Abstract
Objectives
To evaluate the effect of regular physical activity on metabolic risk factors and blood pressure in Inuit with high BMI consuming a western diet (high amount of saturated fatty acids and carbohydrates with a high glycemic index).
Study design
Cross sectional study, comparing Inuit eating a western diet with Inuit eating a traditional diet.
Methods
Two physically active Greenland Inuit groups consuming different diet, 20 eating a traditional diet (Qaanaaq) and 15 eating a western diet (TAB), age (mean (range)); 38, (22–58) yrs, BMI; 28 (20–40) were subjected to an oral glucose tolerance test (OGTT), blood sampling, maximal oxygen uptake test, food interview/collection and monitoring of physical activity.
Results
All Inuit had a normal OGTT. Fasting glucose (mmol/l), HbA1c (%), total cholesterol (mmol/l) and HDL-C (mmol/l) were for Qaanaaq women: 4.8±0.2, 5.3±0.1, 4.96±0.42, 1.34±0.06, for Qaanaaq men: 4.9±0.1, 5.7±0.1, 5.08±0.31, 1.28±0.09, for TAB women: 5.1±0.2, 5.3±0.1, 6.22±0.39, 1.86±0.13, for TAB men: 5.1±0.2, 5.3±0.1, 6.23±0.15, 1.60±0.10. No differences were found in systolic or diastolic blood pressure between the groups. There was a more adverse distribution of small dense LDL-C particles and higher total cholesterol and HDL-C concentration in the western diet group.
Conclusions
Diabetes or impaired glucose tolerance was not found in the Inuit consuming either the western or the traditional diet, and this could, at least partly, be due to the high amount of regular daily physical activity. However, when considering the total cardio vascular risk profile the Inuit consuming a western diet had a less healthy profile than the Inuit consuming a traditional diet.
Keywords: insulin resistance, physical activity, metabolic syndrome
Over the last 50 years the prevalence of lifestyle-related metabolic morbidity and mortality has increased markedly in both westernised societies and societies undergoing a change towards westernised lifestyle (1,2). In Greenlandic Inuit the prevalence of type 2 diabetes was very low prior to the 1980s (3) whereas recent studies of Inuit in Greenlandic (4) as well as in Canada and Alaska have revealed an increasing and very high prevalence of type 2 diabetes and impaired glucose tolerance (IGT) (5). In this modernisation period life-conditions of the Inuit population of Greenland have changed dramatically. The import of “western” food products has gradually increased since 1930 which has led to a decline in the dependence on hunting and fishing (6). The daily consumption of traditional foods has decreased and thereby the consumption of n-3 fatty acids has been significantly reduced from the 1970s to 2004 (7). Thus, the lifestyle changes in the majority of the Greenlandic population include a change from a traditional marine diet to a pre-dominantly “western” diet as well as a decline in the daily physical activity level. Results from studies on Inuit in Canada confirm that the modern lifestyle of Inuit is accompanied by a decrease in aerobic fitness (8,9). The prevalence of metabolic diseases and subsequent morbidity has increased markedly in populations who have recently changed from a traditional hunter-gatherer life style to a modern “western” lifestyle, for example Inuit, American Indians, Australian Aboriginals (1,2).
Changes in daily diet often occur alongside changes in the level of physical activity, and therefore the adverse impact of inactivity versus diet changes are difficult to evaluate when groups within a population drift towards new lifestyle patterns. In the present experiment we had the possibility to study two Inuit groups in the north western part of Greenland unique in their own way and rarely accessible. One Inuit group still lived a traditional Inuit life both in regard to performing hard labour such as fishing and hunting year around and consuming a predominantly marine diet. The other Inuit group had changed to a prototype westernised diet rich in saturated fatty acids and carbohydrates with a high glycemic index, but were still physically active on a daily basis, up to 60 hour work week performing manual jobs at Thule Air Base (TAB). The present study was therefore a unique opportunity to evaluate, the potential effect of physical activity on glucose tolerance, metabolic risk markers and blood pressure in an Inuit population consuming a western diet.
We hypothesised that the substantial daily physical activity level would attenuate the adverse influence of an unhealthy westernised diet, for example maintain a normal glucose tolerance and metabolic risk factor profile.
