiency of PKCδ or Epac1 after 48 h of transfection was verified as shown in Figures 2E and 3B, respectively. Thus, all the experiments were done at 48 h of transfection.
Peptide treatments
After serum starvation for 24 h, cells were first pretreated with or without different. isozyme-selective. PKC peptide inhibitors (myristoylated δV1-1 or control) (Feng et al., 2010), then stimulated with isoprenaline. Cell viability and morphology was not affected by the myristoylated peptides. The inhibition of translocation was confirmed (Supporting Information Figure S2).
Measurement of extracellular LDH concentration
NMCFs were plated in 96-well plates (5000 cells/well), incubated in DMEM supplemented with or without FBS for 24 h. Then cardiac fibroblasts were incubated in DMEM containing various kinase inhibitors for 12 h before harvesting of the supernatant (S). Negative control cells (N) were incubated in DMEM alone. Positive control cells (P) were incubated in DMEM supplemented with Triton X-100 instead of inhibitors. The amount of LDH present in the supernatant harvested from the culture was deemed to be total LDH released from cells. LDH was measured by ELISA (LDH Assay Kit, Bio Vision, Mountain View, CA, USA) with the use of a microplate reader (wavelength: 450 nm). Cytotoxicity rate, reflecting the amount of LDH released into the supernatants of incubated cultures, was calculated by the following formula: Cytotoxicity rate (%) = 100X(S – N/(P – N) according to the manufacturer's instructions.
Rap1 activation assay
Rap1 activation was determined using commercially available kit (Upstate, Waltham, MA, USA) according to the manufacturer's protocol. In brief, treated cells from two 70% confluent 100-mm-diameter plates were lysed in 800 µL of ice-cold Rap1 lysis buffer [50 mM Tris–HCl (pH 8.0), 500 mM NaCl, 1% NP-40, 2.5 mM MgCl2, 10% glycerol, 1 mM PMSF, 1 µM leupeptin, 0.5 mM aprotinin]. Lysates were clarified by low-speed centrifugation, and supernatants containing 2 mg of total protein were incubated with 30 µL of Ral-GDS-PBD agarose beads for 1 h at 4°C. Beads were pelleted and rinsed three times with lysis buffer, and protein was eluted from the beads with Laemmli buffer. The amount of Rap1 bound to beads was detected by Western blotting.
Data analysis
Data are expressed as mean ± SEM. Differences between groups was determined by one-way anova or unpaired two-tailed t-test. All statistical analyses involved use of GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA). P < 0.05 was considered statistically significant.
Materials
Isoprenaline, 8-pCPT-2′-O-Me-cAMP (8-pCPT) and H-89 were from Sigma-Aldrich). PKA inhibitor 14–22 amide (PKI) and phorbol 12-myristate 13-acetate were from Calbiochem (San Diego, CA, USA). Antibodies against phosphorylated p38 MAPK, PKC, PKCα/β and PKCδ were from Cell Signaling (Danvers, MA, USA), and antibodies against Epac1, p38 MAPK, GAPDH, Caveolin-1, PKC and PKCδ were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). δV1-1 ([Myr]-SFNSYELGSL; amino acid 8–17 in PKCδ) was synthesized at the peptide facility at Shanghai Sangon Company. Myristoylated peptides were produced by incorporating a myristoylated lysine during solid-phase synthesis. Peptides were >98% pure. Peptides were dissolved in deionized water and stored in aliquots at −70°C.
Results
Isoprenaline induced PKC activation
In order to study the signalling pathway mediating IL-6 production after activation of β-adrenoceptors, we assessed the role of PKC because PKC can be activated by various extracellular stimuli including G protein-coupled receptors (Markou et al., 2006; Olson et al., 2008). Activation of PKC stimulated its translocation to cellular membranes and increased its phosphorylation status and serine–threonine kinase activity. In this study, we observed that brief incubation (for 5 min) with isoprenaline stimulated serine phosphorylation in PKC and kinase activity (Figure 1A and B). Under basal conditions, PKC was almost exclusively localized in the soluble fraction (Figure 1C, lane 1 and 5). Upon the addition of 10 µM isoprenaline for 5 min, there was a significant translocation of PKC from the soluble to the particulate fraction (Figure 1C, lane 2 and 6). These results indicated that PKC might be involved in β-adrenoceptor-mediated IL-6 production in NMCFs.
Figure 1.
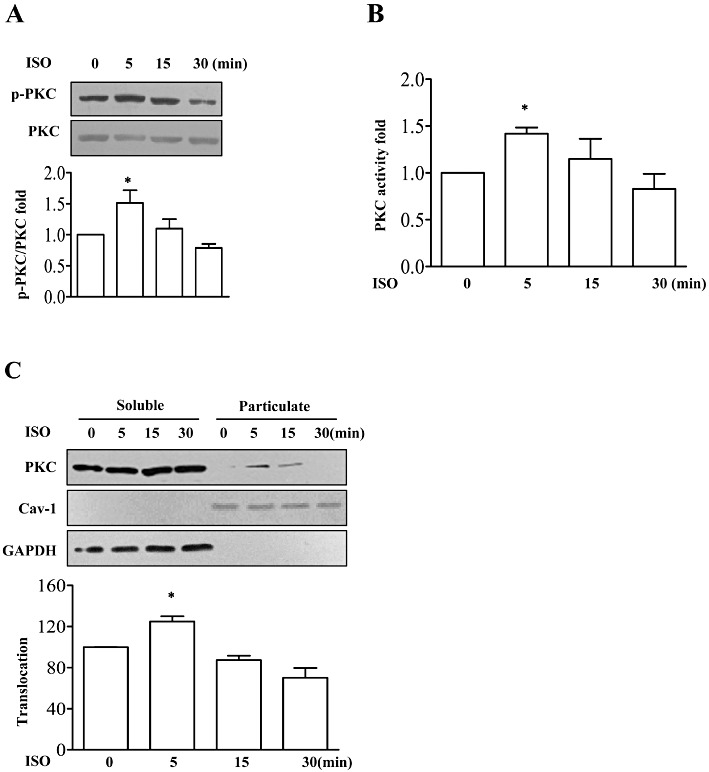
Stimulation of β-adrenoceptors increased protein kinase C (PKC) activation. (A) In the upper half, neonatal mouse cardiac fibroblasts (NMCFs) were treated with isoprenaline (ISO; 10 µM) for 5, 15 and 30 min, and cell lysates were immunoblotted with anti-phospho-PKC pan antibody or anti-PKC antibody as a reference control. In the lower half, summary data showing the fold increase of phospho-PKC/total PKC (p-PKC/PKC). *P < 0.05 vs. value at 0 min, n= 3. (B) The fold increase of PKC activity in these cell lysates. *P < 0.05 vs. value at 0 min, n= 3. (C) NMCFs were treated with isoprenaline (10 µM) for 5, 15 and 30 min, and cell lysates were separated into soluble or particulate fractions, then were immunoblotted with anti-PKC antibody, anti-caveolin-1 (Cav-1) or GAPDH antibody as reference control. In the lower half, mean ± SEM of data from three independent experiments.*P < 0.05 vs. value at 0 min, n= 3.
