Abstract
The E3L gene product found in all poxviruses is required for the lethality of mice in vaccinia virus infection. Both the C-terminal region, consisting of a double-stranded RNA-binding motif, and the N-terminal region (vZE3L), which is similar to the Zα family of Z-DNA-binding proteins, are required for infection. It has recently been demonstrated that the function of the N-terminal domain depends on its ability to bind Z-DNA; Z-DNA-binding domains from unrelated mammalian proteins fully complement an N-terminal deletion of E3L. Mutations that decrease affinity for Z-DNA have similar effects in decreasing pathogenicity. Compounds that block the Z-DNA-binding activity of E3L may also limit infection by the poxvirus. Here we show both an in vitro and an in vivo assay with the potential to be used in screening for such compounds. Using a conformation-specific yeast one-hybrid assay, we compared the results for Z-DNA binding of vZE3L with those for human ZβADAR1, a peptide that has similarity to the Zα motif but does not bind Z-DNA, and with a mutant of hZβADAR1, which binds Z-DNA. The results suggest that this system can be used for high-throughput screening.
The Zα motif, first identified in the vertebrate RNA editing enzyme ADAR1 (double-stranded RNA adenosine deaminase), binds tightly and specifically to left-handed Z-DNA (1-6). This motif has subsequently been found in another mammalian protein, DLM-1, and in the E3L virulence factor of poxviruses (1, 7). In ADAR1 and DLM-1, the Zα motif is separated by an intervening linker from a second related domain called Zβ. The E3L protein of vaccinia virus has only one Zα motif (vZE3L), positioned at the N terminus. The Zα motif is based on a common fold, the winged helix-turn-helix motif, which is also the basis of many B-DNA-binding proteins (8). The crystal structures of human ZαADAR1 (9) and mouse ZαDLM-1 (7), each complexed with Z-DNA, show that Zα uses the recognition helix in combination with a β-loop to form a surface tailored to the zig-zag backbone and protruding syn base of Z-DNA. hZαADAR1 and mZαDLM-1 use a small number of nearly identical amino acids to produce nearly identical binding interfaces (7, 9). These amino acids, which define the Zα motif, are conserved between different members of the Zα family (Fig. 1).
Fig. 1.
Amino acid sequences of ZαADAR1 and ZβADAR1 domains in various species and some related proteins. Residues interacting with Z-DNA based on x-ray crystal structures of hZαADAR1 and mZαDLM1 are underlined and bold. Homologous residues in proteins whose structure has not been determined are shown in bold. h, human; m, mouse; x, Xenopus; dr, zebra fish; fr, Takifugu rubripes. hADAR1, GenBank accession no. P55256; mADAR1, GenBank accession no. AAK17103; xADAR1, GenBank accession no. AAB51688; drADAR1, GenBank accession no. AF124332; frADAR1, GenBank accession no. AAF69764; vE3L, GenBank accession no. AAA02759; mDLM1, GenBank accession no. AAF17234.
Poxviruses generally code for a protein with a single Zα motif linked to a double-stranded RNA-binding motif. The best studied of these proteins is the E3L protein of vaccinia virus, which serves as an immune modulator and is required for viral replication (10, 11). Although the C-terminal half of E3L is sufficient for viral growth in cell culture, the entire protein, including the N-terminal Zα domain, is required for virulence in mice after intranasal or intracerebral inoculation (12). Recently, it has been shown that the ability to recognize the Z conformation is essential for E3L function (13). hZαADAR1 or mZαDLM-1 can replace vZE3L to form a chimeric virus, which is as lethal as wild-type virus. Mutations in the chimeric protein that decrease Z-DNA binding and analogous mutations in wild-type E3L decrease the pathogenicity of the virus. Replacement of the N-terminal domain of E3L with hZβ, which does not bind to Z-DNA, produces a noninfective virus; however, a mutation, I335Y, that creates Z-DNA affinity also creates an infective virus (13).
