Abstract
Aims: Intermittent access (IA) to an alcohol (ethanol) solution can lead rats to higher ethanol intakes than continuous access, and a recent report showed increased drinking in C57BL/6J mice offered 20% ethanol vs. water 3X/week (Prior studies have offered ethanol during 24 h periods, either continuously or intermittently.). Methods: We tested the high-preference C57BL/6J inbred mice: we also studied High Drinking in the Dark (HDID) mice, a line we have selectively bred to reach intoxicating blood ethanol levels after a short period of access to a single bottle of 20% ethanol. Results: Neither HDID or C57BL/6J male mice offered ethanol every other day during only a 4-h access period showed greater daily intake than mice offered ethanol daily for 4 h. There was a small increase in drinking with 24 h IA in C57BL/6J mice. An experiment with HDID mice and their control heterogeneous stock stock modeled closely after a published study with C57BL/6J mice (Hwa, Chu, Levinson SA et al. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res 2011;35:1938–1947) showed no significant elevation with 24 h IA exposure in either sex of any genotype. Finally, a near replication of the Hwa et al. study showed modestly greater intake in C57BL/6J mice, confirming the efficacy of 24 h IA. Conclusion: We conclude that 4 h of IA is likely insufficient to elevate drinking in mice. The lack of effect in HDID mice and their controls further suggests that not all genotypes respond to intermittency.
INTRODUCTION
Amit et al. (1970) and Amit and Stern (1971) studied the effects of hypothalamic stimulation on ethanol preference drinking in male Wistar rats by first exposing animals to a daily choice between escalating concentrations of ethanol vs. water for 30 days with continuous access (CA). When rats were thereafter offered ethanol every other day (EOD), their g/kg/day intake escalated over time. Although there were no control groups offered CA after 30 days, this suggested that intermittent access (IA) promoted escalations in intake. Studies performed by Matt Wayner offered rats a two-bottle preference and then switched them to ethanol IA-EOD or once per 3 days. These animals increased their intakes during the final, IA period (Wayner and Greenberg, 1972; Wayner et al., 1972). Neither of these studies had a concurrently tested CA control group, and all animals had long prior experience with ethanol before being offered IA.
Wise (1973) was the first explicitly to study regular, intermittent exposure to ethanol with the intent of seeing whether animals drank more under IA than CA conditions. Adult male Wistar rats were either forced to drink 20% ethanol as their only fluid for 30 days or were offered only 20% ethanol or only water on alternate days. Other groups were offered ethanol either EOD or daily, but always had the choice of water. A fifth group was offered intermittent choice, but had previously been exposed to a choice between ethanol and water EOD for 36 days as the ethanol concentration was gradually increased to 20%. In this study, all the IA groups came to drink more 20% ethanol than the respective groups with CA. Prior experience did not affect the intake eventually attained, and it did not matter whether IA was forced or with the choice of water available.
Several groups have subsequently adapted this method (e.g. Pinel and Huang, 1976; Pinel et al., 1976). Multiple genotypes of (usually, male) rats have shown this effect. The effect holds for concentrations of ethanol between 7 and 20%, and it does not require either prior experience, gradual acclimation to increasing concentrations of ethanol, or a choice of water. Nearly all such studies have employed 24 h access periods, and used either EOD or Monday–Wednesday–Friday (MWF) schedules of IA. There do not appear to be major differences between EOD and MWF schedules, and the effect of intermittency may obtain when employed at intervals as long as once each 4 days (see Discussion section).
The procedure was recently reported to be effective in male C57BL/6J mice (Hwa et al., 2011). These investigators offered MWF access to naive mice to ascending ethanol concentrations of ethanol in water with a choice of water always available, followed by 20% ethanol vs. water for 3 weeks. The IA group ingested an average of nearly 24 g/kg/day, while the CA group averaged nearly 16 g/kg/day. Females given the same schedule of IA showed a similar pattern, with intakes reaching 32 g/kg/day: there was no CA comparison group of females. Another recent study with C57BL/6J male mice showed that adult mice drinking 15% ethanol EOD in 24 h sessions reached intakes of 12–15 g/kg/day while the CA group drank ∼8 g/kg/day; adolescents given EOD achieved higher intakes than adults on this schedule (Melendez, 2011). Regardless of access schedule, about half of the intake occurred during the first 6 h of the dark period (Melendez, 2011).
