Abstract
EmrE is a small multidrug transporter from Escherichia coli that provides a unique model for the study of polytopic membrane proteins. Here, we show its synthesis in a cell-free system in a fully functional form. The detergent-solubilized protein binds substrates with high affinity and, when reconstituted into proteoliposomes, transports substrate in a Δμ̃H+-dependent fashion. Here, we used the cell-free system to study the oligomeric properties of EmrE. EmrE functions as an oligomer, but the size of the functional oligomer has not been established unequivocally. Coexpression of two plasmids in the cell-free system allowed demonstration of functional complementation and pull-down experiments confirmed that the basic functional unit is the dimer. An additional interaction between dimers has been detected by using crosslinking between unique Cys residues. This finding implies the existence of a dimer of dimers.
Keywords: ion-coupled transporter, drug resistance, membrane protein, oligomer, cell-free protein synthesis
EmrE, a protein from Escherichia coli, provides a unique model for the study of polytopic membrane proteins (1, 2). It is a small (110-aa long) multidrug transporter that extrudes various drugs in exchange for protons, thus rendering bacteria resistant to these drugs (1, 2). The protein has been characterized, purified, and reconstituted in a functional form (3). High-affinity substrate binding has been established as a reliable and sensitive assay for activity of the detergent-solubilized transporter (4, 5). EmrE functions as an oligomer, but the size of the functional oligomer has not been established unequivocally (6, 7). Structural and biochemical evidence suggest that the basic oligomer is a dimer (8, 9). However, negative dominance experiments (6, 7) and substrate-binding measurements (4) suggest the existence of higher oligomeric structures.
Cell-free protein synthesis provides a powerful alternative to in vivo conventional techniques for protein production (10-12). It allows expression of otherwise toxic proteins and provides direct access to the reaction medium as well as control of the reaction conditions. It has been used for high-throughput proteomics and for screening of protein folding and function (13). In addition, labeled amino acids can be incorporated for biophysical and structural studies (14, 15). In the case of polytopic membrane proteins in vitro systems have been used mostly for the study of the mechanism of insertion into the membrane (16-18). However, membrane proteins have not been expressed in amounts that enable a detailed functional analysis, possibly because of their high hydrophobicity, which necessitates the use of detergents and the lack, in general, of quick assays for protein function.
Here, we show in vitro synthesis of a fully functional protein that binds substrate with high affinity. It also transports the substrate in a H+-coupled process after reconstitution into proteoliposomes. The protein concentrations can get as high as 2-3 mg/ml. Coexpression of two plasmids allowed demonstration of functional complementation, and pull-down experiments confirmed that the basic functional unit is the dimer. An additional interaction between dimers has been detected by using crosslinking between unique Cys residues. This finding implies the existence of a dimer of dimers.
Experimental Procedures
Plasmids. The plasmids used for EmrE gene expression were pT7-7 (19) derivatives, with (pT7-7-EmrE-His) (4) or without (pT7-7-EmrE) a six-His tag (3). Vectors used for the expression of homologues of EmrE were pT7-7-BPsmr-His for Bacillus pertussis homologue, pT7-7-Psmr-His for Pseudomonas aeruginosa homologue, and pT7-7-TBsmr-His for Mycobacterium tuberculosis homologue (20); pT7-7-Hsmr-His was used for the expression of the archaeal homologue from Halobacterium salinarum (21).
Mutagenesis. The construction and characterization of the mutants used was as described; we used E14C-His in wild-type background (7), K22C in wild-type background (22), and CLA-His (23). The mutants are named as follows: single amino acid replacements are named with the letter of the original amino acid, followed by its position in the protein and the letter of the new amino acid.
In Vitro Protein Synthesis. Protein was synthesized by using the rapid-translation system 100 E. coli HY kit (Roche Diagnostics), according to the manufacturer's instructions except that the volume of each reaction was 20 μl. For specific experiments (reconstitution and size-exclusion chromatography), the synthesis was carried out by using the rapid-translation system 500 E. coli HY kit. Radiolabeling of EmrE was achieved by addition of 1-3 μCi (>1,000 Ci/mmol; 1 Ci = 37 GBq) of [35S]methionine (Amersham Biosciences).
Determining the Localization of Synthesized Protein. Labeled EmrE-His was synthesized with [35S]methionine supplemented into the reaction mixture, with and without the addition of 0.08% N-dodecyl-β-maltoside (DDM). After the reaction was completed, 100 μl of Na buffer (150 mM NaCl/15 mM Tris·Cl, pH 7.5) with and without 0.08% DDM, correspondingly, was added and then centrifuged for 10 min at 20,800 × g at 4°C. Samples, before and after centrifugation, were analyzed by SDS/PAGE. Radioactive bands were visualized with a FLA-3000 Phosphor-Imager (Fujifilm, Tokyo).
