Abstract
Objectives
Female citizens of Sami (the indigenous people of Norway) municipalities in northern Norway have a low risk of breast cancer. The objective of this study was to describe the attendance rate and outcome of the Norwegian Breast Cancer Screening Program (NBCSP) in the Sami-speaking municipalities and a control group.
Study design
A retrospective registry-based study.
Methods
The 8 municipalities included in the administration area of the Sami language law (Sami) were matched with a control group of 11 municipalities (non-Sami). Population data were accessed from Statistics Norway. Data regarding invitations and outcome in the NBCSP during the period 2001–2010 was derived from the Cancer Registry of Norway (CRN). The NBCSP targets women aged 50–69 years. Rates and percentages were compared using chi-square test with a p-value<0.05 as statistical significant.
Results
The attendance rate in the NBCSP was 78% in the Sami and 75% in the non-Sami population (p< 0.01). The recall rates were 2.4 and 3.3% in the Sami and non-Sami population, respectively (p<0.01). The rate of invasive screen detected cancer was not significantly lower in the Sami group (p=0.14). The percentage of all breast cancers detected in the NBCSP among the Sami (67%) was lower compared with the non-Sami population (86%, p=0.06).
Conclusion
Despite a lower risk of breast cancer, the Sami attended the NBCSP more frequently than the control group. The recall and cancer detection rate was lower among the Sami compared with the non-Sami group.
Keywords: Sami, screening, breast cancer, mammography, Norway
Several investigators have been concerned about the uptake of mammographic screening among ethnic minorities (1–7). The Sami constitute an ethnic minority in the Norwegian community and live in the northern regions of Fennoscandia (the northern area of Norway, Sweden, Finland and Russia's Kola Peninsula). The Norwegian government has ratified the Sami as the indigenous people in Norway (8). The size of the Sami population has been reckoned to be approximately 75,000–100,000, but estimates vary in accordance with criteria used such as genetic heritage, mother tongue and sense of belonging to the Sami. According to the definitions employed by the Sami parliament, a Sami is a person who speaks Sami or one of the parents, grandfathers/grandmothers or great grandfathers/grandmothers spoke Sami language. In addition self-assigned Sami ethnicity is included. The majority of the Sami people in Norway live in the 3 northern counties named Finnmark, Troms and Nordland. The Sami have their own language and culture. Traditionally, their lifestyle diverges from that of the rest of the population in the area, but occupational expansion traditions are changing.
Research aimed at understanding health issues in the Sami peoples has been almost lacking, until recently (9–11). Hassler's thesis (11) (The health conditions in the Sami population of Sweden 1961–2002) from the Umeå University, Sweden, has been one of the major works in this field. In Norway, all inhabitants have equal rights concerning supply of health care independent of ethnical group. Norwegian health care authorities have been concerned about offering the Sami minority and Norwegians in general the same high quality health care service (9,10). Despite the fact that the Sami people are protected by a Sami Act, they have a different native language and culture that may cause several difficulties and challenges when assessing the public health care (12). The services offered by the specialist health care are generally in Norwegian and the knowledge of Sami culture and the access to interpreter service is limited. The challenges experienced by the Sami have often been summarised as threshold, counter, queue and cultural challenges. These hindrances may influence on the attendance rate of the screening programs (5). Furthermore, we have recently documented the Sami municipalities having a significantly reduced risk of breast cancer (13). On this background, we aimed to clarify the attendance and screening outcome in the Norwegian Breast Cancer Screening Program (NBCSP) in the Sami municipalities and a control group.
