Abstract
The anti-tumor effects of calorie restriction (CR) and the possible underlying mechanisms were investigated using ethylnitrosourea (ENU)-induced glioma in rats. ENU was given transplacentally at gestational day 15, and male offspring were used in this experiment. The brain from 4-, 6-, and 8-month-old rats fed either ad libitum (AL) or calorie-restricted diets (40% restriction of total calories compared to AL rats) was studied. Tumor burden was assessed by comparing the number and size of gliomas present in sections of the brain. Immunohistochemical analysis was used to document lipid peroxidation [4-hydroxy-2-nonenal (HNE) and malondialdehyde (MDA)], protein oxidation (nitrotyrosine), glycation and AGE formation [methylglyoxal (MG) and carboxymethyllysine (CML)], cell proliferation activity [proliferating cell nuclear antigen (PCNA)], cell death [single-stranded DNA (ssDNA)], presence of thioredoxin 1 (Trx1), and presence of heme oxygenase-1 (HO-1) associated with the development of gliomas. The results showed that the number of gliomas did not change with age in the AL groups; however, the average size of the gliomas was significantly larger in the 8-month-old group compared to that of the younger groups. Immunopositivity was observed mainly in tumor cells and reactive astrocytes in all histological types of ENU-induced glioma. Immunopositive areas for HNE, MDA, nitrotyrosine, MG, CML, HO-1, and Trx1 increased with the growth of gliomas. The CR group showed both reduced number and size of gliomas, and tumors exhibited less accumulation of oxidative damage, decreased formation of glycated end products, and a decreased presence of HO-1 and Trx1 compared to the AL group. Furthermore, gliomas of the CR group showed less PCNA positive and more ssDNA positive cells, which are correlated to the retarded growth of tumors. Interestingly, we also discovered that the anti-tumor effects of CR were associated with decreased hypoxia-inducible factor-1α (HIF-1α) levels in normal brain tissue. Our results are very exciting because they not only demonstrate the anti-tumor effects of CR in gliomas, but also indicate the possible underlying mechanisms, i.e. anti-tumor effects of CR observed in this investigation are associated with reduced accumulation of oxidative damage, decreased formation of glycated end products, decreased presence of HO-1 and Trx1, reduced cell proliferation and increased apoptosis, and decreased levels of HIF-1α.
Keywords: calorie restriction, ethylnitrosourea, glioma, oxidative stress, HIF-1α
Since the original discovery by McKay et al. that calorie restriction (CR) attenuated aging and age-related diseases, CR has become well known for extending life span and for having robust anti-tumor effects (1–3). Subsequent research has continued to demonstrate that CR is correlated to a reduction in tumor incidence and growth. CR's anti-tumor effects have been confirmed using several experimental model systems, including spontaneous lymphomas in p53-deficient mice (4), breast cancer in DBA mice (5), spontaneous tumors in Fischer 344 (F344) rats (6), transplantations of cultured cells or tumors (7), and induced carcinogenesis (8–13). However, previous studies have been unable to fully uncover the exact underlying mechanisms of the anti-tumor effects of CR.
Among the many putative underlying mechanisms of CR, substantial evidence suggests that reduced oxidative stress could be a critical part of the anti-tumor effects of CR. The assertion that CR reduces oxidative stress and thereby affects carcinogenesis is strongly supported: (1) CR decreases mitochondrial reactive oxygen species (ROS) production (14), enhances antioxidant defense systems (15), improves the repair of oxidatively damaged molecules (16), and promotes the replacement of damaged proteins with newly synthesized ones (17) and (2) oxidative stress is postulated to play an important role in the complex course of multistage carcinogenesis (18–22). It is widely known that many initiators and promoters of carcinogenesis produce ROS that cause oxidative damage to a variety of molecules, including DNA (23). Genetic alterations from DNA damage can lead to abnormal cellular differentiation, followed by neoplastic development (18, 23). In addition, experimental studies have shown that antioxidants have anti-tumor effects, e.g. the inhibitory effect of superoxide dismutase (SOD) and catalase on cellular transformation, and the inhibitory effect of SOD on the tumor-promoting effects of TPA (12-O-tetradecanoyl-phorbol-13-acetate) (19). This combined evidence supports the hypothesis that CR's ability to reduce oxidative stress may play an important role in its anti-tumor effect. However, previous studies have not provided a complete evaluation of the relationship between CR, oxidative stress, and tumor suppression during multistage carcinogenesis using a well-established in vivo system.
Gliomas are the most common brain tumor in humans, and they are difficult to cure because of their infiltrative nature, which makes complete surgical removal challenging, and their resistance to chemotherapy and other treatments. Since glioma patients are typically given an extremely poor prognosis, it is critical to discover an intervention that reduces the incidence and growth of gliomas. Despite this urgency, the effects of CR on glioma development have not been comprehensively tested in vivo. Ethylnitrosourea (ENU)-induced glioma in rats has been extensively utilized as an experimental brain tumor model (24–28). Some advantages of this model are its extremely high rate (100%) of tumor induction and the certainty of the occurrence of multiple tumors per brain. Additionally, the continuous profile and time course of tumor progression in this experimental model have been well documented. Thus, this model is an ideal in vivo model to critically evaluate whether CR attenuates tumor incidence and/or growth. In addition, previous studies have suggested a possible role for oxidative stress in nitrosoamine-induced carcinogenesis (29, 30). Therefore, the ENU-induced glioma model allows us to test whether changes in tumor incidence and/or growth by CR are associated with corresponding changes in oxidative stress, or its physiological consequences, such as changes in redox-sensitive signaling. The purpose of this study is to use the established profile of ENU-induced tumors to identify the point(s) that CR intervenes in tumor development and to explore the underlying mechanisms of CR's anti-tumor effects.
Methods
Animals and ENU administration
Female and male Wistar rats were obtained from Harlan (Indianapolis, IN) at 16 weeks of age. All rats were fed control rat chow and allowed to acclimate to their new surroundings for 2 weeks. Female Wistar rats weighing 200–250 g were caged overnight with males, and the day when sperm was confirmed in vaginal smears was designated as day 1 of gestation. On day 15 of gestation, pregnant rats were injected intravenously with a single dose of N-ethyl-N-nitrosourea (ENU: 50 mg/kg body weight) dissolved in distilled water. Male offspring from the ENU-treated rats were used in these experiments.