The identification of an unexpected function involved in poxvirus pathogenicity raises the hope of new avenues for treatment of the infrequent but potentially deadly complications of vaccination of humans with vaccinia virus. A drug that blocks E3L binding to Z-DNA may be an effective therapy for preventing pathogenicity. Furthermore, the near identity to the E3L of variola, the agent of smallpox, suggests that such drugs may have wider application. High-throughput screening for compounds that block Z-DNA binding and thereby interfere with infection by the virus is an important next step. Although the animal model used to assess the activity of E3L is very sensitive, it is completely unsuitable for testing large numbers of candidate molecules.
To this end, we have developed a highly sensitive in vitro assay for conformation-specific Z-DNA binding, the B-Z equilibrium midpoint assay. In addition, we show that the conformation-specific yeast one-hybrid assay clearly demonstrates the binding of Z-DNA by vZE3L in vivo. With these assays, we can distinguish between vZE3L, which binds Z-DNA specifically, and hZβADAR1, which does not. The assays reported here, especially the conformation-specific yeast one-hybrid assay, will be valuable for high-throughput screening of compounds to find those that interfere with Z-DNA binding. Promising candidates identified in the screening assay can then be tested to see whether they influence the infection of mice with vaccinia virus.
Methods and Materials
Construction, Expression, and Purification of Zα Proteins. Various Zα motifs were cloned into pET28a expression vector (Novagen) by the method described (5). Inserted gene fragments encode the following regions of Zα motifs: hZαADAR1 (GenBank accession no. P55256), amino acids 133-209; hZβADAR1 (GenBank accession no. P55256), amino acids 296-368; drZβADAR1 (GenBank accession no. AF124332), amino acids 410-485; vZE3L (GenBank accession no. AAA02759), amino acids 2-78. The sequences of inserted genes were confirmed by dideoxy sequencing. Site-directed mutations in the hZβADAR1 gene were performed by using the QuikChange site-directed mutagenesis protocol (Stratagene). The expression and purification of Zα proteins were the same as described (5). In brief, expression of Zα proteins was carried out in Escherichia coli strain BL21(DE3). Zα proteins were purified by His-tag affinity chromatography and subsequently further purified to homogeneity by using Mono-S FPLC with the exception of vZE3L, which used Mono Q (Amersham Pharmacia). After thrombin cleavage, the Zα proteins include 5-6 amino acid residues at the N terminus derived from pET28a. The purity of Zα proteins was determined by Coomassie blue staining in SDS/PAGE. The concentration of proteins was determined by using UV absorbance at 280 nm with extinction coefficients determined from the sequence by using the protparam tool (http://us.expasy.org/tools/protparam.html). Sequences are listed in Fig. 1.
Yeast One-Hybrid Analysis with Quantitative β-Galactosidase Activity Assay. The Z conformational specific yeast one-hybrid assay was described in detail (14) and is diagrammed in Fig. 2. The assay was performed as follows. Yeast strain YM 4271 (Clontech) was transformed with the LacZ reporter vector, pLacZcOp-CG9, by using the lithium acetate/polyethylene glycol method described (15). Freshly transformed yeast cells containing pACT2-based expression vectors encoding various Zα domains or mutants were grown on selection plates, then picked and streaked onto fresh plates containing 80 μg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactoside for colorometric assay of β-galactosidase. After 2 days at 30°C, a blue color developed if β-galactosidase is produced. The o-nitrophenyl-β-d-galactose assay was used for a quantitative assay of β-galactosidase. The quantitative measurement of β-galactosidase was carried out according to the manufacturer's instructions (Clontech). Means and standard deviations were calculated from triplicate measurements in Miller's β-galactosidase units (1 unit hydrolyzes 1 μmol of o-nitrophenyl-β-d-galactose per minute per cell).
Fig. 2.