Material and methods
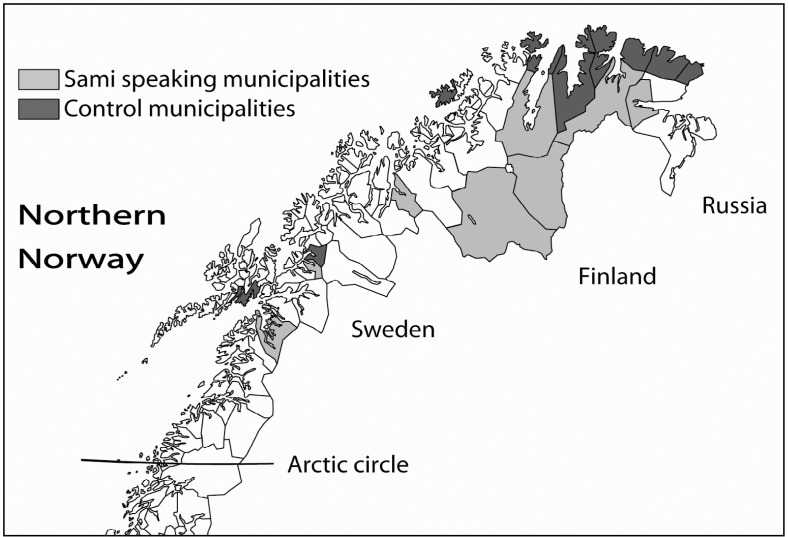
Eight municipalities in northern Norway have been included in the administration area of the Sami language law. These municipalities were selected as the “Sami group” in this study. The coastal municipalities of northern Norway have generally few Sami people and 11 of them were included in the “non-Sami group.” The 11 municipalities in the control group were selected based on having a coastal and rural location and a population of less than 4,500 inhabitants (no Sami municipality had more than 4,100 inhabitants). Furthermore, they were balanced between counties (Nordland 1, Troms 2 and Finnmark the other municipalities) similarly as the Sami group. Municipalities with hospitals were excluded, and consequently, the control group did not represent the northern municipalities in general but had similar characteristics as the Sami municipalities. The location of the 2 groups is shown in Fig. 1. Their names written in Sami language (when employed) and Norwegian were Deatnu/Tana, Unjárga/Nesseby, Porsanger/Porsángu/Porsanki, Kárásjohka/Karasjok, Guovdageaidnu/Kautokeino, Gáivuotna/Kåfjord, Ástávuona/Lavangen and Divtasvuona/Tysfjord.
Fig. 1.
The figure shows the map of northern Norway and the Sami and non-Sami speaking municipalities.
The Cancer Registry of Norway (CRN) is responsible for the administration and quality control of the NBCSP that invites all women aged 50–69 years to a 2-view mammography biennially (14). The program started in 4 counties in 1995/1996 and became nationwide in 2005. The counties in northern Norway, Troms and Finnmark, was included in the program in May 2000 and Nordland in 2001. Stationary screening units were located at the University hospital of North Norway (UNN) in Tromsø and the Nordland Hospital (NH) in Bodø, respectively. In addition there were 2 mobile units included in the screening facilities for the women residing in northern Norway. In the study period, 2001–2010, a total of 10,122 Sami women and 10,358 non-Sami women were invited to the NBCSP.
Data were extracted from the NBCSP-database and the CRN incidence database, both at the CRN. The annual number of invitations and subsequent attendances and recalls (due to mammographic findings, self-reported symptoms and technical inadequate mammograms) were given for the period 2001 to 2010. All numbers were given for each calendar year, in 5-year age groups (50–54 years, 55–59 years, 60–64 years and 65–69 years at screening). Ductal carcinoma in situ (DCIS) was not included. Breast cancer incidence data for women who did not attend the NBCSP were extracted from the CRN incidence database. There is a delay in reporting and updating of the CRN incidence database, and cancer data were available only for the period 2001–2008 for women who did not attend in the NBCSP. Screen detected and interval breast cancers were defined as cancer detected in the NBCSP and breast cancers detected among women who did not attend in the program as cancers detected outside the program.
Statistical analysis and authorisation
Individual data were analyzed at and by the CRN. Anonymous and aggregated data were exported to the first author. Microsoft Excel 2007 version was employed for the final database, calculations and statistical analysis. The comparison between study groups with regard to participation and screening outcome was based on ratios. The attendance rate of northern Norway was set as 1.0. Descriptive statistics and the t-test were employed for the comparison between municipality groups. Significance was set to 5%. The t-test was carried out 2-sided. Collecting information from the screening program in Norway is covered by the regulations on the collection and processing of personal health data in the Cancer Registry (Cancer Registry Regulations) and as the CRN received only aggregated data, no ethical committee or Data Inspectorate approval was necessary. Consequently, no approval from the Regional Committees for Medical and Health Research Ethics (REK) was necessary. Similarly, no approval from the Norwegian Social Science Data Services (NSD) was requested.