We have recently selectively bred mice to reach high blood ethanol concentrations (BECs) by offering them limited access to a single bottle of 20% ethanol during their circadian dark period. The drinking in the dark paradigm usually employs three daily access periods of 2 h followed by a fourth session of 4 h (Rhodes et al., 2005). For selective breeding, the protocol is abbreviated to a single day of 2 h access; the second day offers 4 h access, immediately after which a BEC is determined. The High Drinking in the Dark (HDID) line achieves BECs greater than 1.0 mg/ml and becomes intoxicated, while the unselected (heterogeneous stock) HS/Npt control line drinks much less and achieves BECs of ∼0.3 mg/ml (Crabbe et al., 2009, and unpublished data). HDID mice do not show greater two-bottle preference for ethanol solutions than HS/Npt during a standard preference drinking paradigm with acclimation. However, they do gradually develop greater preference after many weeks of exposure during daily 2 h limited access sessions in the dark (Crabbe et al., 2011). We hypothesized that HDID might be more susceptible than HS to intermittent availability of ethanol solutions. We were also interested in whether greater intake with IA would be seen during limited access sessions, a paradigm that had not been rigorously tested. For these studies, we employed both HDID and C57BL/6J mice.
MATERIALS AND METHODS
Animals and husbandry
All studies were conducted in the Veterinary Medical Unit of the Portland VA Medical Center, an AAALAC-approved facility. Male and female mice were housed 2–5 to a standard polycarbonate or polysulfone (used interchangeably) cage with Bed-o Cob bedding changed weekly. For animals raised in Portland, cages were individually ventilated in Thoren systems racks and animals had ad libitum access to food and water during the tests. Animals were moved to a reversed light-dark cycle and to conventional flat racks 2 weeks before testing, with lights on from 2230 to 1030 or 2200 to 1000 (Experiments 2 and 3). Temperature was maintained at 21 ± 1°C. All animals had constant access to food (Purina 5001), and all animals except those in Experiment 1 had constant access to tap water.
Male and female HDID mice from two replicate selectively bred lines (HDID-1 and HDID-2) were tested as well as their non-selected control heterogeneous stock, HS/Npt (for description of these stocks, see Crabbe et al., 2009). Mice were bred in-house and weaned into same-sex groups at 21 days. Male and female C57BL/6J mice were purchased from The Jackson Laboratory (Davis, CA, USA—Experiments 2 and 3; Bar Harbor, ME, USA— Experiment 5) at 8 weeks of age and allowed to acclimate to the facility for 1 week before the start of testing. All mice were naive and between 48 and 81 days old at the start of testing. All procedures were approved by the local Institutional Animal Care and Use Committee (Protocol Number 2878-11) and adhered to guidelines in the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
Drugs and blood ethanol assays
Ethanol was obtained from Decon Laboratories (King of Prussia, PA, USA). For drinking studies, 200 proof ethanol was added to tap water to the stated concentration(s), all mixed v/v on the day of testing. Blood samples were drawn by removing 20 μl blood from the peri-orbital sinus and were assayed by a gas chromatographic method described elsewhere (Rustay and Crabbe, 2004).
Drinking procedures
During each experiment, fluids were offered from 25 ml tubes fitted with a steel sipper spout with an orifice of 3 mm. In Experiment 1, only a single, 10 ml tube, fitted with a stainless steel sipper spout with a 6 mm orifice with a ball bearing, was offered. For Experiments 2–5, two tubes were offered, one always containing tap water. After all mice had been offered their daily fluid (for 4 or 24 h, depending on the study), all tubes were read. At the end of the drinking access period of 4 or 24 h, all tubes were read, and then both the ethanol tube and the water tubes were replaced with water tubes (IA groups) or ethanol and water tubes (CA groups). The new tube levels were then read. The position of the tube containing ethanol relative to the side of the cage top was switched EOD for the CA groups and every day for the IA groups in Experiments 2 and 3. The position of the tube containing ethanol was switched each day ethanol was presented for Experiments 4 and 5. That is, position of the ethanol tube was switched MWF for IA groups and every day for CA groups.
Statistics
Data were analyzed by t-test or repeated-measures analysis of variance as appropriate. Statistical analyses were generally based on the weekly average across 3 days of intermittent exposure, or the same 3 days’ data for CA groups (see specific experiments).