Methyl Viologen Uptake and Tetraphenylphosphonium (TPP+)-Binding Assays. Uptake of [14C]methyl viologen (11.9 mCi/mmol; Sigma) into proteoliposomes was assayed as described (3) by using 200 ng of EmrE-His per assay. TPP+ binding was assayed as described (4). After the in vitro synthesis reaction was completed, the reaction mixture (20 μl) was diluted in 200 μl of 0.08% DDM/Na buffer and the His-tagged protein was immobilized on Ni2+-nitrilotriacetic acid (Ni-NTA) beads (Qiagen, Hilden, Germany). The beads were washed with 0.08% DDM/Na buffer containing 25 mM imidazole, suspended in 200 μl of 0.08% DDM/Na buffer containing 5 nM [3H]TPP+ (27 Ci/mmol; Amersham Biosciences), and incubated for 15 min at 4°C. In each experiment, the values obtained in a control reaction with 25 μM unlabeled TPP+ were subtracted. All binding reactions were performed in duplicate.
When inhibition of binding was tested, TPP+ binding was done in the absence and presence of the following substrates: ethidium bromide (50 μM), acriflavine (50 μM), and benzalkonium (0.5 μM).
Pull-Down Experiments. A 20-μl sample of the reaction volume, supplemented with [35S]methionine, was diluted in the same buffer to a final volume of 200 μl, added to Ni-NTA beads washed as described above, and incubated overnight at 4°C. The protein that bound to beads was washed three times in 0.08% DDM/Na buffer containing 25 mM imidazole. The bead fraction was then incubated for 15 min at room temperature with sample buffer (100 mM Tris·Cl, pH 6.8/4% SDS/0.2% bromophenol blue/20% glycerol/200 mM 2-mercaptoethanol) plus 450 mM imidazole to release the bound protein from the beads. The radioactive protein was detected as described above and analyzed digitally with imagegauge 3.46 software (Fujifilm).
Crosslinking with o-Phenylene Dimaleimide (o-PDM). When the protein synthesis reaction, supplemented with [35S]methionine, was completed, the reaction was dialyzed against 0.08% DDM/Na buffer overnight. o-PDM was then added to a final concentration of 400 μM, and the reaction was stopped with 15 mM β-mercaptoethanol after 1 h at room temperature. The reaction mixture was then loaded on washed Ni-NTA beads and incubated for 1 h at 4°C. Bound protein was eluted from beads, separated by SDS/PAGE, and visualized as described above. In control experiments, o-PDM (400 μM) was added to the protein already bound to the beads or after it was eluted with 0.08% DDM/Na buffer containing 150 mM imidazole.
Results
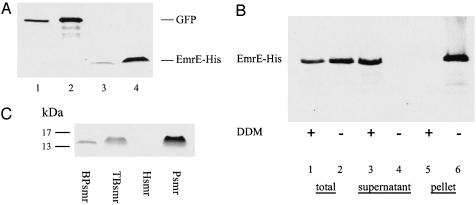
Localization of in Vitro-Synthesized EmrE and the Effect of Detergents. EmrE, a polytopic membrane protein, was synthesized in vitro by using a transcription-translation system. The obtained amounts are easily detectable in SDS/PAGE (Fig. 1A, lanes 3 and 4) and are similar to the amounts of the control reaction of GFP synthesis (Fig. 1 A, lanes 1 and 2). We estimated the amount of EmrE synthesized to be ≈1-2 μg in the miniscale reaction (20-μl reaction volume). In a larger reactor system of 1 ml (rapid-translation system 500 E. coli HY kit), 2-3 mg of EmrE were synthesized (data not shown). Surprisingly, EmrE was expressed at similar amounts with or without the addition of detergent (Fig. 1B, lanes 1 and 2). However, the addition of detergent affected the localization of the protein. When EmrE was synthesized in the presence of detergent (0.08% DDM), it was localized exclusively in the soluble fraction (Fig. 1B, lane 3 and 5), whereas when synthesized without any addition to the reaction mixture provided by manufacturer, it was found within the insoluble fraction only (Fig. 1B, lanes 4 and 6). These results lead us to the conclusion that in vitro-synthesized EmrE is inserted into a particulate fraction, present in the crude lysate, from which it can be removed by addition of detergent. We suggest that this is a membrane fraction because analysis by SDS/PAGE and Coomassie blue staining of this fraction reveals a large number of proteins associated with it that are solubilized with the low concentrations of mild detergents that solubilized EmrE (data not shown).
Fig. 1.
