Abstract
Alzheimer’s disease has been characterized by senile plaque and neurofibrillary tangle in the brain. However, their relation to etiology of this disease has been left unclear. Recently it has been clarified that neurofibrillay tangle consists of highly phosphorylated tau protein. Then we have started to identify the enzyme(s) responsible for this phosphorylation and obtained tau protein kinase I and II. Tau protein kinase I phosphorylated not only tau protein but also pyruvate dehydrogenase, phosphorylation of which caused inactivation of this enzyme and finally led the cell to death. Then we have proved that TPKI is upregulated in AD brain but not in control brain. Upregulation of TPKI was induced by treating the neuronal cells with Aβ protein. Finally we have identified oligomeric aggregation of Aβ protein named Amylospheroid is highly potent to degenerate neuronal cells both in vitro and in vivo systems.
Keywords: Senile plaque, amyloid β, paired helical filaments, tau protein kinase, amylospheroid
Introduction
Alzheimer’s disease can be characterized by two kinds of pathological deposits in specific areas of the brain, i.e. senile plaque and neurofibrillary tangles which would be linked to the etiology of Alzheimer’s disease (AD). However, no clear-cut explanation has been given.
Thus the author has conducted experiments to solve this question from biochemical point of view for about twenty years. This review is his personal view summarizing these works in a logical story. Accordingly the author has no intention to claim that this is the sole explanation for the etiology of Alzheimer’s disease.
Senile plaques contain extracellular deposits of β-amyloid protein (Aβ) associated with degenerating nerve process known as dystrophic neurites. Neurofibrillary tangles (NFTs) exist inside neuronal cells and consist primarily of abnormal paired helical filaments (PHFs). Since large numbers of senile plaques are found in some cognitively normal individuals, it was suggested that neuronal death in AD brain will be related to NFTs.1) Thus, our attention was first directed to the study on the formation and accumulation of PHF.
PHF consists of hyperphosphorylated tau (PHF-tau),2) and contains a small amount of ubiquitin.3) During the course of Alzheimer’s disease, tau becomes hyperphosphorylated at multiple sites and integrated into PHF resulting in loss of physiological functions. No protein kinase phosphorylating tau in PHF-like state was found at that time we started our works from identification of the candidate kinase.
Identification and characterization of Tau Protein Kinases
The characteristics of PHF-tau appeared in early reports were (i) increase of apparent molecular weight (Mr) on SDS-gel responsible for hyperphosphorylation, and (ii) the gain of immuno-reactivity against anti-PHF polyclonal antibody.2) Thus, two criteria were set up for identifying a candidate kinase as mentioned above. The protein kinase activity provided with these criteria was found in the microtubule protein fraction of rat or bovine brain extracts, and partially purified.4) This kinase was a serine/threonine kinase. At that time, this enzyme was admitted as a novel enzyme and designated as tau protein kinase [EC 2.7.1.135] by Nomenclature Committee of the International Union of Biochemistry.5) We have used TPK as an abbreviated name.
Later, two kinds of activities with different mode of phosphorylation of tau were separated and homogeneously purified being designated as TPKI and TPKII, respectively.6)
TPKI can phosphorylate native tau isolated from normal brain which was already phosphorylated to some extent, but not the completely dephosphorylated tau. In contrast, TPKII can phosphorylate both taus. When dephosphorylated tau is prior-phosphorylated by TPKII, however, TPKI can phosphorylate it as well as native tau to generate a PHF epitope, indicating that prior phosphorylation of tau by TPKII enhances phosphorylation by TPKI.7) Thus TPKII may regulate the phosphorylation state of tau not only in normal brain but also in Alzheimer’s disease brain in concert with TPKI.
Later it was proved that TPKI is identical to “glycogen synthase kinase 3β (GSK3β)” by cDNA analysis.
TPKII is a complex composed of two subunits, catalytic subunit of 30 kDa and regulatory subunit of 23 kDa. Catalytic subunit of TPKII was proved from sequence analysis of the cDNA to be identical with a “cdc2-related kinase, PSSALRE/Cdk5”,8) which by itself, was inactive.8) Therefore, 23 kDa protein is regarded as a CDK5 activator in neuronal cell.
Phosphorylation sites of TPKI and TPKII
As the next step, phosphorylation sites in tau protein by TPKI and TPKII were determined. Phosphorylation sites of tau by TPKII were Ser202, Thr205, Ser235, and Ser404,9) while those by TPKI were Ser199, Thr231, Ser396 and Ser413 (Numbering of amino acids are done according to longest human tau).10)
These 8 TPK sites are localized to the amino-and carboxyl-terminal flanking regions of micro-tubule-binding domain of tau. Microtubule-binding domain consists of the tandem 31 or 32 amino acids-repeated region which includes 4 repeats in adult tau and 3 repeats in fetal tau.11) In particular, the pro-line-rich region upstream of the repeats which is the region with a cluster of a Ser/ThrPro phosphorylation sites, appears to be essential for tubulin binding in vivo.12) Thus, phosphorylation by TPKI and TPKII markedly reduces the tubulin-assembly promoting activity.10)
However since the action of TPKI/TPKII cannot explain all phosphorylation sites of PHF it is reasonable to consider that several other kinases are also involved in the hyperphosphorylation of tau to induce PHF-tau.
