Abstract
Molecular biology of mouse and chicken retroviruses had identified oncogenes and provided a revolutionary concept in understanding of cancers. A human retrovirus was established during 1980–1982 in linkage with a unique human leukemia, concurrently in Japan and USA. This review covers our efforts on the discovery of new retrovirus, Human T-cell Leukemia Virus Type 1 (HTLV-1), first introducing to a new class of retroviruses with a unique regulatory factors, Tax and Rex. Then it is followed by analyses of molecular interaction of the vial Tax with cellular machineries involved in the pathogenesis of Adult T-cell Leukemia (ATL). And then a probable mechanism of pathogenesis of ATL is proposed including recent findings on HBZ after our efforts.
Keywords: adult T-cell leukemia, HTLV-1, retrovirus, Tax, viral carcinogenesis, tumor suppressor gene
Introduction
It has been certainly established that cancer is a disease of genes which control cellular proliferation, differentiation and apoptosis. Such molecular concept has been substantiated in 1976 by the discovery of a viral oncogene in Rous sarcoma virus1) followed by identification of the cellular counterpart.2) After the discovery, a number of oncogene has been identified in retroviruses which had been isolated from various animal species in the 1970’s and 1980’s. The oncogene was a conceptual revolution in understanding cancers: the viral oncogenes originated from their host cell “proto-oncogenes”, which are widely conserved in many animal species. Thus the “oncogene” became the “key player” in cancer research linking cancers of animals and humans, and also linking among the different origins of cancers. A human retrovirus was therefore expected to provide a powerful tool in understand human cancers and searched by number of investigators for a long time, however, the trials were all failed.
The discovery of the first human retrovirus proceeded through two independent approaches: One is effort for identification of an etiologic agent of newly described Adult T-cell Leukemia (ATL), in Japan,3) and the other is effort for detection of retrovirus in human cell lines in the USA.4) These independent approaches have reached to discovery of the identical retrovirus, Human T-cell Leukemia Virus Type 1 (HTLV-1). In this review, I first summarize our history of discovery research and then discuss probable pathogenic mechanisms including recent findings by other groups.
1. Prologue to ATLV/HTLV-1
1.1. Discovery of ATL
The first key event for human retrovirus was the discovery of Adult T-cell Leukemia (ATL) by Takatsuki and his colleagues in 1977.5) The leukemia cells in patients were characterized by unusual morphology with lobulated nucleus, which is called “flower-like cell”. The abnormal cells were soon identified as T cell expressing interleukin 2-receptor (IL-2R, CD 25) at abnormally high levels.6) Patients carrying the “flower-like cells” were found mostly in Kyushu (West part of Japan), suggesting its unexpectedly unique nature. The original report by Takatsuki’s group was later confirmed by extensive epidemiology: Most of ATL patients were clustered in Kyushu area and other sporadic cases were mostly originated from Kyushu in their life.7),8) The epidemiology suggested that an etiologic factor hits the patients at the early stage of their lives, and develops ATL after long latency. In fact, possible virus infection or carcinogenic plant factors were seriously discussed at the early stage of the investigation.
1.2. Establishment of cell lines
Cell line which represents specific character of the disease would be critically useful for investigation of the disease. In studies on ATL, cell lines played critical roles in various aspects. A typical cell line, MT-1, was established from ATL by Miyoshi9) by co-cultivation of leukemia cells from patient with cord blood lymphocytes from normal baby. When any purified lymphokines or growth factors were not available, the co-cultivation technique was effectively used for establishing cell lines. In one case, Miyoshi used a combination of cord blood and ATL cells with different sexes, and found by karyotype analysis that the established cell line MT-2 was derived from cord blood cells.10) Nevertheless, MT-1 and -2 cells have greatly contributed to the epidemiology of ATL and identification of the retrovirus (see later discussion).
1.3. Unique antibodies in patient sera with ATL
Discovery of unique serum antibodies in ATL patients was another key event for identification of virus infection. Hinuma and his colleagues applied indirect immuno-fluorescence assay which had been used for diagnosis of Epstein-Barr virus infection in human:7) sera from infected people had specific antibodies against EB-virus and immunologically reacted with cells infected with the EBV. Postulating a viral infection in ATL patients, cell line which positively reacts with ATL patient sera was searched surveying a cell panel randomly accumulated in his laboratory. After laborious assays, a single cell line was identified to give a robustly positive signal. That was the MT-1 cell, established from ATL by Miyoshi.9) The putative antigens in the cell line were termed ATLA (ATL Antigens). MT-1 cell expressing ATLA was then used for screening sera from various types of leukemia, and the results demonstrated that anti-ATLA was detected in all ATL patients and also in a minor fraction of healthy individuals.8) This was a strong suggestion for virus infection in ATL, although the nature of ATLA was unclear at that time.
2. Appearance of the first human retrovirus, HTLV-1
2.1. Identification of retrovirus
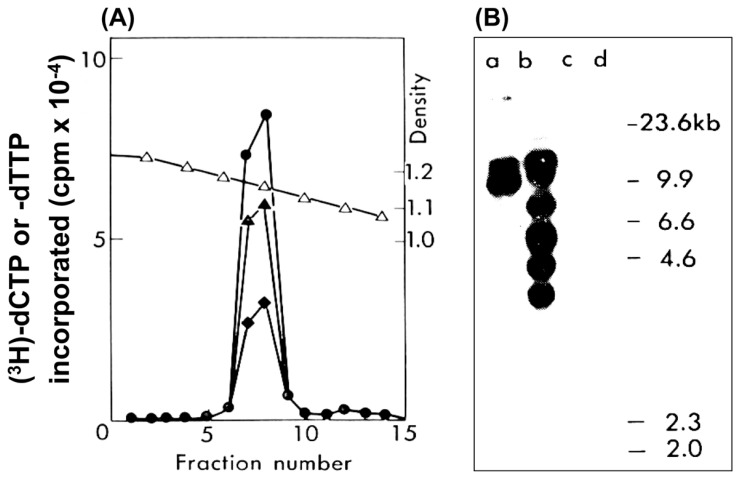
Epidemiology of ATL strongly predicted a viral infection in ATL, and various groups thus challenged identification of the possible virus. We were one of challengers using molecular techniques. Since MT-2 cells were shown to express ATL antigens, an MT-2 cell line was used for further examination. The culture fluid showed reverse transcriptase (RT) activity in the presence of RNA template in particle fractions at similar density to animal retrovirus (Fig. 1).11) More importantly, the RT activity was also detected without exogenous RNA template indicating the presence of endogenous RNA. Thus, the presence of retroviral particles was strongly suggested.11)
Fig. 1.
Identification of HTLV-1 in a cell line MT-2.11) (A) Reverse transcriptase activity. Concentrated culture fluid of MT-2 cells was centrifuged on a gradient of 20–60% sucrose and the fractions were assayed for DNA polymerase activity with poly(A)-oligo(dT) (●), no exogenous template (▲), or in presence of MnCl2 instead of MgCl2 (♦). △ (or open triangle) represents the density of each fraction. (B) Detection of provirus sequences by Southern blot analysis of cellular DNA of ATL-related MT-1 (a) and MT-2 (b), ATL-unrelated A204 (c) and KB cells (d). Cellular DNA was digested with Eco-R1 and probed with cDNA prepared by the endogenous reaction of active fractions in Fig. 1(A).