In vitro synthesis, expression, and localization of EmrE and its homologues. (A) GFP (lanes 1 and 2) and EmrE-His (lanes 3 and 4) were synthesized by using the rapid-translation system 100 E. coli HY kit and then incubated with Ni-NTA beads. After purification and elution from the beads, samples equivalent to 16.5% (lanes 1 and 3) and 50% (lanes 2 and 4) of the reaction volume were separated by SDS/PAGE and detected by Coomassie blue staining. (B) Addition of detergent mobilizes synthesized EmrE from an insoluble fraction to a soluble fraction. We synthesized 35S-labeled EmrE-His with and without addition of 0.08% DDM, as indicated. Samples were taken from the supernatant immediately after reaction (lanes 1 and 2) and after a centrifugation step of 10 min at 20,800 × g (lanes 3 and 4) and pellet fraction (lanes 5 and 6). (C) EmrE homologues BPsmr, TBsmr, Hsmr, and Psmr were synthesized in vitro. A 20-μl sample was then loaded on Ni-NTA beads. Proteins were purified and eluted from beads, separated by SDS/PAGE, and detected after Coomassie blue staining.
Screening of the effect of different detergent concentrations showed no significant effect on expression of EmrE, at detergent concentrations up to 0.08% DDM and 0.4% N-octyl-β-d-glucopyranoside. In the presence of higher detergent concentrations, as high as 2% DDM and 1% N-octyl-β-d-glucopyranoside, expression was significantly lower. In all cases, regardless of the presence or absence of detergent, EmrE was functional, as revealed by the assays described below.
Expression of in Vitro-Synthesized Homologous Membrane Proteins. To check whether other membrane proteins could be synthesized in vitro by using the same system, we tested expression of four other transporters that are homologues of EmrE. TBsmr, BPsmr, Psmr, and Hsmr were previously purified and characterized (20, 21). As shown in Fig. 1C, the three bacterial transporters (TBsmr, BPsmr, and Psmr) were expressed successfully, whereas Hsmr, an archaeal multidrug transporter that was expressed successfully in E. coli BL21 cells, was not expressed to detectable levels in this in vitro system. Relative evaluation of in vivo versus in vitro expression levels of these three proteins shows that Psmr seems to express better in vitro than in vivo, whereas BPsmr demonstrated the opposite pattern. TBsmr showed high expression levels when expressed in either system.
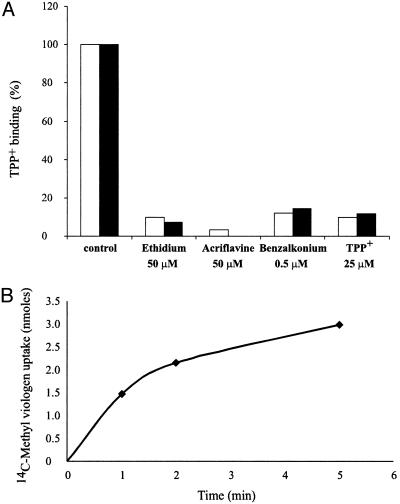
The Synthesized Protein Is Fully Functional. To test whether the in vitro reaction yielded a functional protein, we assayed its ability to bind TPP+, a high-affinity substrate of EmrE (4). In vitro-synthesized EmrE binds [3H]TPP+ as well as EmrE obtained from E. coli membranes, with an affinity (Kd = 2.3 nM) very similar to that of the wild-type protein (23). In addition, [3H]TPP+-binding assays in the absence and presence of different substrates showed that in vitro-synthesized EmrE has the same substrate specificity range as EmrE obtained from E. coli membranes. Thus, ethidium, acriflavine, and benzalkonium inhibit TPP+ binding to native and in vitro EmrE at the same concentration range (Fig. 2A). When synthesized either in the presence or absence of any additional detergent, EmrE proved to be functional and showed, in any case, the ability to bind TPP+.
Fig. 2.
Activity and specificity of in vitro-synthesized protein. (A) In vitro-synthesized (filled bars) and native (open bars) EmrE-His were immobilized on Ni-NTA beads and assayed for TPP+ binding in the absence and presence of different substrates, as described in Experimental Procedures. Control activity was 6.7 pmol and 4.5 pmol of TPP+ per μg of EmrE for in vitro-synthesized and native protein, respectively. (B) In vitro-synthesized EmrE-His was reconstituted into proteoliposomes and assayed for Δμ̃H+-driven methyl viologen uptake, as described in Experimental Procedures.
To test the ability of the protein to transport substrate across a membrane, synthesized EmrE was reconstituted into proteoliposomes and methyl viologen uptake was measured. As shown in Fig. 2B, the in vitro-synthesized protein catalyzes accumulation of methyl viologen against its concentration gradient. Transport depended on the H+ gradient generated across the proteoliposomes because nigericin, an ionophore that collapses the pH gradient, fully inhibited accumulation (data not shown). The rate of uptake was 7.5 nmol/min per μg of EmrE, very similar to the rates reported for the native protein (20).
