Abstract
Although obesity is a major background of life style-related diseases such as diabetes mellitus, lipid disorder, hypertension and cardiovascular disease, the extent of whole body fat accumulation does not necessarily the determinant for the occurrence of these diseases. We developed the method for body fat analysis using CT scan and established the concept of visceral fat obesity, in other word metabolic syndrome in which intra-abdominal visceral fat accumulation has an important role in the development of diabetes, lipid disorder, hypertension and atherosclerosis. In order to clarify the mechanism that visceral fat accumulation causes metabolic and cardiovascular diseases, we have analyzed gene expression profile in subcutaneous adipose tissue and visceral adipose tissue. From the analysis, we found that adipose tissue, especially visceral adipose tissue expressed abundantly the genes encoding bioactive substances such as cytokines, growth factors and complements. In addition to known bioactive substances, we found a novel collagen-like protein which we named adiponectin. Adiponectin is present in plasma at a very high concentration and is inversely associated with visceral fat accumulation. Adiponectin has anti-diabetic, anti-hypertensive and anti-atherogenic properties and recent studies revealed that this protein has an anti-inflammatory and anti-oncogenic function. Therefore hypoadiponectinemia induced by visceral fat accumulation should become a strong risk factor for metabolic and cardiovascular diseases and also some kinds of cancers.
In this review article, I would like to discuss the mechanism of life style-related diseases by focusing on the dysregulation of adiponectin related to obesity, especially visceral obesity.
Keywords: visceral fat, metabolic syndrome, adiponectin, hypoadiponectinemia
Fat distribution and morbidity of obesity
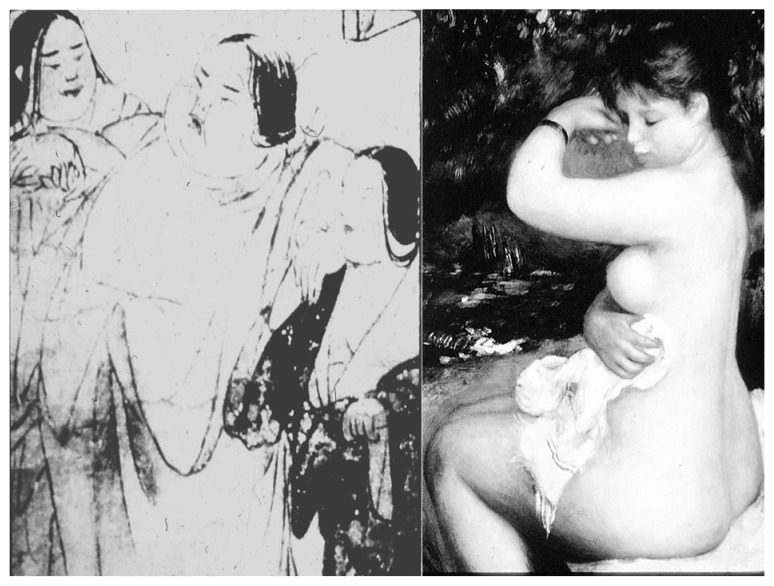
Contemporary civilized countries provide an increasing number of opportunities for overeating and decreased muscular exercise, where common health problems are closely correlated to this over-nutritional state and its typical consequence, obesity. However, previous studies on the morbidity of obesity have indicated that the severity of obesity-related diseases such as diabetes mellitus, lipid disorders and cardiovascular disease does not necessarily correlate to the extent of body fat accumulation, but it closely related to body fat distribution. Several classifications of obesity concerning body fat distribution have been proposed in order to distinguish the possible mechanisms of obesity-related diseases. An ancient Japanese artist showed great insight into the morbidity of obesity 800 years ago when he painted a picture of an obese woman with the title “A very obese woman who can hardly walk” (Fig. 1) in the old Japanese picture scroll “Yamai Zoshi” which means an illustrated scroll for various diseases. Comparing the body figure of an obese girl painted by famous Renoir, she has marked adiposity in her abdomen.
Fig. 1.
High risk obesity (left) and low risk obesity (right) in classical paintings.
In the end of 1940s, Prof. Vague noted that “Fat excess is dangerous because of its metabolic complications and a woman normally has twice a man’s fat mass, i.e. the mass of an obese man. Though she is as often obese a man or is fatter, she dies later and less often from metabolic complications of obesity.” Then he proposed a classification of obesity into android type and gynoid type in 1947.1) His classification was based on the brachio-femoral adipomuscular ratio (BFAMR) and the subjects with higher BFAM were designated to be android type in whom metabolic complications were more prevalent. Although his classification is not exactly the same as current one, he is no doubt a pioneer of recognition for high risk obesity based on fat distribution.