DNA was prepared by the endogenous RT reaction, and was used as probe for hybridization with cellular DNA. The cDNA was demonstrated to hybridize with cellular DNA of MT-1 and MT-2 cells, but not with DNA of cells unrelated to ATL (Fig. 1). This was a clear evidence for the provirus DNA sequence homologous to particulate RNA in MT-2 culture fluid.11) Furthermore, unique proteins with molecular weight 24K and 19K were distributed in particle fractions overlapping with the RT activity. These proteins were shown to react immunologically with patient sera, proofing the 24K and 19K proteins are a part of ATLA.11) Conclusion was that MT-2 cells harbored retroviral provirus DNA and produced the viral particles containing 24K and 19K proteins in addition to RNA, and the viral proteins were a part of ATLA. The retrovirus was originally reported as Adult T-cell Leukemia Virus (ATLV).11)
During corresponding period, Gallo and his group at NIH, USA reported a retrovirus HTLV in HUT102 cell line.12) The cell line HUT102 was established from a patient with Mycosis fungoides. This diagnosis for the patient from whom HUT102 was established was an origin of some confusion for a while. We finally demonstrated the identity of ATLV with HTLV isolates at the sequence levels,13) thus the terminologies were then unified into Human T-cell Leukemia Virus Type 1 (HTLV-1). Diagnosis of the Mycosis fungoides from which HUT102 was established has been changed to ATL later, and all were integrated together.14),16)
2.2. New class of retrovirus with unique genome structure
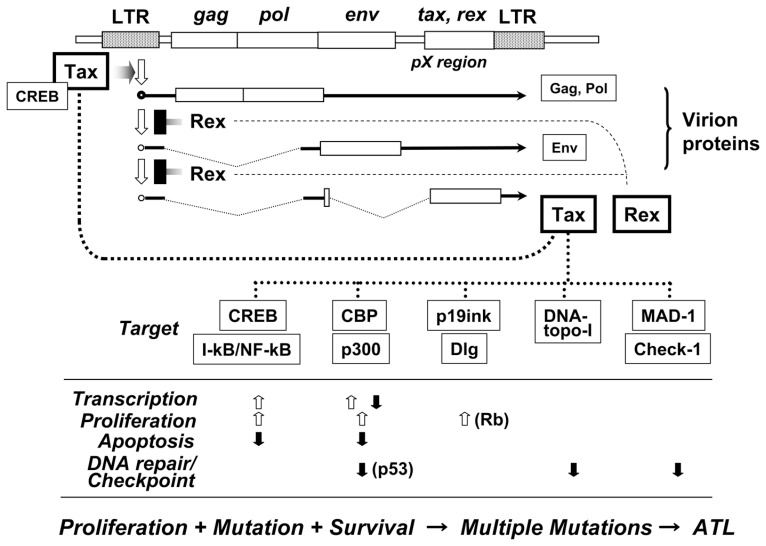
Retroviruses are classified into two categories: chronic and acute leukemia viruses. The retroviral genome is generally illustrated in form of “the provirus”. Chronic retroviruses have a genome structure with LTR-gag-pol-env-LTR; on the other hand, acute viruses have LTR-δgag-onc-δenv-LTR, where has a large deletion spanning δ to δ with variable length and replaced with “oncogene” acquired from cellular “proto-oncogene”. However, genome sequence of newly isolated HTLV-1 was different from either case, but showed LTR-gag-pol-env-pX-LTR, in which pX is 1.6 kb long17),18) (Fig. 2). The pX sequence was not host cell origin, that is, not oncogene. On the other hand, it was reported that HTLV-1 had ability to immortalize primary T cells in vitro.19) These observations clearly indicated that the newly identified HTLV-1 should have a unique transforming gene, because usual chronic retroviruses never immortalize primary cells in vitro. The structure and property were different from any class of the other retroviruses, and thus the newly identified virus was classified into a new group of the retroviruses. Other members of this viral class were later identified including HTLV-2,20) STLV21) and BLV.22)
Fig. 2.
Illustration of the HTLV-1 genome structure, gene expression and function of Tax. In upper half, transient expression by a combination of Tax and Rex is summarized, and in lower half, direct targets of Tax and their effects on cellular functions are summarized. In the lower, open arrows represent “enhancement” and closed arrows “down-regulation”, some arrows with major player in the pathway.
2.3. Association with ATL
Unique antibodies in patient sera were used as marker for HTLV-1 infection, because those reacted with HTLV-1 p24 and p19.11) Using a cell line, MT-1, the serum antibodies were surveyed for HTLV-1 infection in the world-wide scale. The results were rather unique clustering in West Japan, South America and Central Africa. Precisely overlapping with these clustering, ATL was also observed.23)
Furthermore, the overlapping of ATL with HTLV-1 infection was also observed at a level of individuals; vast majority of ATL patients were infected with HTLV-1, but healthy population in the same region are mostly negative.8) However, it should be commented on a paper reporting that some ATL-like patients were free of viral infection.24) These findings on overlapping at geographical and individual levels unequivocally established a close association of HTLV-1 infection and ATL.
Subsequently, HTLV-1 infection was also shown to be associated with tropical spastic paraparesis (TSP) in Jamaica25) and with HTLV-1-associated myelopathy (HAM) in Japan.26) TSP and HAM are today recognized as an identical myelopathy, and termed HAM/TSP.
3. Unique control of HTLV-1 replication
As mentioned in the previous section, HTLV-1 has an extra sequence pX. We had postulated the pX is a transforming gene. From sequence analysis of the pX region, we observed four open reading frames (ORF) possibly coding for proteins.18) Raising antibodies against the C-terminal peptides predicted from each ORF, we successfully identified three proteins, p40Tax, p27Rex and p21.27) These were encoded by two overlapping ORFs, although one ORF encodes both p27Rex and p21 with different initiation codon.28),29)
After trial and error for a while, we found Tax can activate gene expression from the LTR, and then identified it as a transcriptional activator of the viral genome.30)–32) One of the other proteins, p27Rex was identified as a suppressing factor of splicing of un-spliced viral transcripts, which enabled to express un-spliced mRNA transporting in cytoplasm.33) Combination of the Tax and Rex proteins encoded by a single mRNA operated at different levels of viral gene expression, and accomplished a unique control of the viral replication.34)–36)
The control mechanism is very unique and makes the viral replication transient as summarized in the followings (Fig. 2): Transcription of the provirus genome is initiated by cellular machinery at 5′ LTR (Long Terminal Repeat) and terminates at 3′ LTR. The transcripts are fully spliced like cellular transcripts into pX mRNA which encodes p40Tax and p27Rex. One of the products, Tax is a potent transcriptional activator and thus strongly activates the viral transcription, and the transcripts are mostly spliced. After repeating such process, the accumulated Rex protein suppresses splicing of the viral transcripts at visible level. Consequently, unspliced viral RNA, gag- and env-mRNAs are expressed in the cytoplasm and translated into Gag and Env proteins, which are components of viral particles. The suppression of splicing of the viral RNA in turn reduced the level of fully spliced pX mRNA, and thus resulted in down-regulation of viral gene transcription. After such process, the viral expression became moderate quickly and transient. Depend on this regulation, HTLV-1 was able to quickly replicate, but was able to escape form the host immune responses. A similar regulatory mechanism was later identified in HIV with Tat and Rev proteins.37)
Additional accessory proteins to Tax and Rex were also identified including p12, p35 and some others encoded by alternatively spliced RNA of the pX region.38) Some functions of these alternative proteins could be significant in viral replication and pathogenesis, however, mostly are remained to be studied.