We conclude that the protein synthesized in this system is fully active because it binds and transports with kinetic constants practically identical to those of the native one. In addition, the detergent-solubilized in vitro-synthesized EmrE was analyzed in a size-exclusion column (Superdex 200, Amersham Biosciences) and eluted as a single peak with an apparent molecular mass (150 kDa in a 0.08% DDM solution) identical to that of the native EmrE (data not shown). Therefore, the in vitro system provides an experimental paradigm to study various properties of EmrE.
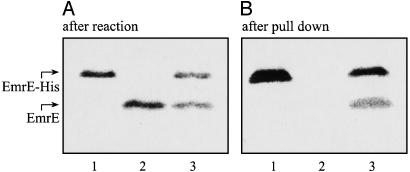
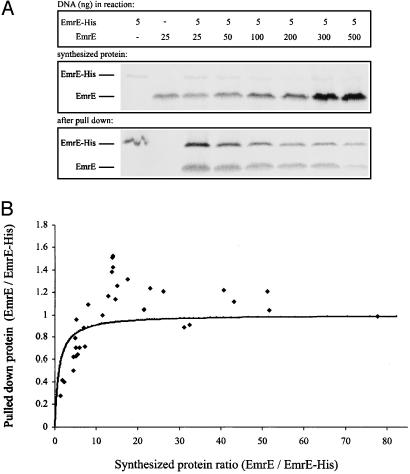
Coexpression of Plasmids and Pull-Down Experiments. We used two plasmids, coding for His-tagged and -untagged proteins (Fig. 3A, lanes 1 and 2, respectively), as template in the same reaction to express both proteins simultaneously (lane 3). To test whether both proteins interact to form an oligomer, tagged and untagged proteins were coexpressed and then immobilized on Ni-NTA beads. Because only His-tagged monomers bind to the Ni-NTA beads (Fig. 3B, lane 1 compared with lane 2), only the untagged monomers that interact with tagged ones were pulled down and detected after separation by SDS/PAGE (lane 3).
Fig. 3.
In vitro coexpression and pull down. Two DNA plasmids, coding for EmrE and EmrE-His, served as templates either singly or together in the same reaction. We supplemented 3 μCi of [35S]methionine into the synthesis-reaction mixture. (A) EmrE (200 ng of DNA) and EmrE-His (200 ng of DNA) were synthesized in vitro. After the reaction was completed, 0.5-μl reaction volume was separated by SDS/PAGE. Radioactive protein was visualized with a FLA-3000 PhosphorImager. When a single plasmid was included in the reaction, a single protein was detected (lanes 1 and 2). A reaction that contained both plasmids produced coexpression of both proteins (lane 3). (B) EmrE (50 ng of DNA) and EmrE-His (350 ng of DNA) were synthesized in vitro. A sample (20 μl) of each reaction was bound to Ni-NTA beads, and pull down was performed, as described in Experimental Procedures. Samples were separated by SDS/PAGE and analyzed. Tagged EmrE-His bound to Ni-NTA beads (lane 1), whereas wild-type EmrE did not (lane 2). When both proteins were coexpressed, EmrE was pulled down by EmrE-His (lane 3).
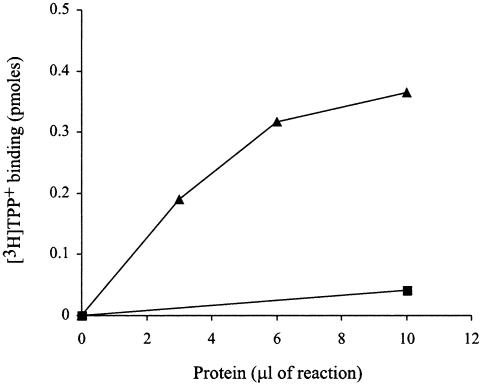
Negative-dominance studies have suggested that the oligomer is the functional unit (7). More recent studies have strongly supported this suggestion, by showing that inactive mutant subunits are functionally complemented when mixed with wild-type subunits (6). This finding provided us an assay to examine whether the physical interactions between EmrE subunits described above have functional consequences also. We, therefore, coexpressed tagged E14C-His and untagged EmrE in vitro. The product of the reaction was immobilized on Ni-NTA beads and washed so that only His-tagged protein, or protein associated with it, would remain bound to the beads. As shown (4), the tagged E14C-His (Fig. 4, ▪) is inactive and does not display any TPP+-binding activity. However, when coexpressed with untagged wild-type protein, TPP+-binding activity is restored (Fig. 4, ▴). We conclude that the two monomers interact with each other to form heterooligomers in which wild-type subunits functionally complement the inactive mutant. This in vitro heterooligomer showed affinity to TPP+ (Kd = 31 nM) lower than that of the wild-type protein (Kd = 2 nM). This affinity is very similar to that of the heterooligomer that was generated by monomer swapping in previous studies (6).