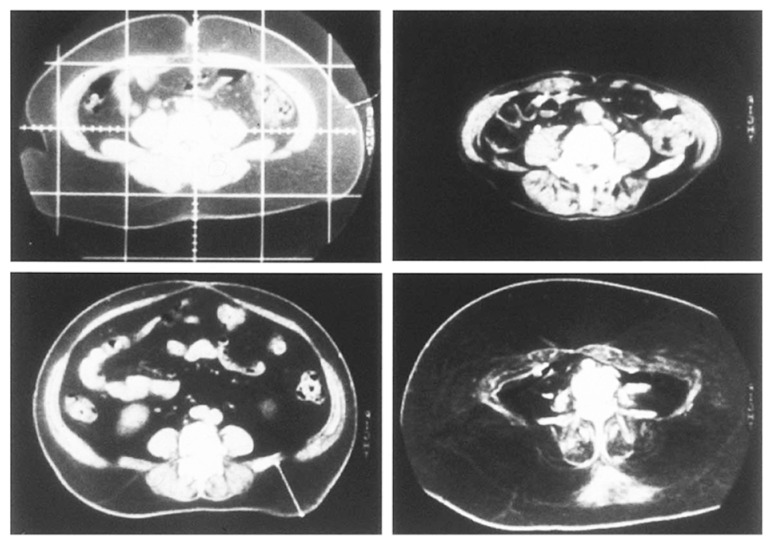
In early 1980s, Prof. Björntorp proposed a classification between central obesity and peripheral obesity and Prof. Kissebah proposed a classification between upper body segment obesity and lower body segment obesity based on waist/hip ratio.2),3) Our group developed the method for fat analysis using CT scan which enabled us to analyze adipose tissues in body cavity in 1983 and we noticed that there is marked variation in fat distribution between subcutaneous fat and intra-abdominal visceral fat. (Fig. 2)4)
Fig. 2.
Marked variation of fat distribution between intra-abdominal cavity and subcutane.4)
Visceral fat accumulation and metabolic or cardiovascular diseases
Using CT scan method for fat analysis, we demonstrated the contribution of visceral fat accumulation to the development of metabolic disorders, including glucose intolerance and hyperlipidemia. For example, visceral fat area determined by CT correlates significantly with glucose area after oral glucose tolerance test, and with cholesterol and triglyceride levels.5),6) Visceral fat accumulation is associated not only with quantitative changes in serum lipids and lipoproteins and but also with qualitative changes in lipoproteins, such as small dense LDL. Studies on muscle glucose uptake reported by Kissebah et al.7) and the steady-state plasma glucose method by our group,8) clearly show that visceral fat obesity has greater insulin resistance than subcutaneous fat obesity.
In addition to these metabolic disorders, we have demonstrated that in premenopausal women visceral fat accumulation correlates closely with systolic blood pressure.9) In hypertensive people, we reported a close correlation between the extent of visceral fat reduction, not subcutaneous fat reduction, and a lowering of blood pressure after weight reduction.
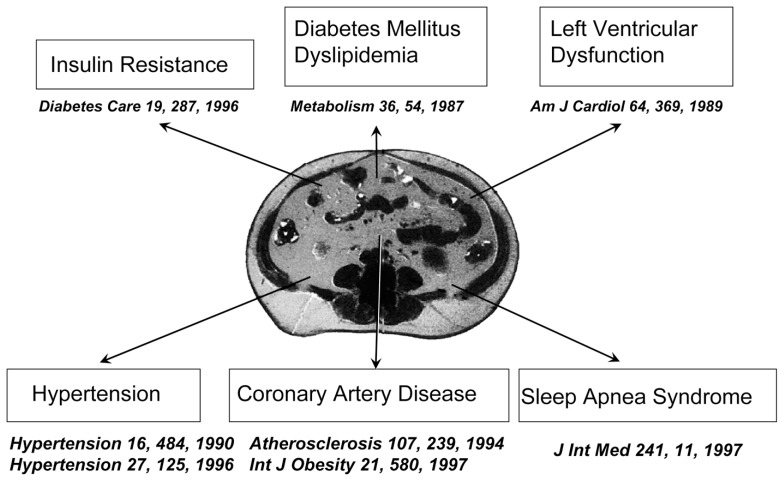
Visceral fat accumulation relates to the development of cardiovascular risks mentioned above and might relate directly to the development of cardiovascular disease. Several studies, including ours, have shown that visceral adiposity determined by CT scanning is related to coronary artery disease even in mildly obese individuals.10) Visceral fat accumulation is also related to the development of cardiac dysfunction and sleep apnea syndrome.11),12) From these evidences, we can conclude that visceral fat accumulation is a major risk of cardiovascular disease as well as metabolic diseases.(Fig. 3)
Fig. 3.
Visceral fat accumulation is related a variety of diseases.
Visceral fat syndrome and metabolic syndrome
In the end of 1980s, the concept of multiple risk factor clustering syndrome has been proposed as a highly atherogenic state independent from hypercholesterolemia.13),14) A variety of common disorders, such as hyperglycemia, hyperlipidemia and hypertension, are seen in individuals with this syndrome, and cardiovascular disease is very prevalent and this syndrome has been called to be the metabolic syndrome. The disorders such as diabetes, dyslipidemia and hypertension are not clustered coincidently, and there is thought to be a key to the simultaneous development within certain individuals along with the associated development of cardiovascular disease. As I showed above, visceral fat accumulation might be present in the upstream of a variety of disorders including cardiovascular disease. Therefore we have proposed the concept of a visceral fat syndrome on the basis of our clinical researches shown above as the same concept of metabolic syndrome.15) An important question is, then, why does visceral fat accumulation causes common disorders; more importantly, why is this syndrome so atherogenic? In order to answer these questions, we have investigated the functions of adipose-tissue, which has been traditionally regarded as a tissue passively storing excess energy in the form of triglycerides.