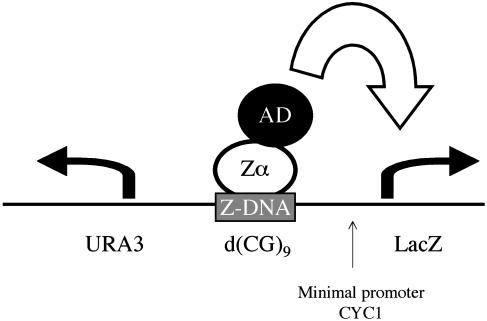
Diagram of the Z-DNA conformation-specific yeast one-hybrid assay. The bait sequence, d(CG)9 in these experiments, was inserted between the reporter gene, LacZ, and the URA3 gene. URA3 is transcribed constitutively in the opposite direction to LacZ. As described in the twin-domain model of Liu and Wang (22), URA3 provides negative supercoils, which stabilize d(CG)9 in the Z conformation. As is shown diagrammatically, transcription of the LacZ reporter gene is activated when a Zα domain is attached to an activation domain (AD) binds to Z-DNA.
Circular Dichroism Measurement. Poly(dG-dC) (Amersham Pharmacia) was purchased and rehydrated with Tris buffer (10 mM Tris·Cl, pH 7.4/50 mM NaCl) and stored at -20°C before use. The conversion of poly(dG-dC) from the B to the Z conformation was monitored by CD. CD spectra were taken at 25°C by using an AVIV model 202 (Aviv Associates, Lakewood, NJ). Measurements were carried out on 50 μg/ml (75 μM base pair) DNA in CD buffer (10 mM Hepes, pH 7.4/10 mM KF/0.1 mM EDTA) in a 2-mm quartz cell. The 30 μM final concentration of protein was then added to the sample from a concentrated stock solution. The maximum volume of protein added to the sample did not exceed 5% of the total volume. Spectra for wavelength scanning were recorded at 1-nm intervals averaged over 3 sec. For kinetic measurements, CD signal changes at 255 nm were recorded at 1-sec intervals up to 1 h.
B-Z Midpoint Assay. The conformational change of poly(dG-dC) to the Z conformation can be effected by a variety of salts including NaCl (16) and cobalt hexamine [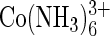 ] (17). NaCl requires a concentration >2 M to convert a measurable portion of the DNA into the Z form. Conformation-specific binding of small intercalating molecules has been tested in 2.5 M NaCl, in which ≈50% of the DNA is in each of the B and the Z conformations (18). Because high salt destabilizes protein-DNA interactions, these conditions are not suitable for testing the interaction between vZE3L and DNA. In contrast, cobalt hexamine can stabilize Z-DNA at submillimolar concentration, making it far more suitable for our needs.
] (17). NaCl requires a concentration >2 M to convert a measurable portion of the DNA into the Z form. Conformation-specific binding of small intercalating molecules has been tested in 2.5 M NaCl, in which ≈50% of the DNA is in each of the B and the Z conformations (18). Because high salt destabilizes protein-DNA interactions, these conditions are not suitable for testing the interaction between vZE3L and DNA. In contrast, cobalt hexamine can stabilize Z-DNA at submillimolar concentration, making it far more suitable for our needs.
Poly(dG-dC) (40 μg/ml; 60 μM base pair, Amersham Pharmacia) was incubated in assay buffer (10 mM Tris, pH 7.4/50 mM NaCl) containing 55-80 μM cobalt hexamine (Sigma) overnight at 25°C. The ratio between the B form and the Z form was determined by CD. Because this assay is very sensitive to small changes in conditions, multiple samples with different concentrations of cobalt hexamine were prepared, and a sample with the desired spectrum was selected. CD spectra were measured as described above. Protein (60 μM) was added to the sample from a concentrated stock solution, not to exceed 5% of the total volume. Protein stocks should not contain EDTA or other chelating agents to prevent chelation of the cobalt ion.
Results and Discussion
The Conformation-Specific Yeast One-Hybrid Assay Shows Binding Between vZE3L and Z-DNA. The conformation-specific yeast one-hybrid assay was developed to detect proteins that bind Z-DNA (14). In this system, expression of β-galactosidase depends on the presence of a Z-DNA-binding protein fused to an activation domain (Fig. 2). To this end, a d(CG)9 sequence was placed upstream of the LacZ promoter, replacing the upstream activation sequence for the minimal promoter CYC1. d(CG)9 adopts the Z conformation under superhelical strain, which is provided by a second gene, URA3, transcribed in a divergent direction from LacZ. Proteins of interest are attached to an activation domain, and binding to Z-DNA results in activation of transcription of LacZ.