Experiment 1. IA vs. CA for 4 h/day in HDID mice
This experiment was designed to see whether IA would increase ethanol drinking using a limited access paradigm during the circadian dark. Naive male HDID-1 mice from the 15th selected generation were divided into two groups of 19 mice each. All mice were first given a variant of the standard, single-bottle drinking in the dark test employed for selective breeding. Drinking each day was timed starting 3 h after the start of the circadian dark cycle. On the first day, a Wednesday, all mice had their water bottle replaced with a bottle of 20% (v/v) ethanol in water for 2 h. On the second day, the ethanol bottle was again offered for 2 h. Starting on Day 3, CA groups were given the single bottle of 20% each day for 4 h, while the IA groups were given 4 h access each Monday, Wednesday and Friday. Blood samples were taken immediately after the drinking session on the last day (Day 31).
Experiment 2. IA vs. CA for 4 h/day in C57BL/6J mice
Because we saw limited effects of 4 h IA in Experiment 1, we sought to assess restricted IA in a genotype known to respond to intermittency. Because most previously published studies employed a choice between ethanol and water, all mice in this experiment were offered a choice of water at all times. Otherwise, this study followed the design of Experiment 1. C57BL/6J male mice were divided into two groups of 12 mice each. After the first 2 days of 2 h access, the CA group was offered 4 h access to 20% ethanol each day for 17 more days starting 3 h after lights off. The IA group was offered ethanol only on MWF each week. BEC was not assessed in this experiment.
Experiment 3. IA vs. CA for 24 h/day in C57BL/6J mice
This study followed the design of Experiment 2 with the exception that groups of 12–13 adult C57BL/6J male mice were offered 24 h access to 20% ethanol each day (CA group) for 19 days starting 3 h after lights off. The IA group was offered ethanol only on MWF each week. BEC was not assessed in this experiment.
Experiment 4. IA vs. CA for 24 h/day in HDID and HS mice
The HDID selection is replicated. Thus, there are two independently selected HDID lines, HDID-1 and HDID-2. HDID-1 mice were from selection generation S21, and HDID-2 mice from generation S14. The HS mice were from filial generation G69. We employed both male and female mice. All mice had a choice of water at all times. CA groups received ethanol each day, while IA groups received ethanol only on MWF each week. Both IA and CA groups were acclimated gradually to ethanol in this experiment, which started on a Monday. On Day 1 (or Days 1 and 2) animals were offered 3% ethanol. On Day 3 (or Days 3 and 4), the concentration was 6%. On Friday (or Days 5, 6 and 7) the concentration was increased to 10%. From Day 8 until the end of the study on Day 24, the concentration of ethanol offered was 20%. A 20 μl blood sample was taken from the peri-orbital sinus at the end of drinking on Day 24.
Experiment 5. Replication of Hwa et al.
To insure that our procedures were effective, this experiment attempted to replicate the conditions described by Hwa et al. (2011), including the source of mice. Male and female C57BL/6J mice were treated as described for Experiment 4. Blood samples were not taken at the end of this study.
RESULTS
Experiment 1. IA vs. CA for 4 h/day in HDID mice
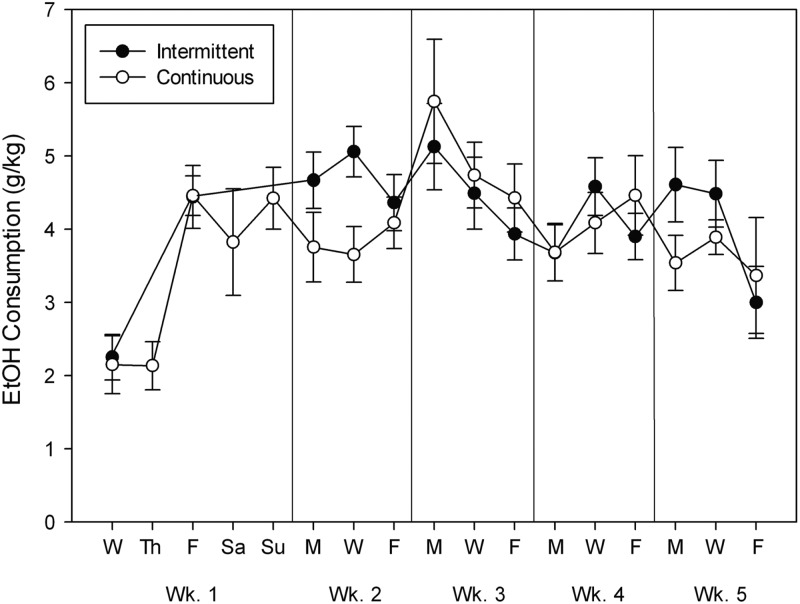
Results are shown in Fig. 1. During their initial 2 h access to 20% ethanol, IA and CA groups did not differ in intake, which was slightly more than 2 g/kg [t(34) < 1)]. On the third day (Friday), 4 h access was offered for the first time, and the intake of both groups increased to >4 g/kg [t(34) < 1]. On the first day following the weekend, both groups were again given 4 h access. The IA group drank more ethanol (4.7 ± 0.4 g/kg) than the CA group (3.8 ± 0.5 g/kg), but not significantly more [t(34) = 1.50, NS: Fig. 1, left panel]. To compare groups for the remainder of the study, average g/kg intake for each mouse was calculated for MWF each week (it was on these days that both groups were given ethanol), and these values are shown in Fig. 1 (right 4 panels). The main effect of groups was not significant [F(1,35) = 1.81, NS], but average drinking differed significantly across weeks [F(3,105) = 8.32, P < 0.001], and there was also a significant interaction of group X week [F(3,105) = 2.97, P < 0.05]. Post hoc tests showed that the groups tended to differ significantly only in Week 2 [F(1,36) = 4.01, P = 0.05]. We conclude that there was a very modest and temporary effect of intermittency, if any, to increase intake with 4 h access periods.