Materials and methods
Thirty-five Greenlandic Inuit from TAB and Qaanaaq, all with Inuit parents and grandparents volunteered. The “westernised” Inuit (7 men and 8 women) were recruited at TAB. Inclusion criteria were (a) living and being employed in physically demanding jobs at TAB for more than 3 years, (b) eating the food at TAB (American staff restaurant and supermarket) and not traditional Arctic food regularly. The traditional Inuit (12 men, 8 women) were recruited in Qaanaaq and nearby settlements. Inclusion criteria were (a) living a traditional Inuit lifestyle. We defined the traditional life style consisting of all year hunting, fishing and gathering of prey (e.g. hunting seal, polar bear and reindeer, deep sea halibut fishing and gathering of berry). With a local habituated interpreter with extensive knowledge of the local community all Qaanaaq Inuit went through a personal interview and only Inuit who could confirm having lived this way for many years and who did not have any other types of job activity were included.
The study was approved by the Commission for Scientific Research in Greenland (J. nr.: 505-89) and was performed according to the Declaration of Helsinki.
Daily physical activity and dietary intake
To estimate the intensity and amount of physical activity during work for the group living at TAB, 5 subjects (age range 39–54) wore a HR monitor on 4–5 different work days (Polar, belt T31, watch S 610i, Kempele, Finland). They were selected through personal interview in order to get activity measurement from the different types of jobs that the Inuit included in the study perform at TAB (cleaning, kitchen and mechanical workshop). In a follow-up session 8 of the subjects, also representing the different types of jobs, wore a pedometer on 2 normal work days to record the number of steps. Five of these 8 subjects were the 5 Inuit that were included in the HR measurement and the last 3 subjects were selected as the other 5 subjects. The Inuit were asked if the days of monitoring differed in regard to activity compared to normal working days. For these 8 subjects complete food consumption during 2 full days where collected for later analysis using the double serving size method (an exact copy of every meal, size and content, were put frozen for later analysis). Furthermore the subjects handed in a written account of the few products not sampled for analysis and these foods were primarily commercially produced candies, biscuits and soda. The composition and energy distribution of these items were added to the total daily energy expenditure (EE).
The traditionally living Inuit completed with a translator present a food frequency questionnaire (FFQ) about the intake of selected specified Arctic and “Danish/western” food items; these were, for example. Arctic locally harvested fish, seal, whale and imported Danish/western goods, such as fresh fruit, frozen vegetables, milk, rice, potatoes, biscuits. To estimate the distribution between Arctic and “Danish/western” food sources the subjects where asked about the frequency of intake of all the food categories; (daily, 3–6 times a week, 1–2 times a week, 2–3 times a month, once a month or less and never. In addition they were asked to estimate the distribution in percentage year round between Arctic and Danish foods. The food questionnaire/interview was similar, to the one used in several surveys by Bjerregaard and colleagues in Greenland (6). Blood samples were analysed for trichina antibodies to verify consumption of traditional Arctic marine mammals (10).
To estimate the intensity and amount of physical activity during hunting and fishing for the Qaanaaq Inuit we tried to measure heart rate during hunting and their everyday life activity.
Unfortunately it was not easy to monitor heart rate because sometimes the hunters travel for many days or weeks without turning back to Qaanaaq and we experienced that they leave without warnings if they hear of good hunting possibilities. Thus it turned out to be logistically impossible to achieve good quality data on physical activity in the hunter population.
Experimental protocol
The investigations were performed at TAB (April–May) and in Qaanaaq (October). The measurements were carried out on 2 separate days. The first day included anthropometric and blood pressure measurements, interview (including FFQ, family origins, smoking and drinking habits), and an oral glucose tolerance test (OGTT). The second day included determination of maximal oxygen uptake (VO2max). For the TAB subjects a third day was included where 1 hour of cycling exercise at 60% of VO2max was performed. The relation between HR and oxygen uptake during submaximal work was used to calculate the daily EE based on the HR recordings obtained during work days at TAB.
Day 1
The subjects arrived at the laboratory in the morning after ten hours of fasting. The subjects were asked to confirm fasting status in the morning. The night before subjects were either called or reminded personally not to eat any breakfast. Body weight and height were measured with the subjects wearing only undergarments. After a 10 min rest in the supine position body composition was measured using bioimpedance (Quantum X, RJL Systems, MI, USA) and blood pressure by auscultation (Heine gamma sphygmomanometer, Herrsching, Germany). The average of the last 2 of 3 measurements was used to calculate mean systolic and diastolic blood pressure.
After this a standard OGTT was performed. The glucose solution was ingested within 5 minutes. Blood samples were taken immediately before and 15, 30, 60, 90 and 120 min. after ingestion. Blood samples were transferred into tubes containing EDTA (10 µm/ml), immediately spun at 8,000 rpm for 6 min. and plasma was frozen at −80°C. Capillary blood was taken from the fingertip, to measure glycosylated haemoglobin (HbA1c).
