Abstract
Chromatin states can be distinguished by differential covalent modifications of histones or by utilization of histone variants. Chromatin associated with transcriptionally active loci becomes enriched for histones with particular lysine modifications and accumulates the H3.3 histone variant, the substrate for replication-independent nucleosome assembly. However, studies of modifications at particular loci have not distinguished between histone variants, so the relationship among modifications, histone variants, and nucleosome assembly pathways is unclear. To address this uncertainty, we have quantified the relative abundance of H3 and H3.3 and their lysine modifications. Using a Drosophila cell line system in which H3.3 has been shown to specifically package active loci, we found that H3.3 accounts for ≈25% of total histone 3 in bulk chromatin, enough to package essentially all actively transcribed genes. MS and antibody characterization of separated histone 3 fractions revealed that H3.3 is relatively enriched in modifications associated with transcriptional activity and deficient in dimethyl lysine-9, which is abundant in heterochromatin. To explain enrichment on alternative variants, we propose that histone modifications are tied to the alternative nucleosome assembly pathways that use primarily H3 at replication forks and H3.3 at actively transcribed genes in a replication-independent manner.
The wrapping of DNA around nucleosomes creates an impediment to active processes, so nucleosomes are mobilized by chromatin remodeling machines to allow for access to DNA by polymerases and other proteins (1). Mobilization or displacement of nucleosomes at active loci leads to enhanced nuclease accessibility relative to silent chromatin (2). Displaced nucleosomes are replaced by a distinct process of nucleosome assembly that can occur in the absence of DNA replication in vivo (3, 4). This replication-independent process uses only the replacement histone 3 variant, H3.3, not canonical H3, and occurs at transcriptionally active loci in Drosophila cells. For example, active rRNA-encoding DNA arrays accumulate H3.3 throughout the cell cycle, and induction of inactive arrays leads to loss of nucleosomes with silencing modifications and their replacement with H3.3-containing nucleosomes (3). These results were obtained by using GFP-tagged versions of H3.3 and H3 in a cytological assay, which was not able to reveal the dynamics and relative abundance of the endogenous forms of H3.3 and H3. Therefore, it was hard to gauge the importance of replication-independent assembly of H3.3 in contributing to chromatin mobility at active loci.
Other features that distinguish active from silent chromatin include covalent modifications of histones. For the histone 3 class, acetylation of lysines is generally correlated with active chromatin, as is methylation of lysine 4, 36, or 79, whereas methylation of either lysine 9 or 27 is correlated with silent chromatin (5, 6). These correlations have motivated a histonecode hypothesis, whereby some of these modifications are essential, perhaps even causal for activity, and combinations of modifications might differentiate multiple chromatin states (7). However, the basis for establishing patterns of histone modifications and for propagating them through the cell cycle remains unknown.
The correlations between histone modifications and active or silent chromatin have been established by using antibodies (5), but no comparable antibodies are available for distinguishing H3.3 from H3. As a result, studies using modification-specific antibodies have not been able to determine whether variants differ as substrates for modification. Here we address this uncertainty by separating histone 3 variants and determining their relative abundance and modifications. Consistent with the hypothesis that H3.3 marks active chromatin (3), we find that the variant is sufficiently abundant to package essentially all actively transcribed genes. In addition, H3.3 is enriched in modifications known to correlate with active chromatin at specific genes and is deficient in H3K9 dimethylation. Our results suggest that modifications found at active and silent chromatin states are tied to alternative nucleosome assembly pathways, which might help explain the generation of complex modification patterns.
Materials and Methods
Preparation of Histones from Nuclei. Drosophila Kc cells were cultured as described (8), dividing 1:10 into fresh medium every 2-3 days and harvesting 1-6 days afterward. To examine histones during differentiation, β-ecdysone (20-hydroxyecdysone, Sigma) was added to a concentration of 0.06 μg/ml, arresting the rapidly dividing Kc cells and causing them to extend neuron-like projections (9). Nuclei were prepared as described (8), except that the hypotonic medium was supplemented with phosphatase and protease inhibitors (2 mM MgCl2/10 mM Tris·HCl, pH 7.4/1 mM sodium pyrophosphate/2 mM sodium-β-glycerophosphate/10 nM sodium orthovanadate/5 mM sodium fluoride/100 nM microcysteine/0.5 mM PMSF), and cells were disrupted by vortexing with Nonidet P-40 (0.5% vol/vol final concentration). Histones were acid-extracted by diluting the nuclear pellet with H2SO4 to a final concentration of 0.2 M with vigorous vortexing. This was transferred to microcentrifuge tubes, left overnight at 4°C, then centrifuged for 10 min at 14,000 rpm (16,000 × g). The supernatant was saved, the pellet was rinsed in 1/2 volume 0.2 M H2SO4, and the supernatants were pooled. The supernatants were then dialyzed overnight against water or 0.1% trifluoroacetic acid before preparative HPLC. For ecdysone-treated cells, the acid extract was diluted at least 20-fold into 0.1% trifluoroacetic acid.