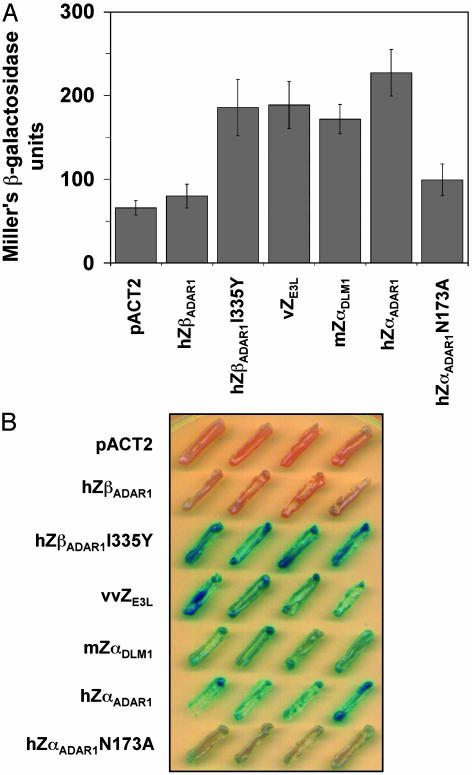
Results of this assay are shown in Fig. 3. A quantitative measurement of β-galactosidase production is shown for several proteins (Fig. 3A). Under these conditions, vZE3Lis as successful at activating gene expression as hZαADAR1 or mZαDLM-1. In contrast, another protein with resemblance to Zα, but without detectable binding activity, hZβADAR1, is as inactive as the pACT2 vector alone. Also shown is the result for a mutation in hZαADAR1, N173A, which decreases the affinity of ZαADAR1 for Z-DNA but does not destroy it, as shown by CD and by an electrophoretic mobility shift assay (ref. 13 and data not shown). This mutation strikingly lowers the amount of β-galactosidase produced. The I335Y mutation in hZβADAR1, which creates limited affinity for Z-DNA in both in vitro CD assays and when introduced into a chimeric virus in the mouse (13), behaves like hZαADAR1 and vZE3L in this yeast one-hybrid assay. Growth of colonies on 5-bromo-4-chloro-3-indolyl-β-d-galactoside plates provides a visual demonstration of β-galactosidase production (Fig. 3B). Strong production is seen for hZαADAR1, mZαDLM-1, vaccinia virus ZE3L, and the hZβADAR1 mutant I335Y.
Fig. 3.
Z-DNA-specific one-hybrid assay of Zα proteins. Yeast containing a LacZ reporter construct was transfected with vectors expressing Zα family proteins fused to an activation domain. (A) The results of quantitative assays for β-galactosidase are shown. Error bars are indicated. (B) When yeast is grown on plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactoside, blue color indicates β-galactosidase synthesis due to binding of the protein to a stretch of Z-DNA near the promoter region of the LacZ reporter gene. Four colonies are shown for each construct. pACTA2 is transfected only with the vector.
Thus, binding between vZE3L and Z-DNA is clearly demonstrated in this in vivo assay. It is striking that, although the assay is sensitive to a decrease in binding affinity, as demonstrated by the hZαADAR1 N173A mutant, the binding of vZE3L is just as robust as that of ZαADAR1. This finding suggests that different factors affect binding in vivo and in vitro. It is possible that subtle conformational changes may differ in proteins expressed in E. coli for purification and in vivo with yeast or virus-infected mice. Z-DNA binding may also be affected by conditions within the cell, such as salt concentration, or the presence of polyions, such as spermine.