Fig. 1.
Drinking in HDID-1 male mice with intermittent (closed symbols) or continuous (open symbols) access to a single bottle of 20% ethanol 4 h/day. Daily intakes during the first week are shown in the left panel. Access was for only 2 h on the first 2 days of Week 1. Average drinking for MWF in Weeks 2–5 is shown in the right panels. Means ± SE are shown.
The absolute levels of intake (3–4 g/kg/4 h) were reflected in the BECs at the end of the last day of drinking. The IA and CA groups did not differ significantly [IA: 0.72 ± 0.10, CA: 0.67 ± 0.14 mg/ml t(36) < 1, NS]. These BECs are consistent with the population average values we found for males of this selected generation (0.8 mg/ml, unpublished data).
Experiment 2. IA vs. CA for 4 h/day in C57BL/6J mice
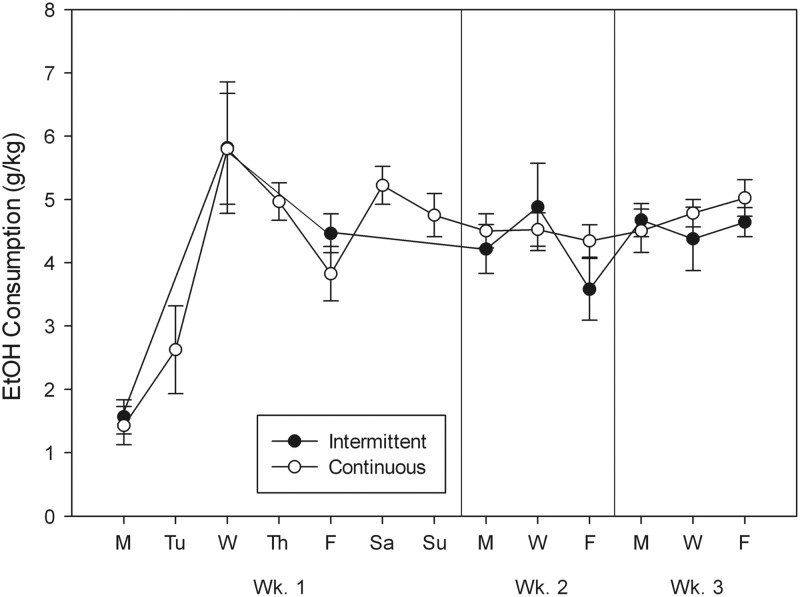
Results are shown in Fig. 2. During their initial 2 h access to 20% ethanol, IA and CA groups did not differ in intake, which was ∼1.5 g/kg in each group [t(22) < 1]. On the third day (Wednesday), 4 h access was offered for the first time, and the intake of both groups increased to nearly 6 g/kg in each group [t(22) < 1]. On the first day following the weekend, the IA group drank slightly less ethanol (4.2 ± 0.4 g/kg) than the CA group [4.5 ± 0.2 g/kg; t(22) < 1, NS]. To compare groups for the remainder of the study, average g/kg intake for each mouse was calculated for MWF each week (it was on these days that both groups were given ethanol), and these values are also shown in Fig. 2 (right 2 panels). The main effect of groups was not significant [F(1,22) < 1]. Average drinking tended to increase slightly between Weeks 1 and 2 [F(1,22) = 2.91, P = 0.10], but there was no significant interaction of group X week [F(2,22) < 1]. We conclude that there was no significant effect of intermittency to increase intake with 4 h access periods.
Fig. 2.