Day 2
On the second day subjects came to the laboratory during the day and at least 2 hours after a light meal. To determine VO2max, subjects performed an incremental exercise test to exhaustion on a bicycle ergometer (Monarch 839E; Monarch Exercise AB, Vansbro, Sweden). After a 5 min warm-up at 100 and 80 watts for men and women, respectively the workload was increased every min. by 20 watts until fatigue. The subjects kept a constant pedal frequency of their own choice within 60–80 revolutions per minute. All subjects received encouragement. To establish whether VO2max was attained, 2 of the following 3 criteria had to be achieved: a HR at fatigue within 10 beats of age-predicted maximal HR (220 – age); a respiratory exchange ratio (RER)>1.15; a levelling off of oxygen uptake despite an increase in workload. During the test, HR was measured with a Polar HR monitor (belt T31) and oxygen uptake and carbon dioxide production determined by an online system (CosMed, Quark b2, Italy).
Analytical methods
Plasma glucose, serum triglyceride (TG), total cholesterol (TC) and HDL cholesterol (HDL) were analysed using an automatic analyzer (Cobas Fara, Roche, Basel, Switzerland). Plasma free fatty acid (FFA) concentration was measured using a Wako NEFA-C test kit (Wako Chemical, Neuss, Germany); Plasma insulin levels were measured using ELISA. (Insulin RIA100, Pharmacia, Sweden). HbA1c was analysed on a Bayer DCA 2000+ (Bayer Healthcare, Elkhart, IN, USA) using a latex immunoagglutination inhibition method. The measurement of trichina antibodies have been described previously (10,11).
Lipoprotein profile was determined using continuous polyacrylamide gel electrophoresis (Lipoprint LDL System, Quantimetrix, Redondo Beach, CA, USA) (12).
Analysis of the food collected
The concentration of fat and fatty acids in the homogenised diets were determined as previously described (13). The nitrogen content in the diets was determined by the conventional Kjeldahl method. To estimate the concentration of protein, the nitrogen content was multiplied by a conversion factor of 6.25, as is used for most foods (14). The amount of dry matter and ash in the diets was measured by gravimetric methods. The content of carbohydrates was calculated as: solids minus the sum of protein, fat, and ash. The energy content of the diets was calculated by the following factors: 17 kJ/g protein or carbohydrate and 37 kJ/g fat (14).
Statistics
Results are given as means±SD, if not otherwise stated. Non-normally distributed variables are given as median and range. Two-way ANOVA measurement was used to test for differences between gender and TAB and Qaanaaq groups. A two-way repeated ANOVA measurement was applied to the OGTT data. If data was not normally distributed, a non-parametric Mann–Whitney test was used to test for differences. The level of significance was reported when (p<0.05). The statistical tests were calculated using Sigma Stat 4.0 (Systat software, Inc. San Jose, USA).
Results
Body composition, blood pressure and Cardio-respiratory fitness
The subjects in the 4 groups had similar age and BMI, weight (kg)/height (m2) (Table I). The women had higher body fat than the men, but no difference was present between the Inuit groups (Table I). The mean systolic and diastolic blood pressures were similar between the Inuit groups. Within the Qaanaaq group the men had higher systolic and diastolic blood pressure than the women (Table I). One out of 15 Inuit from TAB and 2 out of 20 Inuit from Qaanaaq had blood pressures marginally above 140 or 90 mmHg and 1 Inuit from TAB was hypertensive and required follow up and/or medical treatment. Blood haemoglobin content was similar between the Inuit groups, but lower in women than men (Table I). VO2max and maximal HR were similar within each gender in the 2 groups. VO2max was higher in men than women (Table I).
Table I.