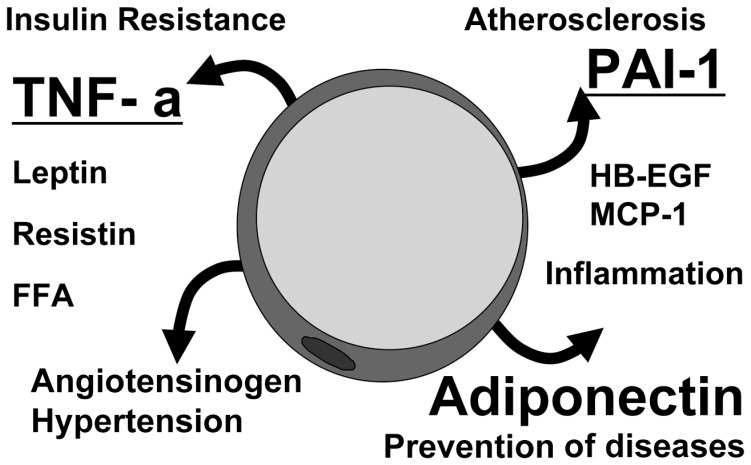
The concept of adipocytokines
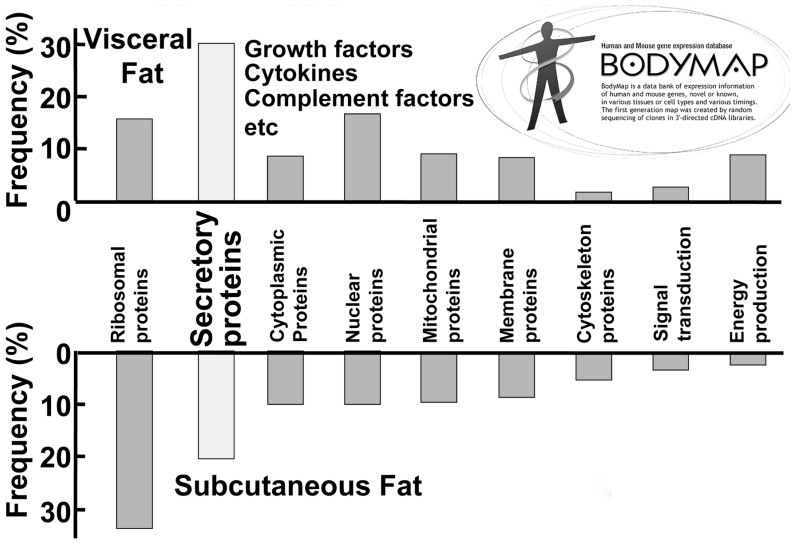
To elucidate the molecular mechanism of visceral fat-related diseases, particularly those in the metabolic syndrome, we have investigated the biological characteristics of visceral adipose tissue and subcutaneous adipose tissue by analysis of the gene-expression profile compared with that of other mesenchymal cells. We systematically analyzed active genes by constructing a 3′-directed complementary DNA library in which the messenger RNA population was faithfully reflected. We found an unexpectedly high frequency of the genes encoding secretary proteins in adipose tissue, most of which are important bioactive substances. Of the gene group classified by functions and subcellular localization, approximately 20% of all genes in subcutaneous adipose tissue encode secretory proteins. This frequency rises to about 30% in visceral adipose tissue. (Fig. 4) We classified these adipose-tissue-derived bioactive substances as adipocytokines. (Fig. 5)
Fig. 4.
Distribution profile of gene groups expressed in visceral fat and subcutaneous fat.44)
Fig. 5.
Concept of adipocytokines.
Adipocytokines and diseases
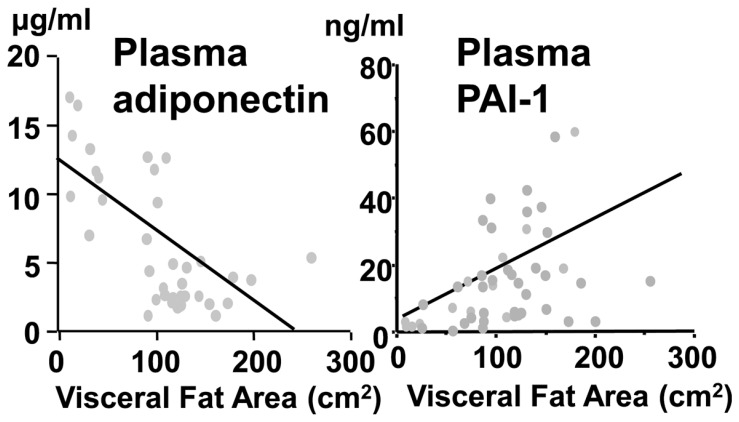
We found that the genes encoding plasminogen activator inhibitor type 1 (PAI-1) and heparin binding epidermal growth factor-like growth factor are highly expressed in adipose tissue.16),17) PAI-1 messenger RNA concentrations increased up to 10-fold in visceral adipose tissue during development of fat accumulation in ventromedial hypothalamic-lesioned rats, which is an experimental animal model of obesity. In subcutaneous adipose tissue, concentrations remained unchanged. In addition to the animal model, we demonstrated that plasma levels of PAI-1 were significantly correlated with visceral adiposity, assessed by CT scanning, in humans. (Fig. 6) Circulating PAI-1 is deemed as a strong risk factor for thrombotic diseases, including acute myocardial infarction, in metabolic syndrome.18) Heparin binding epidermal growth factor-like growth factor, a potent factor for smooth-muscle-cell proliferation, secreted from accumulated adipose tissue could also have some significance for vascular remodeling in obesity. Tumor necrosis factor-α was also reported to be secreted from adipose tissue and to induce insulin resistance by Dr. Hotamisligil.19)
Fig. 6.