Results
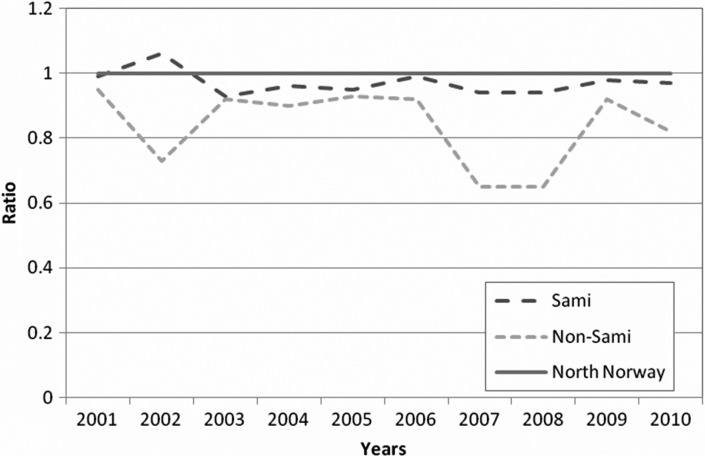
Whereas 10,122 and 10,358 invitations were sent to women in the Sami and non-Sami groups during study period, a total of 237,294 invitations were sent to women in northern Norway (Nordland, Troms and Finnmark) during the study period (Table I). The attendance rate in northern Norway was 81% (192,237/237,294) in the period 2001–2010, whereas it was 78% (7,923/10,122) in the Sami and 75% (7,762/10,358) in the non-Sami group (p<0.01). The annual ratio (attending/invited) is shown in Figure 2. The overall recall rates were 2.4% (188/7,923) and 3.3% (254/7,762), in the Sami and non-Sami population, respectively (p=0.01) (Table II). The recall rates due to mammographic findings were 1.9% (148/7,923) and 2.7% (206/7,762), respectively (p=0.01). Recall due to mammographic findings decreased by age in both the Sami and non-Sami population. Mammographic finding was the most common cause of recalls and contributed to 77 and 81% of the recalls among the Sami and non-Sami, respectively.
Table I.
Number of invitations and attendance rate in the screening program in the Sami and non-Sami group (2001–2010)
| Municipality | Invited | Attended | % | Ratioa |
|---|---|---|---|---|
| Sami group | 10,122 | 7,923 | 78.3 | 0.97 |
| Non-Sami group | 10,358 | 7,762 | 74.9 | 0.93 |
| Total | 20,480 | 15,685 | 76.6 | 0.95 |
When calculating ratio, northern Norway was set to 1.0.
Fig. 2.
The figure shows the annual ratio (attending/invited) in the 2 study groups compared to the total figures of northern Norway (set as 1.0).
Table II.
Attendance, recall and invasive screen detected cancer rates and positive predictive values (PPV) in the Sami and control group, 2001–2010
| Sami group | ||||||
|---|---|---|---|---|---|---|
| 50–59 years n=5,545 | 60–69 years n=4,577 | Total n=10,122 | ||||
| n | % | n | % | n | % | |
| Attending | 4,309 | 77.7 | 3,614 | 79.0 | 7,923 | 78.3 |
| Recall (total) | 116 | 2.7 | 74 | 2.0 | 190 | 2.4 |
| Positive mammography | 91 | 2.1 | 57 | 1.6 | 148 | 1.9 |
| Technical reasons | 11 | 0.3 | 5 | 0.1 | 16 | 0.2 |
| Self defined lump | 14 | 0.3 | 12 | 0.3 | 26 | 0.3 |
| Cancer detection | ||||||
| Invasive screen-detected | 16 | 0.3 | 10 | 0.3 | 26 | 0.3 |
| PPV-1† | 17.6 | 17.5 | 17.6 | |||
| Non-Sami population | ||||||
| n=5,804 | n=4,554 | n=10,358 | ||||
| n | % | n | % | n | % | |
| Attending | 4,425 | 76.2* | 3,338 | 74.9* | 7,762 | 74.9* |
| Recall (total) | 168 | 3.8 | 86 | 2.2 | 254 | 3.3 |
| Positive mammography | 133 | 3.0 | 73 | 1.8 | 206 | 2.7* |
| Technical reasons | 13 | 0.3 | 10 | 0.3 | 24 | 0.3 |
| Self defined lump | 21 | 0.5 | 3 | 0.1 | 24 | 0.3 |
| Cancer detection | ||||||
| Invasive screen-detected | 20 | 0.5 | 18 | 0.3 | 38 | 0.5 |
| PPV-1† | 15.0 | 24.7 | 18.4 | |||
The Norwegian Breast Cancer Screening Program (NBCSP) database was used.