4. Etiology of adult T-cell leukemia
The most critical question on newly identified HTLV-1 was whether it is an etiologic agent of ATL or not. When suitable animal model is not available, the etiology of human disease is hard to answer. We therefore challenged a molecular approach focusing on a character of cancer cells.
The retrovirus infection integrates the pro-viruses into random site of the host chromosomal DNA. In fact, the integration sites in HTLV-1 carriers were confirmed to be mostly random. On the other hand, tumors are known to arise from a single malignantly transformed cell. Therefore, if the virus infection played a causative role in tumorigenesis, the resulted tumor cells would be a clone of the infected original cell. This could be examined by clonality of the provirus integration site in ATL cells. If the viral infection is just passenger such as infection after tumorigenesis, the integration sites would not be clonal.
Leukemia cell DNA was digested with a restriction enzyme, Pst-1, and subjected to Southern blot analysis. Every case of 122 patients gave two distinct bands with cellular franking sequences in addition to the internal fragments, clearly indicating all clonal in the provirus integration.39) We therefore concluded that HTLV-1 is the “causative agent” for ATL.
We had unexpected experience with a few cases of HTLV-1 carriers. These cases were never been diagnosed as any type of ATL, but showed clonal integration site of the provirus. It might have represented antigen stimulation of specific T cell clone, however, not further investigated.
5. Pathogenic mechanism of adult T-cell leukemia
HTLV-1 infection was proposed as an etiology of ATL.39) The most interesting question was its mechanism. Animal retroviruses induce leukemia either through “viral oncogene” or “insertional activation” of a cellular proto-oncogene. However, neither mechanism was applied to HTLV-1 in ATL.39) The reasons were 1) DNA sequence in the pX region did not show any homology to cellular DNA denying the nature of oncogene (MY, unpublished observation), and 2) The sites of provirus integration in ATL cells were clonal in each patient, however, were not common in any specific region among patients.40) These observations suggested the third mechanism in which Tax might be involved. Possible roles of Tax had been suggested by 1) Transforming capacity of Rat-1 cells, a rat fibroblast cell line,41) 2) Immortalizing activity of primary T cells in vitro,42) and 3) Tumorigenic activity in transgenic mice.43) So we focused on molecular function of Tax.
5.1. Multidisciplinary function of Tax
As introduced in the previous section 3, Tax is a activator of the transcription from the HTLV-1 LTR. This finding has triggered the long and wide stories of Tax, which include today multidisciplinary functions44) (Fig. 2): Categorically it covers 1) specific trans-activation and trans-repression of transcription of some sets of genes, 2) inhibition of tumor suppressor proteins, 3) attenuation of cell cycle checkpoints, 4) enhancement of apoptosis-suppressing genes and repression of apoptosis-inducing genes, and 5) repression of DNA repair. These mechanisms and effects are partly discussed in the following sections, #5.1.1, #5.1.2 and #5.1.3.
5.1.1. Transcriptional activation and repression
In addition to LTR activation, Tax was found to activate cellular genes also. Surprisingly furthermore, Tax was also found to repress transcription of another series of cellular genes. Today, a number of cellular genes have been identified as transcriptional targets of Tax. Broadly speaking, through transcriptional regulation Tax seems to enhance expression of many growth-promoting genes and repress another series of growth-suppressing genes. Some examples are discussed in the following sections.
5.1.1.1. CREB/Tax complex in LTR activation
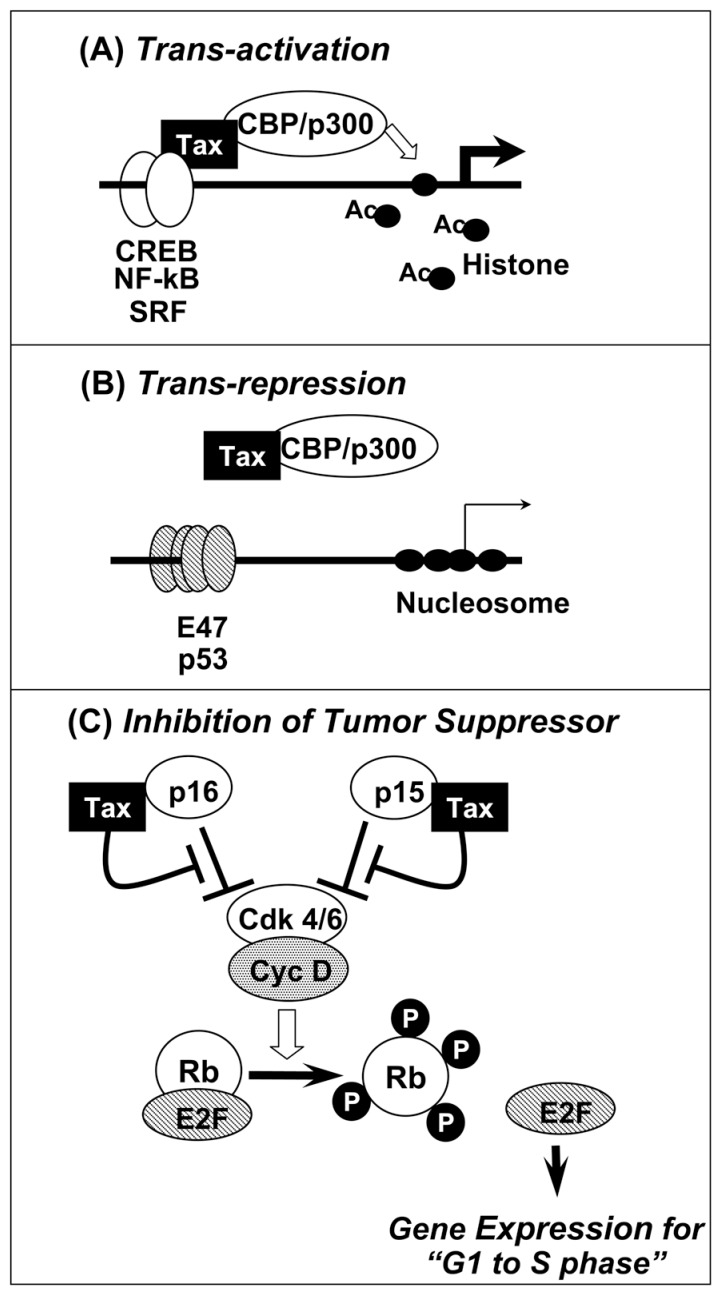
In the activation mechanism of the transcription from the LTR, Tax was demonstrated to bind to CREB (Cyclic AMP Response Element Binding protein) and CBP (CREB Binding Protein) forming ternary complex on the enhancer DNA,45) where CREB is DNA binding protein and CBP is a transcriptional cofactor (Fig. 3A). In normal regulation, CREB is phosphorylated depending upon cellular signal and the p-CREB becomes to interact with CBP on enhancer element in the LTR.46) The CBP in such complex opens nucleasome structure through its histone acetyl transferase activity for transcriptional initiation. In HTLV-1 infected cells, instead, Tax binds to both CREB and CBP without any signal, and forms active complex on the enhancer DNA.45) This signal independent complex formation enables the constitutive activation of LTR as long as Tax is expressed.