HPLC Purification. Acid-extracted nuclear protein from no more than 1 ml of Kc cell pellet in 0.1% trifluoroacetic acid was applied to a 250 × 4.6-mm Jupiter C5 column with 300-Å pore size (Phenomenex, Torrance, CA) equilibrated with 70% 0.1% trifluoroacetic acid/30% acetonitrile (70-30%) at 1 ml/min. Histone 3 was resolved by pumping 70-30% for 10 min, ramping to 55-45% over 5 min, and ramping to 45-55% for 1 h, collecting 0.5-ml fractions at 0.5-min intervals from 25-40 min of retention. A second HPLC fractionation was often performed on the H3.3 fraction to enrich it to ≈90% purity. HPLC fractions were analyzed by SDS/PAGE, staining with Coomassie brilliant blue. Fractions chosen for further analysis contained one band of the correct size for the histone 3 class. For MS, histone 3 fractions were either dried by using a Speed-Vac (Gilson) or excised from an SDS/PAGE gel after equilibration with water.
MS. Coomassie-stained gel bands were subjected to “in-gel” proteolytic digestion as described (10), but with two modifications: gel slices were not reduced or alkylated, and digestions were carried out by using Endoproteinase Arg-C (Roche Diagnostics). Dried HPLC purified histone fractions were resuspended in 20 μl of 50 mM ammonium bicarbonate and digested with 100 ng of Endo Arg-C. Digestions were carried out at 37°C for 16 h. A fraction of the digestion mixture (typically one-fifth of the total volume) was concentrated and purified by using a nanoscale column (11), eluting with 0.1% trifluoroacetic acid in 50% acetonitrile (saturated with α-cyano-4-hydroxycinammic acid) directly onto the matrix-assisted laser desorption/ionization (MALDI) target. Positive-ion MALDI-time-of-flight (TOF) data were collected on a Voyager DE-Pro mass spectrometer (Applied Biosystems) operated in the reflectron mode and calibrated externally. The remainder of the digestion solution was used for in-line liquid chromatography electrospray ionization MS (LC-ESI MS) as described for quantitative measurements (12). Ion species corresponding to peptides of interest were analyzed by tandem MS (LC-ESI MS/MS) in a data-dependent mode.
Antibody Analysis. Separated H3 and H3.3 from HPLC fractions were diluted into PBS for slot blot and ELISA analysis, dried, and redissolved in loading buffer for SDS/PAGE (13). Two- or 4-fold dilution series were used to standardize slot blot and ELISA. Blots were probed with antibodies to H3 dimethyl K4 (1:500, Upstate Biotechnology, Lake Placid, NY); H3 trimethyl K4 (1:500, Abcam, Cambridge, U.K.); H3 dimethyl K9 (1:300, Upstate Biotechnology); H3 acetyl K9 (1:300, Upstate Biotechnology); H3 acetyl K14 (1:300, Upstate Biotechnology); and H3 dimethyl K79 (1:1,000, gift of Fred van Leeuwen, Fred Hutchinson Cancer Research Center, Seattle), and then incubated with anti-rabbit IgG2 horseradish peroxidase-(HRP) conjugated Fab2 fragment (Amersham Pharmacia Life Sciences). Bands were detected on film by chemiluminescence (ECL Western Blotting Detection Reagents, Amersham Pharmacia Biosciences). For ELISA, 96-well plates were probed with antidimethyl K4 at 1:1,000, antitrimethyl K4 at 1:500, antidimethyl K9 at 1:500, antiacetyl K9 at 1:250, antiacetyl K14 at 1:250, and antidimethyl K79 at 1:2,000. Plate wells were then treated with anti-rabbit HRP conjugate at 1:2,000 and visualized by chromogenic detection (ABTS microwell peroxidase substrate, Kirkegaard & Perry Laboratories). The signal was quantified in a plate reader. Signal curves were compared within the linear range for both proteins to determine the fold enrichment on H3.3 for each modification.
