Abstract
Objectives
To describe the essential features of a new Northern Norway mother-and-child contaminant cohort study called MISA, including its rationale, content, implementation and selected findings (mostly dietary).
Study design
Cross-sectional with longitudinal aspects.
Methods
Five hundred and fifteen eligible women were enrolled in early pregnancy, with 391 completing the study protocol that included a self-administrated food frequency questionnaire (FFQ) and donation of biological samples for contaminant analysis in the 2nd trimester, just after delivery, and 6 weeks postpartum. Macronutrient consumption was converted to energy intake, and the amounts of both macro- and micronutrients ingested were estimated. Some of the MISA findings were compared to data available in the Medical Birth Registry of Norway (MBRN).
Results
Compared to all 2004–2006 mothers in Northern Norway, the study cohort women were about 2 years older and smoked less; on average, they had close to 16 years of education. Parity, gestational age and birth weight of the newborn were comparable as well. The estimated average dietary intake of 8.1 MJ per day was less than that recommended by the Nordic Nutritional Recommendations (NNR), but the intake of micronutrients per MJ complied.
Conclusions
Although the final cohort sample size was less than targeted, the generally good comparisons observed between MBRN-registered information for the study cohort and dropouts suggest that this occurrence introduced minimal bias. The agreement of the observed demographic and clinical characteristics of the cohort women and newborns with all births in Northern Norway implied acceptable external validity. Also, the dietary findings aligned well with Norwegian national data and guidelines and other studies, as did the high prevalence of breastfeeding. The MISA database is considered suitable for exploring associations between contaminant exposure and diet, enhancing our knowledge of the interplay of the physiological changes that occur in mothers with contaminant pharmacokinetics (including transfer to the infant before and after birth), and conducting prospective health studies of the children.
Keywords: pregnancy, postpartum, food frequency questionnaire (FFQ), contaminants
Pollutants have been released into the environment for decades and some accumulate in nature and in humans with long half-lives – they are persistent. Worldwide episodes of high-level foetal contamination have led to congenital malformation and impaired cognitive function. Mercury release in Minimata, Japan in the 1950s (1) and PCB-contaminated rice oil in Taiwan (2) are examples. Ongoing international monitoring projects have demonstrated diminishing levels of various contaminants in human blood samples over the last decades (3).
Continuous low-level contamination has been a concern. Biomonitoring studies have reported associations with miscellaneous outcomes such as decrease in birth weight, effect on children's cognitive function, and immune-system impairment (4–10). Many contaminants are transferred from mother to the foetus via the placenta during pregnancy (11), and from mother's milk postpartum (12). Because of their rapid development, foetuses and infants are especially vulnerable to the effects of these substances. Pollutant levels in maternal blood during pregnancy and in mother's milk provide an indication of the exposure risk experienced by the unborn child.
People living in Arctic and sub-Arctic regions are at higher risk of exposure to environmental contaminants due to long range transport, especially individuals who consume traditional diets that include marine mammals and/or fish (either marine or fresh water) (3). Nevertheless, relatively little is known about the situation in Northern Norway.
The MISA1 cohort was established in Northern Norway in 2007 with the purpose of assessing exposure experienced by pregnant women (and postpartum) and their newborns to a suite of environmental pollutants. Detailed information on food intake (past and present), lifetime residency, education, income, obstetrical history and pregnancy outcome was obtained on enrolment and subsequently through questionnaires.
The aim of this article was to describe the MISA project and progress made to date. Details are provided on recruitment, data collection, forms and questionnaires used, the database, the study cohort, and quality control measures taken. Sociodemographic, clinical and dietary characteristics of the mothers and neonates are also described.
Material and methods
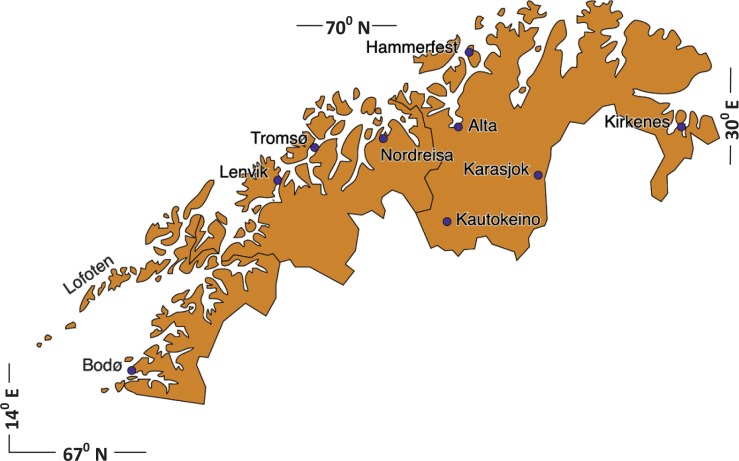
Planning of the project took place in 2006 and approvals were obtained from the Regional Committee for Medical and Health Research Ethics and the Norwegian Data Inspectorate. By May 2007, the recruitment started and continued for the next 25 months (until June 2009). After making a pregnancy ultrasound appointment at 1 of 8 selected delivering units and 2 antenatal centres in Northern Norway, pregnant women were invited by mail to participate in this project. The geographical area and locations of the delivery units are shown in Fig. 1. All women received a letter including a detailed layman explanation of the project's purpose; its voluntarily nature; the clinical, chemical and biological examinations to be conducted; financing; and ethical approval details. After a written consent was received, enrolment in the project was confirmed. A total of 2,600 women were invited to participate, 609 responded of whom 52 avoided further contact. Of the 557 women who received a blood sample collection package and questionnaire, 15 did not donate blood; the latter constituted the criterion for participation. Further, 27 did not hand in their written consent despite reminders. This left 515 eligible study subjects.