Rat maintenance and dietary procedures
After weaning, all male offspring were housed individually under specific pathogen-free conditions, were fed ad libitum (AL), and had free access to water until 6 weeks of age. The composition of the diet, which provides equivalent nutrition but reduces total energy intake, was previously described in detail (31). At 6 weeks of age, 60 rats were randomly assigned to either the AL or calorie-restricted group. The calorie-restricted group was fed approximately 60% of the caloric intake fed to the AL group. Food consumption was measured twice a week as described by Yu et al. (32). All rats were weighed twice a month for the duration of the study. A 12:12 hour light–dark cycle was used. To examine the development of tumors, eight rats were randomly selected and sacrificed at 4, 6, and 8 months of age.
Tissue preparation
At sacrifice, the brains were removed by dissection, sliced into 3-mm-thick sections, and immersed in 4% paraformaldehyde/0.1 M phosphate buffer (PB) at 4 °C overnight and embedded in paraffin. Five-micron-thick sections were placed on glass slides coated with poly-l-lysine (Sigma, Deisenhofen, FRG) and subsequently stained with hematoxylin–eosin (H&E) or used for immunohistochemical examination.
Classification of tumors
All tumors were classified based on the criteria by Koestner et al. (27) and Schiffer et al. (28). According to their size and neoplastic nature, tumors were classified arbitrarily as follows: (1) hyperplasia: a few or several abnormal cells forming a cluster with no destructive nature; (2) early neoplastic proliferation (ENP): a cluster of abnormal cells larger than that for hyperplasia, but less than 500 µm in diameter and with higher cell density; mild destructive nature apparent; (3) microtumor: an apparent destructive nature present, but still measuring less than 1 mm in diameter; (4) medium-sized tumor: from 1 mm to 2 mm in diameter; (5) gross tumor: larger than 2 mm in diameter and are classified into two histological types: (1) oligodendroglioma: isomorphic proliferation of small round cells similar to human oligodendroglioma; (2) anaplastic glioma: showing cellular atypism and pleomorphism, and structural change such as necrosis, hemorrhage, and endothelial proliferation.
Tumor development/image analysis
Tumor development was assessed by measuring both the number and size of brain tumors. The number of tumors in each brain section was counted under a light microscope. The size of each tumor was measured using an image analysis system consisting of a SPOT cooled color digital camera (Diagnostic Instruments Inc., Sterling Heights, MI), an Olympus AX 70 True Research System Microscope (Olympus America Inc., Lake Success, NY), a Dell Dimension XPS M166s (Dell Computer Corp., Round Rock, TX), a FlashiPoint videographics card (Integral Technologies Inc., Indianapolis, IN), and Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). These analyses were performed by a double-blind procedure without knowledge of the animal's age or diet group.
Immunohistochemistry
Tissue distribution and the cellular location of oxidative damage, glycated end products, heme oxygenase-1 (HO-1), thioredoxin 1 (Trx1), proliferating cell nuclear antigen (PCNA), and single-stranded DNA (ssDNA) were assessed by immunohistochemistry using an image analysis workstation after staining with antibodies. The brain sections were pretreated with 0.3% H2O2–methanol for 1 h at room temperature and with normal goat serum for 1 h at room temperature. Each specimen was incubated with a primary antibody overnight at 4 °C. The primary antibodies used in this study and their dilutions were as follows: 4-hydroxy-2-nonenal (HNE)-modified keyhole limpet hemocyanin (KLH) monoclonal antibody (mAbs HNEJ-1-5) (33) [1:400, NOF Co. Ltd., Tokyo, Japan], malondialdehyde (MDA)-modified KLH monoclonal antibody (mAb1F83) (34) [1:200, Dr. Uchida], nitrotyrosine monoclonal antibody (mAb39B6) [1:400, Dr. Shigenaga], methylglyoxal (MG)-modified KLH monoclonal antibody (35) [1:100, Dr. Uchida], carboxymethyllysine (CML) antibody (36) [1:800, Dr. Baynes], PCNA monoclonal antibody [1:200, Novocastra Laboratories], ssDNA polyclonal antibody [1:100, DAKO], HO-1 antibody (H4535) [1:600, Sigma–Aldrich Inc.], and Trx1 antibody (T0803) [1:800, Sigma–Aldrich Inc.]. Immunohistochemistry was performed using the VECTASTAIN ABC System (Vector Laboratories Inc., Burlingame, CA) with the avidin–biotin peroxidase complex (ABC) method (37). Negative controls included replacement of the primary antibodies with normal rabbit serum [1:100-800, DAKO]. The immunoreactivity to rat positive control specimen of the primary antibodies was determined before use.
Western Blot analysis
Hypoxia-inducible factor-1α (HIF-1α) levels were measured by Western Blot analysis using a HIF-1α antibody (Cell Signaling Technology Inc., Danvers, MA) and an ECL Western Blot detection kit (Amersham Biosciences Corp., Piscataway, NJ).
Results
Effects of CR on body and brain weights
Body weight of both AL and CR rats increased progressively with age; however, body weight of CR rats was significantly lower (approximately 40% less) than that of AL rats (data not shown). Brain weight slightly increased with age in both groups. Brain weight was similar between the AL and CR groups at 4 and 6 months; however, brain weight was significantly greater in the AL group than in the CR group at 8 months (p<0.05; data not shown).