The sensitivity of the Z-DNA-binding activity of a given protein to the exact conditions is demonstrated when the size of the d(CG)n insert is varied. Oh et al. (14) studied a variety of constructs containing 0, 4, 5, 9, and 12 d(CG) repeats. When hZαADAR1 is fused to the activation domain, the amount of LacZ expression is roughly proportional to the number of repeats between 4 and 12. In contrast, vZE3L performs poorly in the assay if only five repeats of d(CG) occur. Activation by hZαADAR1-activation domain of LacZ with d(CG)5 as a upstream activation sequence strongly depends on divergent transcription of the URA3 gene. In contrast, in the presence of d(CG)9, activation is nearly equal whether URA3 is transcribed in a divergent or the same direction as LacZ, even though in the later case Z-DNA would not be stabilized by URA3 transcription (14). One explanation is that four hZαADAR1 molecules can bind to d(CG)9, although there is only room for two on d(CG)5, as deduced from the c-crystal structure of hZαADAR1 with d(CG)3 (9). Four hZαADAR1 molecules may be sufficient to stabilize d(CG)9 in the Z conformation without any other factor (4). It is possible that the footprint of vZE3L is larger than that of hZαADAR1 or that the interaction between molecules bound to each strand is different, such that vZE3L binds less well to the smaller target.
vZE3L but not ZβADAR1 Binds Z-DNA in Vitro in a B-Z Equilibrium Midpoint Assay. The binding between ZαADAR1 and Z-DNA can be clearly demonstrated in vitro by several assays, including electrophoretic mobility shift assay and the conversion of the CD spectrum of (dC-dG)6 from that of B-DNA to that of Z-DNA (1, 4, 5). These assays have been used to demonstrate binding by mZαDLM-1,hZαDLM-1, and ZαADAR from all the species shown in Fig. 1 (refs. 1, 4, and 5; Y.-G.K., unpublished data). However, even though the in vivo assay shows that vZE3L binds Z-DNA, this finding cannot be shown in the conventional in vitro assays (data not shown). It is likely that failure of the conventional assay reflects the fact that vZE3L:Z-DNA binding has low affinity under the conditions used for in vitro assays.
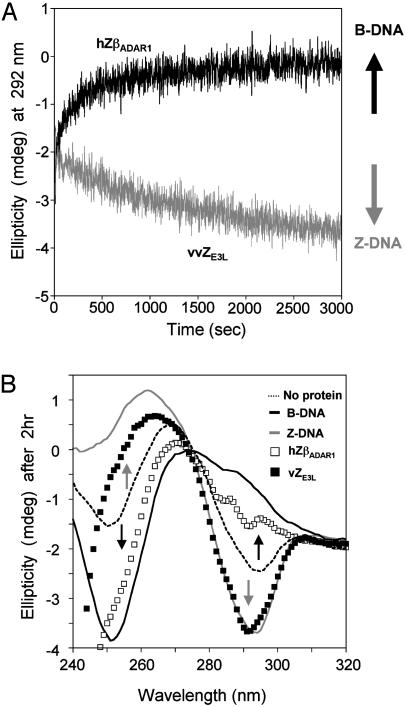
Two steps occur in the conventional in vitro assays: first, conversion of B-DNA to Z-DNA and, second, the binding to Z-DNA. The vZE3L:Z-DNA complex is apparently not robust enough to affect the equilibrium between B-DNA and Z-DNA in a low-salt environment (16). However, if the DNA is poised near the equilibrium between the two forms, a clear result can be seen. Cobalt hexamine is known to be potent at low concentrations in converting poly(dG-dC) from B-DNA to Z-DNA (17). In this assay, cobalt hexamine is added to poly(dG-dC) to a concentration at which the DNA is at approximately a 1:1 ratio of the B form and Z form as assessed by CD. Addition of protein can have no effect (i), or it can drive the equilibrium toward the B conformation (ii) or toward the Z conformation (iii). A protein that binds Z-DNA preferentially will remove free Z-DNA from the mixture, driving the equilibrium toward Z-DNA. A B-DNA binding protein will have the opposite effect. Fig. 4 shows examples of effects ii and iii. In corroboration of the in vivo results, vZE3L drives the equilibrium toward the Z conformation. hZβADAR1 drives the equilibrium toward the B conformation, suggesting that it may be a weak B-DNA-binding protein rather than a Z-DNA-binding protein. To validate the assay, we looked at several well known B-DNA-binding proteins such as human histone 1 (hH1), which also drive the equilibrium to B-DNA, as expected (data not shown). hH1 uses the same winged helixturn-helix fold as Zα (9, 19). A chimeric protein was made containing the N-terminal half (before the recognition helix α3) of hH1 and the C-Terminal half (the recognition helix α3, and the wing) from hZαADAR1. This chimeric protein caused no shift in the spectrum, either toward Z or B (data not shown), which is in agreement with the fact that it shows no Z-DNA binding in a CD experiment. It should be noted that the midpoint assay is less informative about B-DNA-binding proteins than about those with specificity for Z-DNA. A protein that binds cobalt hexamine or the presence of a chelating agent in the solution would reduce the concentration of cobalt hexamine and increase the amount of B-DNA in solution.