Fig. 1.
Map of the Northern Norway study area showing the locations of the delivering units and antenatal centres (Karasjok and Kautokeino).
Sampling
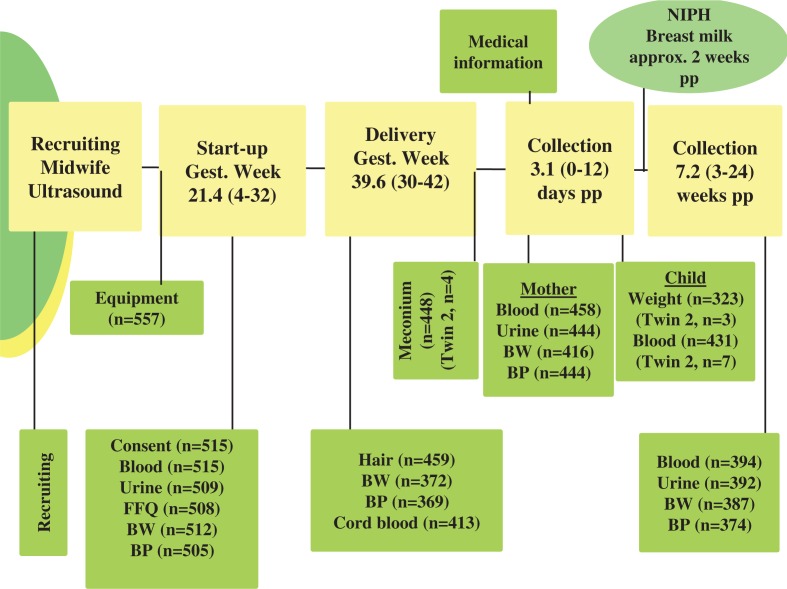
The type of specimens, when collected and number (n) are provided in Fig. 2. All measurements and the sampling were performed with standard equipment. The aim was to start-up before gestational week 20 (as determined by ultrasound). At first contact (see Start-up column in Fig. 2), the following activities took place: completion of a comprehensive self-administrated personal and food-frequency questionnaire (FFQ) (see Table I); donation of blood and urine samples (for which a fasting state was requested); assessment of body weight (BW) while wearing light clothes and without shoes (rounded to the nearest kilo); and measurement of blood pressure (BP) after resting for 15 minutes (3 times, with 1 minute between each). At delivery (see Delivery column in Fig. 2), the mother provided a scalp-hair sample, BW and BP were measured, and umbilical cord blood and meconium samples were collected. Postpartum measurements/collections 3 days after delivery included: maternal BW and BP; urine and blood (fasting requested), and a venous heel blood sample which coincided with the National phenylketonuria (PKU) test. Medical information and details about feeding (breast and/or infant formulae) were also obtained. The fourth contact was targeted to be 6 weeks postpartum (but see last column at right in Fig. 2), during which the measurements of BW and BP and collections of urine and blood were repeated. Information for the previous week's consumption/use of vitamins, fish-oil, medication, tobacco, and alcohol consumption was also obtained through a mini-questionnaire.
Fig. 2.
Protocol flow chart of the MISA Study. Abbreviations: NIPH, Norwegian Institute of Public Health; approx. approximately; pp, postpartum; Gest., gestational; Twin 2, second child of twins; FFQ, food frequency questionnaire; BW, body weight; BP, blood pressure; n, number. Means and ranges of the various intervention times are noted.
Table I.
Overview of the Food Frequency Questionnaire (FFQ)
| Personal information |
| Social status |
| Income level, education and description of working tasks |
| Lifetime residency |
| Family ethnicity; own and child's father's |
| Language skills (grandparents, parents, own and child's father) |
| Ethnic background (parents, own and child's father's) |
| Self perceived ethnicity (own and child's father's) |
| Self perceived health and physical activity |
| Present and previous (primi- and multiparous) lactation history |
| Tobacco and alcohol |
| Six months prior to conception, at conception and today |
| Intake of fish oil products and dietary supplements |
| Winter and rest of the year |
| Type of product and amount |
| Special circumstances for dietary intake |
| Vegetarian/vegan, allergy/intolerance, anorexia/bulimia, try to lose weight, low glycaemic food |
| Dietary intake |
| Beverage |
| Yoghurt/cereals |
| Bread and bread spread |
| Fruit and vegetables, including potatoes |
| Rice, spaghetti, porridge, soup |
| Fish, including seal/whale, shellfish, and sea birds |
| Meat, including game |
| Other food stuff; desserts, berries, including local wild berries, sweets and snacks |
| Lifetime dietary intake |
| Childhood |
| Youth (13–19 years old) |
| Adult life until previous year |
| Fish, roe and fish liver, seal/whale, sea bird eggs, fish oil products |
Breast milk specimens are available for our MISA study participants through the Human Milk Study of Norwegian Institute of Public Health (13), and are to be analysed for environmental contaminants and essential elements. All biological samples were stored at −20 °C in a bio-bank at the University of Tromsø, Norway. Permission was granted to archive the samples until the end of 2022.