Histology of brain tumors
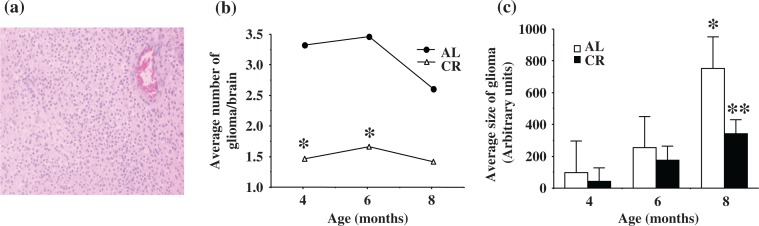
All histological tumor types except hyperplasia were observed in all three age groups of both AL and CR rats. Gross tumors (oligodendroglioma and anaplastic glioma) were more frequent in the 6-month-old (AL: 62.5%; DR: 61.5%) and 8-month-old (AL: 55.5%; DR: 81.8%) groups than in the 4-month-old groups (AL: 21.3%; DR: 0%). Histology of oligodendroglioma (H&E) is shown in Figure 1a. Oligodendroglioma induced by ENU showed isomorphic proliferation of small round cells similar to human oligodendroglioma.
Fig. 1.
Histology (Fig. 1a: left), average number (Fig. 1b: middle), and average size (Fig. 1c: right) of ENU-induced gliomas. a. Oligodendroglioma induced by ENU showed isomorphic proliferation of small round cells similar to human oligodendroglioma (H&E, 200×). b. The average number of gliomas per brain from CR rats was significantly less than AL rats at 4 and 6 months (*p<0.05). c. The average size of glioma in AL rats was not significantly different between 4 and 6 months; however, average size of glioma in AL rats was significantly larger (*p<0.05) at 8 months than at younger ages (4 and 6 months). The size of glioma was not significantly different between AL and CR groups at 4 or 6 months. At 8 months, CR rats showed significantly smaller gliomas than AL rats (**p=0.0010).
Effects of CR on glioma development
Tumor development was assessed by measuring the number of tumors per brain and size of each tumor. In the AL group, the average number of gliomas per brain was similar in all age groups, although the number of gliomas declined slightly in the 8-month-old group (p>0.05; Fig. 1b). The CR group had significantly fewer tumors compared to the AL group at 4 and 6 months (*p<0.05; Fig. 1b). The average size of gliomas in each age group is shown in Figure 1c. The average size of gliomas in the AL group was not significantly different between the 4- and 6-month-old groups; however, the average size of gliomas was significantly larger at 8 months compared to the younger age (4 and 6 months) groups (*p<0.05; Fig. 1c). The average size of gliomas in the CR group was significantly smaller compared to the AL group at 8 months of age (**p=0.0010; Fig. 1c).
Immunohistochemistry
All antibodies used showed an increase in immunoreactivity that corresponded with increasing glioma development. HNE, MDA, MG, CML, HO-1, and Trx1 antibodies showed similar staining patterns and localization. PCNA and ssDNA antibodies showed staining in the nucleus of tumor cells. Gross tumor cells (oligodendroglioma and anaplastic glioma) showed more intense immunostaining compared to the others, and of these, anaplastic gliomas showed the most intense staining among all histological types. Using an image analysis workstation, the stained slides were quantitatively analyzed for differences in the extent of positive staining in each tumor.
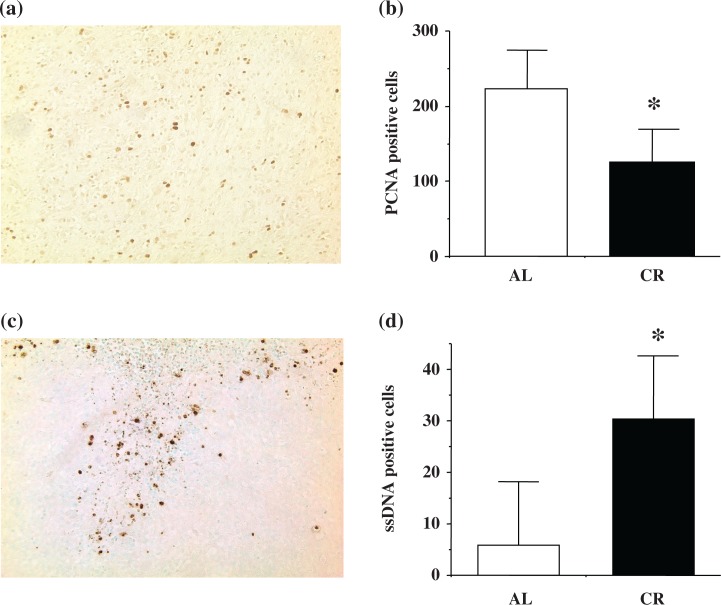
Cell proliferation and cell death
Immunostaining of PCNA and ssDNA was observed in tumor cells. Figure 2 shows the comparison of the number of PCNA and ssDNA immunopositive cells in the AL and CR groups at 8 months of age. Immunostaining of PCNA (Fig. 2a) and ssDNA (Fig. 2c) in gliomas is shown. Gliomas from CR rats showed significantly less immunopositive PCNA cells (*p=0.0157; Fig. 2b) and significantly more ssDNA immunopositive cells (*p=0.0224; Fig. 2d) compared to AL rats.
Fig. 2.
Number of PCNA and ssDNA positive cells in ENU-induced gliomas. a. PCNA staining was present in tumor cells of ENU-induced gliomas (PCNA, 100×). b. Gliomas from CR rats showed a significantly lower PCNA positive cell number (*p=0.0157) compared to the AL group at 8 months of age. c. ssDNA staining was present in tumor cells of ENU-induced gliomas (ssDNA, 100×). d. Gliomas from CR rats showed a significantly higher ssDNA positive cell number (*p=0.0224) compared to the AL group at 8 months of age.
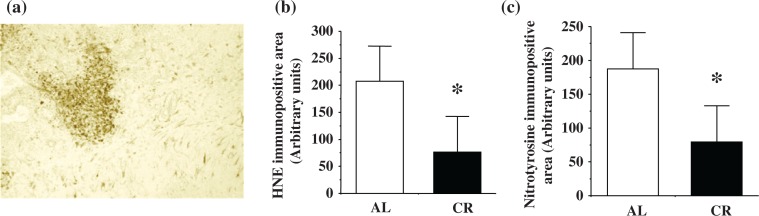
Lipid peroxidation and protein oxidation
Immunostaining was observed in tumor cells and reactive astrocytes, and the gross tumors (oligodendroglioma and anaplastic glioma) showed more intense HNE, MDA, and nitrotyrosine staining. HNE, MDA, and nitrotyrosine showed similar staining patterns. HNE immunostaining of ENU-induced glioma is shown in Figure 3a. Figure 3b shows the comparison of the immunopositive areas for HNE between the AL and CR groups at 8 months of age. Figure 3c shows the comparison of immunopositive areas for nitrotyrosine between the AL and CR groups at 8 months of age. Gliomas from CR rats showed significantly less HNE, MDA, and nitrotyrosine immunopositive areas compared to those from AL rats (HNE: *p=0.0147; Fig. 3b; MDA: *p=0.0248; data not shown; and nitrotyrosine: *p=0.0318; Fig. 3c).