Upregulation of TPKI in AD brain
As shown in Table 1, about 20 sites are involved in PHF-tau13) and especially 10 major sites. Among these sites Ser413 is quite unique because it could be phosphorylated only by TPKI but not by other kinases as could be seen in Table 1. In other words, phosphorylation of Ser413 is the fingerprint of TPKI. Likewise Thr205 is the fingerprint of cyclin dependent kinase (CDK) and TPKII. In order to prove that TPKs are operating in PHF formation, we have examined the staining of AD brain by using anti-PS413 and anti-PT205, which were raised against decapeptides spanning the sequence around phosphorylated Ser-413 and Thr-205, called PS413 and PT205, respectively. Twelve AD brains and nine control brains with no evidence of Alzheimer-like changes were examined by staining with anti-PS413 and anti-PT205 in CA1 region. In serial vibrotome sections of formalin fixed hippocampus, anti-PS413 and anti-PT205 identified three major regions associated with neurofibrillary degeneration (i.e. NFT, NP and NT), in all twelve AD cases.14) On the other hand the brains of non-demented individuals were faintly stained and number of the stained neurons were scarce. These results suggested strongly that both TPKs are operating in AD brain to raise each epitope.
Table 1.
Phosphorylation sites of tau-protein
| T 181 |
S 198 |
S 199 |
S 202 |
T 205 |
S 208 |
S 210 |
T 212 |
S 214 |
T 217 |
T 231 |
S 235 |
S 262 |
S 396 |
S 400 |
T 403 |
S 404 |
S 409 |
S 412 |
S 413 |
S 422 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHF | ○ | ○ | ○ | ◎ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ◎ | ○ | |
| TPKI | ○ | ○ | ○ | ◎ | |||||||||||||||||
| TPKII | ○ | ◎ | ○ | ○ | |||||||||||||||||
| GSK3 α | ○ | ○ | ○ | ||||||||||||||||||
| cdc2 | ○ | ◎ | ○ | ○ | |||||||||||||||||
| MAPK | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |||||||||||||
| 35/41 Kinase | ○ | ○ | |||||||||||||||||||
| Brain Kinase | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ||||||||||||
| PKA | ○ |
The first line shows candidate sites for phosphorylation in tau protein, numbered according to the amino acid sequence from N-terminus. The second line shows the sites phosphorylated in native tau protein. The third line and below show the sites phosphorylated by the kinases indicated in the left column. ○ indicates the site phosphorylated by the kinase shown in the left column. ◎ indicates the site specifically phosphorylated by TPKI or TPKII.
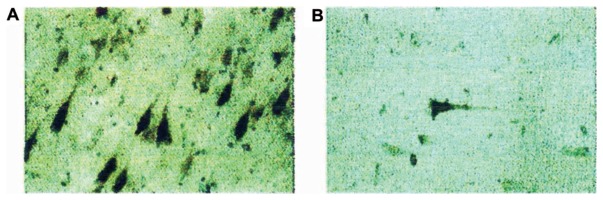
The preferential appearance of epitopes of TPKI and TPKII stained by anti-PS413 and anti-PT205 in AD brain suggested up regulation of these kinases in AD brain. In order to prove this we tried to raise antibody against TPKI/GSK3β. We have raised antibodies against synthetic peptides representing carboxyl-termini of rat TPKI/GSK3β and its isoform GSK3α, where the difference in homology between these isoforms are most eminent. After brief microwave heating intense staining of pyramidal neurons of focal groups in AD brain, and their surrounding neuropil made a vivid contrast against weaker, diffuse reaction of the neighboring cells (Fig. 1A). The same region of the age-matched control brains was subjected to anti-TPKI antibody. The staining was faint and the number of stained cells was scarce (Fig. 1B). Similar strong staining was observed in the prefrontal cortex, entorhinal cortex, and amygdala, but not in the cerebellum, which lacks AD pathology. Preabsorption of the anti-serum with TPKI-peptide inhibited immunostaining completely but preabsorption with GSK3α-peptide had no effect.14)
Fig. 1.
Immunocytochemistry of anti-TPKI in the human hippocampal CA1 subfield.15) (A) AD brain stained with anti-TPKI antibody. (B) Control brain with slight AD-like changes stained with anti-TPKI antibody. In each pyramidal cell, cell body, apical dendrite, basal neurite and proximal axon are stained.