PKCδ mediated the isoprenaline-induced IL-6 secretion
To further identify the involvement of PKC isoforms in isoprenaline-induced IL-6 secretion, PKC isoform-specific inhibitors or knock-down with small hairpin RNAs (shRNA) were used. Mouse cardiac fibroblasts express several PKC isoforms including α, β, ε, η, δ and ζ, and PKCδ has been shown to be involved in IL-6 production (Smyth et al., 2006; Feng et al., 2010). Thus, we focused our study on the PKCδ isoform. Phosphorylation of Ser643 in the turn motif of PKC is important for the activation of PKCδ (Stempka et al., 1999). We found, by Western blot analysis, that phosphorylation at Ser643 in PKCδ was rapidly increased after 5 min treatment with isoprenaline and then phosphorylation was slowly diminished by 30 min (Figure 2A). Meanwhile, phosphorylation of the major isoform, PKC α/β, was not changed over this time. Isoprenaline (10 µM) also caused translocation of the PKCδ isoform, from soluble to particulate fraction at 5 min (Figure 2B). In addition, incubation with myristoylated δV1-1, an isoform-selective translocation inhibitor, dose-dependently inhibited isoprenaline-induced IL-6 release (Figure 2C). Furthermore, we infected cardiac fibroblasts with a recombinant adenovirus expressing shRNAs to specifically knock-down PKCδ (Figure 2D and E) and observed that isoprenaline-induced IL-6 release was markedly suppressed in the infected cells (Figure 2F). These data suggested that PKCδ mediated the IL-6 secretion induced by isoprenaline.
Figure 2.
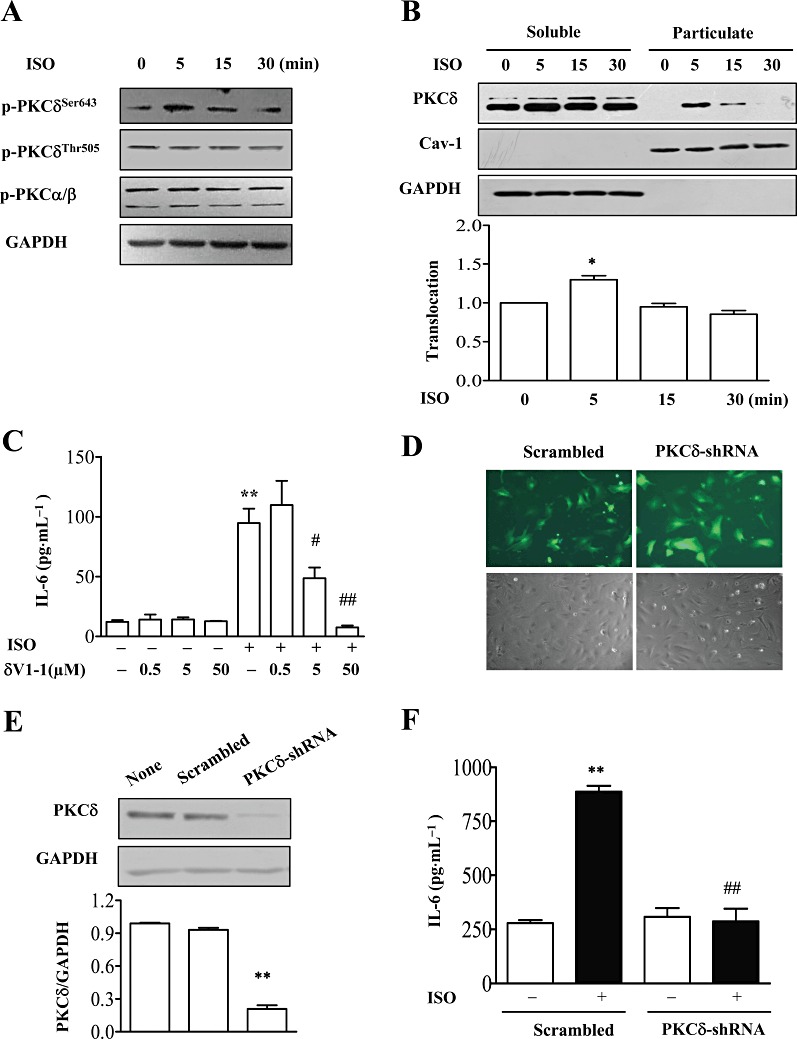
PKCδ mediated isoprenaline (ISO)-induced IL-6 production. (A) NMCFs were stimulated with isoprenaline for different times, and cell lysates were immunoblotted with antibodies against PKCδ phosphorylated at Ser643 or Thr505 (p-PKCδ), p-PKCα/βII phosphorylated at Thr638/641 and GAPDH. A representative image from three independent experiments is shown. (B) NMCFs were treated with isoprenaline (10 µM) for 5, 15 and 30 min, and cell lysates were separated into soluble or particulate fractions that were immunoblotted with anti-PKCδ antibody, anti-caveolin-1 and GAPDH. In the lower graph, mean ± SEM of data from three independent experiments. *P < 0.05 vs. value at 0 min, n= 3. (C) NMCFs were pretreated with the PKCδ translocation inhibitor (δV1-1) at various concentrations for 60 min and incubated with isoprenaline (10 µM) for 12 h. The concentration of IL-6 in cell culture supernatants was assayed by ELISA. **P < 0.01, significant effect of isoprenaline; #P < 0.05, ##P < 0.01, significant effect of δV1-1; n= 3. (D) The transfection efficiency of NMCFs infected with adenovirus expressing PKCδ-shRNAs or scrambled RNA for 48 h. Green, GFP fluorescence. (E) Effect of PKCδ-shRNAs on PKCδ protein levels. NMCFs were infected with adenovirus expressing PKCδ-shRNAs or scrambled RNA for 48 h. Cell lysates were immunoblotted with PKCδ or GAPDH antibody. Data are expressed as mean ± SEM of three independent experiments. **P < 0.01 vs. Scrambled. (F) NMCFs were infected with adenovirus expressing PKCδ-shRNAs or scrambled RNA, then stimulated with isoprenaline (10 µM) for 12 h. The concentration of IL-6 in cell culture supernatants was assayed by ELISA. **P < 0.01 vs. control, ##P < 0.01 PKCδ-shRNAs vs. scrambled. n= 3.