Results
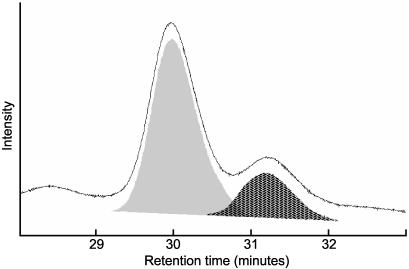
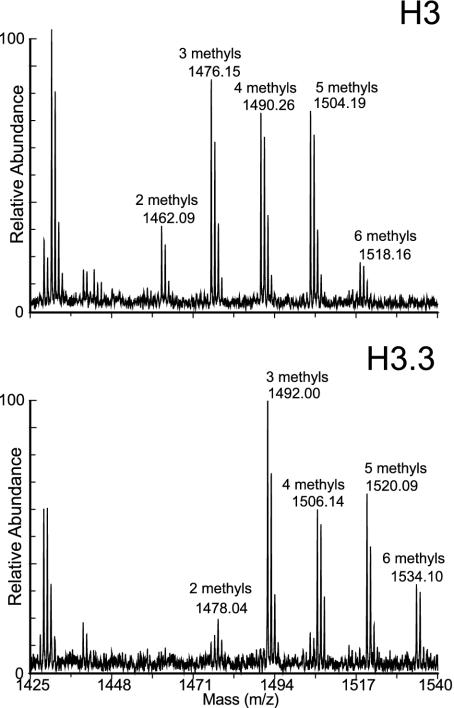
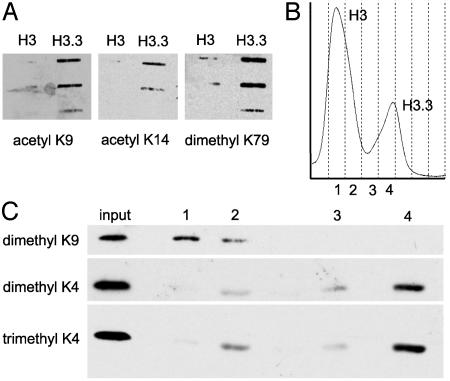
Relative Abundance of H3 and H3.3 in Kc Cells. Until recently, H3.3 was thought to be interchangeable with H3, because there are only four amino acid differences between them. However, our demonstration that H3.3 is found at active loci (3) raises the possibility that all active chromatin is packaged into H3.3-containing nucleosomes. To test whether H3.3 is sufficiently abundant, we used a separation method that would resolve the two variants. HPLC on a reverse-phase column has previously been shown to separate histone 3 variants in plants, despite the abundant tail modifications and the close similarity between the variant sequences (14). When acid-extracted histones from Drosophila Kc nuclei were resolved by reverse-phase HPLC, all H3 histones were found in two partially overlapping peaks well separated from other acid-extractable material (Fig. 1). We pooled peak fractions and subjected them to complete proteolysis with Arg-C protease and MALDI-TOF MS to identify the two HPLC peaks. We were able to identify eight of the 10 expected peptides >500 Da and their known modified forms (15, 16) based on their predicted masses. Examination of peptide 27-40 revealed a 16-Da difference between corresponding mass peaks, identifying the earlier and larger HPLC peak as H3, which has an alanine at position 31, and the later and smaller peak as H3.3, which has a serine at position 31 (Fig. 2).
Fig. 1.
Histone variants are resolved by HPLC separation. Acid-extracted histones from Kc cells were injected onto a reverse-phase HPLC column in 30% acetonitrile/0.1% trifluoroacetic acid. All histone 3 eluted in the two peaks shown here, and the peaks were determined to be composed of pure histone 3 by SDS/PAGE and Coomassie staining. The relative abundance of the two peaks was determined by integrating the area under each curve (gray and hatched areas) and averaging over multiple measurements.
Fig. 2.
Identification of HPLC peaks by MALDI-TOF spectrometry of peptide 27-40. Pooled fractions from early and late histone 3 HPLC peaks were digested to completion with ArgC protease and subjected to MALDI-TOF MS. Peptides were identified based on their masses, which are indicated above the peaks. Peptide 27-40 contains an alanine at position 31 in H3, and serine in H3.3, for a 16-Da difference. The early HPLC peak (Upper) is composed of H3, and the late peak (Lower) is composed of H3.3. Five modified forms, with 2, 3, 4, 5, and 6 methyl groups, were identified on both H3 and H3.3.
To ascertain the relative abundance of H3 and H3.3, we integrated peak areas, and found that H3.3 accounts for about one-fourth of the histone 3 in Kc cells (24 ± 3%, n = 11, Fig. 1). We can compare the abundance of H3.3 in Kc cells to the percentage of the genome that is estimated to be transcribed in the same cells. Microarray analysis has revealed that 68% of a large sample of genes are transcribed in Kc cells (17), which have a tetraploid female karyotype (9). The female Drosophila genome is estimated to encompass 176 Mb (18), of which ≈59 Mb is heterochromatin and 49 Mb is annotated as intergenic (19). This yields an overall estimate of ≈25% of the genome transcribed in Kc cells [0.68 × (176-59-49)/176 = 26%], remarkably close to the relative abundance of H3.3.