As mentioned earlier TPKII consists of two subunits and the larger one is identical with CDK5. By using commercially available anti-CDK5 antibody, we have stained AD brain (data are not shown). The results obtained were very similar to those of TPKI. Especially double staining indicated clearly that tau-1-positive NFT were also positive for CDK5. Neuropil threads were negative for CDK5, in contrast with the case of TPKI.15) Nevertheless our results suggested that the level of TPKI or TPKII are elevated in AD brain by some mechanism.
Induction of TPKI by amyloid β protein
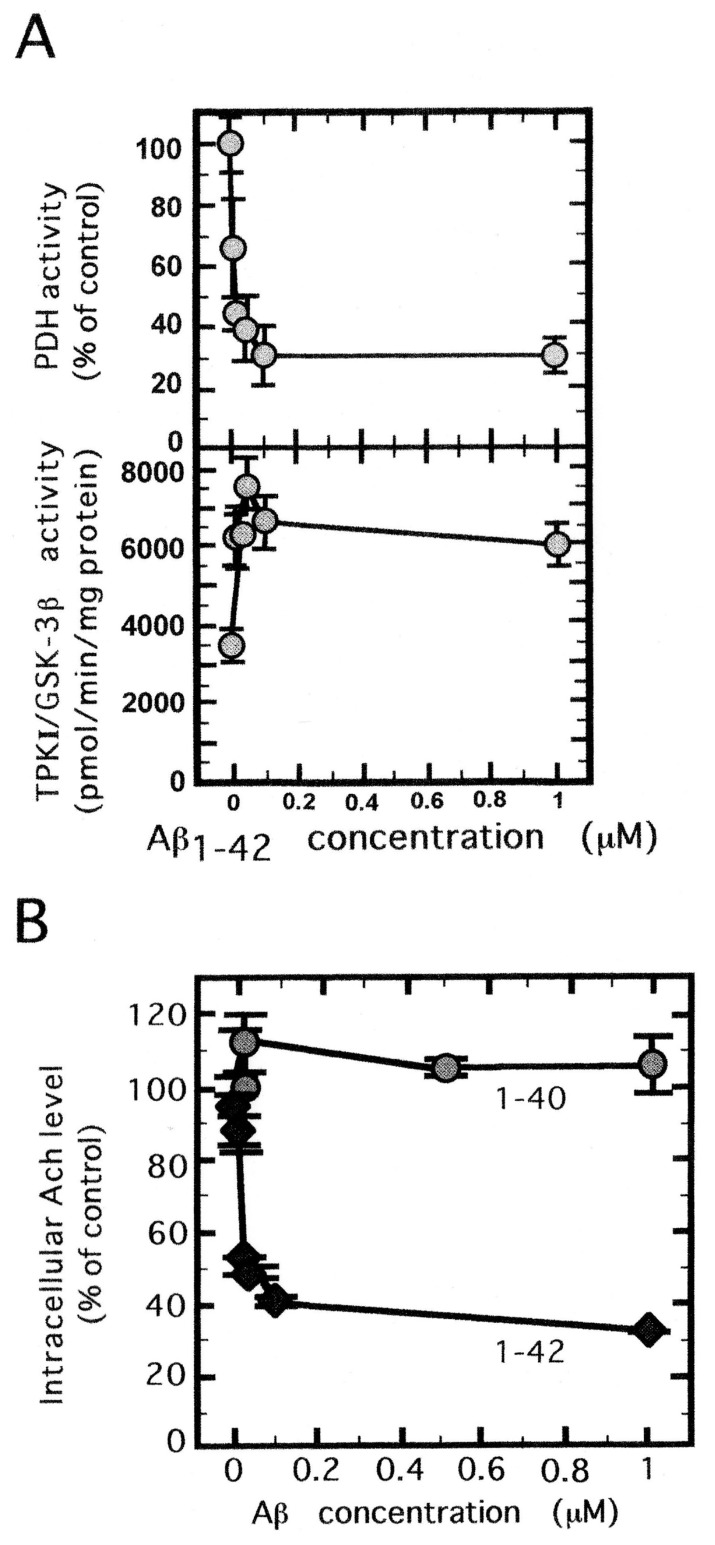
The experimental results above suggest us that some factor(s) bringing induction of TPKs in neuronal cells may appear in AD brain. Although such factor has not been identified clearly, Aβ will be a good candidate since its appearance precedes that of NFT. In order to test this hypothesis we have adopted in vitro approach. Primary culture of embryonic rat hippocampal cells was used for this purpose. This tissue was treated with several concentration of Aβ. As shown in Fig. 2A (below) the activity of TPK1/GSK3β increased remarkably by up to the concentration of 0.1 μM Aβ, but leveled off above this concentration. As will be discussed later, above 0.1 μM the surplus Aβ molecules will aggregate into inactive form.