Fig. 4.
Z-DNA-binding assay using the cobalt hexamine B-Z equilibrium midpoint assay. (A) Time-dependent change of ellipticity at 292 nm from the CD spectrum of duplex poly(dG-dC) in the presence of proteins. A change in the relative amounts of B- and Z-DNA is reflected by a change in ellipticity, upward for an increase in the B form and downward for Z. The result of adding vZE3L is shown in gray, and the result of adding hZβADAR1 is shown in black. (B) CD spectra of poly(dG-dC) in various reaction mixtures after 2 h. B-DNA and Z-DNA spectra were obtained in the absence of cobalt hexamine (black line) and in the presence of 5 M NaCl (gray line), respectively. Dashed line indicates the equilibrium induced by cobalt hexamine with no added protein. □, the effect of adding hZβADAR1; ▪, the effect of adding vZE3L. The proteins alone do not have a CD signal >250 nm.
The midpoint assay is suitable for examining other Zα family members. hZβADAR1 contains an isoleucine (Ile-335) in place of the conserved tyrosine (Fig. 1), which makes a crucial CH-π interaction with a syn-guanine and a hydrogen bond with a backbone phosphate (9). It has also been suggested to interfere with binding to B-DNA (20). A single amino acid change, hZβADAR1I335Y (13), is enough to create Z-DNA binding in the yeast one-hybrid assay (Fig. 2) and the midpoint assay (results not shown). Converting Tyr to Phe retains the aromatic ring but eliminates the hydrogen bond to Z-DNA (9), and hZαADAR1Y177F has decreased affinity for Z-DNA and a slow binding rate (data not shown). An Ile335Phe mutation in hZβADAR1 results in a protein that binds Z-DNA specifically in the CD assay, but with a very low affinity and with extremely slow kinetics.
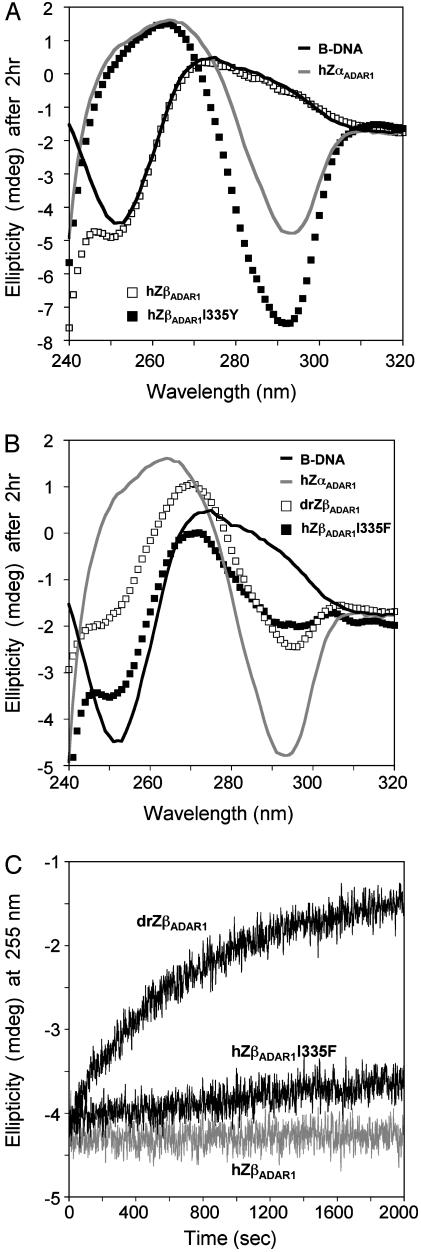
Several proteins have two related Z motifs (Fig. 1). The first, called Zα, binds readily to Z-DNA. In hADAR1, the second motif, Zβ, does not bind Z-DNA unless the isoleucine in 335 is mutated to tyrosine or to phenylalanine. However, some Zβ domains have a tyrosine in that position, for example, the Zβ of Zebra fish ADAR1 (Fig. 1, drZβADAR1). Fig. 5A shows that drZβADAR1 binds to Z-DNA, although ZβADAR1 does not bind. The CD spectrum of poly(dG-dC) in the presence of drZβADAR1 shows a Z-DNA spectrum, but with more modest shifts than that seen with hZαADAR1 (Fig. 5C).