Correlation between visceral adiposity and plasma levels of adiponectin and PAI-1.44)
Discovery of adiponectin and its clinical significance
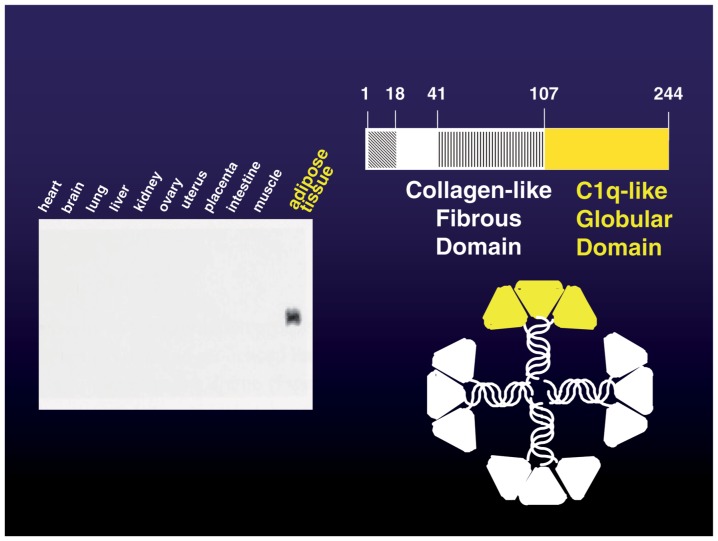
When we started the comprehensive genetic analysis of human adipose tissue, 40% of the expressed genes were previously unknown genes. The gene expressed most abundantly in adipose tissue, which we named adipose most abundant gene transcript-1, apM-1, was a novel gene.20) The molecule encoded by apM-1 possesses a signal peptide, collagen-like motif and globular domain, and has notable homology with collagen X, VIII and complement factor C1q. This protein is present in plasma in a unique multimer form, which is more active than low molecule weight form. (Fig. 7) We termed this collagen-like protein adiponectin. The mouse homolog of adiponectin has been cloned as ACRP30.21) We established the method for the measurement of plasma adiponectin levels using enzyme-linked immunosorbent assay. The average levels of adiponectin in human plasma are extremely high-up to 5–10 μg/ml.22) Plasma concentrations are negatively correlated with visceral adiposity, whereas PAI-1 increases with visceral fat accumulation as mentioned previously. (Fig. 6)
Fig. 7.
Adipose-specific collagen-like protein, adiponectin.
The mechanism by which plasma levels are reduced in individuals with visceral fat accumulation is not yet clarified. Co-culture with visceral fat inhibits adiponectin secretion from subcutaneous adipocytes. This finding suggests that some inhibiting factors for adiponectin synthesis or secretion are secreted from visceral adipose tissue.23) Tumor necrosis factor-α was reported to be a strong inhibitor of adiponectin promoter activity.24) The negative correlation between visceral adiposity and adiponectin levels might be explained by the increased secretion of this cytokine from accumulated visceral fat as at least one mechanism.
Plasma adiponectin concentrations are lower in people who have type 2 diabetes mellitus than in BMI-matched controls.25) The plasma concentrations have been shown to correlate strongly with insulin sensitivity, which suggests that low plasma concentrations are related to insulin resistance.26) In a study of Pima Indians, individuals with high levels of adiponectin were less likely than those with low concentrations to develop type 2 diabetes. High adiponectin concentration was, therefore, a notable protective factor against development of type 2 diabetes.27)
Studies on adiponectin knockout mice support observations in humans. The KO mice showed no specific phenotype when they were fed a normal diet but a high-sucrose and high-fat diet induced a marked elevation of plasma glucose and insulin levels. Notable insulin resistance, estimated by insulin tolerance test during the high-sucrose with high-fat diet, also developed in the knockout mice. The supplementation of adiponectin by adenovirus transfection clearly improved this insulin resistance.28) Adiponectin has been shown to exert its actions on muscle fatty acid oxidation and insulin sensitivity by activation of AMP-activated protein kinase.29)
Plasma levels of adiponectin are also decreased in hypertensive humans, irrespective of the presence of insulin resistance.30) Endothelium-dependent vaso-reactivity is impaired in people with hypoadiponectinemia, which might be at least one mechanism of hypertension in visceral obesity.31)
Most importantly, plasma concentrations of adiponectin are lower in people with coronary heart disease than in controls even when BMI and age are matched.32) A case-control study performed in Japan demonstrated that the group with hypoadiponectinemia with the plasma levels less than 4μg/ml has been shown to have increased risk of CAD and multiple metabolic risk factors, which indicates that hypoadiponectinemia is a key factor in the metabolic syndrome.33) A prospective study by Pischon et al.34) confirmed that high adiponectin concentrations are associated with reduced risk of acute myocardial infarction in men. In addition to hypoadiponectinemia accompanied with visceral fat accumulation, genetic hypoadiponectinemia caused by a missense mutation has been reported, which also exhibit the clinical phenotype of metabolic syndrome.35)
These clinical evidences show that hypoadiponectinemia is a strong risk factor for cardiovascular disease.