Drinking in C57BL/6J male mice with intermittent (closed symbols) or continuous (open symbols) access to a single bottle of 20% ethanol 4 h/day. See caption to Fig. 1.
Experiment 3. IA vs. CA for 24 h/day in C57BL/6J mice
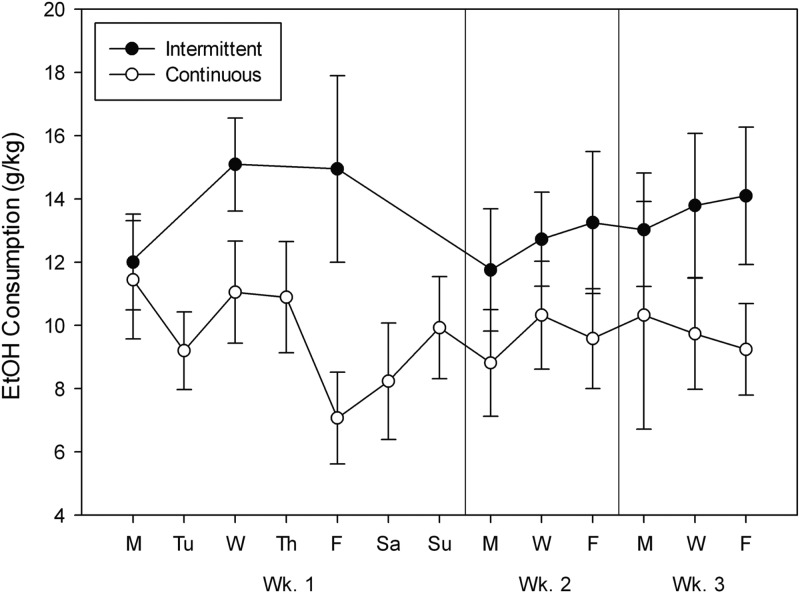
Results are shown in Fig. 3. During their initial 24 h access to 20% ethanol, IA and CA groups did not differ in intake, which was 11–12 g/kg [t(22) < 1]. To compare groups for the remainder of the study, average g/kg intake for each mouse was calculated for MWF each week (it was on these days that both groups were given ethanol), and these values are also shown in Fig. 3 (right 2 panels). The main effect of group was significant [F(1,24) = 4.69, P < 0.05]. Neither the effect of week nor the interaction of group X week was significant [both F(2,48) < 1].
Fig. 3.
Drinking in C57BL/6J male mice with intermittent (closed symbols) or continuous (open symbols) access to a 20% ethanol 24 h/day. Daily intakes during Week 1 are shown in the left panel. Average drinking for MWF in Weeks 2 and 3 is shown in the right panels. Means ± SE are shown.
Experiment 4. IA vs. CA for 24 h/day in HDID and HS mice
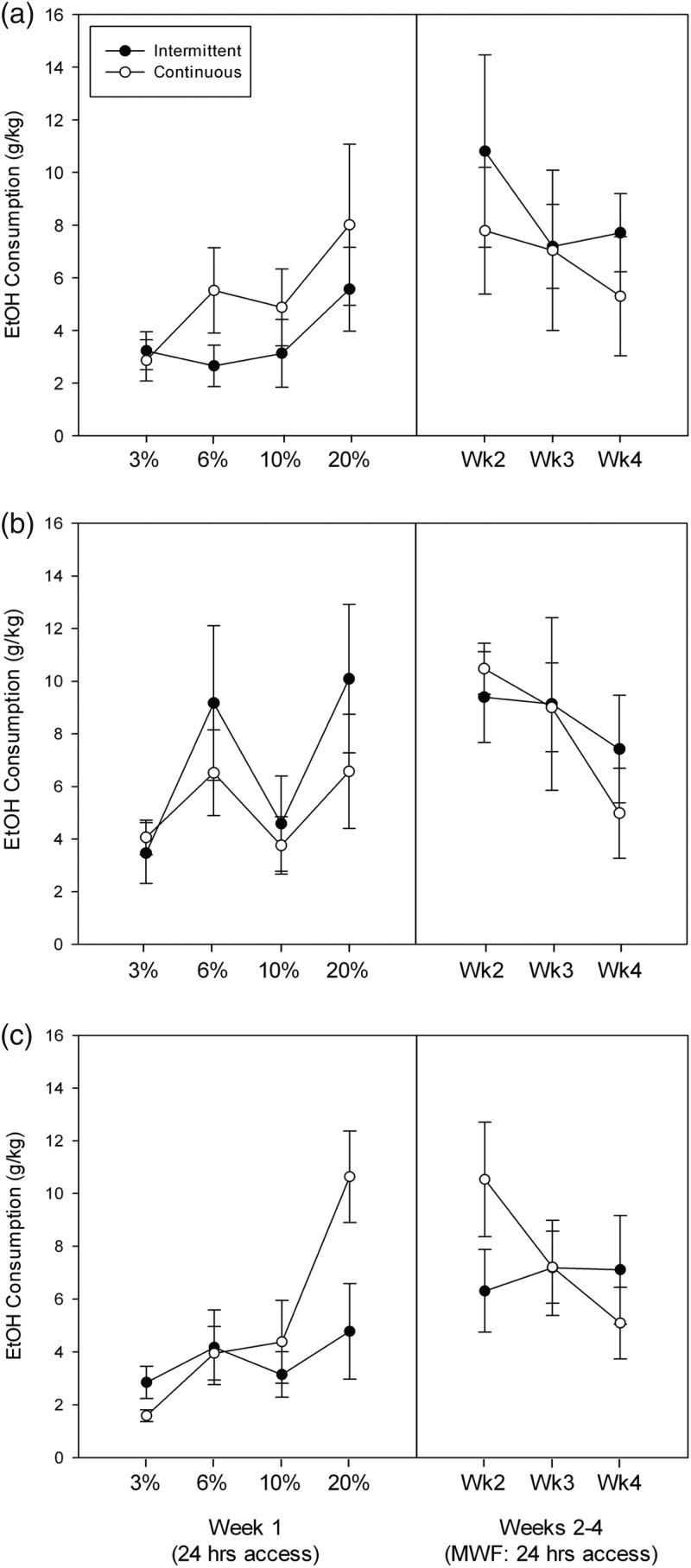
Because it is well-known that female mice drink substantially more alcohol by choice than do males, we analyzed the data from the two sexes separately. Results for females are shown in Fig. 4a–c, and for males in Fig. 5a–c. We first analyzed intakes on the first day that each concentration was presented to the IA group (MWFM: left panels). There was no significant effect of genotype [F(2,42) = 1.29] or intermittency [F(1,42) < 1] or their