Fig. 3.
The effects of CR on accumulation of HNE (Fig. 3a: left and Fig. 3b: middle), and nitrotyrosine (Fig. 3c: right) in ENU-induced gliomas. a. HNE staining was present in tumor cells and reactive astrocytes in ENU-induced glioma (HNE, 100×). b. Gliomas from CR rats showed reduced HNE-positive (*p=0.0147). c. Nitrotyrosine-positive staining compared to AL rats at 8 months (*p=0.0318).
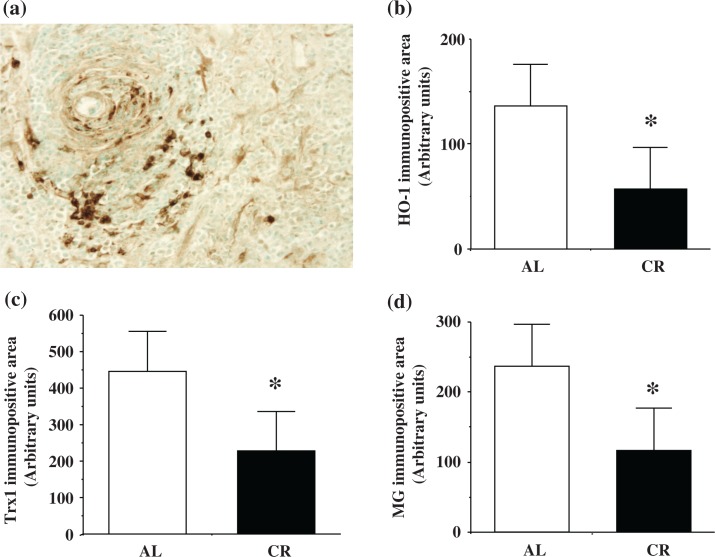
HO-1 and Trx1
Immunostaining of HO-1 and Trx1 was observed in tumors and reactive astrocytes. HO-1 and Trx1 showed similar staining patterns. Immunostaining of HO-1 is shown in Figure 4a. Both HO-1 (Fig. 4b) and Trx1 (Fig. 4c) immunopositivity were significantly lower in CR rats than in AL rats at 8 months of age (*p< 0.05).
Fig. 4.
Presence of heme oxygenase-1 (HO-1) (Fig. 4a: upper left and Fig. 4b: upper right), thioredoxin (Trx1) (Fig. 4c: lower left), and methylglyoxal (MG) (Fig. 4d: lower right) in ENU-induced glioma. a. HO-1 staining was present in tumor cells and reactive astrocytes in ENU-induced glioma (HO-1, 100×). b. The gliomas from CR rats showed significantly lower HO-1 (*p<0.05). c. Trx1 (*p<0.05). d. MG (*p<0.05) accumulation compared to the gliomas of AL rats at 8 months of age.
Glycated end products
Immunostaining of MG and CML was observed in tumors and reactive astrocytes. MG and CML exhibited similar staining patterns. Figure 4d shows the comparison of immunopositive areas for MG between the AL and CR groups at 8 months of age. Immunopositivity of MG in CR rats was significantly lower than in AL rats at 8 months of age (*p< 0.05; Fig. 4d).
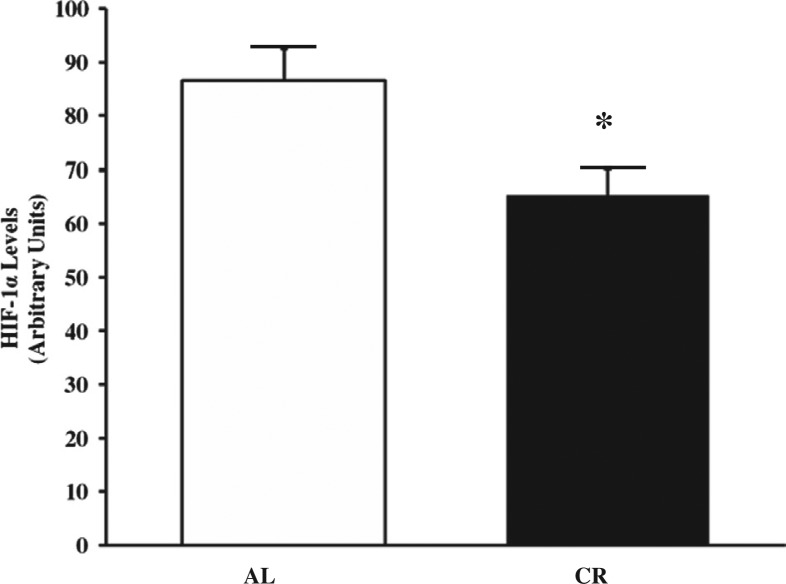
HIF-1α levels
The levels of HIF-1α in ENU-treated normal brain tissue were significantly lower in CR rats than in AL rats (*p=0.0244; Fig. 5).
Fig. 5.
Levels of hypoxia-inducible factor-1 α (HIF-1α) in ENU-treated normal brain. The levels of HIF-1α were significantly lower in CR than in AL rats (*p=0.0244) at 8 months of age. The data are the mean±SEM from five rats.