Fig. 2.
Activation of TPKI/GSK3β, inactivation of PDH and inhibition Ach synthesis in rat septum cells cultured with amyloid β.16) (A) Dependency of TPKI activation and PDH inactivation on the concentration of Aβ1–42. The activity was measured after 12 hours incubation with various concentration of Aβ1–42 shown in the abscissa. (B) Effect of Aβ1–42 and Aβ1–40 on the suppression of Ach synthesis. Intracellular level of Ach was determined by HPLC-electrochemical detector system after 12 hours culture with Aβ.
In parallel to enzyme assay, we have examined the survival of these cells. After 24 hours incubation, the survival of the cells was reduced to less than 10% of the control culture (culture without Aβ). In order to clarify the relation between TPKI activation and cell death, we have synthesized both sense and antisense oligonucleotides of TPKI gene in accordance with its cDNA sequence.16) Sense or antisense oligonucleotide was added to the incubation mixture containing Aβ. After three hour incubation with antisense oligonucleotide, the synthetic activity of TPKI was suppressed to the same level of the control. On the other hand sense oligonucleotide was not effective to rescue the cells from death.
TPKI/GSK3β Inactivates pyruvate dehydrogenase and inhibits acetyl choline syntesis
The above results suggest the existence of another substrate of TPKI and its phosphorylation might be crucial for the survival of the neuronal cells.
Thus we started to identify this substrate of TPKI using yeast two-hybrid system and obtained pyruvate dehydrogenase (PDH), as the candidate. Then we examined if TPKI would phosphorylate PDH.17) As expected, incubation of porcine heart PDH complex with purified TPKI resulted in ATP dependent phosphorylation of PDH. It is quite striking that in parallel to this phosphorylation the activity of PDH decreased. Other kinases including TPKII failed to inactivate PDH.17)
Since these results were obtained in in vitro system we examined to prove these in the in vivo system using primary culture of rat hippocampal neurons. Since PDH exists inside mitochondria we have confirmed that TPKI is also located in mitochondria both biochemically and morphologically.17)
As can be expected from in vitro experiment TPKI activity increased remarkably by adding Aβ in the culture (Fig. 2A below). PDH was inactivated in inverse proportion to the Aβ-induced TPKI activity (Fig. 2A above). The remaining activity of PDH was only 20 per cent of the control. Inactivation of PDH naturally should result in the reduction of acetyl-CoA level, which is the key substance for ATP synthesis through TCA cycle and synthesis of acetyl choline. The above results can explain following phenomena observed for AD patients.
In AD brain 44% reduction in cerebral metabolic rate of glucose and four fold increase of lactate production were observed, whereas cerebral blood flow and cerebral metabolic rate of oxygen were found unaltered.18)
In postmortem studies PDH activity was decreased especially in AD brain.19)
Since acetyl-CoA is the important source of acetyl choline (Ach) production in cholinergic neurons, the inactivation of PDH will cause the reduction of Ach synthesis.
We examined the relation between PDH inactivation and Ach synthesis, using the primary culture of the cholinergic neurons. As expected Ach level in the cultured cells was reduced to 30 per cent relative to the control after Aβ treatment (Fig. 2B). Under this condition Aβ did not affect choline acetyl transferase or choline esterase activity, indicating that the decreased level of acetyl-CoA via inactivation of PDH caused the reduction of Ach synthesis.
In this system aggregation of Aβ in fibrillary state was not necessary and 10 nM concentration was enough to cause significant increase in TPKI activity and reduction in the intracellular Ach level, so long as Aβ1–42 was used. On the other hand Aβ1–40 or 25–35 was completely ineffective in this concentration (Fig. 2B).20)
In summary, Aβ may affect various metabolic pathway. In the first case, Aβ interacts with neurons and activates TPKI, which will lead to extensive phosphorylation of tau and destabilizing micro-tubules, resulting in impaired axonal transport and neuronal death. On the other hand the activated TPKI in mitochondria will phosphorylate and inactivate PDH, resulting in dysfunction of glucose metabolism and also reduction of acetyl-choline production, which also contribute to neuronal death through the failure of the energy production and signal transmission between neuronal cells.
Identification of toxic form of Aβ aggregate in AD brain
Since the previous results indicate that some aggregate of Aβ is not toxic we next tried to clarify the nature of toxic form. We incubated 50 μM chemically synthesized Aβ solution by rotating for 14 hrs. The solution was fractionated by glycerol-gradient centrifugation. The most toxic fraction thus obtained contained spherical aggregates with 10–15 nm in diameter. On the other hand highly aggregated form showed no toxic activity as above-mentioned.