Descriptive data on subjects of both gender of Inuit from Qaanaaq and TAB, including plasma glucose, insulin, lipids and lipoprotein-subfraction
| Qaanaaq | TAB | |||
|---|---|---|---|---|
| Women (n =8) | Men (n=12) | Women (n=8) | Men (n=7) | |
| Age (years) | 40.4 (34–47) | 40.3 (34–56) | 39.1 (23–54) | 35.6 (22–58) |
| Height (cm) | 156 (150–163) | 169 (163–179) b | 159 (150–165) | 165 (160–172) b |
| Weight (kg) | 62.8 (45.6–79.9) | 81.3 (64.8–102.9)b | 72.1 (52.5–105.1) | 78.1 (64.4–90) |
| BMI (kg/m) | 25.9 (19.9–33.1) | 28.4 (23.5–37.0) | 28.1 (22.3–39.6) | 28.8 (22.8–33.6) |
| Fat (%) | 35 (22–48) | 19 (14–24) b | 39 (29–54) | 22 (18–25)b |
| Sys (mmHg) | 104 (92–120) | 119 (102–136)b | 131 (90–200) | 133 (110–150) |
| Dia (mmHg) | 71 (60–80) | 86 (70–103)b | 75 (60–100) | 84 (70–100) |
| Hb (g/l) | 12.7±0.6 | 14.5±1.0b | 12.6±0.6 | 15.0±0.5b |
| VO2max, (ml/kg/min) | 31.2±5.4 | 41.3±5.9 b | 27.4±5.1 | 38.2±8.5b |
| HRmax beat·min 1 | 176±5.4 | 189±9.7 | 184±10.7 | 197±25.9 |
| Glucose (mmol/l) | 4.8±0.6 | 4.9±0.3 | 5.1±0.6 | 5.1±0.5 |
| Insulin (pmol/l) 0 | 4±(2–7) | 10±(2–25) | 17±(5–66)a | 19±(8–40) |
| Insulin (pmol/l) 60 | 201±(60–368) | 202±(13–360) | 285±(83–405) | 279±(181–437) |
| Insulin (pmol/l) 120 | 19±(2–136) | 14±(2–37) | 57±(10–109) | 33±(3–323) |
| HbA1c (mmol/l) | 5.3±0.3 | 5.7±0.3ab | 5.3±0.3 | 5.3±0.3 |
| HOMA-IR | 0.11±0,06 | 0.35±0.28 | 0.86±0.79a | 0.66±0.4 |
| TC (mmol/l) | 4.96±1.19 | 5.08±1.07 | 6.22±1.1a | 6.23±0.4a |
| HDL (mmol/l) | 1.34±0.17 | 1.28±0.31 | 1.86±0.37a | 1.60±0.26a |
| TG (mmol/l) | 0.31±(0.24–0.61) | 0.82±(0.45–1.3)b | 0.87±(0.65–3.4)a | 0.85±(0.63–2.44) |
| NEFA (mol/l) | 0.71±0.29 | 0.57±0.27 | 0.58±0.33 | 0.66±0.27 |
| Subfraction (%) | ||||
| VLDL | 20.8±2.3 | 23.6±4.2 | 16.1±4.8 a | 18.6±7.7a |
| IDL | 30.1±2.8 | 31.2±5.5 | 14.5±2.8a | 16.3±1.6a |
| LDL | 15.0±4.2 | 12.7±5.5 | 37.0±4.2a | 39.8±4.0a |
| HDL | 34.1±5.9 | 32.5±8.0 | 32.4±9.6 | 25.3±9.3 |
| LDL1 | 54.7±9.1 | 60.9±14.5 | 33.3±15.6a | 30.1±22.2a |
| LDL2 | 22.9±3.1 | 20.1±3.8 | 28.0±5.4a | 25.2±7.9a |
| LDL3 | 14.1±4.2 | 13.0±5.9 | 20.5±5.7 a | 21.2±8.2a |
| LDL4 | 5.5±4.0 | 4.0±4.5 | 11.1±6.5a | 11.9±7.4a |
| LDL5 | 1.6±2.0 | 1.4±3.5 | 5.4±5.7a | 7.9±7.7a |
| LDL6 | 1.1±1.7 | 0.5±1.7 | 1.7±2.3 | 3.8±5.3 |
Plasma lipoprotein subfractions. From the top, relative distribution of all fractions, intermediate-density lipoprotein (IDL)-subfraction distribution, low-density lipoprotein (LDL)-subfraction distribution, and high-density lipoprotein (HDL)-subfraction distribution. No differences were found between the two groups in IDL and HDL subfractions (data not shown). Insulin data shown was obtained during OGTT. Data are mean±SD, the first 7 lines are mean and range, only insulin and TC are presented as median and range due to non normally distribution, n=number of subjects.
Significant difference between TAB Inuit (Thule Air Base, on western diet) and Qaanaaq Inuit (on traditional arctic diet).
Significant gender difference within the Qaanaaq and TAB Inuit group, respectively.
Physical activity and food intake
In the TAB group HR was recorded on 4–5 normal work days at TAB, and recording time ranged from 9–16 hours per day. The average time spent for the 5 individuals during 1 work day above 60% of maximal oxygen uptake was 165 min (range; 77–245 min), which corresponds to a mean HR of ~119 bpm. (range; ~100–130 bpm). Based on the time and HR data the average EE was estimated to ~13,000 kJ/day. The pedometer data showed that the TAB Inuit walked between 8,264 and 20,180 steps during a work day, with an average of 13,425 steps per day.