Discussion
Since the initial discoveries in the 1940s (38, 39), CR has been well recognized for its ability to reduce the incidence and attenuate the growth of several types of tumors. In fact, CR is the most widely studied and most potent, broadly acting dietary intervention for preventing carcinogenesis in a variety of experimental models (8–13, 40). Much evidence supports the link between CR, reduced levels of oxidative stress, and inhibition of carcinogenesis, suggesting that reduced oxidative stress could be a major contributor to CR's anti-tumor effects (19, 41, 42). However, the effects of CR on brain tumors and on their underlying regulatory mechanisms remain unclear.
Because of the lack of in vivo studies investigating the role of oxidative stress and CR in brain tumors, to our knowledge this study is the first to demonstrate the overall effects of CR on oxidative stress and brain tumors in their natural state. Of particular importance is that our study makes use of the ENU-induced glioma model, a well-established in vivo model that has many advantages: (1) an extremely high rate (100%) of multiple tumor induction per brain; (2) continuous profile and time course of tumor progression have been well documented; (3) immature glia are initiated simultaneously in utero, allowing for evaluation of CR's effect on tumor promotion/progression by comparing tumor number/size; (4) previous studies suggest a possible role for oxidative stress in nitrosoamine-induced carcinogenesis; and (5) the brain consumes approximately 10% of the total oxygen required by the body, yet the effect of this high oxygen consumption on oxidative stress and brain tumor formation has not been extensively examined. Using this well-suited in vivo brain tumor model (24–28, 43–48), our experiments demonstrated that CR reduces the incidence and growth of gliomas and that CR's anti-tumor effects are associated with reduced levels of oxidative stress, reduced cell proliferation, increased apoptosis, decreased formation of glycated end products, decreased presence of HO-1 and Trx1, and decreased levels of HIF-1α in brain tissues.
The development of tumors, especially chemically induced tumors, is considered a multistage process that can be divided into three distinct stages, i.e. initiation, promotion, and progression (49). In the ENU-induced glioma model, initiation of tumors is controlled by the injection of ENU at day 15 of gestation, but monitoring the incidence and growth of tumors over time can provide insight into the effects of CR on promotion and progression. Thus, the observed reduction in the number and size of tumors in CR rats compared to AL rats at 8 months of age suggests that CR may intervene in both the promotion and progression of carcinogenesis in ENU-induced brain tumors, as it appears to attenuate the growth of existing tumors as well as prolong or prevent tumor onset.
A large number of studies indicate that chronic disease states, including cancer, are associated with increased oxidative stress (50–53). Oxidative stress causes damage to critical cellular biomacromolecules including lipids, proteins, and DNA, which in many cases is shown to be mutagenic. Thus, documenting the accumulation of oxidized biomacromolecules provides an index of cellular oxidative damage that may contribute to malignant cellular transformation (18). Because CR is shown to suppress oxidative stress and thereby reduce oxidative damage to various biomacromolecules (54), CR's anti-tumor effects may be a result of decreased accumulation of oxidative damage to tissues.
Lipids are particularly vulnerable to free radical attack because of their relatively high level of unsaturation compared with other biological molecules. In addition, lipid peroxidation products have a relatively long half-life and can diffuse through membranes, making them capable of damaging other biomacromolecules and affecting cellular homeostasis (55). MDA and HNE are major lipid peroxidation products, and HNE is highly toxic and readily reacts with critical biomolecules (56). The presence of MDA and HNE observed in ENU-induced brain tumors indicates that oxidative damage involved the lipid component of tumor tissue, and that the extent of lipid damage appeared to decrease with attenuated tumor growth in CR rats.
Protein oxidation could also play important roles in the pathophysiology of carcinogenesis. Oxidation of proteins can cause structural modifications resulting in changes in enzyme activity and signaling pathways. In this study, we assessed oxidative damage to proteins by measuring the presence and accumulation of nitrotyrosine in tumors because it is a major product formed when protein is exposed to reactive nitrogen oxides (57). In addition, tyrosine nitration can alter protein and enzyme function (58, 59), thereby potentially affecting cellular homeostasis. The staining pattern of nitrotyrosine was similar to that of the lipid peroxidation products; i.e. there was a significant decrease in nitrotyrosine in the tumors of CR rats that appeared to be associated with a reduction in tumor size and number. Oxidative damage of proteins and lipids appears to be concurrent, suggesting that CR's anti-tumor effects may be associated with decreased oxidative damage to both lipids and proteins. Thus, reduced oxidative stress may be a potential underlying mechanism of CR's anti-tumor effects; however, the downstream consequences of reduced oxidative stress, e.g. redox sensitive signaling pathways, remain to be examined.
We also used immunohistochemistry to determine the distribution of HO-1, one of two isoforms of heme oxygenase, an enzyme that is well known to be responsive to cellular stress levels. HO-1 produces biliverdin, which is subsequently converted to bilirubin by the action of biliverdin reductase, and both biliverdin and bilirubin are potent antioxidants that may contribute to protection against oxidative stress (60–62). Substantial evidence indicates that many cancers overexpress HO-1 (63) and that it may be beneficial for tumor growth because it promotes angiogenesis (64) and limits apoptosis (62). Another protein that protects cells through its antioxidant properties is thioredoxin (Trx). Trx is overexpressed in many types of cancer and has been linked to cancer cell proliferation rates and cancer progression (65). Like HO-1, Trx provides antioxidant protection to cells, but it does so by acting as a hydrogen donor for enzymes involved in reductive reactions and providing protection against oxidative stress (66). Trx also functions as a physiological inhibitor of apoptosis signal-regulating kinase 1 (ASK1) (67, 68). Tumors of CR rats showed a significant decrease in the number of PCNA positive cells and an increase in ssDNA compared to tumors of AL rats at 8 months of age. Tumors of CR rats also showed a significant decrease in HO-1 and Trx levels compared to tumor tissues from the brains of AL rats. Thus, reduced levels of HO-1 and Trx in the tumors of CR rats may have contributed to the reduction in cell proliferation and the increase in apoptosis, which could attenuate tumor growth.
We also examined the effects of CR on glycation and the formation of advanced glycation end products (AGEs) in tumors. AGEs have been implicated in a number of diseases (69), and the majority of work has shown AGEs to be related to oxidative stress (70, 71). We measured MG (35), an intermediate formed during AGE production, and CML (36), an advanced glycation end product, as indices of protein glycation and AGE formation in ENU-induced brain tumors. We found that MG and CML levels were significantly lower in the tumors of CR rats compared to AL rats, suggesting that brain tumors of CR rats were subject to decreased AGE formation and a decrease in the associated production of reactive oxygen intermediates, which could be important in limiting tumor development.