The FFQ was divided into 2 parts. One pertained to personal information and the other to dietary details. It is based on the FFQ employed in the Norwegian Women and Cancer Study (NOWAC) (14). The latter has been validated against the 24-hour recall approach. A minor adjustment to the NOWAC questionnaire involved the expansion of the fish intake section to obtain more details on this topic. A brief overview of the questionnaire is presented in Table I. All food consumption questions had 4 to 7 fixed options ranging from never/seldom to either 5–7 times per week, or 2+ times per day. Details on both frequency and amounts were sought. The women were also asked about past seafood intake during childhood, youth and adult life stages. Consumption of fatty fish, fresh water fish, fish liver, whale and seal, crab meat, sea birds and sea bird eggs were emphasized.
Prior to recruitment, participating personnel from the various delivering units were instructed about the project procedures and protocols. All aspects were discussed, including the sampling and processing of biological samples and the forms to be completed by the health personnel and the study subjects. The 2 researchers responsible for the project were available around the clock during the recruitment and field-work periods by mobile phone and email.
The MISA database
The database was constructed using Microsoft Office Access software, and an accompanying user's manual was prepared. The 2 project leaders employed 3 qualified and trained individuals to assist them in data-entry. The lowest intake was recorded for multiple FFQ answers about quantities. After a complete set of data had been entered, the files were saved and an electronic copy was made for the data-entry quality control step. The database consisted of 1,050 keyed-in variables, including options but excluding biological analysis results.
The dietary information was converted from frequency and amount to daily intake in grams per day using a standardized measurement table (Blaker's Norwegian Weight and Measurement Table) (15). Daily energy and nutrient intakes were calculated using The Norwegian Food Composition Database 2006 (16). Dietary supplements were excluded from this exercise because of limited intake information. Missing answers in the dietary part of the FFQ and “never/seldom” responses were recorded as zero. Ten participants did not complete the dietary questions sufficiently and were excluded from the dietary calculations.
Quality assurance measures
In support of training and for ongoing use during the sampling period, detailed reference manuals were provided. A trained person was also in charge of the University of Tromsø Biobank. The ACCESS database had built-in data-entry blocks to reduce the risk of typing errors and entry of nonsense data. On data entry, every 5th paper file (21%) from each sampling location was checked against the electronically registered data, and any errors were corrected and recorded.
Obstetrical data was obtained from the Medical Birth Registry of Norway (MBRN). This database consists of 200 variables including maternal medical history, previous gestational history and present pregnancy and delivery, as well as medical conditions of the newborn. The registration is nationally mandatory for every pregnancy lasting 12 weeks or more. Permission to access this data was received, and its direct incorporation into to the MISA database minimized data-entry errors.
Statistical analysis
The data in the Access database were analyzed using the SPSS for Windows statistical package (version 17.0; SPSS Inc. Chicago, IL USA). Only descriptive statistics are presented, and the Student's t-test was employed when criteria for normal distributions were fulfilled; if not, non-parametric tests were used. The Kappa (κ) test was applied for assessing strength of agreement and the chi-squared test for categorical variables.
Results
Of the 515 women enrolled at the project outset, 458 participated up to and including delivery, and 394 to 6 weeks postpartum. Subsequently, 11 women formally withdrew from the project. The gestational and postpartum times when samples were collected are indicated in Fig. 2. In the group who completed the sample collection 6 weeks postpartum, 3 women had failed to donate samples at delivery. This reduced the cohort to 391 for whom blood samples were available for all 3 collections. Hereafter this group is referred to as the study cohort. Sociodemographic and clinical profiles are presented for them in Table II.
Table II.
Sociodemographic and clinical characteristics of the study cohort (from the questionnaire or the MBRN)
| Mean, range or % n = 391a | |
|---|---|
| Maternal age mean (range) | 31 (18–43) |
| ≤ 25 | 15.2% |
| 26–30 | 29.7% |
| 31–35 | 37.8% |
| > 35 | 17.3% |
| Child's father mean age (range)b | 34 (19–55) |
| County of residence | |
| Finnmark | 15.5% |
| Troms | 53.6% |
| Nordland | 29.9% |
| Ethnic affiliation | |
| Norwegian | 88.0% |
| Sami | 8.2% |
| Otherc | 3.8% |
| Education, in years (range) (n=375) | 15.9 (8–24) |
| < 13 | 13.6% |
| 13–16 | 42.6% |
| > 16 | 42.3% |
| Household income >600,000 NOK (n = 361) | 59.3% |
| Civil status; married/cohabited | 94.9% |
| Smoking (n = 376) | |
| 6 months prior to conception | 21.5% |
| Beginning of pregnancy | 13.1% |
| Second trimester (mean week 21.4) | 4.7% |
| Alcohol; abstainer before conception (n = 379) | 8.1% |
| Alcohol intake during pregnancy (reported: never/seldom) | |
| Light beer/cider (0.5 L) (n = 335) | 96.7% |
| Beer (0.5 L) (n = 337) | 97.6% |
| Wine (glass) (n = 340) | 95.3% |
| Spirits (drink/shot) (n = 335) | 99.4% |
| Liqueur/fortified wine (n = 336) | 99.7% |
| Dietary supplements (Yes) | |
| Vitamin (n = 378) | 48.6% |
| Fish oil products (n = 381) | 75.3% |
| BMI, mean (range)d | |
| Pre-pregnancy (n = 368) | 24.5 (17–44) |
| Blood pressure %>140/90 and (mean) | |
| Start-up (110/70) | 0% |
| At delivery (124/80) | 9.0% |
| 3 days postpartum (117/78) | 4.9% |
| 6 weeks postpartum (110/75) | 0.5% |
| Pre-eclampsiab | 2.0% |
| Parity (gestational week 12 or more) mean (range)b | 0.98 (0–4) |
| 0 | 37.3% |
| 1 | 36.5% |
| 2 | 18.8% |
| 3 | 5.6% |
| 4 or more | 1.8% |
| Newborn | |
| Preterm delivery (<37 weeks)b | 3.8% |
| Delivery modeb | |
| Vaginal | 88.8% |
| Vacuum | 2.8% |
| Caesarean section – Planned | 2.8% |
| Caesarean section – Acute | 5.6% |
| Pain reliefb | |
| Epidural (vaginal delivery) | 18.5% |
| N2O2 | 57.1% |
| Opiates | 1.5% |
| Narcosis | 1.0% |
| Sex of child (boy/girl)b | 52.3%/47.7% |
| Apgar 5 minutes <7b | 1.3% |
| Lactation 6 weeks postpartum (n = 340) | |
| Exclusive | 81.8% |
| Partly/ substitute | 16.2% |
| Only substitute | 2.1% |
Unless specified otherwise, n = 391.