Fig. 3.
Mode of Tax functions. Typical examples are (a) transcriptional trans-activation with CREB, NF-kB or SRF, (b) transcriptional trans-repression with p53 or E47, and (c) promotion of cells from G1 toward S phase of cell cycle through inhibition of tumor suppressor proteins p16ink4 and p15ink4. Closed circle: histone; Ac: Acetyl residue.
5.1.1.2. I-kB/Tax and NF-kB/Tax in activation of IL-2R
Tax is a potent activator of the viral LTR. This finding led us to postulate activation of cellular gene by Tax and the hypothesis was first examined with IL-2 receptor gene. The IL-2R was reported to be expressed on ATL cell surface at a high level and was supposed essential for its abnormal proliferation. At this time period, only one molecule (CD25, α subunit) was known for IL-2R although α and β subunits were shown later to be required for the activity.
Tax expression vector was found to robustly activate the IL-2R promoter.47)–49) Furthermore, induction of cellular IL-2R gene was demonstrated by Tax induction with Cd++ ion in JPX-9 cell lines. This was an exciting finding because it was thought to represent the mechanism how the viral infection explains abnormal growth of ATL cells. It was unfortunately shown today not to be sufficient for T cell growth, because both α and β expressions were required for the full activity. Nevertheless, our report on IL-2Rα induction triggered number of trials to identify cellular targets of Tax.
Regulation of IL-2Rα expression is under control of NF-kB and Tax was found to activate NF-kB pathway. The mechanism was rather unique since Tax operated at two different levels: NF-kB is bound with I-kB and kept in cytoplasm as inactive complex. The I-kB in the complex is degraded through phosphorylation responding to cellular signals, consequently the released NF-kB is translocated into the nucleus for transcription. The first mechanism is Tax binding to I-kB followed by dissociation of I-kB/NF-kB complex promoting nuclear translocation of NF-kB51) (Fig. 3A). Second mechanism is Tax binding to NF-kB on the enhancer DNA forming complex of NF-kB/Tax and then NF-kB/Tax/ p300.50) Principle for the activation is similar to the mode of CREB/Tax/CBP complex in LTR activation.
Considerably later, another mechanism for activation of NF-kB was reported: Tax enhanced I-kB degradation through activating IKKγ (I-kB kinaseγ) and resulted in nuclear translocation of the released NF-kB for transcriptional activation.52) All together, the NF-kB signaling pathway is activated at several steps by Tax through activation of IKKγ, binding to I-kB dissociating I-kB/NF-kB complex, and enhancement of NF-kB activity on the enhancer DNA through Tax/NF-kB complex formation. The findings on activation of the NF-kB pathway at several steps may be unusual, but it may be a reflection of its importance in survival and proliferation of HTLV-1-infected T cells.
5.1.1.3. p300/Tax complex in repression of p53
During investigation of trans-activation, Tax was found to repress transcription of certain genes. The first report in this category was repression of DNA polymerase β,53) which functions in DNA repair. The interest was two folds: Its significance in pathogenesis and mechanism how a activator protein represses transcription in other hand.
P53 is a tumor suppressor with transcription factor and is a “guardian of chromosomal DNA”. Mutations of p53 were frequently observed in various human cancers, however, not frequently in ATL cases. As one of the reason for this, Tax was found to represses p53-dependent transcription.54) In transcription, p53 interacts with p300 on its binding site in the promoter and initiates transcription. In this system, Tax binds to p300, but not to p53. Consequently, p300 is sequestered from the activation system by binding to Tax, and thus represses p53-dependent transcription (Fig. 3B). The mechanism represents an indirect attenuation of tumor suppressor function of p53 and explains abnormal cell cycle regulation. A similar mechanism is also operating for repression of DNA polymerase β gene, which would enhance mutation rate in infected cells.
The principle of trans-activation and trans-repression by Tax depend on the same mechanism: Tax interaction with two factors A and B and formation of a ternary complex, [A]/Tax/[B], is an activation mechanism, on the other hand, Tax interaction with only one of them interferes the basal activity achieving repression (Fig. 3A and B).
5.1.2. Inhibition of tumor suppressor proteins
Analysis of mode of Tax interaction with NF-kB unexpectedly led us to finding of Tax interaction with tumor suppressor protein. The binding domain of NF-kB to Tax was ankyrin motifs.55) As one of proteins carrying the ankyrin motifs, p16ink4 was focused because it was well known tumor suppressor protein. And we found a new category of Tax function, an inhibition of tumor suppressor proteins. Down-regulation of signaling pathways mediated by Rb, APC and p53 are discussed in the next.
5.1.2.1. Tumor suppressor Rb
p16ink4 and p15ink4 are tumor suppressor proteins keeping Cdk4 inactive, which enables Rb to hold E2Fs as inactive and to arrest cell cycle at G1 phase. Mutations or silencing of both alleles were widely detected in many types of human tumors. We found that Tax bound to p16ink4 and p15ink4 and induced activation of Cdk4. The activated Cdk4 then phosphorylates Rb protein and finally induced cell transition from G1 to S phase of cell cycle56) (Fig. 3C). Therefore, it is conceivable to expect that HTLV-1 infection and Tax expression inactivates both p16ink4 and p15ink4 resulting in activation of Cdk4, which subsequently phosphorylates Rb protein to releases E2F. The released E2F then activates transcription of various genes required for compelling cells into a G1-S transition and promotes abnormal cell cycles. This is an independent growth promoting activity of Tax from transcriptional activation.
5.1.2.2. Tumor suppressor hDLG and APC
To identify a series of targets of Tax, we used yeast two hybrid system. Among various proteins identified, another tumor suppressor protein, hDlg, was focused as a binding target of Tax.57) The Dlg is identified as a tumor suppressor in Drosophila and the human counterpart hDlg is known to interact with several cellular proteins and also with DNA tumor virus transforming proteins. The PDZ domain of hDlg interacted with the C-terminus of Tax and also with that of APC, thus competing with each other. Through such competition, Tax would interrupt hDlg-APC interaction and possibly abrogate growth-suppressing machinery finally enhancing cell growth.57)
5.1.2.3. Tumor suppressor p53
As discussed previous section, #5.1.1.3, a tumor suppressor protein p53 is trans-repressed by Tax through binding to p300. Down-regulation of p53 target genes would induce instability of cellular DNA and increase risk of HTLV-1 infected cells for malignant transformation.
5.1.3. Other functions of Tax
5.1.3.1. Suppression of apoptosis
Apoptosis is a programmed cell death for removing abnormal and undesired cells and plays crucial roles in organ development and protection from undesired events. Failure in apoptosis of abnormal cells is thought to be a significant process toward malignant cells. In accordance with this notion, T cell lines infected with HTLV-1 were reported to be resistant to apoptotic signals.58) Tax was shown to be involved in this mechanism through: 1) NF-kB activation through dissociation of IkB-NF-kB complex in cytoplasm and through complex formation of NF-kB-Tax-p300 in the nucleus50),51) as discussed in the previous section, 2) activation of transcription of Bcl-X gene,59) and 3) trans-repression of Bax gene.60) According to these effects, Tax enables infected cells to survive even under critically harmful alterations.