Hypoxia-inducible factor-1 is a heterodimeric protein that consists of two proteins, one being HIF-1α. HIF-1α has been significantly associated with cancer (72, 73), and it activates the transcription of various genes that are involved in carcinogenesis, including those regulating angiogenesis, cell proliferation, and metastasis (72–74). Because it is redox-sensitive, we believed that HIF-1α might be related to oxidative stress in tumorigenesis. ENU-treated brain tissues of CR rats showed a significant decrease in the levels of HIF-1α that was similar to the decrease in oxidative stress exhibited in tumors of CR rats. This suggests that CR decreases HIF-1α levels, but the effects of CR and oxidative stress on the regulation of HIF-1α have yet to be defined. It is also important to note that because HIF-1α regulates angiogenesis, proliferation, and metastasis, which are essential for tumor growth, decreasing HIF-1α activity could be a direct way of limiting tumor growth. Thus, continuing to investigate the effects of CR on HIF-1α levels in brain tumors is of clear importance.
Using the ENU-induced glioma model, this series of experiments demonstrated that the anti-tumor effects of CR were associated with decreased oxidative stress, decreased glycation and AGE formation, decreased presence of HO-1 and Trx1, decreased cell proliferation, increased apoptosis, and decreased levels of HIF-1α activity. Because CR has been shown to reduce levels of growth factors, such as IGF-I, reduced levels of growth factors and/or energy intake could also play important roles in CR's anti-tumor effects (75). However, we believe reduced oxidative stress could play more important roles in the anti-tumor effects of CR because rats on CR diets typically exhibit food intake per gram of body weight as well as a metabolic rate per lean body mass similar to AL rats (76, 77). Based on our results, the ENU-induced glioma model would appear to be an excellent experimental model to continue to investigate the effects of CR and the role of oxidative stress and its underlying mechanisms in the development and pathophysiology of gliomas. Further exploration of CR's effects on cellular processes, such as changes in the intracellular signal transduction system and in proto-oncogenes, could provide more insight into the pathophysiology and development of a therapeutic treatment to attenuate the growth and development of gliomas.
Acknowledgements
The authors also wish to thank Dr. John Baynes for his generosity in providing us with the carboxymethyllysine antibody.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study. This research was supported by VA Merit Review Grant from the Department of Veteran Affairs, NIH Grant AG13319, The American Federation for Aging Research (AFAR) grant, a Grant from Glenn Foundation, and a San Antonio Nathan Shock Center Pilot Grant.
References
- 1.Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2003;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 2.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–52. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 3.Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Springfield, IL: Charles C. Thomas; 1988. [Google Scholar]
- 4.Hursting SD, Perkins SN, Phang JM, Barrett JC. Diet and cancer prevention studies in p53-deficient mice. J Nutr. 2001;131:3092S–4S. doi: 10.1093/jn/131.11.3092S. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes G, Chandrasekar B, Troyer DA, Venkatraman JT, Good RA. Dietary lipids and calorie restriction affect mammary tumor incidence and gene expression in mouse mammary tumor virus/v-Ha-ras transgenic mice. Proc Natl Acad Sci USA. 1995;92:6494–8. doi: 10.1073/pnas.92.14.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda H, Gleiser CA, Masoro EJ, Murata I, McMahan CA, Yu BP. Nutritional influences on aging of Fischer 344 Rats: II. Pathology. J Gerontol. 1985;40:671–88. doi: 10.1093/geronj/40.6.671. [DOI] [PubMed] [Google Scholar]
- 7.Flory CM, Furth J, Saxton JA, Jr, Reiner L. Chemotherapeutic studies on transmitted mouse leukemia. Cancer Res. 1943;3:729–43. [Google Scholar]
- 8.Cohen LA, Choi K, Wang C-X. Influence of dietary fat, caloric restriction, voluntary exercise on N-nitrosomethylurea-induced mammary tumorigenesis in rats. Cancer Res. 1988;48:4276–83. [PubMed] [Google Scholar]
- 9.Giles TC, Roebuck BD. Effects of voluntary exercise and/or food restriction on pancreatic tumorigenesis in male rats. Adv Exp Med Biol. 1992;322:17–27. doi: 10.1007/978-1-4684-7953-9_3. [DOI] [PubMed] [Google Scholar]
- 10.Klurfeld DM, Weber MM, Kritchevsky D. Inhibition of chemically induced mammary and colon tumor promotion by caloric restriction in rats fed increased dietary fat. Cancer Res. 1987;47:2759–62. [PubMed] [Google Scholar]
- 11.Kritchevsky D, Weber MM, Klurfeld DM. Dietary fat versus caloric content in initiation and promotion of 7,12-dimethylbenz(a)anthracene-induced mammary tumorigenesis in rats. Cancer Res. 1984;44:3174–7. [PubMed] [Google Scholar]
- 12.Lagopoulos L, Stalder R. The influence of food intake on the development of diethylnitrosamine-induced liver tumours in mice. Carcinogenesis. 1987;8:33–7. doi: 10.1093/carcin/8.1.33. [DOI] [PubMed] [Google Scholar]
- 13.Reddy BS, Wang C-X, Maruyama H. Effect of restricted caloric intake on azoxy-methane-induced colon tumor incidence in male F344 rats. Cancer Res. 1987;47:1226–8. [PubMed] [Google Scholar]
- 14.Sohal RS, Dubey A. Mitochondrial oxidative damage, hydrogen peroxide release, and aging. Free Rad Biol Med. 1994;16:921–6. doi: 10.1016/0891-5849(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 15.Armeni T, Pieri C, Marra M, Saccucci F, Principato G. Studies on the life prolonging effect of food restriction: glutathione levels and glyoxalase enzymes in rat liver. Mech Ageing Dev. 1998;101:101–10. doi: 10.1016/s0047-6374(97)00167-x. [DOI] [PubMed] [Google Scholar]