It was impossible to get continuous and objectively recorded data on physical activity in the Qaanaaq Inuit due to the weather conditions and inadequate power supply in the field. Yet based on observation by several of the authors (TMA, JWH, HS) during several weeks in Qaanaaq both in the winter and summer season and during days of sledge travelling including repeated change of camp sites, hunting and fishing, it is beyond doubt, that the Qaanaaq Inuit daily performed a substantial amount of regular physical activities. The Qaanaaq women were responsible for processing of prey and hide and they covered long distances walking accompanying the hunters and gathering berries. In addition, Inuit women only used walking as a means of transport in Qaanaaq.
Analysis of the food collected from the TAB group showed that the total energy intake per day was 9,672±604 kJ and that 29±2 energy% (E%) was from fat, 54±2 E% was from carbohydrate and 17±1 E% was from protein. The relative contribution of the different fatty acids to the total energy consumption was 12±1% from SFA, 11±1% from MUFA, 6±0% from PUFA and 1±0% from TFA. In a prior study it was demonstrated that no trichina antibodies were present in the blood samples from the TAB group (10). The EE calculated by using the HR recordings was approximately 20–40% higher than the energy intake calculated from the food analyses. Despite the difference in energy intake and expenditure we are confident that the macronutrient composition and fatty acid distribution is representative for the food consumed by the TAB Inuit. Overall the food analysis confirmed that the TAB Inuit consumed a typical “western” diet mainly based on food acquired at the American staff restaurant and supermarket at the TAB. The results from the food interview for the Qaanaaq Inuit showed that the average self estimated year round distribution between arctic and Danish/western food items were 65% (20–100%) Arctic and 35% Danish/western (range; 0–80%). In the analysis of the food interview data we focused on seal, whale and fish and fresh fruit, based on experience gained from a similar population in previous studies (4,6). All subjects reported eating a main course containing primarily animal arctic food items at least 3–6 times a week and 17 out of the 20 reported doing it daily. None reported eating fresh fruit on a daily or weekly basis. A prior publication demonstrated very high blood trichina antibody concentrations in Qaanaaq Inuit, which is consistent with a very frequent and steady intake of marine and arctic meat and probably only a lifelong adaptation can explain the absence of pathological symptoms (10).
Fasting glucose, insulin, HbA1c and OGTT
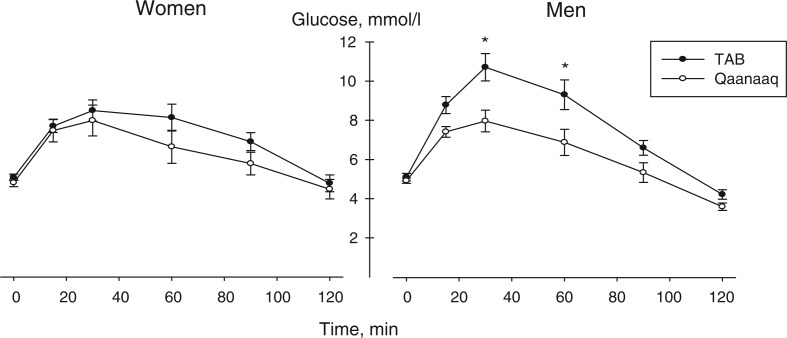
On an individual basis all Inuit had normal fasting glucose, HbA1c and OGTT. Fasting glucose did not differ between the groups or between men and women within each group (Table I). The total area under the OGTT curve was higher (21%) for the men from TAB compared to Qaanaaq, whereas it was similar for the 2 groups of women (Fig. 1). No gender differences could be detected within the Qaanaaq group when comparing the total area under the curve or at any time points. Within the TAB group there was no gender difference when comparing the total area under the curve. However, the glucose concentration at 30 min. was higher for the men compared to the women. The insulin response during the OGTT did not differ between the 2 groups or between men and women (Table I). However, when using a t-test for the fasting pre OGTT concentration, the women from TAB had higher insulin concentration than the women from Qaanaaq (Table I). HbA1c was higher in men from Qaanaaq than in men from TAB and women from Qaanaaq group (Table I). HOMA-IR was calculated as described by Matthews (15) and the results showed that the TAB women had higher HOMA-IR than Qaanaaq women.
Figure 1.
Plasma glucose concentration during an oral glucose tolerance (OGTT) test performed in physically active adult Inuit men (A: TAB: n=7 and Qaanaaq: n=12) and women (B: TAB, n=8 and Qaanaaq: n=8) consuming either a westernised (TAB) or a traditional Inuit (Qaanaaq) diet.
Area under the OGTT curve was significant bigger for the TAB men than the Qaanaaq men, but was similar for the women. *: (p<0.05) 30 and 60 min. TAB vs. Qaanaaq. Error bars are SEM.