interaction [F(2,42) = 1.30]. There was a significant effect of concentration [F(3,126) = 14.6, P < 0.0001], but the interactions of concentration with either genotype or intermittency were not significant [both Fs ≤ 1.05, NS]. There was a trend toward a significant three-way interaction [F(6,126) = 1.96, 0.05 < P < 0.10].
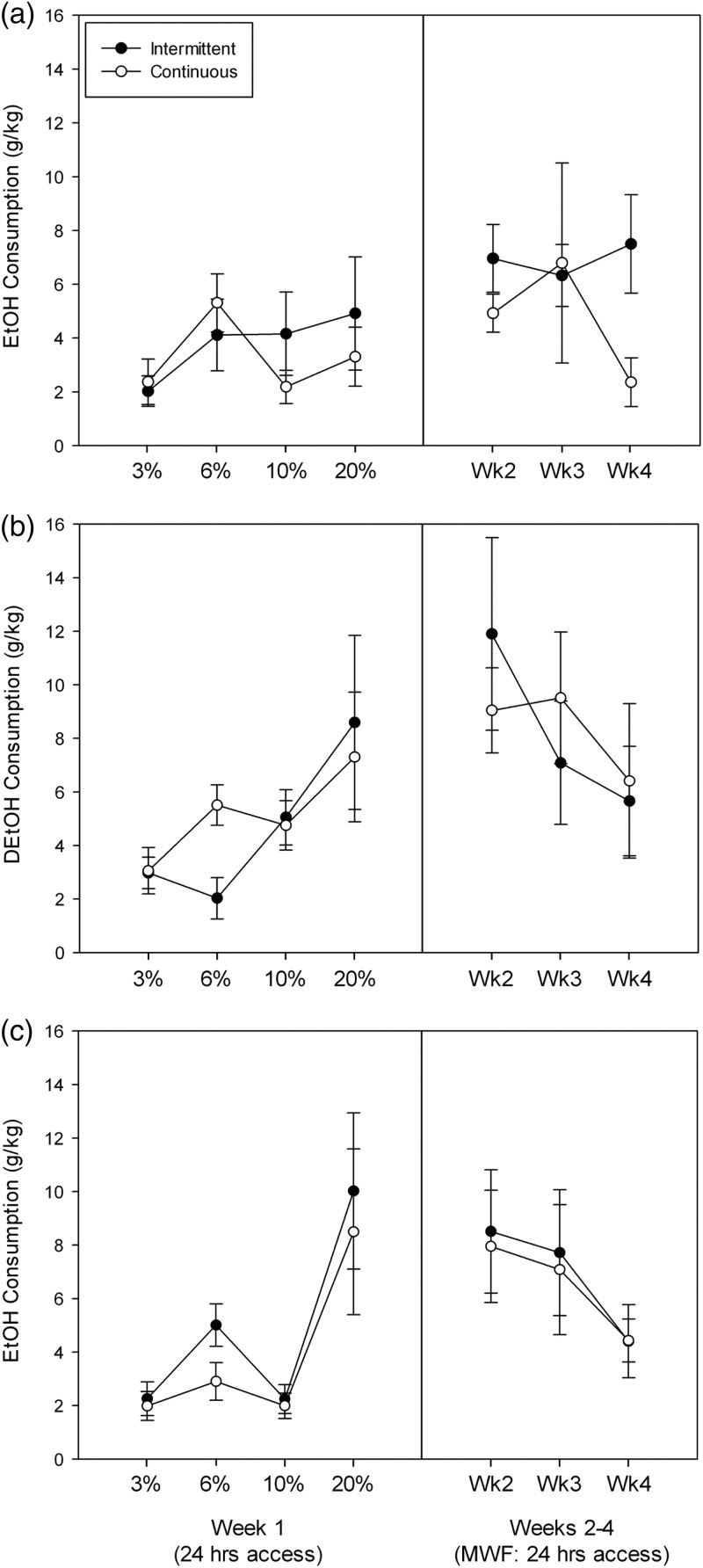
Fig. 4.
Drinking in HDID-1 (a) HDID-2 (b) and heterogeneous stock (HS) (c) female mice with intermittent (closed symbols) or continuous (open symbols) access to ethanol 24 h/day. Daily intakes for MWF during the first week and on M of Week 2 are shown in the left panels. Weekly drinking averaged across MWF in Weeks 2–4 is shown in the right panels. Means ± SE are shown.
Fig. 5.
Drinking in HDID-1 (a) HDID-2 (b) and HS (c) male mice with intermittent (closed symbols) or continuous (open symbols) access to ethanol 24 h/day. See caption to Fig. 4.
We next analyzed the weekly intake across the Weeks 2–4, where 20% ethanol was offered, by averaging the MWF intakes for both IA and CA groups (see Fig. 4a–c, right panels). Statistical outcomes for these data yielded the same results as seen during the initial week at different concentrations (analyses not shown).
Results for males yielded the same pattern of statistical outcomes (Fig. 5a–c) as seen in females for both the initial day at each concentration and for Weeks 2–4 of testing with 20% ethanol. The only difference was there was no trend toward a significant three-way interaction during the initial day at each concentration in males.