- 16.Weraarchakul N, Strong R, Wood WG, Richardson A. Effect of aging and dietary restriction on DNA repair. Exp Cell Res. 1989;181:197–204. doi: 10.1016/0014-4827(89)90193-6. [DOI] [PubMed] [Google Scholar]
- 17.Van Remmen H, Ward WF, Sabia RV, Richardson A. Gene expression and protein degradation. In: Masoro EJ, editor. Handbook of physiology: section 11-Aging. New York: Oxford University Press; 1995. pp. 235–305. [Google Scholar]
- 18.Klaunig JE, Xu Y, Isenberg JS, Bachowski S, Kolaja KL, Jiang J, et al. The role of oxidative stress in chemical carcinogenesis. Environ Health Perspect. 1998;106:289–95. doi: 10.1289/ehp.98106s1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8:583–99. doi: 10.1016/0891-5849(90)90156-d. [DOI] [PubMed] [Google Scholar]
- 20.Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- 21.Kensler TW, Egner PA, Taffe BG, Trush MA. Role of free radicals in tumor promotion and progression. Prog Clin Biol Res. 1989;298:233–48. [PubMed] [Google Scholar]
- 22.Kensler TW, Trush MA. Role of oxygen radicals in tumor promotion. Environ Mutagen. 1984;6:593–616. doi: 10.1002/em.2860060412. [DOI] [PubMed] [Google Scholar]
- 23.Jackson AL, Chen R, Loeb LA. Induction of microsatellite instability by oxidative DNA damage. Proc Natl Acad Sci USA. 1998;95:12468–73. doi: 10.1073/pnas.95.21.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Druckrey H, Ivankovic S, Preussmann R. Teratogenic and carcinogenic effects in the offspring after single injection of ethylnitrosourea to pregnant rats. Nature. 1966;210:1378–9. doi: 10.1038/2101378a0. [DOI] [PubMed] [Google Scholar]
- 25.Ikeno Y. The properties of an immature glial cell line (HITS Glioma) derived from ethylnitrosourea (ENU)-induced rat glioma. Acta Medica Nagasakiensia. 1993;38:2–4. [Google Scholar]
- 26.Ikeno Y, Shimokawa I, Higami Y, Ikeda T. GFAP expression in the subcutaneous tumors of immature glial cell line (HITS glioma) derived from ENU induced rat glioma. J Neuro-Oncology. 1993;17:191–204. doi: 10.1007/BF01049975. [DOI] [PubMed] [Google Scholar]
- 27.Koestner A, Swenberg JA, Wechsler W. Transplacental production with ethylnitrosourea of neoplasms of the nervous system in Sprague-Dawley rats. Am J Pathol. 1971;63:37–50. [PMC free article] [PubMed] [Google Scholar]
- 28.Schiffer D, Giordana MT, Pezzotta S, Lechner C, Paoletti P. Cerebral tumors induced by transplacental ENU: study of the different tumoral stages, particularly of early proliferations. Acta Neuropathol (Berl) 1978;41:27–31. doi: 10.1007/BF00689553. [DOI] [PubMed] [Google Scholar]
- 29.Bartsch H, Hietanen E, Malaveille C. Carcinogenic nitrosoamines: free radical aspects of their action. Free Radic Biol Med. 1989;7:637–44. doi: 10.1016/0891-5849(89)90144-5. [DOI] [PubMed] [Google Scholar]
- 30.Hietanen E, Bartsch H. Gastrointestinal cancers: role of nitrosoamines and free radicals. Eur J Cancer Prev. 1992;1:51–4. [PubMed] [Google Scholar]
- 31.Bertrand HA, Masoro EJ, Yu BP. Maintenance of glucagon-promoted lipolysis in adipocytes by food restriction. Endocrinology. 1980;107:591–5. doi: 10.1210/endo-107-2-591. [DOI] [PubMed] [Google Scholar]
- 32.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol. 1985;40:657–70. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 33.Toyokuni S, Miyake N, Hiai H, Hagiwara M, Kawakishi S, Osawa T, et al. The monoclonal antibody specific for the 4-hydroxy-2-nonenal histidine adduct. FEBS Lett. 1995;359:189–91. doi: 10.1016/0014-5793(95)00033-6. [DOI] [PubMed] [Google Scholar]
- 34.Uchida K, Sakai K, Itakura K, Osawa T, Toyokuni S. Protein modification by lipid peroxidation products. Formation of malondialdehyde-derived N∈-(2propenal)lysine in proteins. Arch Biochem Biophys. 1997;346:45–52. doi: 10.1006/abbi.1997.0266. [DOI] [PubMed] [Google Scholar]
- 35.Oya T, Hattori N, Mizuno Y, Miyata S, Maeda S, Osawa T, et al. Methylglyoxal modification of protein. Chemical and immunochemical characterization of methylglyoxal–arginine adducts. J Biol Chem. 1999;274:18492–502. doi: 10.1074/jbc.274.26.18492. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed MU, Thorpe SR, Baynes JW. Identification of N6-carboxymethyllise as a degradation product of fructoselysine in glycated protein. J Biol Chem. 1986;261:4889–94. [PubMed] [Google Scholar]
- 37.Hsu S-M, Raine L, Fanger H. Use of avidin–biotin–peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–80. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 38.Tannenbaum A. The initiation and growth of tumors. Introduction. I. Effects of underfeeding. Am J Cancer. 1940;38:335–50. [Google Scholar]
- 39.Tannenbaum A. The dependence of the genesis of induced skin tumors in the caloric intake during different stages of carcinogenesis. Cancer Res. 1944;4:673–79. [Google Scholar]
- 40.Hursting SD, Kari FW. The anti-carcinogenic effects of dietary restriction: mechanisms and future directions. Mutat Res. 1999;443:235–49. doi: 10.1016/s1383-5742(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 41.Ames BN. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983;221:1256–64. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- 42.Weindruch R. Dietary restriction, tumors, and aging in rodents. J Gerontol. 1989;44:B67–71. doi: 10.1093/geronj/44.6.67. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda T, Matsuo T, Kohno S, Tashiro T, Maeda H. Early stage of development of transplacentally induced glioma with ethylnitrosourea in rats. Sequential historadioautographic and electron microscopic studies. Acta Pathol Jpn. 1983;33:237–47. doi: 10.1111/j.1440-1827.1983.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 44.Claisse PJ, Roscoe JP, Lantos PL. Cellular heterogeneity in an ethylnitrosourea-induced glioma: malignancy, karyology and other properties of tumor cell types. Br J Exp Path. 1979;60:209–24. [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida J, Cravioto H. Nitrosourea-induced brain tumors: An In Vivo and In Vitro Tumor Model System. J Natl Cancer Inst. 1978;61:365–74. [PubMed] [Google Scholar]