Epac1 was involved in IL-6 production by NMCFs, stimulated with isoprenaline
We had previously reported that isoprenaline-induced IL-6 production was cAMP-dependent but PKA-independent and recent studies have shown that Epac1 mediated PKA-independent effects of cAMP. To assess the role of Epac1 in isoprenaline-induced IL-6 production, we designed adenovirus to express shRNAs to knock-down Epac1 expression. We first confirmed the transfection and knock-down efficiency of Epac1 expression by Western blot (Figure 3A and B). To check that knock-down of Epac1 did affect its signalling pathway, the activity of Rap1, a direct downstream effector of Epac1, was measured after knock-down of Epac1. Activation of β-adrenoceptors activated Rap1 activity in cells transfected with adenovirus expressing scrambled shRNAs (Figure 3C) but isoprenaline failed to increase Rap1 activity in the cells transfected with adenovirus expressing Epac1-shRNAs (Figure 3C). These results confirmed that knock-down of Epac1 also blocked the activation of its direct target Rap1. In cardiac fibroblasts transfected with scrambled adenovirus, isoprenaline markedly induced IL-6 production but after knock-down of Epac1, isoprenaline failed to up-regulate IL-6 production (Figure 3D), implying that Epac1 was involved in the IL-6 production induced by isoprenaline.
Figure 3.
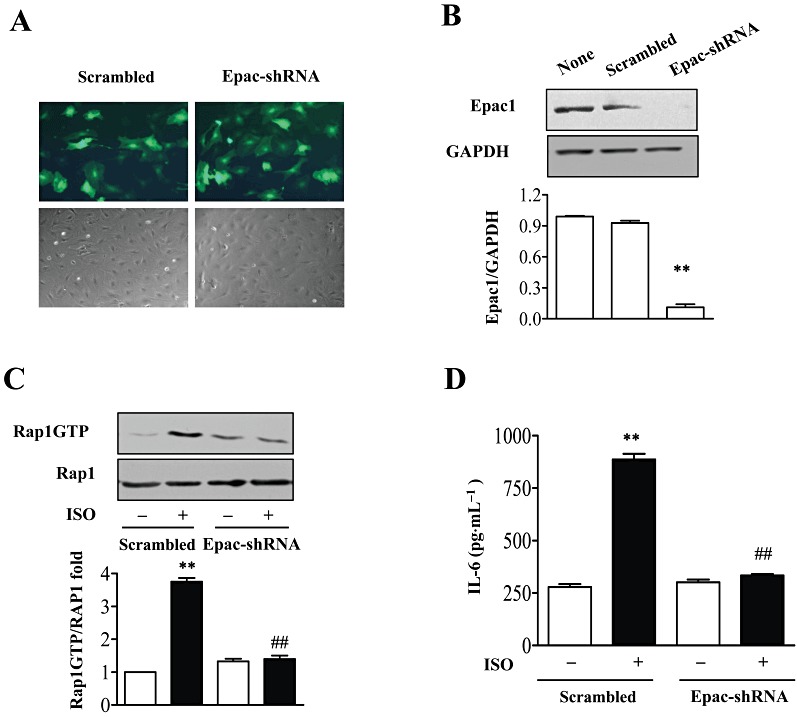
Inhibition of Epac repressed β-adrenoceptor-induced IL-6 secretion. (A) The infection efficiency of NMCFs with adenovirus expressing Epac-shRNAs or scrambled RNA for 48 h. Green, GFP inflorescence. (B) Effect of Epac-shRNAs on Epac protein levels. Cells were infected with adenovirus expressing Epac-shRNAs or scrambled RNA for 48 h. Cell lysates were immunoblotted with Epac antibody or GAPDH as loading control. Data are expressed as mean ± SEM of three independent experiments. **P < 0.01, PKCδ-shRNA vs. scrambled, n= 3. (C) Effect of Epac-shRNA on Rap1 activity after isoprenaline (ISO) by using GST-RalGDS-Ras binding domain as an activation-specific probe. Data are expressed as mean ± SEM of three independent experiments. **P < 0.01 vs. control, ##P < 0.01 PKCδ-shRNAs vs. scrambled, n= 3. (D) NMCFs were infected with adenovirus expressing Epac-shRNAs or scrambled RNAs for 48 h, then stimulated with isoprenaline (10 µM) for 12 h. IL-6 in the supernatant was determined by ELISA. **P < 0.01 vs. control, ##P < 0.01 PKCδ-shRNAs vs. scrambled, n= 3.
Epac pathway mediated isoprenaline-induced PKC activity
To find the link between cAMP and PKCδ, we hypothesized that Epac might mediate isoprenaline-induced PKCδ activation. Isoprenaline significantly induced PKCδ phosphorylation (at Ser643) in cells after transfection with scrambled adenovirus but after knock-down of Epac1, phosphorylation of PKCδ was not affected by isoprenaline incubation (Figure 4A). The translocation of PKCδ was also inhibited after knock-down of Epac1 (Figure 4B). Furthermore, assay of total PKC activity (Figure 4C) showed that Epac1 knock-down decreased isoprenaline-induced PKC activity, suggesting that the PKCδ isoform contributed most of the isoprenaline-dependent increase of PKC activity. These data suggested that Epac1 functioned upstream of PKCδ.
Figure 4.
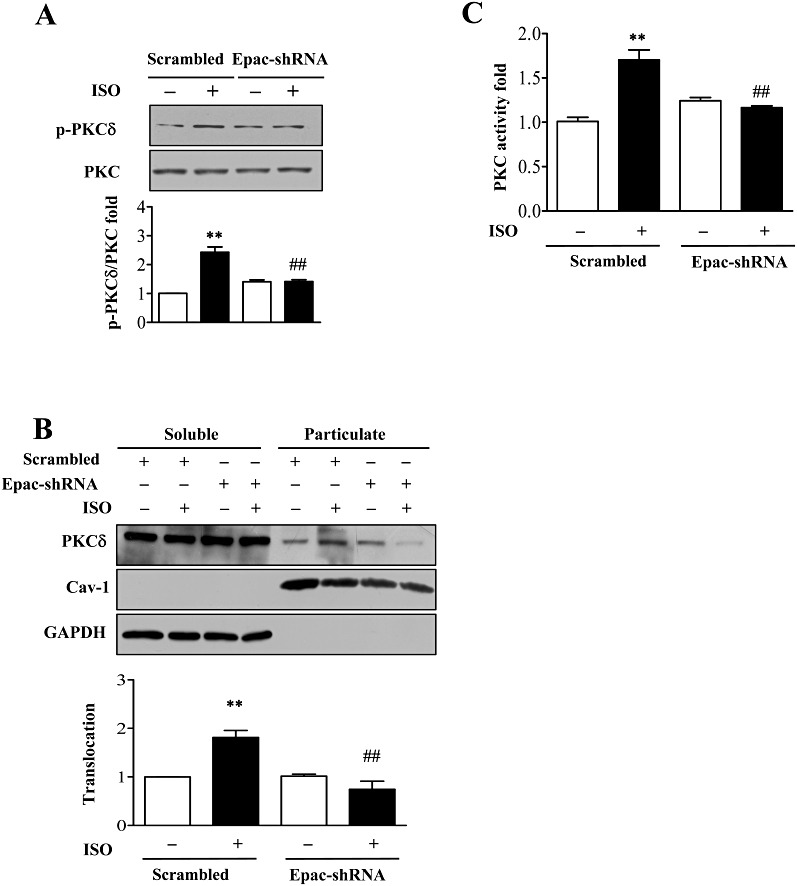
Inhibition of Epac depressed β-adrenoceptor-induced PKCδ activation. (A) After knock-down of Epac1 by adenovirus, cells were stimulated with isoprenaline (10 µM; ISO) for 5 min and PKCδ Ser643 phosphorylation was determined. **P < 0.01 vs. control, ##P < 0.01 PKCδ-shRNAs vs. scrambled, n= 3. (B) After knock-down of Epac1 by adenovirus, cells were stimulated with isoprenaline (10 µM) for 5 min.. Cell lysates were separated into soluble or particulate fractions and PKCδ translocation was determined by Western blot. **P < 0.01 vs. control, ##P < 0.01 PKCδ-shRNAs vs. scrambled, n= 3. (C) After knock-down of Epac1 by adenovirus, cells were stimulated with isoprenaline (10 µM) for 5 min. and PKC activity measured by kinase assay. **P < 0.01 vs. control, ##P < 0.01 PKCδ-shRNAs vs. scrambled, n= 3.