Chi square p-value<0.05 between Sami and Non-Sami populations.
Positive predictive value-1: invasive breast cancers detected among women recalled due to mammographic findings.
The rate of invasive screen detected cancers was lower in the Sami (0.33%) compared with the non-Sami population (0.49%), but the difference did not reach statistical significant difference (p=0.14). A statistical significant difference was seen only for the age group 65–69 years (0.25% vs. 0.83%). Details are shown in Table II.
Discussion
In this study, we have revealed that women aged 50–69 years living in Sami-speaking municipalities took more frequently part in the NBCSP than women in the control group. The overall recall rate in the non-Sami group was 1.4 times higher than the Sami group. The percentage of breast cancers detected as a result of attending the NBCSP was lower in the Sami group, but the difference did not reach statistically significant level.
Data quality
We included results only from 2001 to assure complete years offering screening in the actual population. Data were extracted by the CRN. The Nordic cancer registries are known for their high quality (15). In Norway, data on minorities (such as the Sami people) are not available in registries/databases as we are not allowed to register people based on ethnicity. Those living in the administration area of the Sami language law were selected as a surrogate for the Sami people. The exact percentage of Sami in each group is not known. However, a Gallup poll back in October 2000 asked people in Finnmark if they could speak Sami and included 5 municipalities from the Sami group and 8 from our control group. The result was 71 and 6%, respectively.
Do the study cohort and the control group differ in any other way than being mostly Sami and mostly non-Sami, which possibly could affect the attendance rate? They differ in geographical setting. Whereas the Sami group is mainly located in the inland, the control group is only located in the coastal areas. However, both groups are living in rural areas.
The County of Nordland entered the national screening program the 7th of May 2001, about 4 months after the starting point of our study. However, both study groups were balanced between counties. In Nordland County, the municipalities of Tysfjord (Sami group) and Lødingen (non-Sami group) were matched. They had during study period a mean of 269 and 305 women invited per screening round, respectively.
Ethnic minorities and mammography screening
Whereas we expected a lower attendance rate among the Sami people in the national breast cancer screening program due to a known reduced risk of cancer and threshold, counter, queue and cultural challenges (13,16,17), this was not confirmed in this study. During the study, we had a meeting with the Sami Medical Association in Norway and disclosed that the low risk of breast cancer among the Sami people was well known among their members. Other investigators have revealed ethnic minorities having lower participation rates (1,3–5). A meta-analysis revealed that African-Americans, Hispanics and Asian/Pacific Islander were screened less than non-Hispanic white women (3). However, when controlling for socioeconomic status, ethnic differences in mammography screening were no longer significant. An English study (4) pointed especially on the fact that women in the Muslim population were less likely to continue to participate in mammography than those in other South Asian groups. Furthermore, Chinese American women have been reported experiencing low cancer screening rates (5). Masi et al. (5) reported several screening barriers as knowledge deficits, foreign language, culture barriers, lack of health insurance, low income and reduced access to transportation. Furthermore, they mentioned that interventions addressing financial and logistical concerns had increased mammography in patient populations that were diverse with respect to race, ethnicity and insurance status. The use of “mammography busses” in our region and the national health insurance system in Norway (all inhabitants are members) may have facilitated logistics and may have influenced positively on the participation rate. Furthermore, the Northern Norway Regional Health Authority trust's focus on interpreter services (Sami language), and Sami signposts in several institutions may also have contributed to the attendance rate among the Sami.