5.1.3.2. DNA repair and cell cycle check point
DNA repairs are crucial function to protect DNA from mutations, and thus lower activity of the repair would increase mutation rate. Surprisingly, Tax was shown to enhance accumulation of mutations. This was demonstrated using a rat cell line carrying an indicator gene in presence or absence of Tax.61) Various mechanisms were proposed for this phenotype: As examples, 1) Tax represses transcription of DNA polymerase β which is involved in DNA repair;53) 2) Tax inhibits DNA topoisomerase I required for DNA replication and repair;62) 3) Tax suppresses Mad1 function and attenuates G2/M cell cycle check point.63) These functions of Tax all together enhance mutation rates and make the abnormal cells possible to go through checkpoint fixing the mutations.
5.2. Possible molecular mechanism of ATL
5.2.1. Malignant transformation of infected cells
It is well established that cancer formation proceeds through multiple processes. In fact, multiple abnormalities are detected in a single cancer cell. This would be rational from the point of aspect that cells are protected through multiple mechanisms, so that, normal cells are able to tolerate against a single or just few damages. The multifold functions of Tax (discussed in the previous sections) may correspond to multiple alterations observed in cancer cells (Fig. 2): Tax expression upon HTLV-1 infection induces abnormal cell cycle through transcriptional activation and repression, inhibition of tumor suppressor proteins and attenuation of cell cycle checkpoints. Concurrently, Tax enhances mutation rates through inhibition of DNA repair. Because of Tax-mediated suppression of apoptosis and cell cycle checkpoint capacity, the mutated cells are now enforced to continue abnormal cell proliferation. Consequently, mutations are gradually accumulated in infected T cells, and after repeating these cycles, a cell will fortuitously accumulate the right combination of mutated DNA which would trigger malignant transformation and progress into malignant conversion.
5.2.2. Mode of pathogenesis in ATL
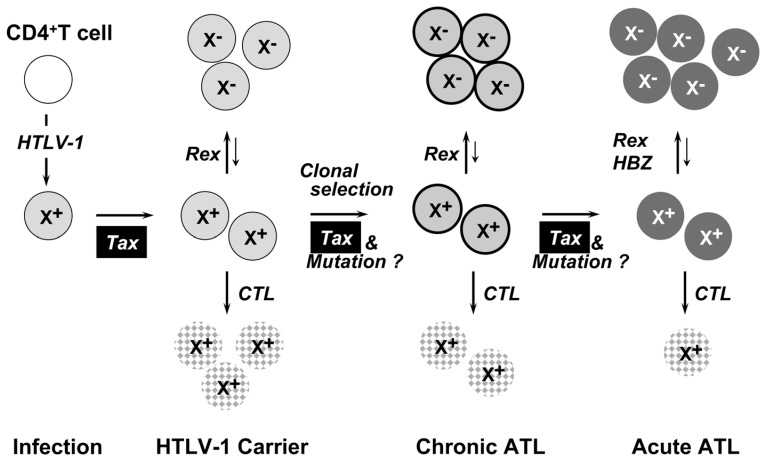
Tax is a potent modulator targeting multiple molecules. Nevertheless the prevalence of ATL shows long latency for 40~60 years after infection in rate of 3~5% among HTLV-1 carriers. The long latent period does not sound in good accordance with potent multiple functions of Tax. This apparent discrepancy would be probably due to extremely limited expression of HTLV-1 in vivo. In fact, it is reported that Tax expression is absolutely negative in the vast majority of ATL cells64) and also in non-malignant infected cell in carriers. Our proposal for possible mechanism of ATL pathogenesis is as follows (Fig. 4):
Fig. 4.
Probable mechanism of pathogenesis in ATL. Circles with X+ represents Tax expressing T cell and those with X− represents Tax expression negative infected T cell. CTL represents cytotoxic T cell against virus expressing cells and dotted circle represents infected cell injured.
Upon infection into T cell, the original expression of HTLV-1 genome is initiated by cellular machinery and then transiently amplified by the viral regulatory proteins as discussed above. The expressed viral Tax stimulates cell growth through multiple pathways and also stimulate virus production and infection, but the expression of Tax is transient and quickly turned off. Cells failed to be transient or delayed in shutting off the viral expression would be rejected by host immune response and only the latent cells for the viral expression would survive. After certain period for possibly rather long, any signal for infected T cells such as immune stimulation may re-initiate the viral expression and repeat the same cycle, but slowly increasing a population of infected T cells through their own replication and new infection. Repeating such cycles for long period, infected individuals stay at “virus carrier” state, but Tax-induced mutation would be accumulated in infected T cells.
Tax-mediated mutation, however, would finally hit right combination of genes for malignant transformation. This would be the critical event for ATL that triggers a clonal selection of infected T cells as a malignant leukemia cell. Even after such event, the selected cell clone might be frequently benign or limited in its expansion, but transient expression of the viral Tax would be repeated at similar levels to the carrier cells, and finally abrupt aggressive ATL. The rate of ATL is 3–5% of HTLV-1 carriers in their life span.
5.2.3. Transformation strategy similar to DNA tumor viruses
Through strenuous efforts on Tax function including ours and others, Tax has been turned out to be multi-functional interacting with cellular regulatory factors including Rb, p53, APC, CBP/p300, and some others. It is interesting to note that those factors referred above are also known as targets of T antigens of SV40 and Poliomaviruses, E6/E7 of Papillomaviruses, or E1A/B of Adenoviruses. Firstly, these similarities among evolutionally unrelated tumor viruses suggest strongly that the targets of Tax would play significant roles in the pathogenesis of ATL. Secondly, the viral similarity suggests that multiple functions of Tax might be a unique surviving strategy of HTLV-1. Retroviruses replicate its genome through transcription, but HTLV-1 dominantly through replication of infected cells. This has been achieved by transient viral expression by Rex and Tax acquired cell growth promotion activity similarly to DNA tumor virus replication. HTLV-1 thus is able to survival more effectively under strong immune response of the host.
6. Epilogue
Since HTLV-1 was isolated, molecular and epidemiologic understanding on the virus itself and ATL progressed rapidly and comprehensively during subsequent two decades. Epidemiology, clinical and basic sciences on ATL and HTLV-1 established prevention of HTLV-1 infection through bottle feeding and removal of sero-positive blood from the bank. Molecular biology of Tax greatly improved understanding of ATL and provided a probable mechanism for the leukemogenesis. However, we still have obstacles to be overcome. I like to introduce here issues left and add an additional tail which has been extended according to recent studies by other investigators.
6.1. Contradiction in Tax hypothesis
As discussed in the previous section, Tax can modulate cellular activities for proliferation, survival, and mutation, and thus strongly indicated to play central roles in leukemogenesis. On the other hand, however, it has been also known that viral expression is extremely limited at low levels in vivo. Some reports described that a vast majority (over 95%) of ATL cells is absolutely negative in HTLV-1 expression.64) Furthermore, Tax sequence in some cases of ATL had a termination mutation in the coding region, clearly indicating Tax function is no longer required for maintenance of the ATL cell phenotypes.65) Nevertheless, ATL cells in such patients kept tumor phenotypes such as an expression of IL-2Rα. It was therefore apparent additional abnormality should be identified for better understanding of ATL.