Information was obtained from the Medical Birth Registry of Norway (MBRN).
15 Women describe themselves as “other” ethnic background: 12 from Europe and 3 outside Europe.
Maternal height and weight are self-reported.
It is evident from Table II that the majority of the prospective mothers were aged 26–35; 12% considered themselves as Sami, or of ethnic background other than Norwegian (17). A good proportion (42.3%) had more than 16 years of education. Note that in Norway primary and secondary education takes 13 years. Prior to conception, 21.5% of the mothers smoked; this dropped to 4.7% during the 2nd trimester. Even though the study subjects consumed wine and beer prior to conception (data not shown), alcohol intake during pregnancy is reported to be low – 8% declared themselves to be teetotallers. The majority of the group rated their physical activity to be 8 during child hood, on a scale of 1–10; 7 before this pregnancy, and 5 during pregnancy. Nearly all (98%) stated their health as good or very good.
Features of note related to pregnancy, delivery and the neonate were (see Table II and III): nullipara and primipara were most common; close to 4% were preterm births; 8.4% of the deliveries were by caesarean section; protracted parturition was the most frequent (22.8%) of the pathological occurrences; mean Apgar score at (5 minutes) was 9.5; exclusive breast feeding was practised by most mothers (81.8%); 72% among primiparous and 87% among multiparous mothers. Mothers with previous deliveries reported average exclusive breast feeding for 5 months and, on average, continued breastfeeding with additional supplements for another 7 months. The study sample was too small to report birth defects.
Table III.
Comparison of selected characteristics of the study cohort and dropouts (based on the MBRN database)
| Item | Dropouts mean, n (%), n = 113 | Study cohort mean, n (%), n = 391 | p-value |
|---|---|---|---|
| Maternal age (years) | 29.2 | 31.0 | 0.002 |
| Years at school (years)a | 14.7 | 15.9 | 0.002 |
| Parity (previous deliveries) | 0.80 | 0.97 | NS |
| Gestational age (weeks) | 39.4 | 39.6 | NS |
| Birth weight (g) | 3502 | 3623 | NS |
| Apgar 5 minutes | 9.2 | 9.5 | NS |
| Smoking, beginning of pregnancy | 34 (32.1) | 45 (13.1) | <0.001 |
| Smoking, end of pregnancy | 17 (17.3) | 16 (5.2) | <0.001 |
| Instrumental deliveryb | 21 (18.6) | 43 (11.0) | 0.03 |
| Dystocia: prolonged delivery, inertia, disproportion | 28 (24.8) | 89 (22.8) | NS |
| Meconium stained amniotic liquid | 16 (14.2) | 35 (9.0) | NS |
| Haemorrhage | 6 (5.3) | 26 (6.6) | NS |
| Medical pain relief | 87 (77.0) | 273 (69.8) | NS |
| Induced delivery | 5 (4.4) | 26 (6.6) | NS |
| Twins | 3 (2.7) | 6 (1.5) | NS |
| Vitamins during pregnancy | 57 (50.4) | 183 (46.8) | NS |
| Folic acid during pregnancy | 78 (69.0) | 278 (71.1) | NS |
From the FFQ.
Caesarean section (elective and acute) and vacuum.
A comparison of personal and clinical characteristics for the study cohort and the dropout group is provided in Table III, and of dietary practices in Supplementary Table S1. It shows that the individuals in the dropout group were younger, less schooled, had lighter babies, smoked more, and instrumental delivery was more common. Statistically significant differences between macro- and micro-nutrient intakes were few, and this also applied to fish, fish products and the miscellaneous food items (lower intakes for the dropout group in %): carbohydrates (5%, p=0.06); riboflavin (7%, p=0.004); vitamin B12 (6%, p=0.03); calcium (13%, p=0.007); zinc (7%, p=0.02); selenium (7%, p=0.04); fatty fish (22%, p=0.02); shell fish (7%, p=0.02); bread and cereals (10%, p=0.02); milk and milk products (15%, p=0.05); and meat (1%, p=0.05). Not surprisingly, the energy intake was also somewhat (5%) lower (p=0.03) (Table S1). Note that the number of participants reported in Table II and S1 differ slightly because a small number failed to complete some sections of the FFQ (despite reminders and requests).
On the whole, fish intake generally declined with age, including fatty fish (data not shown). Whale meat, fish liver and sea bird eggs decreased significantly. Consumption of fish spread increased somewhat. In general, use of fish oil supplements was more common during the winter season compared to the rest of the year, and was comparable in childhood (<13 years of age) and adult-life (>19 years of age). In both age groups 27% reported daily use during the winter season, while during the remainder of the year it was 15% (childhood) and 20% (adults). By comparison, 9% indicated daily winter use of fish oil products during their youth (13–19 years) and 4% during the rest of the year. In terms of current use, close to 50% of the respondents stated they used fish oil products regularly (2–7 times a week), and more frequently during the winter season (65%).