This toxic aggregate was named Amylospheroid (ASPD).21) Then we have raised anti-body against ASPD in mice system. The obtained antibody, which we will call mASD3 hereafter, was quite specific to ASPD and showed no cross-reactivity with Aβ monomer, or Aβ fibrils. This mASD3 antibody was quite potent to neutralize apoptosis activity of ASPD.
Then, to elucidate whether ASPD-like aggregates are present in vivo brain, sections of patients with clinico-pathologically confirmed AD and those of non-cognitively impaired (NCI) people.22) The binding of the mASD3 antibody in AD brain was associated with regions where prominent neurodegeneration had occurred, whereas the binding of mASD3 was negligible in NCI brains. This clearly suggested ASPD-like aggregates exist specifically in AD brain and we named this aggregate native ASPD.22)
Isolation and characterization of native ASPD from AD brain22)
We prepared soluble fraction of AD brains under non-denaturing condition, using solutions of physiological ionic strength and pH without detergent. Then we passed the fraction through 0.22-μm filter and the filtrate was applied to 100-kDa molecular weight cut off (MWCO) filters to avoid smaller Aβ aggregates and to concentrate native ASPD. We then purified native ASPD using mASD3 antibody. Native ASPD thus derived was analyzed by mass spectroscopy, which showed single charged ions of Aβ.
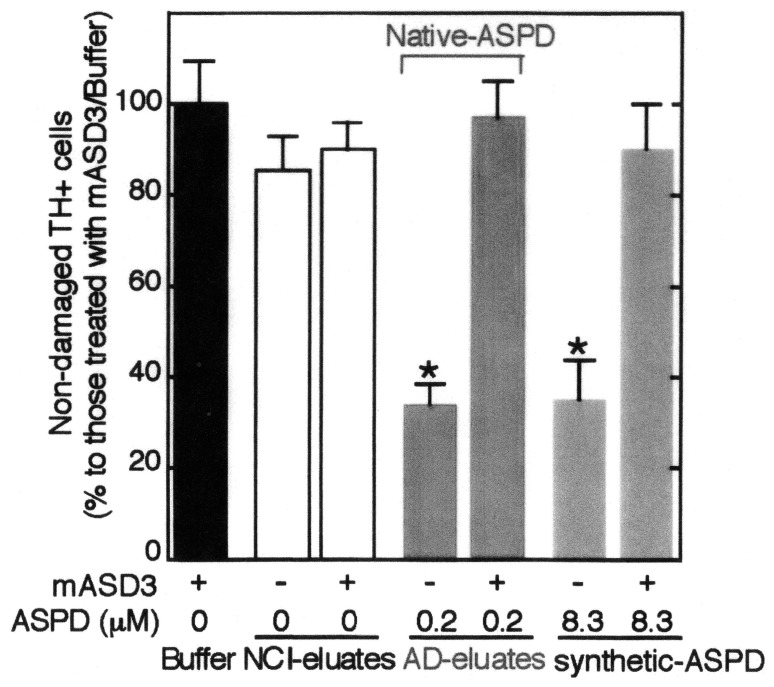
We next examined if this native ASPD will induce neuronal death. As shown in Fig. 3 native ASPD showed strong neurotoxicity as compared with synthetic ASPD, which is strongly suppressed by the addition of anti-ASPD antibody (mASD3). However it is worthy to note that toxicity of native ASPD is much stronger than synthetic ASPD. As can be seen in Fig. 3, 0.2 μM of native ASPD showed the same toxicity as 8.3 μM of synthetic ASPD. Eluates from NCI brain or mASD3 had no neurotoxicity (Fig. 3).
Fig. 3.
Characterization of native and synthetic ASPD-induced toxicity.22) Human neuronal cells were treated for 2 days with the eluates of NCI-brain, AD-brain and synthetic ASPD, with or without 2-hrs mASD3 pretreatment. Non-damaged cells after treatment to control cells were counted after tyrosine hydrogenase and Hoechst3328 staining. The ratio of damaged cells to neuronal cells treated with buffer alone is shown.
Both synthetic and native ASPDs showed activation of TPKI, inactivation of PDH and inhibition of Ach synthesis in similar ways to those shown in Fig. 2.