Fig. 4.
Functional complementation of E14C-His by EmrE. E14C-His (50 ng of DNA) and EmrE (300 ng of DNA) were coexpressed in vitro. After the synthesis reaction was completed, DDM was added to 0.08%, and the reaction was loaded on Ni-NTA beads. The protein that bound to beads was washed twice with 0.08% DDM/Na buffer, and samples (▴) were assayed for TPP+-binding activity, as described in Experimental Procedures. TPP+-binding activity was measured also for E14C-His (▪) that was synthesized in vitro in a separate reaction. TPP+ concentration used for binding was 5 nM for E14C-His/EmrE and 20 nM for the inactive mutant E14C-His alone.
Establishing that monomers interact to form oligomers during in vitro synthesis provided a new tool to examine the oligomeric state of EmrE further. A clear advantage of this system is that the ratios of both species can be manipulated easily and accurately. We coexpressed in vitro EmrE-His and EmrE at different DNA ratios and determined two factors for each reaction: the ratio of total EmrE: EmrE-His synthesized observed immediately at the end of the reaction and the same ratio observed after the pull-down assay (Fig. 5A). Increase in the amount of DNA coding for untagged EmrE brought about a corresponding increase in the amount of EmrE synthesized (Fig. 5A). Excess in EmrE DNA has a deleterious effect on the synthesis of EmrE-His in the same reaction, probably because of competition and maximum capacity of the system. Pull down of untagged EmrE was fully dependent on the presence of EmrE-His. The amounts of pulled-down EmrE decreased with the increase in the amount of DNA coding for EmrE because of the decrease in the amount of EmrE-His (Fig. 5A). The ratio of pulled-down EmrE to EmrE-His was quantitated as a function of the total amount synthesized of both species, and the results are shown in Fig. 5B. Our results confirm that EmrE forms an oligomer. The EmrE/EmrE-His ratio after pull down increases with the total EmrE/EmrE-His ratio (Fig. 5B). The solid line in Fig. 5B is the theoretical ratio to be expected from a randomly forming dimer. As seen in the initial part of the curve, the ratio tends to be lower than expected, suggesting a certain preference for homodimer formation. Furthermore, at the higher EmrE/EmrE-His ratios, the pulled-down product seems in most cases to have more untagged protein than expected from a dimer. Although there is some variability, the majority of the experimental points are above the expected ratio. We suggest that there is a high-affinity interaction to form a dimer that withstands extensive washes of the bead-bound protein and a weaker interaction that cannot be fully detected because of the technical limitations in the design of our experiments.
Fig. 5.
Pull down of untagged EmrE by EmrE-His. (A) EmrE and EmrE-His were coexpressed in vitro at different DNA ratios, as indicated (Top). The amount of plasmid coding for EmrE-His was kept constant, whereas the DNA plasmid coding for the untagged EmrE was at varying amounts and always in excess compared with the DNA plasmid coding for the tagged protein form (up to 100-fold). After the reaction was completed, 0.5-μl samples were analyzed by SDS/PAGE (Middle). Samples (20 μl) were then bound to Ni-NTA beads. Bound protein was washed three times in 0.08% DDM/Na buffer containing 25 mM imidazole and then eluted from beads and separated by SDS/PAGE (Bottom). (B) Data from seven different experiments, as described in A, were quantitated as follows. Amounts of total synthesized and pulled-down protein were estimated by using image gauge 3.46 software (Fujifilm). The ratio of pulled-down EmrE/EmrE-His was then plotted as a function of the ratio of total synthesized EmrE/EmrE-His.