Fig. 5.
CD spectra showing the binding of hZβADAR1I335Y and drZβADAR1 to Z-DNA. (A) Time-dependent change at 255 nm in the CD spectrum of poly(dG-dC) in the presence of proteins. Comparison of Zebra fish drZβADAR1 with human ZβADAR1 and a mutant drZβADAR1 results in a slow accumulation of Z-DNA, whereas hZβADAR1I335F has a much slower effect. (B and C) CD spectra of reaction mixtures after 2 h. All reactions have reached equilibrium at this time. (B) The spectrum in the presence of hZαADAR1 (gray line) is the same as that for Z-DNA stabilized by 5 M NaCl (not shown) in the range of 250-320 nm. hZβADAR1 (□) has no effect on the CD spectrum. The black line indicates DNA with no protein. The mutant hZβADAR1I335Y (▪) produces a larger negative ellipticity of Z-DNA at 292 nm than does hZαADAR1, which converts >90% of the DNA to the Z form under these conditions. This difference in spectrum may reflect a slight change in the conformation of the DNA in the presence of hZβADAR1I335Y. (C) Changing the isoleucine of hZβADAR1 to phenylalanine (I335F) also converts poly(dG-dC) to Z-DNA, but with a more modest lowering of ellipticity at 290 nm than that seen in the I335Y mutant in B. drZβADAR1 yields a similar conversion to Z-DNA but with smaller spectral changes.
The CD spectrum of Z-DNA induced by hZβADAR1I335Y differs somewhat from that induced by hZαADAR1. The minimum at 292 nm is more negative relative to the peak at 255 nm induced by hZβADAR1I335Y than that produced by hZαADAR1 (Fig. 5B). This effect is not seen in the spectrum of poly(dC-dG) in the presence of the phenylalanine mutant hZβADAR1I335F (Fig. 5C). CD spectra reflect molecular conformation in a way not well explained by theory. However, the hZβADAR1I335Y-induced Z conformation of poly(dG-dC) may be somewhat different from the salt-induced or hZαADAR1-induced Z conformations. It will be of interest if the structure of the hZβADAR1I335Y:Z-DNA complex can be determined at atomic resolution.
The development of these relatively simple assays, which allow us to look at the binding between vZE3L and Z-DNA, will greatly accelerate the search for compounds that interfere with this interaction. The yeast assay is particularly well suited for high-throughput screening. For example, Young et al. (21) used a counterselection yeast two-hybrid assay to identify small molecules that modulate calcium channel activity. By using a reporter gene, CYH2, which inhibits growth, cells would only grow when the interaction between the bait and the prey was disrupted. A similar system is being developed by using the conformation-specific yeast one-hybrid assay to test for small molecules that disrupt the interaction between vZE3L and Z-DNA.
Proteins that bind to Z-DNA can thus be characterized in two general methods involving in vitro and in vivo assays. The parameters of the in vivo assay usually have to be optimized for each individual protein. This is clearly illustrated for vZE3L, which produces a robust signal when the bait sequence is d(CG)9 (Fig. 3A), but a weaker signal when the bait sequence is d(CG)5. It is likely that the various proteins and mutants described in Fig. 5 would also require signal optimization before a screening procedure could be undertaken. This finding may become relevant in the future if other Z-DNA-binding proteins become of interest for clinical applications.
Acknowledgments
This research was supported by grants from the National Institutes of Health.