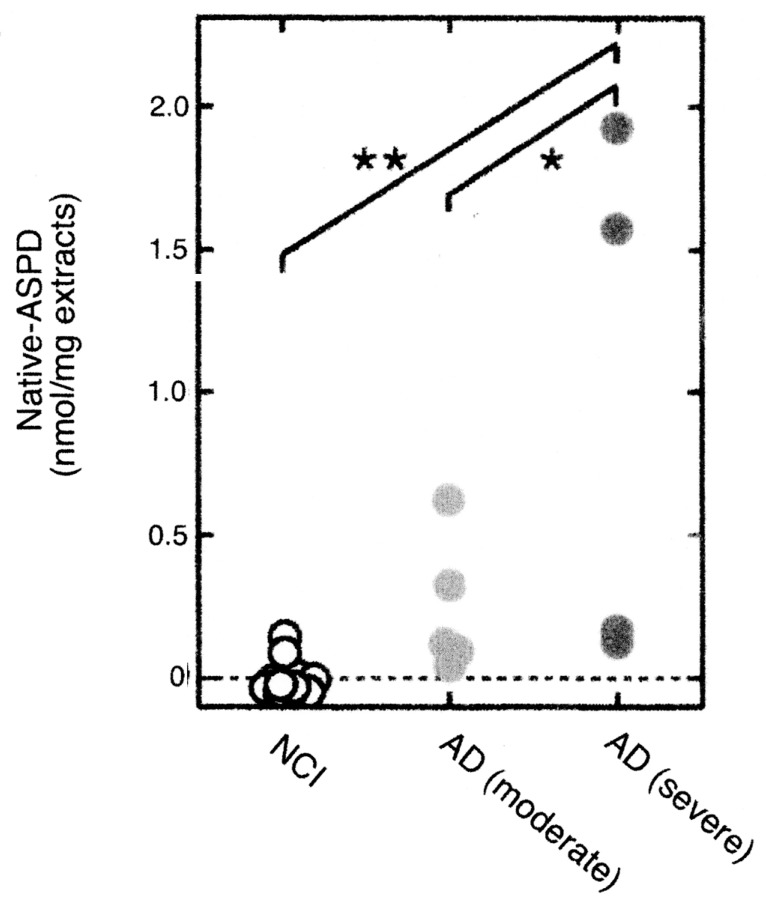
As can be expected from the above results, the concentration of native ASPD was highest in the brain extract of severe AD patients lower in moderate AD patient and none in NCI patient (Fig. 4). These results suggest strongly that native ASPD plays important role in neuronal degeneration of AD patient.
Fig. 4.
The contents of native ASPD in NCI, moderate AD and severe AD brain.22) The contents of ASPD in the brain extracts of NCI brains and AD brains of different stages were determined. Each extract was treated with mASD3 and the precipitate was assayed by dot blotting.
Discussion
Alzheimer’s disease has been characterized by two intra-cellular and extra-cellular deposits, neurofibrillary tangle and senile plaque. However relation between these deposits has not been clarified. In addition, it has not been completely explained how these deposits might be related to neuronal cell death in AD patients. It is well known that senile plaque, which is highly aggregated Aβ protein, appears in normal (non-demented) brain. Even in the case of AD patients appearance of senile plaque precedes the onset of dementia. On the other hand, appearance of neurofibirillar tangle proceeds almost in parallel with the progress of dementia. Since it was demonstrated by Ihara that paired helical filaments, which are the main component of neurofibrillary tangle, consist of hyper-phophorylated tau protein2) we have assumed that the phosphorylation of tau may be the key to solve the etiology of this disease.
Thus we have purified TPKI and TPKII as the candidate enzymes of this phosphorylation. These enzymes phosphorylated 8 sites of PHF but not all of them. We then examined either enzyme may have another substrate, phosphorylation of which will be crucial for the cell death. We have found that pyruvate dehydrogenase (PDH) can be phosphorylated by TPKI resulting disappearance of its activity. The in-activation of PDH will result in the shortage of ATP and acetyl choline, which are crucial for the survival of neuronal cells. Then the problem is how TPKI will be up-regulated in AD brain.
Although another candidate for the key of the etiology of AD may exist, it is known that deposition of senile plaque occurs even in normal (non-demented) individuals. So we assumed that soluble form of Aβ but not highly aggregated Aβ proteins may play the key role. Truly as shown in Fig. 2, solution of Aβ has potent ability to activate TPKI, inactivate of PDH, resulting in the shortage of acetyl choline. However, since this solution was somewhat turbid, toxic moiety is thought to be oligomeric aggregation of Aβ. Final problem for us was to characterize this aggregate. So we have fractionated the components in the solution by assaying the strength of cell toxicity, and isolated spherical aggregate of Aβ with a diameter of 10–15 nm, which we called Amylospheroid (ASPD). In order to examine if this ASPD may exist in the brain of AD patients we raised the antibody which is strictly specific to ASPD. As expectedly this antibody stained strongly the brain of AD patients but not those of normal adult or even of NCI patients.
Finally we have tried to separate ASPD in the brain of AD patients, by immunoprecipitation, using the specific antibody mentioned above. The obtained ASPD, native ASPD was mostly identical with the synthetic ASPD, except that native ASPD was much higher in the neurotoxicity than the synthetic one.
Although several works are proceeding,23),24) reporting other kinds of Aβ aggregates showing neurotoxicity, present report will give self-consistent story to explain the mechanism of neuro-degeneration in AD brain.