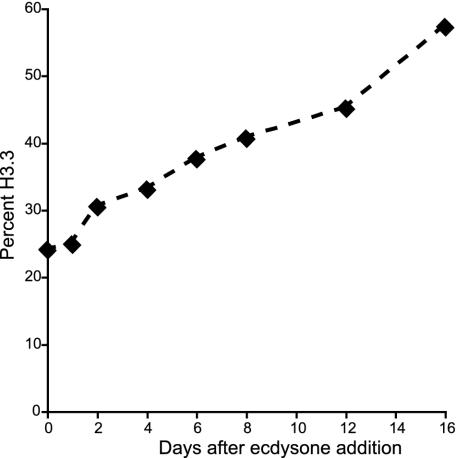
H3.3 is known to increase in abundance in nondividing cells in vertebrates (20, 21), presumably because H3 is not deposited outside of S phase, whereas H3.3 is deposited constitutively. We asked whether replication-independent assembly of H3.3 in Kc cells (3) also leads to accumulation of H3.3 when cells are induced to exit the cell cycle and differentiate. Kc cells are known to cease dividing and differentiate in response to the moulting hormone, ecdysone, and so we added ecdysone and harvested cells at various time intervals up to 16 days. Indeed, a gradual increase from 24% to >50% H3.3 was seen (Fig. 3), whereas untreated cells remained close to 25% H3.3 as they reached stationary phase over a 6-day period. Thus, the close correspondence between H3.3 abundance and active transcription does not apply to differentiating cells, where accumulation of H3.3 likely represents replacement of nucleosomes that are lost (20, 21). The increase of endogenous H3.3 levels in differentiating Kc cells confirms our previous results showing replication-independent deposition of tagged H3.3 introduced into this cell line (3).
Fig. 3.
H3.3 levels increase during Kc cell differentiation after ecdysone addition. After the indicated time at 25°C, histones were extracted and analyzed by HPLC. Curves were fit to HPLC traces (see Fig. 1), and peak areas were measured and averaged for two experiments.
Differential Enrichment of Covalent Modifications on H3 and H3.3 Detected by MS. The deposition of H3.3 at active loci in Kc cells (3), taken together with our demonstration that H3.3 is sufficiently abundant to account for all active transcription (Fig. 1), is consistent with the notion that H3.3-containing nucleosomes comprise all active chromatin in dividing cells. If this were the case, we would expect covalent histone 3 modifications that have been correlated with active transcription to be enriched on H3.3 relative to H3. Our separation of these two forms allowed for identification and quantification of their modifications.
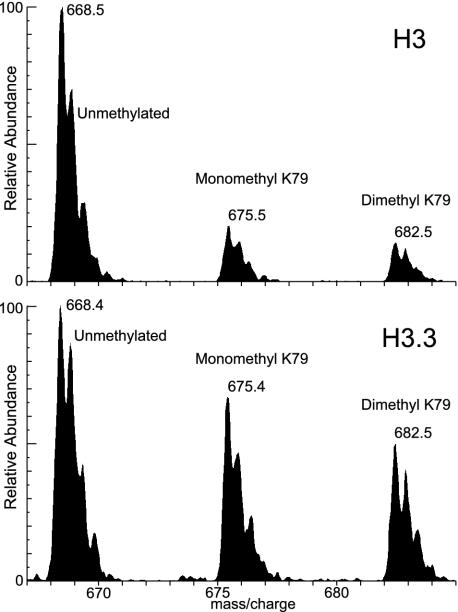
We quantified modifications on the variants using LC-ESI MS on ArgC-digested peptides. The area of each LC-ESI MS peak was measured and expressed as the ratio of that modified form to the total areas of all of the modified forms of that peptide. Fig. 4 shows an example of LC-ESI MS data for peptide 73-83 in both H3 and H3.3. This peptide can be methylated at lysine 79, which has been associated with active chromatin regions (12). Consistent with our expectation that active modifications would be enriched on H3.3, we find that dimethylated H3.3K79 is enriched 2-fold on H3.3 relative to H3 (Fig. 5A). H3.3 was also enriched for monomethyl K79, whereas trimethylation of this residue, known to occur on active chromatin in budding yeast (12), was not observed in Kc cells on either histone.
Fig. 4.