For information entry, the quality assurance protocol revealed that 70% of the files re-checked had no or minor punching errors. For the FFQ, typically 1 consumption unit over or under what had actually been recorded by the subject had been entered. Similarly on maternal blood sampling information forms, time or name of sampling location were missing occasionally. Such errors were limited to 1 per form in the majority of cases. However, the missing information could be obtained from other forms through the participant's ID number.
Discussion
The main feature and strength of the MISA study is the wide range of biological specimens collected from mothers during pregnancy, just after delivery, and 6 weeks postpartum; and from the newborns at birth. This is supplemented by the construction of a database that also includes maternal food-frequency questionnaire information and sociodemographic characteristics, as well as clinical information for both the mother and child. Once the available data is fully analyzed, the understanding gained should enhance our knowledge of the interplay between maternal diet, the physiological changes that occur in mothers during pregnancy and postpartum, and contaminant pharmacokinetics (including transfer to the infant before and after birth).
The average total energy intake by the study cohort was 8.1 MJ/day (median 8.1; range 3.1–16.4) with an average fish intake of 80 g/day (median 72 g/day; range 0–252 g/day), including 11.5 g/day (median 9 g/day, range 0–66 g/day) of fatty fish and 19.8 g/day (median 15.8 g/day, range 0–136 g/day) of lean fish (Table S1). One woman reported not to consume fish or fish products. For the study cohort and dropouts, the daily total average energy intake was below the Nordic Nutritional Recommendations (NNR) (18): 73% were below 9.3 MJ, which is the recommended intake for women in the age group 18–60 with an average physical stature. Women with normal weight are recommended to increase their intake by 2.1 MJ during the first trimester, or 10.3–10.5 MJ per day. The average intakes reported here are also lower than that observed (mean of 9.8 MJ; 1999–2007 study period) in the Norwegian Mother and Child Cohort (MoBa) Study (19). It is to be noted that the FFQ in the present study probed fish consumption in greater detail than its NOWAC parent did, but was nevertheless considerably shorter than the FFQ employed in the MoBa study (5 vs. 14 pages) (20). However, nutrient intake per MJ (i.e. nutrient density) was in good compliance with NNR recommendations (18) and a more recent Danish report (21). Daily dietary intake among Norwegian and Sami women was comparable for most food items (22).
Generally speaking, fish consumption has declined over the last decades. In a 1980 Norwegian study, 2.6% of the women aged 20–49 reported intakes of fish dinners 5–7 times a week (23), compared to 0.5% by mothers in the MISA study. It is worth mentioning that during childhood, 8.9% reported fish intake 4 times or more per week. Young women are reported to consume less fish compared to the general public average (24). Average total fish consumption in the MISA group was more than double that reported in the MoBa cohort (n=62,099, for the period 2002–2008), namely 80 g/day vs. 36 g/day (or 42 g/day for a subgroup of 119 who participated in the validation of the MoBa food frequency questionnaire) (24,25). However seafood intake is indeed reported to be higher in Northern Norway compared to the rest of the country (26). Our findings align well with those of another study performed above the Arctic Circle (27); it reported annual fatty-fish consumption of 4200 g/year, compared to our assessment of 4138 g/year.
A comparison of the MBRN-registered data for the cohort subjects and the dropouts (Table III) indicated that there were no significant differences in gestational age at delivery, parity or induced delivery and mean birth weight of the newborns (although lower for the dropout mothers). The latter were also more likely to smoke, and this constitutes a rational explanation for lighter babies. Furthermore, compatibility extended to the consumption of most macronutrients and micronutrients, but somewhat lower intakes by the dropouts. In line with this, total energy intake was also lower (Table S1).
Average household income for families with children aged 0–5 in Norway in 2008 was 601,000 NOK (28), and this is reflected in the current study. It also concurs with the observed high (95%) married/cohabited status, as well as the average parity of 0.98 and advanced (bachelor level) education (see Table II).
Distribution of pre-pregnancy BMI in the study cohort is similar to what is generally found in Norway for women in their 30s (29): 10.4% in the MISA study were obese (BMI > 30) vs. 13.2% in the general Norwegian female population, and 1.0% reported being underweight (BMI < 18.5) vs. 1.7%.
No woman had diagnosed hypertension prior to pregnancy, whereas 9.0% had elevated blood pressure (BP) at delivery. This is a little higher than reported previously in American women aged 18–39 (7.2%) (30), although it should be emphasized that in the MISA study BP was measured when the women entered the delivery unit (at 3 days postpartum, the proportion with high BP had declined to 4.9%). Only 2% were diagnosed with pre-eclampsia. In Norway, the prevalence in first-time delivering women is 5.1%, and the crude risk is 2.5% in women with 1 previous delivery (31). Two thirds of the cases in the present study also occurred among the nullipara.
Breast-feeding is common in Norway. A study by the Norwegian Directorate of Health and others showed that only 1% of newborns were never nourished with mother's milk, and 4 weeks postpartum a total of 95% were breastfed (82% exclusively so) (32). Corresponding proportions were reported in the MoBa study, with 84.6% and 79.1% being fully breast-fed 1 and 2 months after delivery respectively (33). We observed comparable proportions (Table II). However, the proportion of any breastfeeding was somewhat higher in our study (98.0% at 7 weeks, data not shown) than in the MoBa study (96.6% at 1 month and 94.0% at 2 months). No data were available beyond this period, but mothers with previous deliveries reported exclusively breastfeeding their children for 6 months (38%), or 4 months (26%). Most of these continued breastfeeding independent of the number of previous deliveries with supplements for 6 months or longer (73%); 16% continued for at least 12 months.