Blood lipids
TC and HDL-C for men and women were higher in the TAB compared to the Qaanaaq group (Table I). No difference in the TC/HDL ratio between the groups was found for either gender. For the women TG concentration was higher in the TAB compared to the Qaanaaq group (Table I). Within the Qaanaaq group, the men had higher TG concentration than the women (Table I). The TAB group had more smaller dense LDL particles than the Qaanaaq group (Table I). The HDL and IDL subfractions were similar between all groups (data not shown).
Discussion
In this study of 2 groups of physically active Inuit with high BMI, consuming either a traditional Inuit or a typical western diet we did not find any individuals with glucose intolerance or type 2 diabetes. Some significant differences in metabolic risk profile between the 2 groups were found. In Inuit consuming western diet TC and HDL-C were significantly higher and there was a distribution towards smaller dense LDL-C. Non-significant but probably clinically relevant differences were observed for systolic blood pressure and the insulin and glucose response during OGTT tended to be less favourable in the TAB group compared to the Qaanaaq group. Especially the Inuit women from TAB tended to have higher BMI, insulin and TG levels than Inuit women from Qaanaaq.
During the last 30–40 years the prevalence of glucose intolerance and type 2 diabetes has increased markedly in the indigenous populations in the northern polar regions (5) as well as in the Inuit population in Greenland (4). Furthermore, based on the expected progression of obesity it has been predicted that the prevalence of type 2 diabetes in female Inuit will increase up to 23% whereas it will remain unchanged in male Inuit (16). It is clear that lifestyle has a major impact on this development and decreased physical activity and even inactivity has been put forward as one of the major contributors (4). In the present study we focused on the glucose tolerance and metabolic risk factors in 2 distinct groups both very physically active due primarily to their daily occupational work/lifestyle but consuming very different diets, either a westernised diet or the traditional Inuit diet. Interestingly we did not observe any subjects with either glucose intolerance based on OGTT data evaluated according to 1999 WHO criteria (17) or impaired fasting glucose. In addition the measurement of HbA1c confirmed according to recently suggested guidelines (18) that none of the subjects had type 2 diabetes.
In the present study maximal oxygen uptake was similar in the 2 groups within each gender, thus demonstrating similar aerobic fitness and degree of training. It is noteworthy that the aerobic fitness (women 27–31, men 38–41 mL O2/kg/min) is somewhat low considering that the TAB Inuit performed about 55–60 hours of manual work every week and the Qaanaaq Inuit also had a high daily physical activity level. However, the main part of the physical activity performed by both Inuit groups was low intensity work and as such this does not stimulate any training effect on aerobic fitness. There is strong evidence that daily physical activity is correlated to improved insulin sensitivity and this effect is independent of the effect of physical activity on body composition (19,20). Based on these findings it is likely that the high amount of daily low intensity physical activity exert a significant contribution to maintain normal glucose tolerance despite the dietary macronutrient composition and the high BMI of the Inuit in the present study.
In this study the Inuit who consumed the westernised diet with a high content of high glycaemic carbohydrates and high content of saturated fat had a higher TC and HDL-C concentration than the Qaanaaq Inuit. Furthermore, there was a sub distribution of LDL-C towards a higher percentage of the more atherogenic low density LDL-C particles after the westernised compared to the traditional Inuit diet. These findings are not surprising as prior studies in healthy Caucasian subjects where a westernised diet was consumed found dyslipidaemia, including high levels of TCl, high LDL-C/ HDL-C ratio and an atherogenic LDL-C sub-fraction distribution (21,22). We emphasise that in the present study the effect on metabolic risk factors after consumption of a westernised diet is compared to consumption of the traditional Inuit hunter-fisher diet. It is very probable that the Qaanaaq Inuit group constitutes a rare but true control group, when evaluating the effect of lifestyle modifications in Inuit. Studies on the effect of physical activity on blood lipids have shown that regular physical activity will lead to an increased HDL-C content, lowering of plasma TG content, and a decreased LDL-C level (23). In 2 recent studies we demonstrated that repeated daily very prolonged low intensity exercise improved the blood lipid profile causing a decrease in TC and a shift towards a less atherogenic LDL-cholesterol profile (24,25). Interestingly the beneficial effect of physical activity on metabolic risk factors, e.g. improved metabolic fitness occurred without any change in aerobic fitness (24,25). In line with these observations the present study demonstrates a rather low maximal oxygen uptake e.g. aerobic fitness in both Inuit groups considering the level of daily physical activity and therefore the effect of the daily physical activity in the 2 Inuit groups is probably mainly observed in the metabolic profile. In this study male and females of both Inuit groups were investigated and we did observe the expected gender differences in body fat content, haemoglobin content, maximal oxygen uptake and height. However, in terms of metabolic risk markers and glucose tolerance there was an almost complete absence of gender differences. Although we did not a priori expect major gender differences in metabolic risk markers it is not possible to exclude that the number of subjects included is insufficient to discriminate possible gender differences.