Identification of modified forms by LC-ESI MS. Pooled fractions from the H3 or H3.3 HPLC peaks were digested to completion with the ArgC protease and subjected to LC-ESI MS. Peptides and their modified forms were identified by their masses, as indicated above the peaks. For peptide 73-83, doubly charged ions of unmethylated, monomethylated, and dimethylated forms were found in both the H3 and H3.3 spectra, corresponding to methylation at lysine 79. H3.3 is enriched for lysine 79 methylation.
Fig. 5.
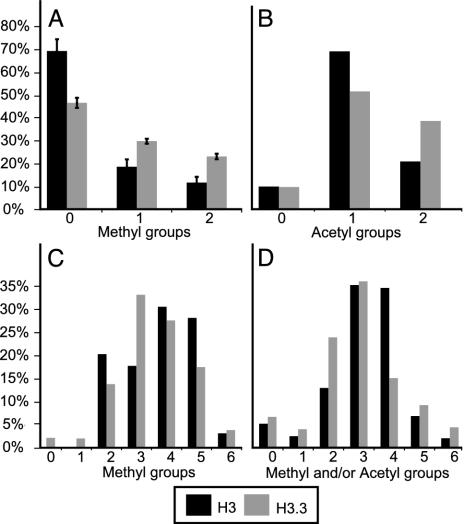
LC-ESI MS determinations of relative abundances of lysine modifications on H3 and H3.3. Histograms show the averages for two to four experiments. Percentages of H3 (black bars) or H3.3 (gray bars) are shown for each modification. (A) Methyls on K79 (peptide 73-83), integrating areas under the first three isotopic peaks (e.g., Fig. 4). (B) Acetyl groups on K18 and/or K23 (peptide 18-26). (C) Methyl groups on K27, K36 and/or K37 (peptide 27-40). (D) Methyl groups and/or acetyl groups on K9 and/or K14 (peptide 9-17).
Active chromatin is also correlated with the hyperacetylation of histone tails (22, 23). Two acetylation sites are known to be present on peptide 18-26, at lysines 18 and 23. LC-ESI MS shows that H3.3 has more total acetylation, with more diacetyl (both 18 and 23) and less mono-acetyl (either 18 or 23, but not both) than H3 (Fig. 5B). Therefore, modifications to lysine residues in both the core (K79) and tail (K18 and 23) that correlate with active chromatin are enriched on H3.3.
Other peptides had too many possible modifications on lysines to readily ascertain relative abundances. Nevertheless, we were able to determine the spectrum of modifications by using tandem MS, in which individual ions are collected, fragmented, and reanalyzed, to assign modifications to specific lysines. In this way, we found that peptide 27-40 displays complex methylation patterns for both H3 and H3.3, with different peptides containing 0, 1, 2, or 3 methyl groups on K27, combined with 0, 1, or 2 methyl groups on K36 and K37 (Table 1). These complex patterns precluded our assignment of specific modifications to particular peptide masses, where LC-ESI MS spectra showed 0-6 methyl groups overall (Fig. 5C). Likewise, we identified numerous and abundant modifications on peptide 9-17, including 0-3 methyl groups and/or single acetyl groups on K9 and K14, with only low levels of unmodified peptide 9-17 (Fig. 5D).
Table 1. Summary of modifications detected in this study.
| Residue | Modification | H3 | H3.3 |
|---|---|---|---|
| K4 | Monomethyl | + | + |
| Dimethyl | + | ++* | |
| Trimethyl | + | ++* | |
| K9 | Monomethyl | + | + |
| Dimethyl | ++* | + | |
| Trimethyl | |||
| Acetyl | + | ++* | |
| K14 | Monomethyl | + | + |
| Dimethyl | + | + | |
| Trimethyl | |||
| Acetyl | + | ++* | |
| K18 + K23 | Acetyl | + | ++† |
| K27 | Monomethyl | + | + |
| Dimethyl | + | + | |
| Trimethyl | + | + | |
| K36 | Monomethyl | + | + |
| Dimethyl | + | + | |
| Trimethyl | |||
| K37‡ | Monomethyl | + | + |
| Dimethyl | + | + | |
| Trimethyl | |||
| K79 | Monomethyl | + | ++*† |
| Dimethyl | + | ++*† | |
| Trimethyl |
+, observed; ++, relatively enriched.