References
- 1.Herbert, A., Alfken, J., Kim, Y. G., Mian, I. S., Nishikura, K. & Rich, A. (1997) Proc. Natl. Acad. Sci. USA 94, 8421-8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger, I., Winston, W., Manoharan, R., Schwartz, T., Alfken, J., Kim, Y. G., Lowenhaupt, K., Herbert, A. & Rich, A. (1998) Biochemistry 37, 13313-13321. [DOI] [PubMed] [Google Scholar]
- 3.Kim, Y. G., Lowenhaupt, K., Schwartz, T. & Rich, A. (1999) J. Biol. Chem. 274, 19081-19086. [DOI] [PubMed] [Google Scholar]
- 4.Kim, Y. G., Lowenhaupt, K., Maas, S., Herbert, A., Schwartz, T. & Rich, A. (2000) J. Biol. Chem. 275, 26828-26833. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz, T., Lowenhaupt, K., Kim, Y. G., Li, L., Brown, B. A., II, Herbert, A. & Rich, A. (1999) J. Biol. Chem. 274, 2899-2906. [DOI] [PubMed] [Google Scholar]
- 6.Kim, Y. G., Kim, P. S., Herbert, A. & Rich, A. (1997) Proc. Natl. Acad. Sci. USA 94, 12875-12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz, T., Behlke, J., Lowenhaupt, K., Heinemann, U. & Rich, A. (2001) Nat. Struct. Biol 8, 761-765. [DOI] [PubMed] [Google Scholar]
- 8.Gajiwala, K. S. & Burley, S. K. (2000) Curr. Opin. Struct. Biol. 10, 110-116. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz, T., Rould, M. A., Lowenhaupt, K., Herbert, A. & Rich, A. (1999) Science 284, 1841-1845. [DOI] [PubMed] [Google Scholar]
- 10.Smith, E. J., Marie, I., Prakash, A., Garcia-Sastre, A. & Levy, D. E. (2001) J. Biol. Chem. 276, 8951-8957. [DOI] [PubMed] [Google Scholar]
- 11.Xiang, Y., Condit, R. C., Vijaysri, S., Jacobs, B., Williams, B. R. & Silverman, R. H. (2002) J. Virol. 76, 5251-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandt, T. A. & Jacobs, B. L. (2001) J. Virol. 75, 850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, Y. G., Muralinath, M., Brandt, T., Pearcy, M., Hauns, K., Lowenhaupt, K., Jacobs, B. L. & Rich, A. (2003) Proc. Natl. Acad. Sci. USA 100, 6974-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh, D. B., Kim, Y. G. & Rich, A. (2002) Proc. Natl. Acad. Sci. USA 99, 16666-16671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gietz, R. D., Schiestl, R. H., Willems, A. R. & Woods, R. A. (1995) Yeast 11, 355-360. [DOI] [PubMed] [Google Scholar]
- 16.Pohl, F. M. & Jovin, T. M. (1972) J. Mol. Biol. 67, 375-396. [DOI] [PubMed] [Google Scholar]
- 17.Behe, M. & Felsenfeld, G. (1981) Proc. Natl. Acad. Sci. USA 78, 1619-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qu, X., Trent, J. O., Fokt, I., Priebe, W. & Chaires, J. B. (2000) Proc. Natl. Acad. Sci. USA 97, 12032-12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garner, M. M. & Felsenfeld, G. (1987) J. Mol. Biol. 196, 581-590. [DOI] [PubMed] [Google Scholar]
- 20.Schade, M., Turner, C. J., Kuhne, R., Schmieder, P., Lowenhaupt, K., Herbert, A., Rich, A. & Oschkinat, H. (1999) Proc. Natl. Acad. Sci. USA 96, 12465-12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young, K., Lin, S., Sun, L., Lee, E., Modi, M., Hellings, S., Husbands, M., Ozenberger, B. & Franco, R. (1998) Nat. Biotechnol. 16, 946-950. [DOI] [PubMed] [Google Scholar]
- 22.Liu, L. F. & Wang, J. C. (1987) Proc. Natl. Acad. Sci. USA 84, 7024-7027. [DOI] [PMC free article] [PubMed] [Google Scholar]