In conclusion, glucose intolerance and type 2 diabetes were absent in 2 physically active Inuit groups, despite high levels of BMI and, in the case of the TAB Inuit, the consumption of a typical western diet. We propose that the regular and rather large amount of daily physical activity performed plays an important role in these findings. We note, however, that the metabolic profile of traditionally living Inuit were more favourable than in those who had changed to a westernised diet containing high glycemic carbohydrates sources and high amounts of saturated fatty acids.
Limitations to the study
The relatively small numbers of subjects limits the power of the study. This is particularly apparent in the comparison between groups were non-significant differences in metabolic risk factors with a higher power might have become significant. Due to the limited number of subjects we did not perform adjusted analysis and did not perform continuous metabolic risk cluster analysis. It was not feasible to get reliable data on physical activity in the Qaanaaq Inuit population and it is difficult to directly compare the amount and type of activity performed in the 2 groups. The food intake in the Qaanaaq group was determined by FFQ and this probably is a less precise way to estimate the food intake compared to the double portion method used for the TAB Inuit. Overall both the TAB and Qaanaaq Inuit were a selected, physically and mentally fit group that were able to perform the 55 working hours per week at the air base or endure the hunting lifestyle over long periods. How representative they are for the general Inuit population in Greenland is difficult to determine and selection bias can therefore not be excluded. Smoking and drinking were not recorded and we did not try to classify the socio-economic status in the 2 groups.
Acknowledgements
The study was supported by the Meyer Foundation and the Danish National Research Foundation (504-14). Mogens Morgen (architect and many times Greenland traveller) and Jeppe Handwerk (Copenhagen Contractors, and organiser of several arctic expeditions as well as Jens Peter Jensen (Air Greenland, Greenland executive) are acknowledged for helping with logistics. Mogens Morgen also provided invaluable help with local translators in obtaining the dietary information from the Inuit in Qaanaaq. The skilled technical assistance of Lene Foged, the late Carsten Bo Nielsen, Nina Pluszek (Copenhagen Muscle Research Center, Rigshospitalet) is kindly acknowledged. Support was provided from the Hunter Society in Qaanaaq and Hans Jensen at Hotel Qaanaaq. The Danish and American staff at the Thule Air Base and the staff at the hospital in Qaanaaq and at Thule Air Base kindly provided support.
Conflict of interest and funding
The authors declare they have no conflicts of interest.
References
- 1.Jorgensen ME, Young TK. Cardiovascular diseases. Diabetes, and obesity. In: Young TK, Bjerregaard P, editors. Health transitions in Artic populations. Toronto: University of Toronto Press; 2008. pp. 291–307. [Google Scholar]
- 2.Hegele RA, Young TK, Connelly PW. Are Canadian Inuit at increased genetic risk for coronary heart disease? J Mol Med. 1997;75:364–70. doi: 10.1007/s001090050122. [DOI] [PubMed] [Google Scholar]
- 3.Kromann N, Green A. Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950–1974. Acta Med Scand. 1980;208:401–6. [PubMed] [Google Scholar]
- 4.Jorgensen ME, Bjerregaard P, Borch-Johnsen K. Diabetes and impaired glucose tolerance among the inuit population of Greenland. Diab Care. 2002;25:1766–71. doi: 10.2337/diacare.25.10.1766. [DOI] [PubMed] [Google Scholar]
- 5.Ebbesson SO, Schraer CD, Risica PM, Adler AI, Ebbesson L, Mayer AM, et al. Diabetes and impaired glucose tolerance in three Alaskan Eskimo populations. The Alaska-Siberia Project. Diab Care. 1998;21:563–9. doi: 10.2337/diacare.21.4.563. [DOI] [PubMed] [Google Scholar]
- 6.Bjerregaard P. Public health research and practice in Greenland. Int J Circumpolar Health. 2004;63:210–1. doi: 10.3402/ijch.v63i3.17713. [DOI] [PubMed] [Google Scholar]