BECs after the final day of drinking were negligible, averaging 0.12 mg/ml across all mice. The main effects of intermittency, genotype and sex, and their interactions, were all non-significant [all F(1–2,87) < 1, NS].
Experiment 5. IA vs. CA for 24 h/day in C57BL/6J mice
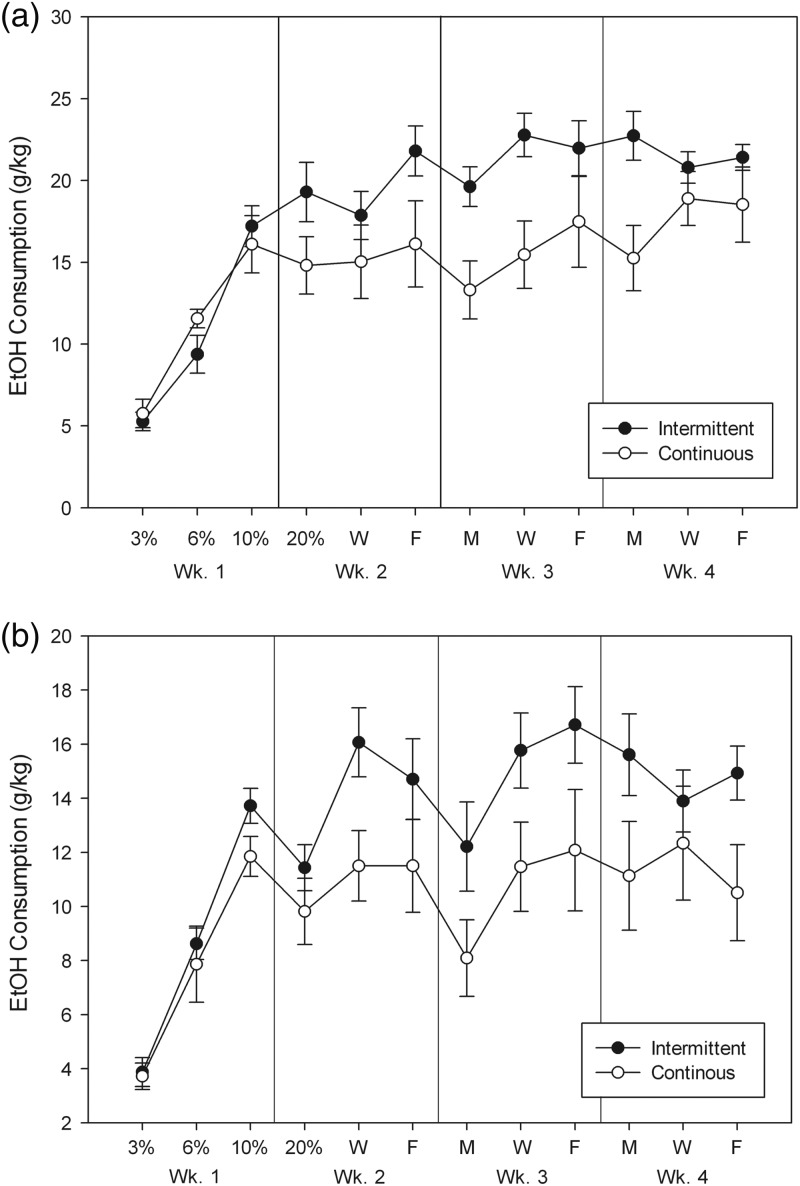
Results for females are shown in Fig. 6a and for males in Fig. 6b. We first analyzed intakes on the first day that each concentration was presented to the IA group (MWFM: left panels). For females, there was a significant effect of concentration [F(3,72) = 33.4, P < 0.0001] but no significant main effect of intermittency [F(1,24) < 1, NS]. IA interacted significantly with concentration [F(3,72) = 3.5, P < 0.05]. Post hoc tests showed that the IA group tended to drink more ethanol than the CA group only at the 20% concentration [F(1,27) = 3.1, P = 0.09]. For males, only the effect of concentration was significant [F(3,72) = 55.0, P < 0.0001; both other Fs < 1, NS]. We next analyzed the weekly intake across Weeks 2–4, where 20% ethanol was offered, by averaging the MWF intakes for both IA and CA groups (right panels). For both females and males, the IA groups ingested more ethanol than the CA groups [Fs(1, 28) = 7.8, Ps < 0.01] and the interactions of intermittency and week were not significant (both Fs < 1, NS).
Fig. 6.
Average drinking on MWF each week in C57BL/6J female (a) and male (b) mice with intermittent (closed symbols) or continuous (open symbols) access to ethanol 24 h/day. EtOH concentration remained at 20% after M of Week 2. Means ± SE are shown.
DISCUSSION