Cell biological functions of adiponectin
Antiatherogenicity of adiponectin is also demonstrated in animal experiments. Adiponectin knockout mice developed more-severe intimal thickening by endothelial injury than did wild-type mice.36) In addition, overexpression of human adiponectin by adenovirus transfection attenuated plaque formation in apolipoprotein E-KO mice.37)
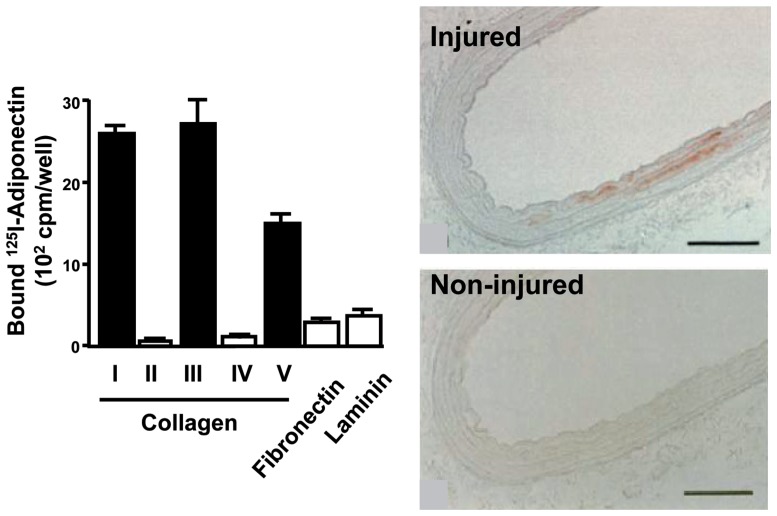
A large amount of adiponectin flows with the blood stream and, therefore, comes into contact with the vascular walls all over the body. The ways in which adiponectin interacts with vascular cells would be important to know. Immunohistochemical examination with antibodies to adiponectin showed no adiponectin protein in the untreated normal vascular walls in rabbits. Markedly positive immunohistochemical staining was detected, however, in balloon-injured vascular walls. Since adiponectin has the ability to bind subendothelial collagens such as collagen I, III, and V, endothelial injury may induce the adiponectin from entering into the subendothelial space through binding to these collagens. (Fig. 8)38)
Fig. 8.
Adiponectin accumulate injured vascular walls by binding subendothelial collagens.38)
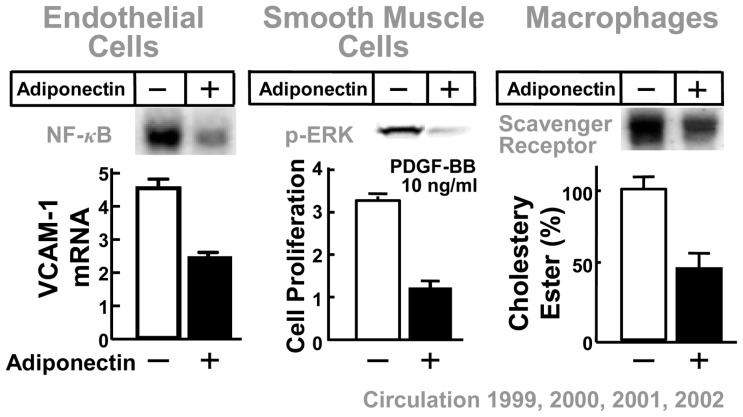
Cell biological studies have demonstrated that adiponectin has multiple, potent antiatherogenic functions. When the endothelial barrier is injured by attacking factors such as oxidized LDL, chemical substances and mechanical stress, adiponectin accumulates in the subendothelial space of vascular walls by binding to subendothelial collagen, at which point antiatherogenic properties of adiponectin become apparent.38) The protein suppresses monocyte attachment to vascular endothelial cells by inhibiting the expression of adhesion molecules, such as vascular cell adhesion molecule 1, intracellular-adhesion molecule 1 and E-selectin via the inhibition of NF-κB activation.39) Adiponectin also attenuates growth-factor-induced proliferation of vascular smooth-muscle cells by the inhibition of mitogen-activated protein kinase.40) Adiponectin suppresses foam-cell formation by the inhibition of expression of scavenger receptor class A. (Fig. 9)41)
Fig. 9.
Cell biological mechanism of anti-atherogenicity of adiponectin.
Acute coronary syndromes are considered to determine the prognosis of cardiovascular disease in which vulnerability of plaque is the important determinant of plaque rupture. In this process, matrix metalloproteinase secreted from macrophages is thought to play an important part in plaque vulnerability. Tissue inhibitor of metalloproteinase is thought to act as a protector of plaque rapture by inhibition of matrix metalloproteinase. Adiponectin increases the expression of messenger RNA and protein production of tissue inhibitor of metalloproteinase in macrophages via the induction of interleukin-10 synthesis. This finding suggests that adiponectin protects plaque rapture by the inhibition of matrix metalloproteinase function, through the induction of interleukin-10-dependent production of tissue inhibitor of metalloproteinase.42) Shibata et al. have demonstrated that adiponectin-deficient mice shows enhanced concentric hypertrophy and increased mortality under pressure overload. These phenomena were associated with increased extracellular signal-regulated kinase and diminished AMP-activated protein kinase signaling in the myocardium.