Epac/PKCδ pathway is involved in p38 MAPK activation in isoprenaline-stimulated NMCFs
Activation of p38 MAPK was known to be crucial for isoprenaline-induced IL-6 release (Yin et al., 2006) and we therefore sought a link between p38 MAPK and Epac activation (Ster et al., 2007; 2009). In cardiac fibroblasts transfected with the scrambled adenovirus, isoprenaline clearly activated p38 MAPK, by phosphorylation, whereas this effect was lost in cells with knock-down of Epac1 (Figure 5A).
Figure 5.
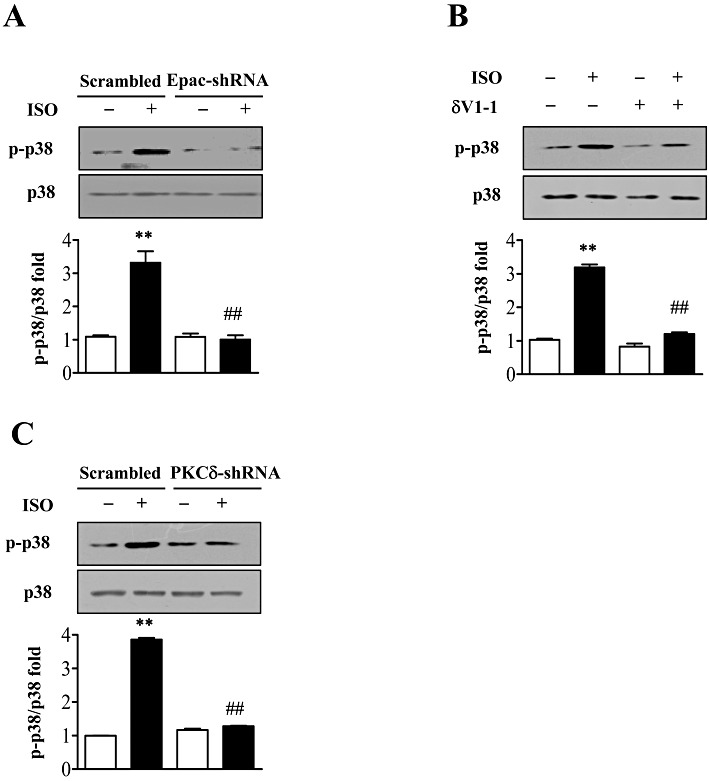
Inhibition of Epac/PKCδ pathway prevented β-adrenoceptor-induced p38 MAPK activation. (A) After knock-down of Epac1 by adenovirus, cells were stimulated with isoprenaline (10 µM; ISO) for 5 min and p38 MAPK phosphorylation was measured by immunoblot analysis. **P < 0.01 vs. control, ##P < 0.01 PKCδ-shRNAs vs. scrambled, n= 3. (B) NMCFs were pretreated with PKCδ translocation inhibitor (δV1-1, 5 µM), or control peptide for 30 min and incubated with isoprenaline (10 µM) for 5 min. p38 MAPK phosphorylation was measured by immunoblot analysis. **P < 0.01 vs. Con. NS, isoprenaline vs. Con in δV1-1 group, n= 3. (C) After knock-down of PKCδ by adenovirus, cells were stimulated with isoprenaline (10 µM) for 5 min and p38 MAPK phosphorylation was measured by immunoblot analysis. **P < 0.01 vs. control, ##P < 0.01 PKCδ-shRNAs vs. scrambled, n= 3.
PKCδ is upstream of p38 MAPK (Feng et al., 2010; Furundzija et al., 2010) and we therefore assessed the role of PKCδ in p38 MAPK activation in our cells. Cardiac fibroblasts pretreated with a PKCδ-specific translocation inhibitor or specific knock-down of PKCδ showed no increase in p38 MAPK phosphorylation following isoprenaline stimulation (Figure 5B and C), implying that PKCδ was involved in this iosprenaline-induced activation of p38 MAPK.
Discussion
IL-6 plays crucial roles in cardiac hypertrophy, cardiac fibrosis and heart failure. We have reported that β-adrenoceptor agonists induced IL-6 production in NMCFs through a Gs/AC/cAMP/p38 MAPK pathway that was independent of PKA (Yin et al., 2006). In the present study, we investigated the role of Epac, as a directly downstream messenger of cAMP, and PKCδ in the p38 MAPK activation and IL-6 production that followed activation of β-adrenoceptors in NMCFs. The PKCδ isoform rather than PKC α/β was activated by isoprenaline and specific inhibition or knock-down of the PKCδ isoform markedly depressed IL-6 production by the β-adrenoceptor/Gs/AC/cAMP pathway. This is the first report where PKCδ is involved in β-adrenoceptor-mediated p38 MAPK activation and IL-6 induction in cardiac fibroblasts. We also demonstrated that Epac activation was required for isoprenaline-induced PKCδ activation and increased output of IL-6, suggesting a novel role of Epac in cardiac inflammation and an involvement in cardiac remodelling. Taking all the data together, we have proposed a more detailed mechanism of β2-adrenoceptor-induced IL-6 production which is schematically represented in Figure 6.
Figure 6.