The prevalence of smoking in the study cohort was lower than that reported in the 2009 national Norwegian statistics (34): 11.5% compared to 19% at the beginning of pregnancy, and 4.1% vs. 8% at the end. In this national report, on average 85% gave information about smoking habits, whereas in the current study 88% did so at the onset of pregnancy and 78% at the end. Only 2% of our respondents failed to report on tobacco use in the FFQ, which is consistent with the data in the MBRN (beginning of pregnancy; κ=0.72, p < 0.001; 95% CI [0.61;0.83]). Underreporting about smoking is common (35). Since in our study no information is available for 22% at the end of pregnancy, the actual prevalence might be higher. Nevertheless, the national numbers quoted probably constitute an upper value.
Smoking appears most dominant in the younger groups. Among those aged 25 or less 32.1% reported smoking at the beginning of pregnancy, 12.7% among those aged 26–30, and 5.3% among the oldest group (36 years or more). Again, this is consistent with the national Norwegian statistics (34).
Even though only few women describe themselves as teetotallers, alcohol consumption during pregnancy was minimal. It was statistically independent of the reported smoking habits. In fact among the few who reported any intake of alcohol, the majority were non-smokers. Prior to conception, beer was the most common alcoholic drink among the younger age-groups (up to and around 30 years) and declined with age. By contrast, the proportion of women who reported intake of wine increased as they got older. Intake of other types of spirits was limited. Habitual drinking seems not to be an issue for the study cohort.
Strengths and limitations
Some operational bases for the reduced size of the study cohort were pointed out in the Results section. From the feedback received from the field workers and participants, another frequent reason was complications at delivery or postpartum. Others were that the business of the delivery units discouraged the mothers, as well as study tiredness. The latter reflects multiple requests to participate in other research projects that focus on the North. Based on the comparison of the MBRN registered data for the cohort subjects and the dropouts (Table III), we conclude that this turn of events has introduced minimal bias.
Even though the project is comprehensive, detailed planning prior to its implementation, continuous follow-up, and minor punching errors all helped the internal validity. Several attempts to increase interest and awareness of the project were carried out, but recruitment remained sluggish and the final sample size was less than targeted. Therefore the study is likely less representative than planned, although the generally good comparisons with the MBRN observed is encouraging.
The annual number of deliveries in Northern Norway is around 5,500 and are distributed between 3 counties. In Finnmark County, all 3 delivering units are represented in the study cohort. In Troms County, 1 delivering unit was not included and it represented close to 20% of deliveries there. In Nordland County there are a total of 9 delivery units, but only 2 were included in the study cohort. However, the participating units represented almost half of the total number of deliveries. It should be mentioned that in Bodø (the largest unit and city in the county) recruitment was limited to 1 obstetrical unit. County participation rates varied somewhat. Comparisons with averages for the all deliveries registered in the MBRN for the Northern Norway counties (live born, gestational age 30–42, n=15,571) for 2004–2006 is helpful. The women in the MISA cohort were on average 1–2 years older, and smoke less. However, many parameters were of comparable magnitude between the Northern Norway 2004–2006 mothers and the MISA group: mean birth weight (3,557 vs. 3,623 g) and the Apgar score at 5 minutes were the same (9.5); Apgar score% <7 at 5 minutes (1.2 vs. 1.3 for the MISA group); gestational age (39.3 vs. 39.6); parity (1.02 vs. 0.98); haemorrhage >1500 mL (0.9% vs. 0.8%); and polyhydramnion (1.3% vs. 1.3%).
The fact that the MISA cohort women were somewhat older and perhaps better schooled than the dropouts might well explain the lower smoking rate. Food consumption might also have been more selective in terms of healthy food choices. Not surprisingly, fish intake did not vary substantially between cohort members and dropouts, and this agrees well with the consumption of most micronutrients and macronutrients. Although the parent NOWAC FFQ has been validated, this did not involve pregnant women. This reduces the confidence in our findings somewhat. However, as indicated earlier, the consumption data obtained do align with other studies.
To date, studies of changes in the maternal concentrations during pregnancy and postpartum of organochlorines and of toxic and essential elements have been initiated (22,36). The observed trends were interpreted in the context of underlying metabolic, haematological and physiological changes that occur in mothers. The MISA database also offers opportunities to explore the interplay of the physiological changes that occur in mothers with contaminant pharmacokinetics, including transfer to the infant before and after birth. Future investigations will also focus on associations between diet and contaminant levels for both the women and their offspring, and prospective studies are contemplated for exploring prenatal exposure and the developmental health of the children.
Acknowledgements
Provision of data by the Medical Birth Registry of Norway (MBRN) is acknowledged. We would like to thank the women for their willingness to participate. Additionally we thank the health-care personnel and all participating delivery units for their contributions. Special thanks for professional input is extended to Bente Augdal (analyst), and Guri Skeie and Magritt Brustad of the NOWAC and SAMINOR research groups.
To access the supplementary material to this article please see Supplementary files under Article Tools online
Footnotes
1The Norwegian title of the project is: Miljøgifter i svangerskapet og i ammeperioden (MISA).