Acknowledgements
The present work was carried in collaboration with many researchers in Tokyo Metropolitan Institute of Gerontology and Mitsubishi Kagaku Institute of Life Sciences, to whom the author’s thanks are due. Among these the author is indebted especially to late Prof. Masanori Tomonaga at Univeristy of Tokyo, who guided the author to the research on AD and to Drs. Tsuneko Uchida and Koichi Ishiguro for isolation and characterization of TPKs. The author appreciates the great contribution of Dr. Minako Hoshi, who has proved the story that ASPD kills the neuronal cells through the activation of TPKI.
Abbreviations
- Aβ
β-amyloid protein
- Ach
acetyl choline
- AD
Alzheimer’s disease
- ASPD
amylospheroid
- CDK
cycline dependent kinase
- GSK
glycogen synthase kinase
- MAPK
Mapkinase
- mASD
antibody raised agaist ASPD in mouse
- NCI
non-cognitively impaired
- NFT
neurofibrillary tangle
- NP
neurite plaque
- NT
neuropil thread
- PKA
protein kinase A
- PDH
pyruvate dehydrogenase
- tau-1
commercially available anitibody raised against tau-protein.
Profile
Kazutomo Imahori was born in Osaka, Japan in 1920. After graduated from the Faculty of Science, The University of Tokyo he joined the laboratory of late Professor San-ichiro Mizushima as a research fellow and was trained as physico-chemist. In 1950 he moved to College of General Education of The University as an associate professor and started his earlier part of life works, “The Structure and Function of the Proteins.” He was the research associate at Harvard University from 1956 to 1958, where he was involved in the research on the conformation of polypeptides. He made a big discovery that remarkable decrease optical absorbance (hypochromism) takes place when the polypeptide chain is transformed from random coil to α-helical conformation. This study led to subsequent works which elucidate the conformation of the protein from its optical properties.
In 1961 he was promoted to Professor and made another big findings that β-structure exists not only in fibrous proteins but also in globular proteins. In 1968 he moved to Department of Agricultural Chemistry, Faculty of Agriculture at the University of Tokyo, where he has clarified the bacteriocidal mechanism of colicin E3 from its sub-unit structure. In 1975 he moved to Faculty of Medicine at the University of Tokyo, where he has first isolated and characterized Calcium-dependent Protease (Calpain). In 1981 he retired from The University of Tokyo and became a Director of Tokyo Metropolitan Institute Gerontology and started his later half of life works, Biochemical Studies on the Etiology of Alzheimer’s Disease, which is summarized in the present review. He continued these works after he has moved to Mitsubishi Kagaku Institute of Life Sciences, as a Chairman. In 1999 he retired from this Institute.

References
- 1).Arriagada P.A., Growdon J.H., Hedley-White E.T., Hyman B.T. (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42, 631–639 [DOI] [PubMed] [Google Scholar]
- 2).Ihara Y., Nukina N., Miura R., Ogawara M. (1986) Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer’s disease. J. Biochem. (Tokyo) 99, 1807–1810 [DOI] [PubMed] [Google Scholar]
- 3).Mori H., Kondo J., Ihara Y. (1987) Ubiquitin is a component of paired helical filament in Alzheimer’s disease. Science 235, 1641–1644 [DOI] [PubMed] [Google Scholar]
- 4).Ishiguro K., Ihara Y., Uchida T., Imahori K. (1988) A novel tubulin-dependent protein kinase forming a paired helical filament epitope on tau. J. Biochem. (Tokyo) 104, 319–321 [DOI] [PubMed] [Google Scholar]
- 5).Webb E.C. (1990) Enzyme nomenclature. Recommendations 1984. . Supplement 3: corrections and additions. Eur. J. Biochem. 187, 263–281 [DOI] [PubMed] [Google Scholar]
- 6).Ishiguro K., Takamatsu M., Tomizawa K., Omori A., Takahashi M., Arioka M., Uchida T., et al. (1992) Tau protein kinase I converts normal tau protein into A68-like component of paired helical filaments. J. Biol. Chem. 267, 10897–10901 [PubMed] [Google Scholar]
- 7).Arioka M., Tsukamoto M., Ishiguro K., Kato R., Sato K., Imahori K., Uchida T. (1993) τ protein kinase II is involved in the regulation of normal phosphorylation state of τ protein. J. Neuro-chem. 60, 461–468 [DOI] [PubMed] [Google Scholar]