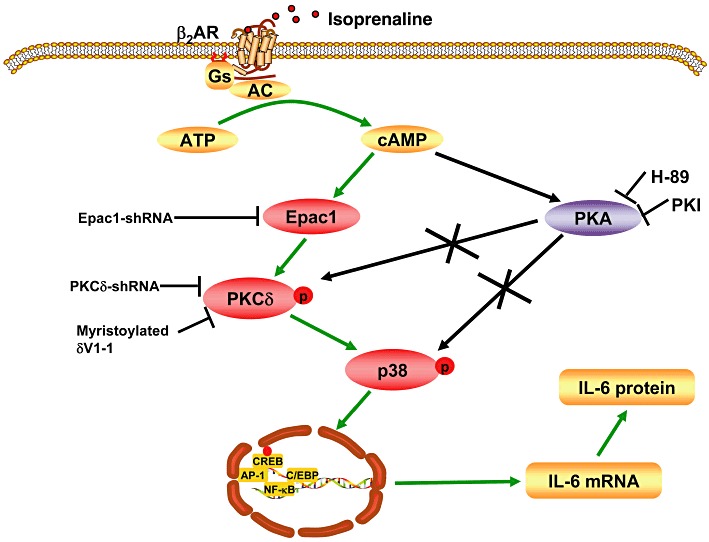
Schematic presentation of the signalling pathway involved in isoprenaline-induced up-regulation of IL-6 production in NMCFs. Binding of isoprenaline to β2-adrenoceptors (β2AR) activates adenylate cyclase (AC), then AC converts ATP to cAMP. Increased cAMP binds and activates Epac1, and Epac1 stimulates PKCδ phosphorylation and translocation through indirect mechanisms. PKCδ phosphorylates the key player, p38 MAPK, which activates transcription factors to stimulate IL-6 transcription and production. Kinase inhibitors and molecular biological methods of interfering with signalling molecules are also shown in the Figure.
PKC comprises a family of serine/threonine kinases that are involved in growth, differentiation, inflammatory responses and regulation of other cellular processes. PKC-dependent regulation of IL-6 release has been reported in several other cell types, including astrocytoma cells (Lieb et al., 2005), adipocytes (Ohashi et al., 2005), fibroblasts (Smyth et al., 2006), mast cells (Fehrenbach et al., 2009) and cardiac fibroblasts (Feng et al., 2010), although different PKC isoforms have been reported in various cell types upon diverse stimuli. Accumulating evidence indicates that β-adrenoceptor agonists can activate specific PKC isoforms in the heart. In rat isolated hearts, β-adrenoceptor stimulation changed the subcellular distribution of PKCδ (Yabe et al., 1998). In adult rat cardiomyocytes, isoprenaline caused the membrane translocation of PKCε rather than that of the α or δ isoforms (Shizukuda and Buttrick, 2001). Chronic β-adrenoceptor stimulation with isoprenaline for 2 days increased PKC activity and up-regulated the expression of PKCα, β and δ isoforms in the myocardium (Braun et al., 2003). Mouse cardiac fibroblasts express various PKC isoforms including α, β, ε, η, δ and ζ (Braun et al., 2003). Gö 6976, a potent inhibitor of Ca2+-dependent PKC isoforms, had no effect on isoprenaline-induced IL-6 release up to a concentration of 100 nM (Supporting Information Figure S3). By using PKCδ translocation inhibitors and shRNA for PKCδ, our study has been able to show that PKCδ was the primary isoform contributing to β-adrenoceptor-mediated IL-6 induction in NMCFs.
In this study, β-adrenoceptor activation significantly increased PKCδ serine–threonine kinase activity as well as its phosphorylation and translocation. There are several phosphorylation sites in PKCδ. Phosphorylation of the V3 region (Tyr311, Tyr332), the activation loop (Thr505), the turn motif (Ser643) and the hydrophobic motif (Ser662) are considered as essential steps in the activation of PKCδ (Steinberg, 2004). In contrast to the PKCδ-Thr505 phosphorylation induced by the α1-adrenoceptor agonist noradrenaline in cardiomyocytes (Rybin et al., 2003) or angiotensin II-induced PKCδ-Tyr311 phosphorylation in vascular smooth muscle cells (Nakashima et al., 2008), our study found that β-adrenoceptor stimulation significantly enhanced PKCδ-Ser643 phosphorylation in NMCFs but had no effect on PKCδ-Thr505 phosphorylation (Figure 2A). Ser643 is regarded as a major PKCδ autophosphorylation site (Li et al., 1997) but it is still not clear if this phosphorylation site is essential for the kinase activity of PKCδ (Li et al., 1997; Stempka et al., 1999). Thus, it was important to demonstrate that phosphorylation of Ser643 in PKCδ was sufficient for β-adrenoceptor-induced IL-6 release in our experimental system. Translocation of PKC to subcellular targets is a pivotal signalling step in PKC activation. Our data showed that PKCδ Ser643 phosphorylation and translocation both rapidly peaked after 5 min stimulation with isoprenaline but, as there is no selective inhibitor of PKCδ phosphorylation, we cannot exclude the possibility that phosphorylation of PKCδ is a prerequisite for its translocation. Other papers have reported that translocation of PKCδ is inhibited by rottlerin (Tapia et al., 2003; Uecker et al., 2003), but there is considerable controversy over the non-specific effects of rottlerin (Soltoff, 2007).
However, we did not know how cAMP activated PKCδ. In NMCFs, inhibition of PKA either by H-89 or PKI did not inhibit isoprenaline-induced PKCδ phosphorylation (Supporting Information Figure S4). Analogues of cAMP activate PKCζ and PKCε in PC-12 cells (Wooten et al., 1996; Graness et al., 1997)and, in macrophages, db-cAMP induced IL-6 production through PKA-dependent activation of PKC (Chio et al., 2004). Recently, evidence has emerged that Epac1, another cAMP effector, played a critical role in several cellular events mediated by cAMP in the heart (Metrich et al., 2010). We have shown that isoprenaline-induced IL-6 increase was cAMP-dependent but PKA-independent. 8-pCPT, which was used as a Epac-specific activator to discriminate between PKA- and Epac-mediated effects, had no effect on IL-6 release. This apparent ineffectiveness of 8-pCPT was not due to the lack of compound efficacy as it was able to significantly activate Rap1, a downstream effector of Epac (data not shown). These results were interpreted to show that Epac was not involved in isoprenaline-induced IL-6 production. However, here, we observed that PKCδ activation and IL-6 increase induced by isoprenaline were repressed by Epac knock-down. One possible explanation of the results is to postulate that 8-pCPT might also act as a substrate or inhibitor for various phosphodiesterases (Poppe et al., 2008; Enyeart and Enyeart, 2009). Here, we demonstrated that 8-pCPT inhibited p38 MAPK phosphorylation (Supporting Information Figure S5), a step which was crucial for β-adrenoceptor-induced IL-6 secretion. Overall, our study showed that activation of Epac was necessary to mediate the IL-6 release induced by isoprenaline.