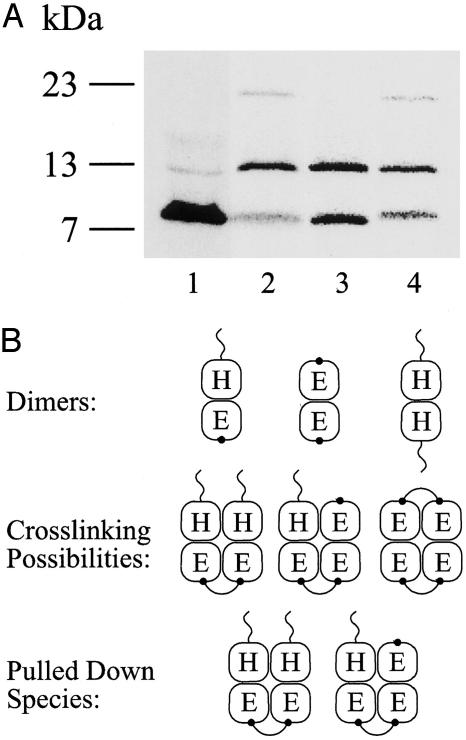
The findings described above led us to consider the possibility of two oligomer populations that exist in equilibrium: the basic dimer unit and a higher-degree oligomer formed, possibly, by two dimers. Efforts were made to detect the population with a lower affinity by lowering the stringency of the washes (smaller volumes of buffer and lower imidazole concentration) to no avail. Therefore, to test the above contention, we designed an experimental protocol by using irreversible crosslinking between monomers. We have shown (9) that o-PDM, which is a dimaleimide crosslinker, reacts with single Cys residues engineered in the EmrE monomer and generates dimers. Among the residues that crosslinked, we chose to focus here on K22C, a replacement in a hydrophilic, relatively long, and exposed loop. In the following experiments, the tagged EmrE is without Cys and, therefore, does not crosslink with o-PDM (9). The untagged protein contains a single Cys residue and can crosslink with another untagged protein in a different dimer (Fig. 6B). We coexpressed in vitro untagged K22C (in background without Cys) and tagged CLA-His, in a reaction in which K22C was in excess to optimize the formation of heterodimers of CLA-His:K22C (Fig. 6A, lane 1). When in vitro protein synthesis was completed, the reaction was dialyzed to stop it and to remove reducing agents, and then it was subjected to o-PDM crosslinking before immobilization on beads. As seen after SDS/PAGE analysis, when comparing samples treated with o-PDM (Fig. 6, lane 2) to a control sample (Fig. 6, lane 3), the amount of tagged CLA-His does not change. The amount of untagged K22C monomer, in contrast, decreases, and a polypeptide with a lower mobility (apparent molecular mass ≈20 kDa) appears in an amount that corresponds quantitatively with the disappearance of the monomer (Fig. 6, lane 2 compared with lane 3). The 20-kDa polypeptide corresponds to the untagged K22C dimer, as has been determined in experiments (9). The only Cys residue in each heterodimer was a Cys replacement in position 22 of the untagged monomer (Fig. 6B). Because anything bound to the beads has to have at least one tagged subunit (which cannot crosslink), the crosslinked dimers on the beads must have been in a complex with at least three subunits.
Fig. 6.
Crosslinking of CLA-EmrE-His/K22C heterodimers. (A) CLA-EmrE-His/K22C heterodimers were generated in vitro by coexpressing both proteins. K22C was expressed in excess to optimize formation of heterodimers over CLA-EmrE-His homodimers. A sample (0.5 μl) was separated by SDS/PAGE immediately after in vitro-synthesis reaction was completed. K22C was expressed in large excess compared with CLA-EmrE-His (lane 1). A 20-μl sample was bound to Ni-NTA beads immediately after synthesis was completed (lane 3) or dialyzed against 0.08% DDM/Na buffer and crosslinked with o-PDM (lane 2). Final o-PDM concentration was 400 μM. The reaction was stopped after 1 h at room temperature with 15 mM 2-mercaptoethanol, loaded on Ni-NTA beads, washed, and eluted, as described in Experimental Procedures. A sample of the eluted protein was then separated by SDS/PAGE (lane 2). An identical untreated sample was bound to Ni-NTA beads, and crosslinking with o-PDM was performed, whereas the protein was already bound to beads as described in Experimental Procedures (lane 4). (B) Schematic representation of the forms of the dimers, crosslinking possibilities, and pulled-down species. H, EmrE-His; E, EmrE-CLA-K22C. The “tail” on the H subunit symbolizes the His-tag, and the dot on EmrE-CLA-K22C stands for the crosslinking site (K22C).
Because the basic oligomer observed by cryo-electron microscopy is a dimer (8, 24), the results described implicate the possibility of two dimers being in very close proximity, enough to interact and form a tetramer. The percentage of K22C monomers that reacted to form the dimer was ≈30% of the K22C monomers pulled down by CLA-His. The crosslinking is performed after the synthesis is finished and the heterodimers are already formed in a very stable state. When formed, the heterodimers do not dissociate unless treated with denaturing agents, such as SDS, or temperatures as high as 80°C (6). Because the amount of K22C in these experiments was in great excess, we tested whether the pull down was artifactually removing polypeptide that was interacting nonspecifically. Crosslinking was repeated under different conditions. The crosslinker was added to the protein that bound to beads after all of the excess K22C was removed (Fig. 6, lane 4). The same results were observed when the protein was treated with o-PDM after elution from the beads (data not shown). Interestingly, the degree of crosslinking was similar whether the protein was on the beads or was free in the detergent, suggesting that the dimers are close enough to each other on the beads also. Moreover, crosslinking was practically identical in the crude (Fig. 6, lane 2) and in the purified (Fig. 6, lane 4) preparation, demonstrating the high specificity of the reaction. In this context, it is noteworthy also that, under all of the conditions tested, the crosslinking was inhibited when the reaction was performed in the presence of a denaturant, such as SDS (data not shown). Therefore, we conclude that the crosslinking reported here reflects a specific interaction between dimers.