- 8).Kobayashi S., Ishiguro K., Omori A., Takamatsu M., Arioka M., Imahori K., Uchida T. (1993) A cdc2-related kinase PSSALRE/cdk5 is homologous with the 30kD subunit of tasu protein kinase II, a proline-directed protein kinase associated with microtubule. FEBS Lett. 335, 171–175 [DOI] [PubMed] [Google Scholar]
- 9).Ishiguro K., Omori A., Sato K., Tomizawa K., Imahori K., Uchida T. (1991) A serine/threonine protein kinase activity is included in the tau protein kinase fraction forming a paired helical filament epitope. Neurosci. Lett. 128, 195–198 [DOI] [PubMed] [Google Scholar]
- 10).Ishiguro K., Omori A., Takamatsu M., Sato K., Arioka M., Uchida T., Imahori K. (1992) Phosphorylation sites on tau by tau protein kinase I, a bovine derived kinase generating an epitope of paired helical filaments. Neurosci. Lett. 148, 202–206 [DOI] [PubMed] [Google Scholar]
- 11).Goedert M. (1993) Tau protein and the neurofibrillary pathology of Alzheimer’s disease. TINS 16, 460–465 [DOI] [PubMed] [Google Scholar]
- 12).Kanai Y., Chen J., Hirokawa N. (1992) Micro-tuble bundling by tau protein in vivo. EMBO J. 11, 3953–3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Morishima-Kawashima M., Hasegawa M., Takio K., Suzuki M., Yoshida H., Titani K., Ihara Y. (1995) Proline-directed and non-proline-directed phosphorylation of PHF-tau. J. Biol. Chem. 270, 823–829 [DOI] [PubMed] [Google Scholar]
- 14).Shiurba R.A., Ishiguro K., Takahashi M., Sato K., Spooner E.T., Mercken M., et al. (1996) Immuno-cytochemistry of tau phosphoserine 413 and tau protein kinase I in Alzheimer pathology. Brain Res. 737, 119–132 [DOI] [PubMed] [Google Scholar]
- 15).Yamaguchi H., Ishiguro K., Uchida T., Takashima A., Lemere C.A., Imahori K. (1996) Preferential labeling of Alzheimer neurofibrillary tangle with antisera for protein kinase (TPK)I/GSK3β and cdk5, a component of TPKII. Acta Neuro-pathol. 92, 232–241 [DOI] [PubMed] [Google Scholar]
- 16).Takashima A., Noguchi K., Sato K., Hoshino T., Imahori K. (1993) Tau protein kinase I is essential for amyloid β-protein-induced neurotoxicity. Proc. Natl. Acad. Sci. USA 90, 7789–7793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Hoshi M., Sato M., Kondo S., Takashima A., Noguchi K., Takahashi M., et al. (1995) Different localization of tau protein kinaseI/glycogen synthase kinase-3β from glycogen synthase-3α in cerebrum mitochondoria. J. Biochem. (Tokyo) 118, 683–685 [DOI] [PubMed] [Google Scholar]
- 18).Hoyer S., Oesterreich K., Wagner O. (1988) Glucose metabolism as the site of the primary abnormality in early-onset dementia of Alzheimer type? J. Neurol. 235, 143–148 [DOI] [PubMed] [Google Scholar]
- 19).Sorbi S., Bird E.D., Blass J.P. (1982) Decreased pyruvate dehydroenase complex activity in Huntington and Alzheimer brain. Ann. Neurol. 13, 72– 78 [DOI] [PubMed] [Google Scholar]
- 20).Hoshi M., Murayama M., Yasutake K., Hoshino T., Ishiguro K., Takashima A., Imahori K. (1997) Effect of amyloid β-peptide1–42 on cholinergic neurons: Supppression of acetyl choline syththesis precedes neurodegeneration. J. Biol. Chem. 272, 2038–2041 [DOI] [PubMed] [Google Scholar]
- 21).Hoshi M., Sato M., Matsumoto S., Noguchi A., Yasutake K. (2003) Spherical aggregates of β-amyloid (amylospheroid) show high neurotoxicity, activate tau protein kinaseI/glycogen synthase kinase-3β. Proc. Natl. Acad. Sci. USA 100, 6370–6375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Noguchi A., Matsumura S., Denzawa M., Kikuchi S., Yanazawa M., Akioka M, et al. (2009) Isolation and characterization of patient-specific, toxic, high-mass amyloid β-protein (Aβ) assembly from Alzheimer’s disease brain. J. Biol. Chem. (in press). [DOI] [PMC free article] [PubMed]
- 23).Hung L.V., Cistosto G.D., Gllanako E., Tew D.J., Prez K., Muslers C.C., et al. (2008) Amyloid-beta peptide (Aβ) neurotoxicity is modulated by the role of peptide aggregation; Amyloid β dimmer and trimer correlate with neurotoxicity. Arch. Neuro. 66, 190–199 [Google Scholar]
- 24).Lcor P.N., Bunel M.C., Furlow P.W., Clemente A., Sanz Velasco P.T., et al. (2008) Abeta oligomer induced aberration of synapse compositiom, shape and density. J. Neurosci. 27, 796–807 [DOI] [PMC free article] [PubMed] [Google Scholar]