The usual model of PKC activation focuses on allosteric activation by calcium and diacylglycerol and how Epac could induced PKCδ activation after β-adrenoceptor stimulation is still not determined. Epac has been implicated in several biochemical and biological processes in the cardiovascular system, such as vascular cell migration and cardiac hypertrophy (Metrich et al., 2010). Interestingly, the hypertrophic effects of Epac1 are independent of its classic effector, Rap1, but involve the small GTPase Ras, the phosphatase calcineurin and Ca2+/CaMK II (Metrich et al., 2008). In our study, Epac might regulate PKCδ through phospholipase Cε, which raises intracellular diacylglycerol concentrations (Schmidt et al., 2001). Epac might directly or indirectly phosphorylate and activate PKCδ because PKCδ was phosphorylated and activated by Src kinase or epidermal growth factor receptor, without increased calcium concentrations (Konishi et al., 2001; Morita et al., 2008). These possibilities need further assessment.
We have previously demonstrated that p38 MAPK played a crucial role in the isoprenaline-induced IL-6 release in cardiac fibroblasts via a cAMP-dependent but PKA-independent pathway (Yin et al., 2006). In this study, PKCδ acted as an upstream regulator of p38 MAPK in this process because of the activation time and the inhibition of p38 MAPK by PKCδ-specific kinase inhibitors, translocation peptide inhibitor and PKCδ knock-down. The cross-talk between PKC and p38 MAPK by agonists has been extensively studied in various cell systems. Our data are consistent with previous reports that the PKCδ/p38 MAPK pathway mediated IFN-γ-induced inflammatory cytokines in mast cells (Seo et al., 2009). However, p38 MAPK activation has been reported to be independent of PKC activation (Shigemoto-Mogami et al., 2001; Tsiani et al., 2002; Lee et al., 2005), which may be due to differences in the cell types and stimuli used in the experimental conditions.
In summary, our data clearly showed that Epac, as a directly downstream messenger of cAMP, mediated β-adrenoceptor-induced IL-6 secretion. These results also add a novel downstream effector, PKCδ, to the cAMP/Epac pathway. This study extends our understanding of β-adrenoceptor signalling in the heart and provides potential therapeutic targets for the treatment of heart diseases.
Acknowledgments
This work was supported by the Projects of International Cooperation and Exchanges NSFC (30910103902), the Natural Science Foundation of China (81030001, 30821001) and the National Key Basic Research Program of the People's Republic of China (2011CB503903).
Glossary
- NMCFs
neonatal mouse cardiac fibroblasts
- PKCδ
protein kinase Cδ
- PKI
PKA inhibitor 14–22 amide
- shRNA
small hairpin RNA
Conflicts of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Cytotoxic effects of various kinase inhibitors and adenovirus infection. (A) NMCFs were incubated with various kinase inhibitors used in the experiments. LDH in the supernatant was measured and cytotoxicity rate was calculated. n = 3. A representative image of each treatment from three independent experiments is shown in the below. (B) NMCFs were infected with adenovirus expressing Epac-shRNA, PKCδ-shRNA or scrambled RNA. LDH in the supernatant was measured and cytotoxicity rate was calculated. n = 3. A representative image of each treatment from three independent experiments was shown. All the images were collected at 100-fold magnification; all the treated cells showed no significant difference comparing with control group.
Figure S2 Isoprenaline (ISO)-induced PKCδ translocation is inhibited by PKCδ translocation inhibitor. (Upper) NMCFs were pre-incubated with PKCδ translocation inhibitor (δV1-1;5 μM) for 30 min, then stimulated with isoprenaline (10 μM) for 5 min, cell lysates were separated into soluble and particulate fractions, PKCδ translocation was quantified by Western blot. A representative image from three independent experiments was shown. (Lower) Mean ± SEM of data from three independent experiments. **P < 0.01 isoprenaline vs. Con. ##P < 0.01 ISO+δV1-1 vs. ISO. n = 3.
Figure S3 PKCα/β is not involved in ISO-induced IL-6 production. NMCFs were pre-incubated with Gö 6976 for 30 min, then stimulated with isoprenaline (10 μM) for 12 h, IL-6 in the supernatant was determined by ELISA. **P< 0.01 isoprenaline vs. Con. n = 3.
Figure S4 PKA is not involved in ISO-induced PKCδ phosphorylation. NMCFs were pre-incubated with H-89 (10μM) for 30 min (left) or PKI (100 nM) for 30 min (right), then stimulated with isoprenaline (10 μM) for 15 min, PKCδ phosphorylation was measured by Western blot. A representative image from three independent experiments was shown. **P <0.01 isoprenaline vs. Con. n = 3.
Figure S5 Activation of Epac by 8-pCPT inhibits p38 MAPK phosphorylation. NMCFs were pre-incubated with 8-pCPT (50μM) for 30 min, then stimulated with isoprenaline (10 μM), Adenosine-5′-N-ethyluronamide (NECA) (1 μM), angiotensin II (AngII) (1 μM) for 5 min. Cell lysates were immunoblotted with anti-phospho-p38 MAPK or anti-p38 MAPK antibody. A representative image from three independent experiments was shown. **P < 0.01 ISO, NECA or AngII vs. Con.#P < 0.05, 8-pCPT vs. Con in isoprenaline group. ##P < 0.01, 8-pCPT vs. Con in NECA or AngII group. n = 3.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- Braun M, Simonis G, Birkner K, Pauke B, Strasser RH. Regulation of protein kinase C isozyme and calcineurin expression in isoproterenol induced cardiac hypertrophy. J Cardiovasc Pharmacol. 2003;41:946–954. doi: 10.1097/00005344-200306000-00018. [DOI] [PubMed] [Google Scholar]
- Cazorla O, Lucas A, Poirier F, Lacampagne A, Lezoualc’h F. The cAMP binding protein Epac regulates cardiac myofilament function. Proc Natl Acad Sci USA. 2009;106:14144–14149. doi: 10.1073/pnas.0812536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio CC, Chang YH, Hsu YW, Chi KH, Lin WW. PKA-dependent activation of PKC, p38 MAPK and IKK in macrophage: implication in the induction of inducible nitric oxide synthase and interleukin-6 by dibutyryl cAMP. Cell Signal. 2004;16:565–575. doi: 10.1016/j.cellsig.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Dao KK, Teigen K, Kopperud R, Hodneland E, Schwede F, Christensen AE, et al. Epac1 and cAMP-dependent protein kinase holoenzyme have similar cAMP affinity, but their cAMP domains have distinct structural features and cyclic nucleotide recognition. J Biol Chem. 2006;281:21500–21511. doi: 10.1074/jbc.M603116200. [DOI] [PubMed] [Google Scholar]