6.2. Anti-sense HBZ of HTLV-1
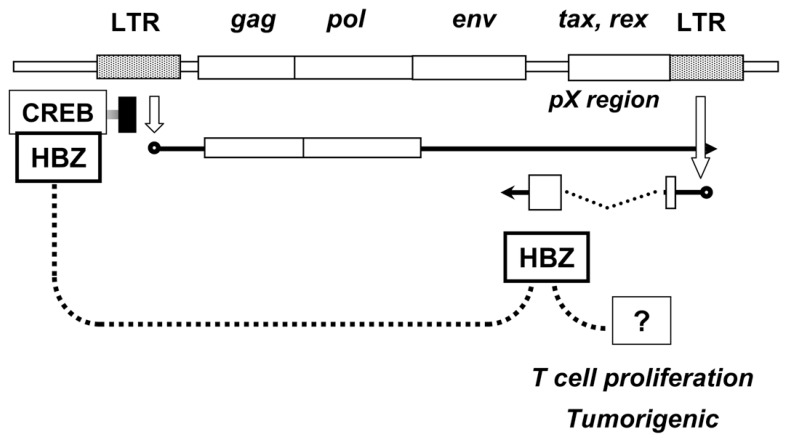
One solution of the contradiction in Tax hypothesis could be a “hit-and-run” mechanism. But, it apparently needs an additional player after Tax. Other possibility has been proposed by recent studies identifying a new viral gene. It is transcribed from the pX region by initiating at the 3′ LTR in the form of anti-sense RNA. The RNA is translated into a protein, HTLV-1 bZIP factor (HBZ), with basic leucine zipper motif (Fig. 5).
Fig. 5.
Illustration of HBZ expression and the functions.
The function of HBZ protein was first reported as transcription factor that repressed the viral expression, competing with Tax-mediated LTR activation.66) More strikingly, it was then shown to stimulate proliferation of T cells without translation into protein.67) Furthermore, transgenic mice expressing HBZ by T cell specific promoter developed lymphoma indicating HBZ alone is oncogenic.68)
Another striking feature of the HBZ is constitutive expression in vivo:67) Expression was detected by PCR in most cases of ATL patients and also in viral carriers so far tested. Taking all these observations into account, HBZ has been expected to be responsible for inducing and persisting tumor state even after Tax was shut off. Although the mechanism how HBZ induces tumors in transgenic mice is yet unknown, it could be speculate that HBZ suppresses Tax function protecting infected cells from immune response and concurrently contributes to persistence of the tumor phenotypes through continuous expression (Fig. 4).
6.3. Questions remained in pathogenesis
6.3.1. Clonal expansion of infected cell
As discussed in previous section #5.2 and #6.2, issue on Tax expression seemed to be solved by HBZ. However, HBZ is still unable to explain a clonal selection of infected cell toward malignant transformation because it would express in all infected cells. A key question how a given T cell infected with HTLV-1 is selected for clonal expansion for triggering ATL is still remained.
6.3.2. Specificity to CD4+ T cells
Receptor for HTLV-1 infection was identified as Glut-1, which is ubiquitously expressed as a glucose transporter; nevertheless, HTLV-1 infection is restricted to T cells in vivo. Furthermore, ATL cells are always CD4+ T cells. HTLV-1 infects both CD4+ and CD8+ T cells, but immortalizes only CD4+ T cells. Mechanism of such specific infection and transformation is totally unknown. The functions of Tax and HBZ are unlikely to explain the cell type specificity; therefore, we need an additional event for malignant transformation in ATL.
During the last three decades since discovery of ATL, the HTLV-1/ATL research has provided great opportunities for scientists to study tumor initiation and progression directly in humans. Even though the ATL is only in small population, it would provide an insight into oncogenesis, immune response mechanisms, and treatment and prevention strategies for cancer in humans. Efforts for further understanding and better treatment of ATL seemed to be curtailed recently, however, it should be emphasized to re-visit remained issues and prematurely derived conclusion. Recent technology advances in dealing with cancer stem cell, micro RNA, molecular profiling and individual genome sequencing would make it possible to lead complete solution of ATL.
Abbreviations
- HTLV-1
Human T-cell Leukemia Virus Type 1
- ATL
Adult T-cell Leukemia
- pX
Functionary Unknown X Region with 1.6 Kb long in HTLV-1 Genome
- Tax
Trans-activator Encoded by pX Region of HTLV-1
- Rex
Regulator Encoded by pX Region of HTLV-1
Profile
Mitsuaki Yoshida was born in 1939 and started his research career in 1961 at Faculty of Pharmaceutical Science, the University of Tokyo on structure and function of transfer RNA. Being nominated in 1975 for a Head of Viral Oncology at Cancer Institute (Japanese Foundation of Cancer Research), his interest has been focused on molecular mechanism of viral oncogenesis. He discovered there a new oncogene “yes” in 1980 from Y73 retrovirus which had been isolated from chicken sarcoma in Japan. Almost in parallel, he initiated retrovirus hunting in human cancer in 1981. His most profound contribution to oncology is isolation and molecular characterization of the first human retrovirus, Human T-cell Leukemia Virus type 1 (HTLV-1). Through discovery of the viral oncogenic gene “tax” in HTLV-1 followed by continuous effort for elucidation of its pleiotropic functions, he has proposed a plausible molecular mechanism how the viral infection induces Adult T-cell Leukemia (ATL) in human being. He was promoted to Professor at the University of Tokyo in 1989 and was Director of the Institute of Medical Science, the University of Tokyo between 1986 and 1988. He was awarded Science Award of Princess Takamatsu Cancer Research Fund in 1984, Takeda Medical Science Prize in 1985, Asahi Prize in 1987 and Medal with Purple Ribbon in 2000. He moved to Banyu Pharmaceutical Co. Ltd, a subsidiary of Merck Corporation, USA in 1999 and served as Director of the Research Institute, where he experienced drug discovery in industry. He was elected as an honorary member of Japanese Cancer Association. In 2009, he was assigned to Director of Cancer Chemotherapy Center, Cancer Institute (Japanese Foundation of Cancer Research).