Discussion
In this article, we present the in vitro synthesis of a fully functional polytopic membrane protein, EmrE, a well characterized multidrug transporter from E. coli. The amounts synthesized are large enough for biochemical and structural studies. EmrE is synthesized in the presence or absence of detergents. In the latter case, it inserts into a membrane-like fraction from which it can be solubilized at low concentrations of detergents, such as DDM. In vitro-synthesized EmrE shows TPP+-binding properties similar to those of the in vivo EmrE, and it has the same substrate specificity. In addition, the in vitro-synthesized EmrE was reconstituted in proteoliposomes, and it shows Δμ̃H+-dependent accumulation of substrate.
The ability to synthesize a functional protein in vitro provides a tool to numerous options for further research, including specific labeling of amino acids and incorporation of amino acids homologues. Here, we describe one of its applications, namely, the study of oligomerization of membrane proteins.
Negative-dominance studies, crosslinking, ligand binding, and heterooligomer formation all confirm that EmrE functions as an oligomer (4, 6, 7, 9, 25). A projection structure of EmrE determined by cryo-electron microscopy of two-dimensional crystals showed that the repetitive unit in the crystal was composed of eight α-helices arranged in an asymmetric manner, indicating that the minimal functional unit for substrate binding is a dimer (8, 25). Available data do not preclude the existence or functionality of higher oligomers. Our original ligand-binding measurements indicated a stoichiometry of about three monomers per bound substrate (4). These data were challenged, and it was suggested that in our measurements performed at very low EmrE concentrations, part of the population is in the monomeric form and, thereby, inactive in the binding assay (25). In our findings, however, the stoichiometry is three monomers per TPP+ bound as reported and does not change with the EmrE concentration (M. Soskine and S.S., unpublished data). The simplest interpretation is that the protein functions as a trimer. However, this interpretation does not agree with the structural data mentioned above (8, 25). One possible explanation for the ligand stoichiometry that we find is the equilibrium between a dimer and a tetramer, the latter form binding only one TPP+ at a time. However, the functional implications of tetramer formation still remain to be elucidated.
The functional complementation demonstrated in this work implies also that the presence of two Glu-14 residues per dimer is not an essential requisite for full activity. The formed heterooligomer binds TPP+ with lower affinity in a pH-dependent mode (i.e., binds and releases protons with a PK in a similar range). This finding suggests that all Glu-14 residues in the oligomer are equivalent and that the whole set improves the affinity.
The EmrE dimers are very stable in the detergent-solubilized preparations, and dissociation was achieved only after exposure to high temperature or strong denaturing agents (20). This strong interaction between EmrE monomers turns out to be a major advantage in this system that allows the use of the pull-down assay. Very extensive washes of the heterooligomers do not decrease the stoichiometry between tagged and untagged subunits below one. The in vitro synthesis system allows coexpression of different constructs and the control of the expression levels of each one of the proteins.
The pull-down assay that we used provided us with results that can be quantitated more accurately than the results provided by our previous monomer-swapping system. Our results exceeded, to some extent, the 1:1 ratio expected from a purely dimeric structure as determined by cryo-electron microscopy of two-dimensional crystals (8). The possibility of a higher organized oligomeric form could not be excluded, but because the affinity of the interaction seemed to be too low to detect directly, we had to resort to crosslinking, an approach that provides a snapshot of short-lived intermediates or low-affinity interaction. In the experiments presented here, the tagged monomer was without Cys so that it could not crosslink, whereas the untagged EmrE had a single Cys in an exposed long and hydrophilic loop of EmrE. Because the species isolated on the beads had to have at least one tagged species, crosslinking was a result of the interaction of at least three subunits. The specificity of the crosslinking is best illustrated by the fact that the same result was obtained when the reaction was performed in the crude lysate containing an excess of untagged protein and a large number of other proteins, including membrane proteins, and after purification in which all of the excess untagged protein and all of the other proteins were removed. Our results revealed interaction between two untagged monomers that could also imply a trimeric or a larger oligomeric structure. However, because the dimer is the basic oligomeric form detected by various means, we suggest that our findings reveal interaction between pairs of heterodimers being in close proximity (close enough to interact and form a tetramer under some conditions).