- Duquesnes N, Derangeon M, Metrich M, Lucas A, Mateo P, Li L, et al. Epac stimulation induces rapid increases in connexin43 phosphorylation and function without preconditioning effect. Pflugers Arch. 2010;460:731–741. doi: 10.1007/s00424-010-0854-9. [DOI] [PubMed] [Google Scholar]
- Enyeart JA, Enyeart JJ. Metabolites of an Epac-selective cAMP analog induce cortisol synthesis by adrenocortical cells through a cAMP-independent pathway. PLoS ONE. 2009;4:e6088. doi: 10.1371/journal.pone.0006088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach K, Lessmann E, Zorn CN, Kuhny M, Grochowy G, Krystal G, et al. Steel factor enhances supraoptimal antigen-induced IL-6 production from mast cells via activation of protein kinase C-beta. J Immunol. 2009;182:7897–7905. doi: 10.4049/jimmunol.0801773. [DOI] [PubMed] [Google Scholar]
- Feng W, Song Y, Chen C, Lu ZZ, Zhang Y. Stimulation of adenosine A(2B) receptors induces interleukin-6 secretion in cardiac fibroblasts via the PKC-delta-P38 signalling pathway. Br J Pharmacol. 2010;159:1598–1607. doi: 10.1111/j.1476-5381.2009.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furundzija V, Fritzsche J, Kaufmann J, Meyborg H, Fleck E, Kappert K, et al. IGF-1 increases macrophage motility via PKC/p38-dependent alphavbeta3-integrin inside-out signaling. Biochem Biophys Res Commun. 2010;394:786–791. doi: 10.1016/j.bbrc.2010.03.072. [DOI] [PubMed] [Google Scholar]
- Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–375. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- Graness A, Adomeit A, Ludwig B, Muller WD, Kaufmann R, Liebmann C. Novel bradykinin signalling events in PC-12 cells: stimulation of the cAMP pathway leads to cAMP-mediated translocation of protein kinase Cepsilon. Biochem J. 1997;327:147–154. doi: 10.1042/bj3270147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao F, Tan M, Wu DD, Xu X, Cui MZ. LPA induces IL-6 secretion from aortic smooth muscle cells via an LPA1-regulated, PKC-dependent, and p38alpha-mediated pathway. Am J Physiol Heart Circ Physiol. 2010;298:H974–H983. doi: 10.1152/ajpheart.00895.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen E, Gan LM, Isic A, Skommevik T, Fu M. Increased interleukin-6 but not tumour necrosis factor-alpha predicts mortality in the population of elderly heart failure patients. Exp Clin Cardiol. 2008;13:19–24. [PMC free article] [PubMed] [Google Scholar]
- He TC, Zhou S, Da CL, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobara M, Noda K, Kitamura M, Okamoto A, Shiraishi T, Toba H, et al. Antibody against interleukin-6 receptor attenuates left ventricular remodelling after myocardial infarction in mice. Cardiovasc Res. 2010;87:424–430. doi: 10.1093/cvr/cvq078. [DOI] [PubMed] [Google Scholar]
- Konishi H, Yamauchi E, Taniguchi H, Yamamoto T, Matsuzaki H, Takemura Y, et al. Phosphorylation sites of protein kinase C delta in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc Natl Acad Sci USA. 2001;98:6587–6592. doi: 10.1073/pnas.111158798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Park SH, Jeung TO, Kim KW, Lee JH, Han HJ. Effect of adenosine triphosphate on phosphate uptake in renal proximal tubule cells: involvement of PKC and p38 MAPK. J Cell Physiol. 2005;205:68–76. doi: 10.1002/jcp.20367. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang J, Bottaro DP, Pierce JH. Identification of serine 643 of protein kinase C-delta as an important autophosphorylation site for its enzymatic activity. J Biol Chem. 1997;272:24550–24555. doi: 10.1074/jbc.272.39.24550. [DOI] [PubMed] [Google Scholar]
- Lieb K, Biersack L, Waschbisch A, Orlikowski S, Akundi RS, Candelario-Jalil E, et al. Serotonin via 5-HT7 receptors activates p38 mitogen-activated protein kinase and protein kinase C epsilon resulting in interleukin-6 synthesis in human U373 MG astrocytoma cells. J Neurochem. 2005;93:549–559. doi: 10.1111/j.1471-4159.2005.03079.x. [DOI] [PubMed] [Google Scholar]
- Mabuchi N, Tsutamoto T, Kinoshita M. Therapeutic use of dopamine and beta-blockers modulates plasma interleukin-6 levels in patients with congestive heart failure. J Cardiovasc Pharmacol. 2000;36(Suppl. 2):S87–S91. doi: 10.1097/00005344-200000006-00019. [DOI] [PubMed] [Google Scholar]
- Markou T, Yong CS, Sugden PH, Clerk A. Regulation of protein kinase C delta by phorbol ester, endothelin-1, and platelet-derived growth factor in cardiac myocytes. J Biol Chem. 2006;281:8321–8331. doi: 10.1074/jbc.M508398200. [DOI] [PubMed] [Google Scholar]
- Melendez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56:225–231. doi: 10.1161/HYPERTENSIONAHA.109.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metrich M, Lucas A, Gastineau M, Samuel JL, Heymes C, Morel E, et al. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res. 2008;102:959–965. doi: 10.1161/CIRCRESAHA.107.164947. [DOI] [PubMed] [Google Scholar]
- Metrich M, Berthouze M, Morel E, Crozatier B, Gomez AM, Lezoualc’h F. Role of the cAMP-binding protein Epac in cardiovascular physiology and pathophysiology. Pflugers Arch. 2010;459:535–546. doi: 10.1007/s00424-009-0747-y. [DOI] [PubMed] [Google Scholar]
- Morel E, Marcantoni A, Gastineau M, Birkedal R, Rochais F, Garnier A, et al. cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ Res. 2005;97:1296–1304. doi: 10.1161/01.RES.0000194325.31359.86. [DOI] [PubMed] [Google Scholar]
- Morita M, Matsuzaki H, Yamamoto T, Fukami Y, Kikkawa U. Epidermal growth factor receptor phosphorylates protein kinase C {delta} at Tyr332 to form a trimeric complex with p66Shc in the H2O2-stimulated cells. J Biochem. 2008;143:31–38. doi: 10.1093/jb/mvm190. [DOI] [PubMed] [Google Scholar]
- Murray DR, Prabhu SD, Chandrasekar B. Chronic beta-adrenergic stimulation induces myocardial proinflammatory cytokine expression. Circulation. 2000;101:2338–2341. doi: 10.1161/01.cir.101.20.2338. [DOI] [PubMed] [Google Scholar]