Adenovirus-mediated supplementation of adiponectin attenuated cardiac hypertrophy in response to pressure overload.43)
Molecular mechanism of adiponectin functions has not been fully clarified and is considered to be very complicated. Not like other bioactive substances such as cytokines and hormones, adiponectin is present abundantly in plasma. In addition, bioactivities of adiponectin are displayed more potently in multi-merized high molecule form than monomer or trimer type. With respect to the studies on adiponectin receptor, two kinds of concept have been proposed adiponectin receptors, AdipoR1 and AdipoR2 were identified by Kadowaki et al.44) They showed that both receptors have roles in activation of AMP activated kinase, AMPK and PPARγ. Lodish et al. suggested that T-cadherin may act as a coreceptor for an-yet-unidentified signaling receptor through which adiponectin transmits metabolic signals.45) As I mentioned above, the mode of action adiponectin may be dierent from that of other bioactive substances. It accumulates in injured tissue primarily by binding with extracellular collagens and may bind to some adhesion molecule such as T-cadherin or some signaling receptors such as AdipoRs which are expressed in target cells Further studies for molecular mechanism of adiponectin action are necessary to know physiological role of this unique protein.
Establishment of a disease entity-hypoadiponectinemia
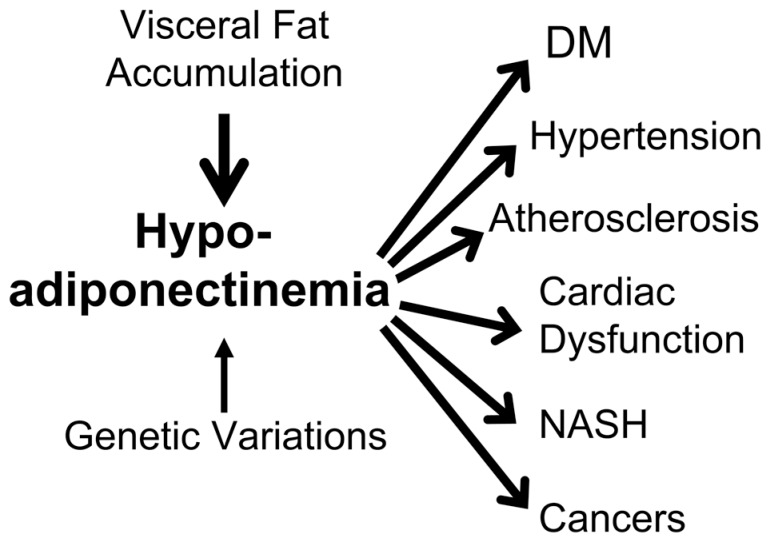
As shown above, it is no doubt that adiponectin is the most important adipocytokine which prevent cardiovascular disease as well as metabolic diseases including type 2 diabetes. In other words, hypoadiponectinemia has been demonstrated to be related to a variety of major diseases such as cardiovascular disease and metabolic disease, namely metabolic syndrome which may threaten life.46) In addition to the metabolic syndrome, recently hypoadiponectinemia has been reported to be related to non-alcoholic stea-tohepatitis and some kinds of cancer such as breast cancer and endometrial cancer. (Fig. 10) Therefore I would like to propose a disease entity named hypoadiponectinemia. Hypoadiponectinemia may be classified into two types; one is primary hypoadiponectinemia which may be caused by genetic disorders and the other is secondary hypoadiponectinemia which is caused by visceral fat accumulation. The later is corresponding to metabolic syndrome and much more frequent than primary one. Then I expect the development of therapeutic strategy which can elevate plasma levels of adiponectin, as statin was developed for hypercholesterolemia.
Fig. 10.
A disease entity of hypoadiponectinemia
Conclusion
Adipocytes secrete various adipocytokines to control the functions of other organs and cells. Production and secretion of adipocytokines are considered to be dynamically regulated mainly by the nutritional condition. Lifestyle factors, such as overeating and physical inactivity, induce visceral fat accumulation, which results in the dysfunction of adipocytes. Oversecretion of oensive adipocytokines, such as PAI-1, tumor necrosis factor-α and hyposecretion of defensive adipocytokines, such as adiponectin, might be major mechanisms of lifestyle-related diseases, including diabetes mellitus, hyperlipidemia, hypertension and atherosclerosis, comprising the so-called metabolic syndrome. The reduction of visceral fat might be, therefore, an essential preventive measure for metabolic syndrome and its consequence, cardiovascular disease. The regulation of key adipocytokines such as adiponectin might be considered as an efficient therapeutic procedure.46)
Profile
Yuji Matsuzawa was born in 1941 in Tanabe City, Wakayama Prefecture. He graduated Osaka University Medical School in 1966 and received PhD in 1977. He became Professor of the Second Department of Internal Medicine in 1993 and had been Director of Osaka University Hospital from 2000 to 2002. Since 2003, he is Director of Sumitomo Hospital. He has been working on lifestyle-related diseases such as obesity, hyperlipidemia and atherosclerosis. He established a concept of visceral fat syndrome which is corresponding to so-called metabolic syndrome and discovered adiponectin, a key molecule of lifestyle-related diseases. By these achievements, he received the medical award of Japan Medical Association in 2000, Takeda Award in 2004 and Willendorf’s Award from International Association for the Study of Obesity in 2006. He is now President of Asian Pacific Atherosclerosis Federation and also the President of Asia Oceania Association for the Study of Obesity.