Conflict of interest and funding
The authors declare that they have no conflicts of interest. This study was financed by The Norwegian Women's Public Health Association, The Research Council of Norway, The Northern Norway Regional Health Authority, The Arctic Monitoring and Assessment Programme, and The Centre for Sámi Health Research, University of Tromsø.
References
- 1.Eto K, Marumoto M, Takeya M. The pathology of methylmercury poisoning (Minamata disease) Neuropathology. 2010;30:471–9. doi: 10.1111/j.1440-1789.2010.01119.x. [DOI] [PubMed] [Google Scholar]
- 2.Hsu ST, Ma CI, Hsu SK, Wu SS, Hsu NH, Yeh CC, et al. Discovery and epidemiology of PCB poisoning in Taiwan: a four-year follow-up. Environ Health Perspect. 1985;59:5–10. doi: 10.1289/ehp.59-1568088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AMAP. AMAP assessment 2009: human health in the Arctic. Oslo: Arctic Monitoring and Assessment Programme; 2009. [cited 2011 Dec 5]. Available from: http://www.amap.no/ [Google Scholar]
- 4.Odland JO, Nieboer E, Romanova N, Thomassen Y, Lund E. Blood lead and cadmium and birth weight among sub-Arctic and Arctic populations of Norway and Russia. Acta Obstet Gynecol Scand. 1999;78:852–60. [PubMed] [Google Scholar]
- 5.Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–28. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 6.Grandjean P, Murata K, Budtz-Jørgensen E, Weihe P. Cardiac autonomic activity in methylmercury neurotoxicity: 14-year follow-up of a Faroese birth cohort. J Pediatr. 2004;144:169–76. doi: 10.1016/j.jpeds.2003.10.058. [DOI] [PubMed] [Google Scholar]
- 7.Saint-Amour D, Roy MS, Bastien C, Ayotte P, Dewailly E, Després C, et al. Alterations of visual evoked potentials in preschool Inuit children exposed to methylmercury and biphenyls from a marine diet. Neurotoxicology. 2006;27:567–78. doi: 10.1016/j.neuro.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Plusquellec P, Muckle G, Dewailly E, Ayotte P, Jacobson SW, Jacobson JL. The relation of low-level prenatal lead exposure to behavioural indicators of attention in Inuit infants in Arctic Quebec. Neurotoxicol Teratol. 2007;29:527–37. doi: 10.1016/j.ntt.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaktskjold A, Talykova LV, Chashchin VP, Odland JO, Nieboer E. Maternal nickel exposure and congenital musculoskeletal defects. Am J Ind Med. 2008;51:825–33. doi: 10.1002/ajim.20609. [DOI] [PubMed] [Google Scholar]
- 10.Heilmann C, Budtz-Jørgensen E, Nielsen F, Heinzow B, Weihe P, Grandjean P. Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environ Health Perspect. 2010;118:1434–8. doi: 10.1289/ehp.1001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudge CV, Röllin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JØ. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J Environ Monit. 2009;11:1322–30. doi: 10.1039/b903805a. [DOI] [PubMed] [Google Scholar]
- 12.Fängström B, Strid A, Grandjean P, Weihe P, Bergman A. A retrospective study of PBDEs and PCBs in human milk from the Faroe Islands. Environ Health. 2005;4:12. doi: 10.1186/1476-069X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eggesbø M, Stigum H, Polder A, Lindstrøm G, Becher G, Skaare JU, et al. The Human Milk Study, HUMIS. Presentation of a birth cohort study which aims to collect milk samples from 6000 mothers, of the assessment of persistent organic pollutants (POPS), relating it to exposure factors and health outcomes. Organohalogen Compounds. 2004;66:2669–75. [cited 2011 Dec 5]. Available from: http://www.dioxin20xx.org/pdfs/2004/04-444.pdf. [Google Scholar]
- 14.Hjartåker A, Andersen LF, Lund E. Comparison of diet measures from a food-frequency questionnaire with measures from repeated 24-hour dietary recalls. The Norwegian Women and Cancer Study. Public Health Nutr. 2007;10:1094–103. doi: 10.1017/S1368980007702872. [DOI] [PubMed] [Google Scholar]
- 15.Blaker B, Aarsland M. Mål og vekt for matvarer. 2nd edition. [Measures and weights for foodstuffs]. Oslo: National Association for Nutrition and Health; 1995. pp. 6–42. [in Norwegian] [Google Scholar]
- 16.Matportalen. Matvaretabellen. [Food composition table]. Oslo: Matportalen; 2006. [cited 2011 Dec 5]. Available from: http://matportalen.no/matvaretabellen/ [Google Scholar]
- 17.Lund E, Melhus M, Hansen KL, Nystad T, Broderstad AR, Selmer R, et al. Population based study of health and living conditions in areas with both Sámi and Norwegian populations – The SAMINOR Study. Int J Circumpolar Health. 2007;66:113–28. doi: 10.3402/ijch.v66i2.18241. [DOI] [PubMed] [Google Scholar]
- 18.NCM. Integrating nutrition and physical activity. 4th edition. Copenhagen: Nordic Council of Ministers; 2005. Nordic Nutrition Recommendations 2004; pp. 13–22.pp. 109–138. [Google Scholar]