Quantified by ELISA
Quantified by MS
Axel Imhof, personal communication
Our detection of abundant lysine modifications on H3 and H3.3 tails contrasts with our inability to detect any phosphorylated serines or threonines. This failure to detect phosphates does not appear to have resulted from their removal during acid extraction, because salt-extracted and SDS gel-purified histone 3 that was not exposed to acidic pH yielded MALDI-TOF spectra that were indistinguishable from those obtained by acid extraction followed by HPLC and/or SDS gel purification (data not shown). We also found that phosphatase treatment (24) before a second round of HPLC did not alter the mass spectra (data not shown). Furthermore, it is unlikely that changes occurred during nuclear isolation, because histones extracted from whole cells produced H3 and H3.3 spectra that were indistinguishable from those obtained from nuclei. Therefore, it appears that phosphates on H3 and H3.3 are not sufficiently abundant in Kc cells to be detected in bulk chromatin, and that our isolation procedures had no effect on modification patterns. Our inability to detect phosphates by MS might simply be that phosphates on histones, such as H3S10 phosphate, are restricted to regulatory regions at interphase (25), and to mitotic chromosomes (26), so would be undetectable in bulk histone 3 by our methods. Phosphates on histone 3 have been seen in bulk chromatin only after in vivo inhibition of phosphatases by okadaic acid (24) or after colchicine treatment, which arrests Kc cells after mitosis (unpublished results).
Differential Enrichment of Covalent Modifications on H3 and H3.3 Detected by Using Antibodies. The limitations of peptide mass analysis that we encountered in trying to quantify certain modifications, including complexity and low abundance, led us to use modification-specific antibodies to further examine correlations between histone 3 subtypes and covalent modifications. Indeed, the links between particular modifications and active vs. inactive chromatin that motivated our study have been almost entirely based on such antibodies.
We first used slot and Western blot analyses to look for modification differences between H3 and H3.3. Fig. 6A shows an example of a slot blot in which equal amounts of HPLC-purified H3 and H3.3 were applied and probed with antiacetyl-K9, antiacetyl-K14, and antidimethyl-K79. All three of these modifications are enriched in active chromatin, and it is clear that all three are enriched on H3.3. Fig. 6B shows an example of an HPLC spectrum, where individual fractions were resolved by SDS/PAGE, transferred to nitrocellulose, and the blot was probed successively by using antidimethyl-K9, antidimethyl-K4, and antitrimethyl-K4. Dimethyl-K9 is known to be enriched in silent chromatin, and it is clearly enriched on H3. Di- and trimethyl-K4 are known to be enriched in active chromatin, and they are clearly enriched on H3.3. Such enrichments were also seen by LC-ESI MS (data not shown).
Fig. 6.
Differential enrichment of modifications on H3 and H3.3 detected by using antibodies. (A) Examples of “active” modifications detected by using antibodies to probe slot blot filters after applying equal protein amounts of H3 and H3.3 from pooled fractions, ascertained by Coomassie blue staining of gel samples (not shown). Two-fold dilutions from top to bottom are shown for acetyl-K9 and dimethyl-K79, and 4-fold dilutions are shown for acetyl-K14 modifications. (B) HPLC trace showing fractions (designated 1-4) used for the Western blot analysis in C, where acid-extracted histone from the column input was loaded in the first lane, and HPLC fractions in equal volumes (protein amounts differ) were loaded in remaining lanes. The blot was successively probed with antibodies to the indicated modifications.
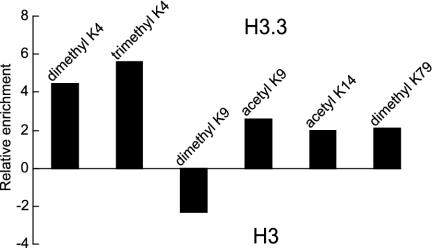
We used ELISA to more effectively quantify the enrichment or depletion of specific modifications on H3.3 relative to H3. We found that H3.3 is enriched in both di- and trimethyl-K4 ≈5-fold and in dimethyl-K79, acetyl-K9, and acetyl-K14 ≈2-fold. H3.3 is depleted in dimethyl-K9 ≈2-fold (Fig. 7). These enrichments are unlikely to be caused by differential binding to the variants, because none of the sites assayed are close to sequence differences between H3 and H3.3. Therefore, these well established “silent” and “active” modifications correlate with H3 and H3.3, respectively, consistent with the hypothesis that active chromatin consists of H3.3-containing nucleosomes (3).
Fig. 7.
Relative enrichment of modifications on H3.3 vs. H3 determined by ELISA. Histogram bars show enrichment on H3.3 (above zero) or on H3 (below zero) of each indicated modification.
Discussion
Active and silent chromatin have been distinguished both by differential histone modification and by the incorporation of alternative histone 3 variants. The lack of antibodies able to distinguish H3.3 from H3 has left open the question of whether modifications are specific for these alternative variants. We have shown that modifications are indeed enriched on H3.3 or H3 in accordance with previously demonstrated associations with active or silent chromatin. Specifically, we found that “active” modifications, including methylated K4 and K79 and acetylated K9, K14, and K18 + K23, are enriched on H3.3, and a “silent” modification, methylated K9, is enriched on H3. Similarly, Waterborg (14) showed enrichment of “active” modifications in alfalfa replacement histone 3, even before they were found to correlate with activity of specific loci. Therefore, our results using a Drosophila system appear to be general for plants and animals.