- 7.Deutch B, Dyerberg J, Pedersen HS, Asmund G, Moller P, Hansen JC. Dietary composition and contaminants in north Greenland, in the 1970sand 2004. Sci Total Environ. 2006;370:372–81. doi: 10.1016/j.scitotenv.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Rode A, Shephard RJ. Physiological consequences of acculturation: a 20-year study of fitness in an Inuit community. Eur J Appl Physiol Occup Physiol. 1994;69:516–24. doi: 10.1007/BF00239869. [DOI] [PubMed] [Google Scholar]
- 9.Rode A, Shephard RJ. Ten years of “civilization”: fitness of Canadian Inuit. J Appl Physiol. 1984;56:1472–7. doi: 10.1152/jappl.1984.56.6.1472. [DOI] [PubMed] [Google Scholar]
- 10.Moller LN, Krause TG, Koch A, Melbye M, Kapel CM, Petersen E. Human antibody recognition of Anisakidae and Trichinella spp. in Greenland. Clin Microbiol Infect. 2007;13:702–8. doi: 10.1111/j.1469-0691.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- 11.Moller LN. Epidemiology of Trichinella in Greenland – occurrence in animals and man. Int J Circumpolar Health. 2007;66:77–9. doi: 10.3402/ijch.v66i1.18230. [DOI] [PubMed] [Google Scholar]
- 12.Hoefner DM, Hodel SD, O'Brien JF, Branum EL, Sun D, Meissner I, et al. Development of a rapid, quantitative method for LDL subfractionation with use of the Quantimetrix Lipoprint LDL System. Clin Chem. 2001;47:266–74. [PubMed] [Google Scholar]
- 13.Bysted A, Mikkelsen AÆ, Leth T. Substitution of trans fatty acids in foods on the Danish market. Eur J Lipid Sci Technol. 2009;111:574–83. [Google Scholar]
- 14.Alexander J, Anderssen SA, Aro A, Becker W, Fogelholm M, Lyhne N, et al. 4th ed. Copenhagen: Nord 2004:013, Nordic Council of Ministers; 2005. Nordic nutrition recommendations 2004. Integrating nutrition and physical activity; p. 436. [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Martinsen N, Jorgensen ME, Bjerregaard P, Krasnik A, Carstensen B, Borch-Johnsen K. Predictions of type 2 diabetes and complications in Greenland in 2014. Int J Circumpolar Health. 2006;65:243–52. doi: 10.3402/ijch.v65i3.18106. [DOI] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diab Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Selvin E, Zhu H, Brancati FL. Elevated A1C in adults without a history of diabetes in the U.S. Diab Care. 2009;32:828–33. doi: 10.2337/dc08-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kriska AM, Hanley AJ, Harris SB, Zinman B. Physical activity, physical fitness, and insulin and glucose concentrations in an isolated Native Canadian population experiencing rapid lifestyle change. Diab Care. 2001;24:1787–92. doi: 10.2337/diacare.24.10.1787. [DOI] [PubMed] [Google Scholar]
- 20.Kriska AM, Pereira MA, Hanson RL, Court de, Zimmet PZ, Alberti KG, et al. Association of physical activity and serum insulin concentrations in two populations at high risk for type 2 diabetes but differing by BMI. Diab Care. 2001;24:1175–80. doi: 10.2337/diacare.24.7.1175. [DOI] [PubMed] [Google Scholar]
- 21.Brouwer IA, Wanders AJ, Katan MB. Effect of animal and industrial trans fatty acids on HDL and LDL cholesterol levels in humans – a quantitative review. PLoS One. 2010;5:e9434. doi: 10.1371/journal.pone.0009434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKeown NM, Meigs JB, Liu S, Rogers G, Yoshida M, Saltzman E, et al. Dietary carbohydrates and cardiovascular disease risk factors in the Framingham offspring cohort. J Am Coll Nutr. 2009;28:150–8. doi: 10.1080/07315724.2009.10719766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraus WE, Slentz CA. Exercise training, lipid regulation, and insulin action: a tangled web of cause and effect. Obesity (Silver Spring) 2009;17:S21–6. doi: 10.1038/oby.2009.384. [DOI] [PubMed] [Google Scholar]
- 24.Helge JW, Damsgaard R, Overgaard K, Andersen JL, Donsmark M, Dyrskog SE, et al. Low-intensity training dissociates metabolic from aerobic fitness. Scand J Med Sci Sports. 2008;18:86–94. doi: 10.1111/j.1600-0838.2006.00604.x. [DOI] [PubMed] [Google Scholar]
- 25.Helge JW. Arm and leg substrate utilization and muscle adaptation after prolonged low-intensity training. Acta Physiol (Oxf) 2010;199:519–28. doi: 10.1111/j.1748-1716.2010.02123.x. [DOI] [PubMed] [Google Scholar]