- 19.Meltzer HM, Brantsaeter AL, Ydersbond TA, Alexander J, Haugen M. Methodological challenges when monitoring the diet of pregnant women in a large study: experiences from the Norwegian Mother and Child Cohort Study (MoBa) Matern Child Nutr. 2008;4:14–27. doi: 10.1111/j.1740-8709.2007.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magnus P, Irgens LM, Haug K, Nystad W, Skjærven R, Stoltenberg C, et al. Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35:1146–50. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 21.DTU Food. Søborg: National Food Institute, Technical University of Denmark; 2010. Danskernes kostvaner 2003–2008 [Dietary habits in Denmark 2003–2008] [cited 2012 Feb 15]. Available from: http://www.food.dtu.dk/upload/f%C3%B8devareinstituttet/food.dtu.dk/publikationer/2010/danskernes%20kostvaner%202003–2008.pdf [in Danish] [Google Scholar]
- 22.Hansen S, Nieboer E, Sandanger TM, Wilsgaard T, Thomassen Y, Veyhe AS, et al. Changes in maternal blood concentrations of selected essential and toxic elements during and after pregnancy. J Environ Monit. 2011;13:2143–52. doi: 10.1039/c1em10051c. [DOI] [PubMed] [Google Scholar]
- 23.Jacobsen BK, Thelle DS. The Tromsø Heart Study: food habits, serum total cholesterol, HDL cholesterol, and triglycerides. Am J Epidemiol. 1987;125:622–30. doi: 10.1093/oxfordjournals.aje.a114575. [DOI] [PubMed] [Google Scholar]
- 24.Brantsæter AL, Birgisdottir BE, Meltzer HM, Kvalem HE, Alexander J, Magnus P, et al. Maternal seafood consumption and infant birth weight, length and head circumference in the Norwegian Mother and Child Cohort Study; Br J Nutr; 2012. pp. 436–44. [DOI] [PubMed] [Google Scholar]
- 25.Brantsaeter AL, Haugen M, Thomassen Y, Ellingsen DG, Ydersbond TA, Hagve TA, et al. Exploration of biomarkers for total fish intake in pregnant Norwegian women. Public Health Nutr. 2010;13:54–62. doi: 10.1017/S1368980009005904. [DOI] [PubMed] [Google Scholar]
- 26.VKM. Et helhetssyn på fisk og annen sjømat i norsk kosthold [A comprehensive assessment of fish and other seafood in the Norwegian diet] Oslo: Vitenskapskomiteen for mattrygghed [Norwegian scientific Committee for Food Safety]; 2006. [cited 2011 Dec 5]. Available from: http://www.vkm.no/dav/a2805d6a8c.pdf. [in Norwegian] [Google Scholar]
- 27.Rylander C, Sandanger TM, Brustad M. Associations between marine food consumption and plasma concentrations of POPs in a Norwegian costal population. J Environ Monit. 2009;11:370–6. doi: 10.1039/b811868j. [DOI] [PubMed] [Google Scholar]
- 28.Statistics Norway. Inntekt. [Income]. Oslo: Statistics Norway; 2009. [cited 2011 Dec 5]. Available from: http://www.ssb.no/inntekt/ [Google Scholar]
- 29.NIPH. Oslo: Norwegian Institute of Public Health; 2011. Overweight and obesity in Norway – fact sheet. [cited 2011 Dec 5]. Avaliable from: http://www.fhi.no/eway/default.aspx?pid=238&trg=MainLeft_5976&MainArea_5811=5976:0:15,5012:1:0:0:::0:0&MainLeft_5976=5825:74991::1:5977:20:::0:0. [Google Scholar]
- 30.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 31.Trogstad L, Magnus P, Stoltenberg C. Pre-eclampsia: risk factors and causal models. Best Pract Res Clin Obstet Gynaecol. 2011;25:329–42. doi: 10.1016/j.bpobgyn.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Helsedirektoratet. Oslo: Norwegian Directorate for Health, University of Oslo & Norwegian Food Safety Authority; 2008. Spedkost – 6 måneder Landsomfattende kostholdsundersøkelse. [cited 2011 Dec 5]. Available from: http://www.matportalen.no/kosthold_og_helse/article3345.ece/BINARY/Helsedirektoratet+Landsomfattende+kostholdsunders%C3%B8kelse+blant+6+m%C3%A5neder+gamle+barn [in Norwegian] [Google Scholar]
- 33.Häggkvist AP, Brantsæter AL, Grjibovski AM, Helsing E, Meltzer HM, Haugen M. Prevalence of breast-feeding in the Norwegian Mother and Child Cohort Study and health service-related correlates of cessation of full breast-feeding. Public Health Nutr. 2010;13:2076–86. doi: 10.1017/S1368980010001771. [DOI] [PubMed] [Google Scholar]
- 34.NIPH. Oslo: Norwegian Institute of Public Health; 2011. Røyking og snus – fakta og statiskik. [cited 2011 Dec 5]. Available from: http://www.fhi.no/eway/default.aspx?pid=233&trg=MainLeft_6039&MainArea_5661=6039:0:15,4578:1:0:0:::0:0&MainLeft_6039=6041:70823::1:6043:5:::0:0 [in Norwegian] [Google Scholar]
- 35.Hovengen R, Nordhagen R. Av-og-til-røyking – et økende problem. [[Occasional smoking – an increasing problem]. Tidsskr Nor Laegeforen. 2004;124:3222–3. in Norwegian. [PubMed] [Google Scholar]
- 36.Hansen S, Nieboer E, Odland JØ, Wilsgaard T, Veyhe AS, Sandanger TM. Levels of organochlorines and lipids across pregnancy, delivery and postpartum periods in women from Northern Norway. J Environ Monit. 2010;12:2128–37. doi: 10.1039/c0em00346h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
To access the supplementary material to this article please see Supplementary files under Article Tools online