In no case were modifications found to be exclusive to H3.3 or H3 (Table 1). All sites of known modification are shared between the two variants. Furthermore, none of the modified residues for which we saw differential enrichment is close to any of the four sequence differences. Therefore, binding-site preferences of modification enzymes are unlikely to account for these differences. Rather, alternative nucleosome assembly pathways may be responsible, whereby modifying enzymes would be primarily associated with alternative assembly machines (27). These machines are separated in both space and time, with replication-coupled assembly depositing primarily H3 and replication-independent assembly depositing exclusively H3.3 (3). If modification enzymes are preferentially associated with alternative assembly machines, they could efficiently propagate the modification status of chromatin. “Silent” modifications would occur as the replication fork passes through, whereas “active” modifications would occur as H3.3-containing nucleosomes are assembled at transcriptionally active loci throughout the cell cycle.
The active modification of histone 3 and the replication-independent deposition of H3.3 may be independent events. For example, the association of both processes with transcription could explain their correlation. However, the possibility that modifications are primarily associated with alternative assembly pathways not only can account for this correlation but also can reconcile discrepancies in the burgeoning histone modification literature. A case in point is a finding by Labrador and Corces (25) that challenges the assumption that a modification classified as active by chromatin immunoprecipitation is relevant to gene activity. They showed that H3K14 acetylation is enriched in polytene bands in Drosophila, rather than in interbands, which are sites of active transcription. We note that the polytene banding pattern reflects the chromatin density, so any marker that is uniformly distributed in chromatin will be enriched in bands. The enrichments that we saw for active modifications on H3.3 ranged from 2- to 5-fold, increases not likely to be sufficient to show an interband pattern even if H3.3 packages all active chromatin. Thus, the polytene banding pattern seen for H3K14 acetyl is consistent with its 2-fold enrichment on H3.3. Likewise, the widespread euchromatic localization of H3K4 dimethylation on polytene chromosomes (28), rather than a band-interband pattern, is consistent with its 5-fold enrichment on H3.3. Therefore, enrichment of active modifications on H3.3 can explain both the enrichment of these modifications on active chromatin detected by chromatin immunoprecipitation (29) and the localization patterns seen on polytene chromosomes.
The failure of stable modification patterns to correlate with transcription and silencing genome-wide (25, 28) makes them unlikely candidates for the propagation of epigenetic information. Rather, the nucleosome assembly process provides a straightforward mechanism for inheritance of an active chromatin state through multiple rounds of cell division (30, 31). H3.3 deposits at active regions (3), and we have shown that its abundance corresponds closely to the extent of transcription; therefore, modifications that generally follow H3.3 deposition patterns would be classified as active. This active chromatin would be diluted 2-fold by new H3-containing nucleosomes during DNA replication, but the remaining H3.3 might be enough to maintain the active state. Reinitiation of transcription would lead to replication-independent replacement, restoring H3.3-containing nucleosomes with their active modifications over entire transcription units.
Acknowledgments
We thank Brian Milless for performing HPLC separations, Xiaohong Wang and Elizabeth Wayner for help with ELISAs, Pavel Hradecky for gene and intergene size information, and Axel Imhof for unpublished information alerting us to the presence of methylated H3K37. E.M. was supported by a Howard Hughes Medical Institute predoctoral fellowship.
Abbreviations: MALDI-TOF, matrix-assisted laser desorption/ionization-time-of-flight; LC-ESI, liquid chromatography electrospray ionization.
See Commentary on page 1429.
References
- 1.Workman, J. L. & Kingston, R. E. (1998) Annu. Rev. Biochem. 67, 545-579. [DOI] [PubMed] [Google Scholar]
- 2.Weintraub, H. & Groudine, M. (1976) Science 193, 848-856. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad, K. & Henikoff, S. (2002) Mol. Cell 9, 1191-1200. [DOI] [PubMed] [Google Scholar]
- 4.Smith, M. M. (2002) Mol. Cell 9, 1158-1160. [DOI] [PubMed] [Google Scholar]
- 5.Turner, B. M. (2002) Cell 111, 285-291. [DOI] [PubMed] [Google Scholar]
- 6.Richards, E. J. & Elgin, S. C. (2002) Cell 108, 489-500. [DOI] [PubMed] [Google Scholar]
- 7.Strahl, B. D. & Allis, C. D. (2000) Nature 403, 41-45. [DOI] [PubMed] [Google Scholar]
- 8.Henikoff, S., Ahmad, K., Platero, J. S. & van Steensel, B. (2000) Proc. Natl. Acad. Sci. USA 97, 716-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Echalier, G. (1997) Drosophila Cells in Culture (Academic, New York), 1st Ed.