The attendance rate in our study groups were 78 and 75%, respectively. The difference could be due to use of a private clinic for mammography. Clinics located in Bødø and Tromsø have offered mammography during study period. The private alternative might have been a choice for some more of the non-Sami compared with the Sami women, but this can only be speculated. Knowing that rural areas experience lower participation rates (6), we were satisfied with the attendance rate. The rate is comparable with the rate achieved in the NBCSP in total (14). Within Europe, figures have been ranging between 43 and 89% (6,7,18,19). The average in 6 European countries was 78% (19). The recall rate was 5.4% (range 3.3–17.7%). In this setting, the recall rate in the Sami group of 2.4% seems low. However, high recall rates due to false positive results may have different consequences in various ethnic groups. Jafri et al. (1) observed that, following a false-positive result, black (80%) and Hispanic women (71%) were significantly less likely to continue in the screening program than white women (93%). The Norwegian women pay a fee of about 30 Euro for the screening examination. However, the fee includes eventual recall examination and treatment. This might be of influence for almost 100% compliance in recall examinations in Norway. The Sami population did not differ from the rest of Norway, and the results were comparable with previous results for the nationwide program (14).
The percentage of breast cancers detected in the NBCSP was significantly higher in the non-Sami versus the Sami group. This was somewhat surprising but may be due to small numbers. Goldman et al. (2) documented no difference in mammography sensitivity between facilities serving vulnerable (racial/ethnic minority) and not vulnerable populations. On the other hand, the difference in detection rate between the 2 groups should be reanalysed after several screening rounds and adjusted for possible risk factors. Taking different risk factors (hormonal related factors as age at first birth, number of births, use of hormonal treatment, in addition to other life style factors as physical activity, smoking and alcohol habits) into account, in addition to including the DCIS, would make us able to make more precise estimates of the cancer detection.
Conclusion
Women in Sami speaking municipalities have a higher attendance rate than the non-Sami women, but a lower percentage of their breast cancer cases were detected in the screening program. The Sami women did also experience a lower recall rate compared with the non-Sami women.
Acknowledgements
The authors wish to thank Jann Georg Falck at the Northern Norway Regional Health Authority (NNRHA) for his support with regard to population data for the municipalities in northern Norway. Furthermore, the authors wish to thank the staff at the library at the Faculty of Health Sciences at the University of Tromsø for their kind assistance.
Conflict of interest and funding
No conflict of interest. The study was funded by the North Norway Regional Health Authority trust. Ethical approval is not required.
References
- 1.Jafri NF, Ayyala RS, Ozonoff A. Screening mammography. Does ethnicity influence patient preferences for higher recall rates given the potential for earlier detection of breast cancer? Radiology. 2008;249:785–91. doi: 10.1148/radiol.2493072176. [DOI] [PubMed] [Google Scholar]
- 2.Goldman LE, Haneuse SJPA, Miglioretti DL, Kerlikowske K, Buist DSM, Yankaskas B, et al. An assessment of the quality of mammography care at facilities treating medically vulnerable populations. Med Care. 2008;46:701–8. doi: 10.1097/MLR.0b013e3181789329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purc-Stephenson R, Gorey KM. Lower adherence to screening mammography guidelines among ethnic minority women in America. A meta-analytic review. Prev Med. 2008;46:479–88. doi: 10.1016/j.ypmed.