- Nakashima H, Frank GD, Shirai H, Hinoki A, Higuchi S, Ohtsu H, et al. Novel role of protein kinase C-delta Tyr 311 phosphorylation in vascular smooth muscle cell hypertrophy by angiotensin II. Hypertension. 2008;51:232–238. doi: 10.1161/HYPERTENSIONAHA.107.101253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Kanazawa A, Tsukada S, Maeda S. PKCepsilon induces interleukin-6 expression through the MAPK pathway in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;327:707–712. doi: 10.1016/j.bbrc.2004.12.072. [DOI] [PubMed] [Google Scholar]
- Olson ER, Shamhart PE, Naugle JE, Meszaros JG. Angiotensin II-induced extracellular signal-regulated kinase 1/2 activation is mediated by protein kinase C delta and intracellular calcium in adult rat cardiac fibroblasts. Hypertension. 2008;51:704–711. doi: 10.1161/HYPERTENSIONAHA.107.098459. [DOI] [PubMed] [Google Scholar]
- Pager CT, Dutch RE. Cathepsin L is involved in proteolytic processing of the Hendra virus fusion protein. J Virol. 2005;79:12714–12720. doi: 10.1128/JVI.79.20.12714-12720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Metrich M, Fernandez-Velasco M, Lucas A, Leroy J, Perrier R, et al. The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J Physiol. 2007;583:685–694. doi: 10.1113/jphysiol.2007.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, et al. Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods. 2008;5:277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- Rybin VO, Sabri A, Short J, Braz JC, Molkentin JD, Steinberg SF. Cross-regulation of novel protein kinase C (PKC) isoform function in cardiomyocytes. Role of PKC epsilon in activation loop phosphorylations and PKC delta in hydrophobic motif phosphorylations. J Biol Chem. 2003;278:14555–14564. doi: 10.1074/jbc.M212644200. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, et al. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol. 2001;3:1020–1024. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- Seo JY, Kim DY, Lee YS, Ro JY. Cytokine production through PKC/p38 signaling pathways, not through JAK/STAT1 pathway, in mast cells stimulated with IFNgamma. Cytokine. 2009;46:51–60. doi: 10.1016/j.cyto.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Koizumi S, Tsuda M, Ohsawa K, Kohsaka S, Inoue K. Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. J Neurochem. 2001;78:1339–1349. doi: 10.1046/j.1471-4159.2001.00514.x. [DOI] [PubMed] [Google Scholar]
- Shizukuda Y, Buttrick PM. Protein kinase C(epsilon) modulates apoptosis induced by beta-adrenergic stimulation in adult rat ventricular myocytes via extracellular signal-regulated kinase (ERK) activity. J Mol Cell Cardiol. 2001;33:1791–1803. doi: 10.1006/jmcc.2001.1442. [DOI] [PubMed] [Google Scholar]
- Smyth DC, Kerr C, Richards CD. Oncostatin M-induced IL-6 expression in murine fibroblasts requires the activation of protein kinase C delta. J Immunol. 2006;177:8740–8747. doi: 10.4049/jimmunol.177.12.8740. [DOI] [PubMed] [Google Scholar]
- Soltoff SP. Rottlerin: an inappropriate and ineffective inhibitor of PKC delta. Trends Pharmacol Sci. 2007;28:453–458. doi: 10.1016/j.tips.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Steinberg SF. Distinctive activation mechanisms and functions for protein kinase C delta. Biochem J. 2004;384:449–459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempka L, Schnolzer M, Radke S, Rincke G, Marks F, Gschwendt M. Requirements of protein kinase cdelta for catalytic function. Role of glutamic acid 500 and autophosphorylation on serine 643. J Biol Chem. 1999;274:8886–8892. doi: 10.1074/jbc.274.13.8886. [DOI] [PubMed] [Google Scholar]
- Ster J, De Bock F, Guerineau NC, Janossy A, Barrere-Lemaire S, Bos JL, et al. Exchange protein activated by cAMP (Epac) mediates cAMP activation of p38 MAPK and modulation of Ca2+-dependent K+ channels in cerebellar neurons. Proc Natl Acad Sci USA. 2007;104:2519–2524. doi: 10.1073/pnas.0611031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ster J, de Bock F, Bertaso F, Abitbol K, Daniel H, Bockaert J, et al. Epac mediates PACAP-dependent long-term depression in the hippocampus. J Physiol. 2009;587:101–113. doi: 10.1113/jphysiol.2008.157461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia JA, Garcia-Marin LJ, Jensen RT. Cholecystokinin-stimulated protein kinase C-delta kinase activation, tyrosine phosphorylation, and translocation are mediated by Src tyrosine kinases in pancreatic acinar cells. J Biol Chem. 2003;278:35220–35230. doi: 10.1074/jbc.M303119200. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Tsiani E, Lekas P, Fantus IG, Dlugosz J, Whiteside C. High glucose-enhanced activation of mesangial cell p38 MAPK by ET-1, ANG II, and platelet-derived growth factor. Am J Physiol Endocrinol Metab. 2002;282:E161–E169. doi: 10.1152/ajpendo.2002.282.1.E161. [DOI] [PubMed] [Google Scholar]
- Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:391–398. doi: 10.1016/s0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- Uecker M, Da SR, Grampp T, Pasch T, Schaub MC, Zaugg M. Translocation of protein kinase C isoforms to subcellular targets in ischemic and anesthetic preconditioning. Anesthesiology. 2003;99:138–147. doi: 10.1097/00000542-200307000-00023. [DOI] [PubMed] [Google Scholar]
- Wooten MW, Seibenhener ML, Matthews LH, Zhou G, Coleman ES. Modulation of zeta-protein kinase C by cyclic AMP in PC12 cells occurs through phosphorylation by protein kinase A. J Neurochem. 1996;67:1023–1031. doi: 10.1046/j.1471-4159.1996.67031023.x. [DOI] [PubMed] [Google Scholar]
- Yabe K, Ishishita H, Tanonaka K, Takeo S. Pharmacologic preconditioning induced by beta-adrenergic stimulation is mediated by activation of protein kinase C. J Cardiovasc Pharmacol. 1998;32:962–968. doi: 10.1097/00005344-199812000-00013. [DOI] [PubMed] [Google Scholar]
- Yin F, Li P, Zheng M, Chen L, Xu Q, Chen K, et al. Interleukin-6 family of cytokines mediates isoproterenol-induced delayed STAT3 activation in mouse heart. J Biol Chem. 2003;278:21070–21075. doi: 10.1074/jbc.M211028200. [DOI] [PubMed] [Google Scholar]
- Yin F, Wang YY, Du JH, Li C, Lu ZZ, Han C, et al. Noncanonical cAMP pathway and p38 MAPK mediate beta2-adrenergic receptor-induced IL-6 production in neonatal mouse cardiac fibroblasts. J Mol Cell Cardiol. 2006;40:384–393. doi: 10.1016/j.yjmcc.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Zhao LJ, Jian H, Zhu H. Specific gene inhibition by adenovirus-mediated expression of small interfering RNA. Gene. 2003;316:137–141. doi: 10.1016/s0378-1119(03)00750-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