- 10.Shevchenko, A., Wilm, M., Vorm, O. & Mann, M. (1996) Anal. Chem. 68, 850-858. [DOI] [PubMed] [Google Scholar]
- 11.Gobom, J., Nordhoff, E., Mirgorodskaya, E., Ekman, R. & Roepstorff, P. (1999) J. Mass Spectrom. 34, 105-116. [DOI] [PubMed] [Google Scholar]
- 12.van Leeuwen, F., Gafken, P. R. & Gottschling, D. E. (2002) Cell 109, 745-756. [DOI] [PubMed] [Google Scholar]
- 13.Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A. & Struhl, K., eds. (1994) Current Protocols in Molecular Biology (Wiley, New York).
- 14.Waterborg, J. H. (1990) J. Biol. Chem. 265, 17157-17161. [PubMed] [Google Scholar]
- 15.Zhang, K., Tang, H., Huang, L., Blankenship, J. W., Jones, P. R., Xiang, F., Yau, P. M. & Burlingame, A. L. (2002) Anal. Biochem. 306, 259-269. [DOI] [PubMed] [Google Scholar]
- 16.Zhang, L., Eugeni, E. E., Parthun, M. R. & Freitas, M. A. (2003) Chromosoma 112, 77-86. [DOI] [PubMed] [Google Scholar]
- 17.Schubeler, D., Scalzo, D., Kooperberg, C., van Steensel, B., Delrow, J. & Groudine, M. (2002) Nat. Genet. 32, 438-442. [DOI] [PubMed] [Google Scholar]
- 18.Hoskins, R. A., Smith, C. D., Carlson, J. W., Carvalho, A. B., Halpern, A., Kaminker, J. S., Kennedy, C., Mungall, C. J., Sullivan, B. A., Sutton, G. G., et al. (2002) Genome Biol. 3, RESEARCH0085. [DOI] [PMC free article] [PubMed]
- 19.Misra, S., Crosby, M. A., Mungall, C. J., Matthews, B. B., Campbell, K. S., Hradecky, P., Huang, Y., Kaminker, J. S. & Millburn, G. H. (2002) Genome Biol. 3, RESEARCH0083. [DOI] [PMC free article] [PubMed]
- 20.Urban, M. K. & Zweidler, A. (1983) Dev. Biol. 95, 421-428. [DOI] [PubMed] [Google Scholar]
- 21.Pina, B. & Suau, P. (1987) Dev. Biol. 123, 51-58. [DOI] [PubMed] [Google Scholar]
- 22.Allfrey, V. G., Faulkner, R. M. & Mirsky, A. E. (1964) Proc. Natl. Acad. Sci. USA 51, 786-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waterborg, J. H. (2002) Biochem. Cell Biol. 80, 363-378. [DOI] [PubMed] [Google Scholar]
- 24.Galasinski, S. C., Louie, D. F., Gloor, K. K., Resing, K. A. & Ahn, N. G. (2002) J. Biol. Chem. 277, 2579-2588. [DOI] [PubMed] [Google Scholar]
- 25.Labrador, M. & Corces, V. G. (2003) Genes Dev. 17, 43-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendzel, M. J., Wei, Y., Mancini, M. A., Van Hooser, A., Ranalli, T., Brinkley, B. R., Bazett-Jones, D. P. & Allis, C. D. (1997) Chromosoma 106, 348-360. [DOI] [PubMed] [Google Scholar]
- 27.Vermaak, D., Ahmad, K. & Henikoff, S. (2003) Curr. Opin. Cell Biol. 15, 266-274. [DOI] [PubMed] [Google Scholar]
- 28.Byrd, K. N. & Shearn, A. (2003) Proc. Natl. Acad. Sci. USA 100, 11535-11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bulger, M., Schubeler, D., Bender, M. A., Hamilton, J., Farrell, C. M., Hardison, R. C. & Groudine, M. (2003) Mol. Cell. Biol. 23, 5234-5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goll, M. G. & Bestor, T. H. (2002) Genes Dev. 16, 1739-1742. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad, K. & Henikoff, S. (2002) Proc. Natl. Acad. Sci. USA 99, Suppl. 4, 16477-16484. [DOI] [PMC free article] [PubMed] [Google Scholar]