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price CL, Szczepura AK, Gumber AK, Patnick J. Comparison of breast and bowel cancer screening uptake patterns in a common cohort of South Asian women in England. BMC Health Serv Res. 2010;10:103. doi: 10.1186/1472-6963-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masi CM, Blackman DJ, Peek ME. Interventions to enhance breast cancer screening, diagnosis and treatment among racial and ethnic minority women. Med Care Res Rev. 2007;64(5 Suppl.):195S–242S. doi: 10.1177/1077558707305410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borda A, Sanz B, Otero L, Blasco T, Garcia-Gómez FJ, de Andrés F. Travel time and participation in breast cancer screening in a region with high population dispersion. Gac Sanit. 2011;25:151–6. doi: 10.1016/j.gaceta.2010.10.010. [In Spanish] [DOI] [PubMed] [Google Scholar]
- 7.Aarts MJ, Voogd AC, Duijm LE, Coebergh JW, Louwman WJ. Socioeconomic inequalities in attending the mass screening for breast cancer in the south of the Netherlands – associations with stage at diagnosis and survival. Breast Cancer Res Treat. 2011;128:517–25. doi: 10.1007/s10549-011-1363-z. [DOI] [PubMed] [Google Scholar]
- 8.International Labour Organisation (ILO) Geneva: International Labour Organisation (ILO); 1989. C169 Indigenous and Tribal Peoples Convention, 1989. [cited 2011 Jan 19]. Available from: http://www.ilo.org/ilolex/cgi-lex/convde.pl?C169. [Google Scholar]
- 9.Ministry of Health and Care services. Norwegian Public Reports – NOU 1995:6. Oslo: Ministry of Health and Care services; 1995. Plan for health and social services to the Sámi population in Norway; p. 502. [Google Scholar]
- 10.Department of Health. The Northern Norway Regional Health Authority's mission 2009; Oslo: Department of Health, Departments’ service centre; 2009. p. 34. [Google Scholar]
- 11.Hassler S. Causes of death and incidences of cancer and cardiovascular diseases [Dissertation] Umeå: Umeå University, Department of Public Health and Clinical Medicine; 2005. The health conditions in the Sami population of Sweden 1961–2002. [cited 2011 Sept 11]. 81 p. Available from: http://www.hv.se/dynamaster/file_archive/110323/d1bbfe45b1704e7c3da5540269f59c8f/Avhandling%20Hassler.pdf. [Google Scholar]
- 12.Nystad T, Melhus M, Lund E. The monolingual Sámi population is less satisfied with the primary health care. Tidsskr Nor Laegeforen. 2006;126:738–40. [In Norwegian] [PubMed] [Google Scholar]
- 13.Norum J, Olsen A, Småstuen M, Nieder C, Broderstad AR. Health consumption in Sami-speaking municipalities with regard to cancer and radiotherapy. Int J Cirumpolar Health. 2011;70:319–28. doi: 10.3402/ijch.v70i3.17832. [DOI] [PubMed] [Google Scholar]
- 14.Hofvind S, Geller B, Vacek PM, Thoresen S, Skaane P. Using the European guidelines to evaluate the Norwegian Breast Cancer Screening Program. Eur J Epidemiol. 2007;22:447–55. doi: 10.1007/s10654-007-9137-y. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Registry of Norway. Cancer in Norway 2006. Cancer incidence, mortality, survival and prevalence in Norway. Oslo: Cancer Registry of Norway; 2007. Data quality at the Cancer Registry of Norway. In: Cancer Registry of Norway; pp. S1–S77. [Google Scholar]
- 16.Hedlund M, Moe A. De forstår ikke hva som er viktig for oss. Helsetjenester og sørsamer [They do not understand what is important to us. Health care services and southern Lapps]. NTF-rapport. 2000;2:1–116. [cited 2011 Jan 19].Available from: http://generator.firmanett.no/t/tforsk/doc/Ra_2_2000.pdf. [In Norwegian] [Google Scholar]
- 17.Haldorsen T, Tynes T. Cancer in the Sámi population of North-Norway 1970–97. Eur J Cancer Prev. 2005;14:63–8. doi: 10.1097/00008469-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Ventura L, Stefanini V, Senore C, Castagno R, Paci E, Segnan N. Time trends of process and impact indicators in Italian breast screening programmes: 1998–2008. Epidemiol Prev. 2010;34(5–6 Suppl. 4):27–34. [PubMed] [Google Scholar]
- 19.Törnberg S, Kemetli L, Ascunce N, Hofvind S, Anttila A, Sèradour B, et al. A pooled analysis of interval cancer rates in six European countries. Eur J Cancer Prev. 2010;19:87–93. doi: 10.1097/CEJ.0b013e32833548ed. [DOI] [PubMed] [Google Scholar]