References
- 1).Vague J. (1947) La differenciation sexuelle, feateur determinant des formes de l’obesite. Presse Med. 55, 339–340 [PubMed] [Google Scholar]
- 2).Kissebah A.H., Vydelingum N., Murray R., Evans D.J., Hartz A.J., Kalkhoff R.K., et al. (1982) Relation of body fat distribution in metabolic complication of obesity. J. Clin. Endocrinol. Metab. 54, 254–260 [DOI] [PubMed] [Google Scholar]
- 3).Björntorp P. (1987) Classification of obese patients and complications related to the distribution of surplus fat. Am. J. Clin. Nutr. 45, 112–125 [DOI] [PubMed] [Google Scholar]
- 4).Tokunaga K., Matsuzawa Y., Ishikawa K., Tarui S. (1983) A novel technique for the determination of body fat by computed tomography. Int. J. Obes. 7, 437–445 [PubMed] [Google Scholar]
- 5).Després J.P., Nadeau A., Tremblay A., Ferland M., Moorjani S., Lupien P.J., et al. (1989) Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes 38, 304–309 [DOI] [PubMed] [Google Scholar]
- 6).Fujioka S., Matsuzawa Y., Tokunaga K., Tarui S. (1987) Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism. Metabolism 36, 54–59 [DOI] [PubMed] [Google Scholar]
- 7).Evans D.J., Murray R., Kissebah A.H. (1984) Relationship between skeletal muscle insulin resistance, insulin-mediated glucose disposal and insulin binding: Effect of obesity and body fat topography. J. Clin. Invest. 74, 1515–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Yamashita S., Nakamura T., Shimomura I., Nishida M., Yoshida S., Kotani K., et al. (1996) Insulin resistance and body fat distribution. Diabetes Care 19, 287–291 [DOI] [PubMed] [Google Scholar]
- 9).Kanai H., Matsuzawa Y., Kotani K., Keno Y., Kobatake T., Nagai Y., et al. (1990) Close correlation of intra-abdominal fat accumulation to hypertension in obese women. Hypertension 16, 484–490 [DOI] [PubMed] [Google Scholar]
- 10).Nakamura T., Tokunaga K., Shimomura I., Nishida M., Yoshida S., Kotani K., et al. (1994) Contribution of visceral fat accumulation to the development of coronary artery disease in non-obese men. Atherosclerosis 107, 239–246 [DOI] [PubMed] [Google Scholar]
- 11).Nakajima T., Fujioka S., Tokunaga K., Matsuzawa Y., Tarui S. (1989) Correlation of intraabdominal fat accumulation and left ventricular performance in obesity. Am. J. Cardiol. 64(5), 369–373 [DOI] [PubMed] [Google Scholar]
- 12).Shinohara E., Kihara S., Yamashita S., Yamane M., Nishida M., Arai T., et al. (1997) Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J. Intern. Med. 241, 11–18 [DOI] [PubMed] [Google Scholar]
- 13).Reaven G.M. (1988) Role of insulin resistance in human disease. Diabetes 37, 1595–1607 [DOI] [PubMed] [Google Scholar]
- 14).Kaplan N.M. (1989) The deadly quartet. Arch. Intern. Med. 149, 1514–1520 [DOI] [PubMed] [Google Scholar]
- 15).Matsuzawa Y. (1997) Pathophysiology and molecular mechanism of visceral fat syndrome: The Japanese experience. Diabetes Metab. Rev. 13, 3–13 [DOI] [PubMed] [Google Scholar]
- 16).Shimomura I., Funahashi T., Takahashi M., Maeda K., Kotani K., Nakamura T., et al. (1997) Enhanced expression of PAI-1 in visceral fat: Possible contribution to vascular disease in obesity. Nature Med. 2, 800–803 [DOI] [PubMed] [Google Scholar]
- 17).Matsumoto S., Kishida K., Shimomura I., Maeda N., Nagaretani H., Matsuda M., et al. (2000) Increased plasma HB-EGF associated with obesity and coronary artery disease. Biochem. Biophys. Res. Commun. 292, 781–786 [DOI] [PubMed] [Google Scholar]
- 18).Matsuzawa Y. (2005) Adipocytokines and metabolic syndrome. Semin. Vasc. Med. 5, 34–39 [DOI] [PubMed] [Google Scholar]
- 19).Uysal K.T., Wiesbrock S.M., Marino M.W., Hotamisligil G.S. (1997) Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389, 610–614 [DOI] [PubMed] [Google Scholar]
- 20).Maeda K., Okubo K., Shimomura I., Funahashi T., Matsuzawa Y., Matsubara K. (1996) cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem. Biophys. Res. Commun. 221, 286–289 [DOI] [PubMed] [Google Scholar]
- 21).Scherer P.E., Williams S., Fogliano M., Baldini G., Lodish H.F. (1995) A novel serum protein similar to C1q produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 [DOI] [PubMed] [Google Scholar]
- 22).Arita Y., Kihara S., Ouchi N., Takahashi M., Maeda K., Miyagawa J., et al. (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 257, 79–83 [DOI] [PubMed] [Google Scholar]
- 23).Halleux C.M., Takahashi M., Delporte M.L., Detry R., Funahashi T., Matsuzawa Y., et al. (2001) Secretion of adiponectin and regulation of apM1 gene expression in human visceral adipose tissue. Biochem. Biophys. Res. Commun. 288, 1102–1107 [DOI] [PubMed] [Google Scholar]
- 24).Maeda N., Takahashi M., Funahashi T., Kihara S., Nishizawa H., Kishida K., et al. (2001) PPARγ ligands increase expression and plasma concentration of adiponectin, an adipose-derived protein. Diabetes 50, 2094–2099 [DOI] [PubMed] [Google Scholar]