References
- 1).Stehelin D., Guntaka R.V., Varmus H.E., Bishop J.M. (1976) Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma viruses. J. Mol. Biol. 101, 349–365 [DOI] [PubMed] [Google Scholar]
- 2).Spector D.H., Baker B., Varmus H.E., Bishop J.M. (1978) Characteristics of cellular RNA related to the transforming gene of avian sarcoma viruses. Cell 13, 381–386 [DOI] [PubMed] [Google Scholar]
- 3).Yoshida M. (2005) Discovery of HTLV–1, the first human retrovirus, its unique regulatory mechanisms, and insights into pathogenesis. Oncogene 24, 5931–5937 [DOI] [PubMed] [Google Scholar]
- 4).Gallo R.C. (2005) History of the discoveries of the first human retroviruses: HTLV-1 and HTLV-2. Oncogene 24, 5926–5930 [DOI] [PubMed] [Google Scholar]
- 5).Uchiyama T., Yodoi J., Sagawa K., Takatsuki K., Uchino H. (1977) Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood 50, 481–492 [PubMed] [Google Scholar]
- 6).Hattori T., Uchiyama T., Toibana T., Takatsuki K., Uchino H. (1981) Surface phenotype of Japanese adult T-cell leukemia cells characterized by monoclonal antibodies. Blood 58, 645–647 [PubMed] [Google Scholar]
- 7).Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K.I., et al. (1981) Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 78, 6476–6480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Hinuma Y., Komoda H., Chosa T., Kondo T., Kohakura M., Takenaka T., et al. (1982) Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide sero-epidemiologic study. Int. J. Cancer 29, 631–635 [DOI] [PubMed] [Google Scholar]
- 9).Miyoshi I., Miyamoto K., Sumida M., Nishihara R., Lai M., Yoshimoto S., et al. (1981) Chromosome 14q+ in adult T-cell leukemia. Cancer Genet. Cytogenet. 3, 251–259 [DOI] [PubMed] [Google Scholar]
- 10).Miyoshi I., Kubonishi I., Yoshimoto S., Shiraishi Y. (1981) A T-cell line derived from normal human cord leukocytes by co-culturing with human leukemic T-cells. Gann 72, 978–981 [PubMed] [Google Scholar]
- 11).Yoshida M., Miyoshi I., Hinuma Y. (1982) Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79, 2031–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Poiesz B.J., Ruscetti F.W., Gazdar A.F., Bunn P.A., Minna J.D., Gallo R.C. (1980) HTLV Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77, 7415–7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Watanabe T., Seiki M., Yoshida M. (1984) HTLV type I (US isolate) and ATLV (Japanese isolate) are the same species of human retrovirus. Virology 133, 238–241 [DOI] [PubMed] [Google Scholar]
- 14).Watanabe T., Seiki M., Yoshida M. (1983). Retrovirus terminology. Science 222, 1178. [DOI] [PubMed] [Google Scholar]
- 15).Gallo R., Wong-Staal F., Montagnier L., Haseltine W.A., Yoshida M. (1988) HIV/HTLV gene nomenclature. Nature 333, 504. [DOI] [PubMed] [Google Scholar]
- 16).Gallo R.C. (2005) The discovery of the first human retrovirus: HTLV-1 and HTLV-2. Retrovirology 2, 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Seiki M., Hattori S., Yoshida M. (1982) Human adult T-cell leukemia virus: Molecular cloning of the provirus DNA and the unique terminal structure. Proc. Natl. Acad. Sci. USA 79, 6899–6902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Seiki M., Hattori S., Hirayama Y., Yoshida M. (1983) Human adult T-cell leukemia virus: Complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc. Natl. Acad. Sci. USA 80, 3618–3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Grassmann R., Dengler C., Muller-Fleckenstein I., Fleckenstein B., McGuire K., Dokhelar M.C. (1989) Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a Herpesvirus saimiri vector. Proc. Nat. Acad. Sci. USA 86, 3351–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Shimotohno K., Takahashi Y., Shimizu N., Gojobori T., Golde D.W., Chen I.S., et al. (1985) Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: an open reading frame for the protease gene. Proc. Natl. Acad. Sci. USA 82, 3101–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Watanabe T., Seiki M., Tsujimoto H., Miyoshi I., Hayami M., Yoshida M. (1985) Sequence homology of the simian retrovirus (STLV) genome with human T-cell leukemia virus type I (HTLV-I). Virology 144, 59–65 [DOI] [PubMed] [Google Scholar]
- 22).Sagata N., Yasunaga T., Ohishi K., Suzuku-Kawamura J., Onuma M., Ikawa Y. (1984) Comparison of the entire genomes of bovine leukemia virus and human T-cell leukemia virus and characterization of their unidentified open reading frames. EMBO J. 20, 3231–3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Blayney D.W., Blattner W.A., Jaffe E.S., Gallo R.C. (1983) Retroviruses in human leukemia. Hematol. Oncol. 1, 193–204 [DOI] [PubMed] [Google Scholar]
- 24).Shimoyama M., Kagami Y., Shimotohno K., Miwa M., Minato K., Tobinai K., et al. (1986) Adult T-cell leukemia/lymphoma not associated with human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 83, 4524–4528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Gessain A., Barin F., Vernant J.C., Gout O., Maurs L., Calender A., et al. (1985) Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet 326, 407–410 [DOI] [PubMed] [Google Scholar]
- 26).Osame M., Usuku K., Izumo S., Ijichi N., Amitani H., Igata A., et al. (1986) HTLV-I associated myelopathy, a new clinical entity. Lancet 327, 1031– 1032 [DOI] [PubMed] [Google Scholar]
- 27).Kiyokawa T., Seiki M., Imagawa K., Shimizu F, Yoshida M. (1984) Identification of a protein (p40x) encoded by a unique sequence pX of human T-cell leukemia virus type I. Gann 75, 747–751 [PubMed] [Google Scholar]
- 28).Seiki M., Hikikoshi A., Taniguchi T., Yoshida M. (1985) Expression of the pX gene of HTLV-1: General splicing mechanism in the HTLV family. Science 228, 1532–1534 [DOI] [PubMed] [Google Scholar]
- 29).Kiyokawa T., Seiki M., Iwashita S., Imagawa K., Shimizu F., Yoshida M. (1985) p27x–III and p21x–III, proteins encoded by the pX sequence of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 82, 8359–8363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Sodroski J.G., Rosen C.A., Haseltine W.A. (1984) Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science 225, 381–385 [DOI] [PubMed] [Google Scholar]
- 31).Fujisawa J., Seiki M., Kiyokawa T., Yoshida M. (1985) Functional activation of long terminal repeat of human T-cell leukemia virus type I by trans-acting factor. Proc. Natl. Acad. Sci. USA 82, 2277–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Seiki M., Inoue J., Takeda T., Hikikoshi A., Sato M., Yoshida M. (1985) The p40x of human T-cell leukemia virus type I is a trans-acting activator or viral gene transcription. Jpn. J. Cancer Res. (Gann) 76, 1127–1131 [PubMed] [Google Scholar]
- 33).Inoue J., Seiki M., Yoshida M. (1986) The second pX product p27x–III of HTLV-1 is required for gag gene expression. FEBS 209, 187–190 [DOI] [PubMed] [Google Scholar]
- 34).Hidaka M., Inoue J., Yoshida M., Seiki M. (1988) Post-transcriptional regulator (rex) of HTLV-1 initiates expression of Viral structural proteins but suppresses expression of regulatory proteins. EMBO J. 7, 519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Inoue J., Yoshida M., Seiki M. (1987) Transcriptional (p40x) and post-transcriptional (p27x-III) regulator are required for the expression and replication of human T-cell leukemia virus type I genes. Proc. Natl. Acad. Sci. USA 84, 3653–3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Yoshida M. (1987) Expression of the HTLV-1 genome and its association with a unique T-cell malignancy. Biochem. Biophys. Acta Cancer Rev. 907, 145–161 [DOI] [PubMed] [Google Scholar]