- 46.Zook BC, Simmens SJ, Jones RV. Evaluation of ENU-induced gliomas in rats: nomenclature, immunohistochemistry, and malignancy. Toxicol Path. 2000;28:193–201. doi: 10.1177/019262330002800124. [DOI] [PubMed] [Google Scholar]
- 47.Yoshino T, Motoi M, Ogawa K. Morphological maturation of tumor cells induced by ethylnitrosourea (ENU) in rat brains. I. On the tumors by administration of ENU in the late gestational stage. Acta Pathol Jpn. 1985;35:1385–96. doi: 10.1111/j.1440-1827.1985.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 48.Yoshino T. Morphological maturation of tumor cells induced by ethylnitrosourea (ENU) in rat brains. II. On the tumors by administration of ENU in the mid-gestational stage. Acta Pathol Jpn. 1985;35:1397–408. doi: 10.1111/j.1440-1827.1985.tb01437.x. [DOI] [PubMed] [Google Scholar]
- 49.Ames BE, Gold LS. Animal cancer test and prevention. Natl Cancer Inst Monogr. 1992;12:125–32. [PubMed] [Google Scholar]
- 50.Trush MA, Kensler TW. An overview of the relationship between oxidative stress and chemical carcinogenesis. Free Radic Biol Med. 1991;10:201–9. doi: 10.1016/0891-5849(91)90077-g. [DOI] [PubMed] [Google Scholar]
- 51.Willett WC, MacMahon B. Diet and cancer – an overview (second of two parts) N Engl J Med. 1984;310:697–703. doi: 10.1056/NEJM198403153101106. [DOI] [PubMed] [Google Scholar]
- 52.Nelson RL. Dietary iron and colorectal cancer risk. Free Radic Biol Med. 1992;12:161–68. doi: 10.1016/0891-5849(92)90010-e. [DOI] [PubMed] [Google Scholar]
- 53.Stevens RG, Nerishi K. Iron and oxidative damage in human cancer. In: Spats L, Bloom AD, editors. Biological consequences of oxidative stress. Implications for cardiovascular disease and carcinogenesis. New York: Oxford University Press; 1992. pp. 138–61. [Google Scholar]
- 54.Yu BP. Aging and oxidative stress: modulation by dietary restriction. Free Radic Biol Med. 1996;21:651–68. doi: 10.1016/0891-5849(96)00162-1. [DOI] [PubMed] [Google Scholar]
- 55.Rice-Evans C, Burdon R. Free radical-lipid interactions and their pathological consequences. Prog Lipid Res. 1993;32:71–110. doi: 10.1016/0163-7827(93)90006-i. [DOI] [PubMed] [Google Scholar]
- 56.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 57.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, et al. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–7. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 58.MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch Biochem Biophys. 1999;366:82–8. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- 59.Zou M, Martin C, Ullrich V. Tyrosine nitration as a mechanism of selective inactivation of prostacyclin synthase by peroxynitrite. Biol Chem. 1997;378:707–13. doi: 10.1515/bchm.1997.378.7.707. [DOI] [PubMed] [Google Scholar]
- 60.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA. 1997;94:10925–30. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediatesan adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci USA. 1994;91:2607–10. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–55. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 63.Was H, Dulak J, Jozkowicz A. Heme oxygenase-1 in tumor biology and therapy. Curr Drug Targets. 2010;11:1551–70. doi: 10.2174/1389450111009011551. [DOI] [PubMed] [Google Scholar]
- 64.Sunamura M, Duda DG, Ghattas MH, Lozonsci L, Motoi F, Yamauchi J, et al. Heme oxygenase-1 accelerates tumor angiogenesis of human pancreatic cancer. Angiogenesis. 2003;6:15–24. doi: 10.1023/a:1025803600840. [DOI] [PubMed] [Google Scholar]
- 65.Powis G, Mustacich D, Coon A. The role of the redox protein thioredoxin in cell growth and cancer. Free Radic Biol Med. 2000;29:312–22. doi: 10.1016/s0891-5849(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 66.Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–71. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 67.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baker A, Payne CM, Briehl MM, Powis G. Thioredoxin, a gene found overexpressed in human cancer, inhibits apoptosis in vitro and in vivo . Cancer Res. 1997;57:5162–7. [PubMed] [Google Scholar]
- 69.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–46. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 70.Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–97. [PubMed] [Google Scholar]
- 71.Kristal BS, Yu BP. An emerging hypothesis: synergistic induction of aging by free radicals and Maillard reactions. J Gerontol. 1992;47:B107–14. doi: 10.1093/geronj/47.4.b107. [DOI] [PubMed] [Google Scholar]
- 72.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–90. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 73.Semenza GL. Targeting HIF-1 for cancer therapy. Nature Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 74.Harris AL. Hypoxia-a key regulatory factor in tumor growth. Nature Rev Cancer. 2001;2:38–46. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 75.Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–7. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 76.Masoro EJ, Yu BP, Bertrand HA. Action of food restriction in delaying the aging process. Proc Natl Acad Sci USA. 1982;79:4239–41. doi: 10.1073/pnas.79.13.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCarter RJM, Palmer J. Energy metabolism and aging: a lifelong study in Fischer 344 rats. Am J Physiol. 1992;263:E448–52. doi: 10.1152/ajpendo.1992.263.3.E448. [DOI] [PubMed] [Google Scholar]