- 25).Hotta K., Funahashi T., Arita Y., Takahashi M., Matsuda M., Okamoto Y., et al. (2000) Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 20, 1595–1599 [DOI] [PubMed] [Google Scholar]
- 26).Stefan N., Vozarova B., Funahashi T., Matsuzawa Y., Weyer C., Lindsay R.S., et al. (2002) Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation and low plasma concentration precedes a decrease in whole body insulin sensitivity in humans. Diabetes 51, 1884–1888 [DOI] [PubMed] [Google Scholar]
- 27).Lindsay R.S., Funahashi T., Hanson R.L., Matsuzawa Y., Tanaka S., Tataranni P.A., et al. (2002) Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 360, 57–58 [DOI] [PubMed] [Google Scholar]
- 28).Maeda N., Shimomura I., Kishida K., Nishizawa H., Matsuda M., Nagaretani H., et al. (2002) Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 8, 731–737 [DOI] [PubMed] [Google Scholar]
- 29).Tomas E., Tsao T.S., Saha A.K., Murrey H.E., Zhang Cc. C., Itani S.I., et al. (2002) Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc. Natl. Acad. Sci. U.S.A. 90, 16309–16313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Iwashima Y., Katsuya T., Ishikawa K., Ouchi N., Ohishi M., Sugimoto K., et al. (2004) Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension 43, 1318–1323 [DOI] [PubMed] [Google Scholar]
- 31).Ouchi N., Ohishi M., Kihara S., Funahashi T., Nakamura T., Nagaretani H., et al. (2003) Association of hypoadiponectinemia with impaired vaso-reactivity. Hypertension 42, 231–234 [DOI] [PubMed] [Google Scholar]
- 32).Ouchi N., Kihara S., Arita Y., Maeda K., Kuriyama H., Okamoto Y., et al. (1999) Novel modulator for endothelial adhesion molecules. Circulation 100, 2473–2476 [DOI] [PubMed] [Google Scholar]
- 33).Kumada M., Kihara S., Sumitsuji S., Kawamoto T., Matsumoto S., Ouchi N., et al. (2003) Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler. Thromb. Vasc. Biol. 23, 85–89 [DOI] [PubMed] [Google Scholar]
- 34).Pischon T., Girman C.J., Hotamisligil G.S., Rifai N., Hu F.B., Rimm E.B. (2004) Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 291, 1730–1737 [DOI] [PubMed] [Google Scholar]
- 35).Kondo H., Shimomura I., Matsukawa Y., Kumada M., Takahashi M., Matsuda M., et al. (2002) Association of adiponectin mutation with type 2 diabetes: a candidate gene for the insulin resistance syndrome. Diabetes 51, 2325–2328 [DOI] [PubMed] [Google Scholar]
- 36).Matsuda M., Shimomura I., Sata M., Arita Y., Nishida M., Maeda N., et al. (2002) Role of adiponectin in preventing vascular stenosis: the missing link of adipovascular axis. J. Biol. Chem. 277, 37487–37491 [DOI] [PubMed] [Google Scholar]
- 37).Okamoto Y., Kihara S., Ouchi N., Nishida M., Arita Y., Kumada M., et al. (2002) Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 26, 2767–2770 [DOI] [PubMed] [Google Scholar]
- 38).Okamoto Y., Arita Y., Nishida M., Muraguchi M., Ouchi N., Takahashi M., et al. (2000) An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm. Metab. Res. 32, 47–50 [DOI] [PubMed] [Google Scholar]
- 39).Ouchi N., Kihara S., Arita Y., Okamoto Y., Maeda K., Kuriyama H., et al. (2000) Adiponectin, adiocyte-derived plasma protein, inhibits endothelial NF-κB signaling through c-AMP dependent pathway. Circulation 102, 1296–1301 [DOI] [PubMed] [Google Scholar]
- 40).Arita Y., Kihara S., Ouchi N., Maeda K., Kuriyama H., Okamoto Y., et al. (2002) Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation 105, 2893–2898 [DOI] [PubMed] [Google Scholar]
- 41).Ouchi N., Kihara S., Arita Y., Nishida M., Matsuyama A., Okamoto Y., et al. (2001) Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation 103, 1057–1063 [DOI] [PubMed] [Google Scholar]
- 42).Kumada M., Kihara S., Ouchi N., Kobayashi H., Okamoto Y., Ohashi K., et al. (2004) Adiponectin specifically increases tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation 109, 2046–2049 [DOI] [PubMed] [Google Scholar]
- 43).Shibata R., Ouchi N., Ito M., Kihara S., Shiojima I., Pimentel D.R., et al. (2004) Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med. 10, 1384–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Yamauchi T., Kamon J., Ito Y., Tsuchida A., Yokomizo T., Kita S., et al. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762–769 [DOI] [PubMed] [Google Scholar]
- 45).Hug C., Wang J., Ahmad N.S., Bogan J.S., Tsao T.S., Lodish H.F. (2004) T-cadherin is a receptor for hexameric and high molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci. USA 101, 10308–10313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Matsuzawa Y. (2006) Therapy Insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nature Clin. Prac. Cardiovasc. Med. 3, 35–42 [DOI] [PubMed] [Google Scholar]