The work described here documents the cell-free synthesis of a fully functional polytopic membrane protein. This system provides a powerful tool for incorporation of unnatural or labeled amino acids and for study of the oligomerization properties of a membrane protein.
Acknowledgments
We thank Nataly Gutman for performing the reconstitution experiment, Misha Soskine for the size-exclusion chromatography, and Hila Frenkel (Dyn Diagnostics, Caesarea, Israel) for excellent support. This work was supported by National Institutes of Health Grant NS16708 and Israel Science Foundation Grant 463/00.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DDM, N-dodecyl-β-maltoside; TPP+, tetraphenylphosphonium; Ni-NTA, Ni2+-nitrilotriacetic acid; o-PDM, o-phenylene dimaleimide.
References
- 1.Schuldiner, S., Granot, D., Mordoch, S. S., Ninio, S., Rotem, D., Soskin, M., Tate, C. G. & Yerushalmi, H. (2001) News Physiol. Sci. 16 130-134. [DOI] [PubMed] [Google Scholar]
- 2.Schuldiner, S., Granot, D., Steiner, S., Ninio, S., Rotem, D., Soskin, M. & Yerushalmi, H. (2001) J. Mol. Microbiol. Biotechnol. 3 155-162. [PubMed] [Google Scholar]
- 3.Yerushalmi, H., Lebendiker, M. & Schuldiner, S. (1995) J. Biol. Chem. 270 6856-6863. [DOI] [PubMed] [Google Scholar]
- 4.Muth, T. R. & Schuldiner, S. (2000) EMBO J. 19 234-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yerushalmi, H. & Schuldiner, S. (2000) Biochemistry 39 14711-14719. [DOI] [PubMed] [Google Scholar]
- 6.Rotem, D., Sal-man, N. & Schuldiner, S. (2001) J. Biol. Chem. 276 48243-48249. [DOI] [PubMed] [Google Scholar]
- 7.Yerushalmi, H., Lebendiker, M. & Schuldiner, S. (1996) J. Biol. Chem. 271 31044-31048. [DOI] [PubMed] [Google Scholar]
- 8.Tate, C., Kunji, E., Lebendiker, M. & Schuldiner, S. (2001) EMBO J. 20 77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soskine, M., Steiner-Mordoch, S. & Schuldiner, S. (2002) Proc. Natl. Acad. Sci. USA 99 12043-12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betton, J. M. (2003) Curr. Protein Pept. Sci. 4 73-80. [DOI] [PubMed] [Google Scholar]
- 11.Zubay, G. (1973) Annu. Rev. Genet. 7 267-287. [DOI] [PubMed] [Google Scholar]
- 12.Spirin, A. (2002) Cell-Free Translation Systems (Springer, Berlin).
- 13.Sawasaki, T., Ogasawara, T., Morishita, R. & Endo, Y. (2002) Proc. Natl. Acad. Sci. USA 99 14652-14657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morita, E. H., Sawasaki, T., Tanaka, R., Endo, Y. & Kohno, T. (2003) Protein Sci. 12 1216-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doi, N., Takashima, H., Kinjo, M., Sakata, K., Kawahashi, Y., Oishi, Y., Oyama, R., Miyamoto-Sato, E., Sawasaki, T., Endo, Y. & Yanagawa, H. (2002) Genome Res. 12 487-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang, J. (1996) Mol. Biol. Cell 7 1709-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinrich, S. U. & Rapoport, T. A. (2003) EMBO J. 22 3654-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagamori, S., Vazquez-Ibar, J. L., Weinglass, A. B. & Kaback, H. R. (2003) J. Biol. Chem. 278 14820-14826. [DOI] [PubMed] [Google Scholar]
- 19.Tabor, S. & Richardson, C. (1985) Proc. Natl. Acad. Sci. USA 82 1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ninio, S., Rotem, D. & Schuldiner, S. (2001) J. Biol. Chem. 276 48250-48256. [DOI] [PubMed] [Google Scholar]
- 21.Ninio, S. & Schuldiner, S. (2003) J. Biol. Chem. 278 12000-12005. [DOI] [PubMed] [Google Scholar]
- 22.Yerushalmi, H. & Schuldiner, S. (2000) J. Biol. Chem. 275 5264-5269. [DOI] [PubMed] [Google Scholar]
- 23.Gutman, N., Steiner-Mordoch, S. & Schuldiner, S. (2003) J. Biol. Chem. 278 16082-16087. [DOI] [PubMed] [Google Scholar]
- 24.Ubarretxena-Belandia, I., Baldwin, J. M., Schuldiner, S. & Tate, C. G. (2003) EMBO J. 22 6175-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tate, C. G., Ubarretxena-Belandia, I. & Baldwin, J. M. (2003) J. Mol. Biol. 332 229-242. [DOI] [PubMed] [Google Scholar]