- 37).Klotman M.E., Kim S., Buchbinder A., DeRossi A., Baltimore D., Wong-Staal F. (1991) Kinetics of expression of multiply spliced RNA in early human immunodeficiency virus type 1 infection of lymphocytes and monocytes. Proc. Natl. Acad. Sci. USA 88, 5011–5015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Johnson J.M., Nicot C., Fullen J., Ciminale V., Casareto L., Mulloy J.C., et al. (2001) Free major histocompatibility complex class I heavy chain is preferentially targeted for degradation by human T-cell leukemia/lymphotropic virus type 1 p12(I) protein. J. Virol. 75, 6086–6094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Yoshida M., Seiki M., Yamaguchi K., Takatsuki K. (1984) Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc. Natl. Acad. Sci. USA 81, 2543–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Seiki M., Eddy R., Shows T.B., Yoshida M. (1984) Nonspecific integration of the HTLV pro-virus genome into adult T-cell leukemia cells. Nature (London) 309, 640–642 [DOI] [PubMed] [Google Scholar]
- 41).Tanaka A., Takahashi C., Yamaoka S., Nosaka T., Maki M., Hatanaka M. (1990) Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc. Natl. Acad. Sci. USA 87, 1071–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Pozzatti R., Vogel J., Jay G. (1990) The human T-lymphotropic virus type I tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol. Cell Biol. 10, 413–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Nerenberg M., Hinrichs S.H., Reynolds R.K., Khoury G., Jay G. (1987) The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 237, 1324–1329 [DOI] [PubMed] [Google Scholar]
- 44).Yoshida M., Seiki M. (1987) Recent advances in molecular biology of HTLV-1: trans-activation of viral and cellular genes. Ann. Rev. Immunol. 5, 541–559 [DOI] [PubMed] [Google Scholar]
- 45).Fujisawa J., Toita M., Yoshida M. (1989) A unique enhancer element for the trans activator (p40tax) of human T-cell leukemia virus type I that is distinct from cyclic AMP- and 12-O-Tetra-decanoylphorbol-13-acetate-responsive elements. J. Virol. 63, 3234–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Kwok R.P., Lundblad J.R., Chrivia J.C., Richards J.P., Bachinger H.P., Brennan R.G., et al. (1994) Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature 370, 223–226 [DOI] [PubMed] [Google Scholar]
- 47).Yoshida M., Fujisawa J., Inoue J., Seiki M. (1986) Mechanism of the gene expression of HTLV-I and its association with ATL. AIDS Res. 2, 71–78 [PubMed] [Google Scholar]
- 48).Inoue J., Seiki M., Taniguchi T., Tsuru S., Yoshida M. (1986) Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 5, 2883–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Maruyama M., Shibuya H., Harada H., Hatakeyama M., Seiki M., Fujita T., et al. (1987) Evidence for aberrant activation of the interleukin-2 autocrine loop by HTLV-1-encoded p40x and T3/ Ti complex triggering. Cell 48, 343–350 [DOI] [PubMed] [Google Scholar]
- 50).Hirai H., Fujisawa J., Suzuki T., Ueda K., Muramatsu M., Tsuboi A., et al. (1992) Transcriptional activator Tax of HTLV-1 binds to the NF-kB precursor p105. Oncogene 7, 1737–1742 [PubMed] [Google Scholar]
- 51).Suzuki T., Hirai H., Murakami T., Yoshida M. (1995). Tax protein of HTLV-1 destabilizes the complexes of NF-kB and IkB-a and induces nuclear translocation of NF-kB for transcriptional activation. Oncogene 10, 1199–1207 [PubMed] [Google Scholar]
- 52).Jin D.Y., Giordano V., Kibler K.V., Nakano H., Jeang K.T. (1999). Role of adapter function in oncoprotein-mediated activation of NF-κB. Human T-cell leukemia virus type I tax interacts directly with IκB kinase γ. J. Biol. Chem. 274, 17402–17405 [DOI] [PubMed] [Google Scholar]
- 53).Jeang K.T., Widen S.G., Semmes O.J., 4th, Wilson S.H. (1990) HTLV-I trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science 247, 1082–1084 [DOI] [PubMed] [Google Scholar]
- 54).Suzuki T., Kitao S., Matsushime H., Yoshida M. (1999) Tax protein of HTLV-1 inhibits CBP/ p300-mediated transcription by interfering with recruitment of CBP/p300 onto DNA element of E-box or p53 binding site. Oncogene 18, 4137–4143 [DOI] [PubMed] [Google Scholar]
- 55).Hirai H., Suzuki T., Fujisawa J., Inoue J., Yoshida M. (1994) Tax protein of human T-cell leukemia virus type I binds to the ankyrin motifs of inhibitory factor κB and induces nuclear translocation of transcription factor NF-κB proteins for transcriptional activation. Proc. Natl. Acad. Sci. USA 91, 3584–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Suzuki T., Kitao S., Matsushime H., Yoshida M. (1996) HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 15, 1607–1614 [PMC free article] [PubMed] [Google Scholar]
- 57).Suzuki T., Ohsugi Y., Uchida-Toita M., Akiyama T., Yoshida M. (1999) Tax oncoprotein of HTLV-1 binds to the human homologue of Drosophila discs large tumor suppressor protein, hDLG, and perturbs its function in cell growth control. Oncogene 18, 5967–5972 [DOI] [PubMed] [Google Scholar]
- 58).Copeland K.F., Haaksma A.G., Goudsmit J., Krammer P.H., Heeney J.L. (1994) Inhibition of apoptosis in T cells expressing human T cell leukemia virus type I Tax. AIDS Res. Hum. Retro-viruses 10, 1259–1268 [DOI] [PubMed] [Google Scholar]
- 59).Tsukahara T., Kannagi M., Ohashi T., Kato H., Arai M., Nunez G., et al. (1999) Induction of Bcl-x(L) expression by human T-cell leukemia virus type 1 Tax through NF-κB in apoptosis-resistant T-cell transfectants with Tax. J. Virol. 73, 7981–7987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Brauweiler A., Garrus J.E., Reed J.C., Nyborg J.K. (1997) Repression of bax gene expression by the HTLV-1 Tax protein: implications for suppression of apoptosis in virally infected cells. Virology 23, 135–140 [DOI] [PubMed] [Google Scholar]
- 61).Miyake H., Suzuki T., Hirai H., Yoshida M. (1999) Trans-activator tax of human T-cell leukemia virus type 1 enhances mutation frequency of the cellular genome. Virology 253, 155–161 [DOI] [PubMed] [Google Scholar]
- 62).Suzuki T., Uchida-Toita M., Andoh T., Yoshida M. (2000) HTLV-1 Tax oncoprotein binds to DNA topoisomerase I and inhibits its catalytic activity. Virology 270, 291–298 [DOI] [PubMed] [Google Scholar]
- 63).Jin D.Y., Spencer F., Jeang K.T. (1998) Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell 93, 81–91 [DOI] [PubMed] [Google Scholar]
- 64).Shimoyama M., Tobinai K., Ito M., Ito S., Ikeda S., Tajima K. (1989) Detection of mRNA for the tax1/rex1 gene of human T-cell leukemia virus type I in fresh peripheral blood mononuclear cells of adult T-cell leukemia patients and viral carriers by using the polymerase chain reaction. Proc. Natl. Acad. Sci. USA 86, 5620–5624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Takeda S., Maeda M., Morikawa S., Taniguchi Y., Yasunaga J., Nosaka K., et al. (2004) Genetic and epigenetic inactivation of tax gene in adult T-cell leukemia cells. Int. J. Cancer 109, 559–567 [DOI] [PubMed] [Google Scholar]
- 66).Gaudray G., Gachon F., Basbous J., Biard-Piechaczyk M., Devaux C., Mesnard J.M. (2002) The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J. Virol. 76, 12813–12822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Satou Y., Yasunaga J., Yoshida M., Matsuoka M. (2006) HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc. Natl. Acad. Sci. USA 103, 720–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Matsuoka M., Green P.L. (2009) The HBZ gene, a key player in HTLV-1 pathogenesis. Retrovirology 6, 71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]