These experiments, and prior work, allow us to draw several tentative conclusions about the effect of IA on ethanol consumption. First, when access is limited to 4 h per day, the effect of IA to increase ethanol consumption is not seen in mice. Although there was a significant difference between IA and CA groups of HDID-1 male mice in Experiment 1 during the first week after IA was initiated, this appeared to result more from a reduction in the CA group than an increase in the IA group (see Fig. 1). No hint of a difference in intake between IA and CA groups in Experiment 2 was seen in C57BL/6J mice, even though IA can lead to increased access in this genotype when offered in 24 h-periods (Experiment 5; Hwa et al., 2011; Melendez, 2011). We did not test periods of intermittent availability between 4 and 24 h, and only tested four genotypes, so we cannot determine where in this interval the effect begins to appear for mice. We also cannot rule out the possibility that 4 h-access MWF would be effective in female C57BL/6J mice, or in rats.
A second conclusion, drawn from the literature, is that prior experience with ethanol is not necessary for IA to yield increased intakes. Wise's original study and several since have found this (Wise, 1973; Melendez, 2011), although some of them lack a CA comparison group (Pinel and Mucha, 1975; Simms et al., 2008).
A third conclusion we draw is that neither the HDID-1 nor the HDID-2 selectively bred mouse line responded to IA by increasing their ethanol intake vs. CA groups; neither did the HS controls. This suggests that if there are genetic contributors to IA escalation, they differ from those leading to high BEC during limited access drinking in the dark. There are no current systematic data that suggest that IA drinking is genetically influenced, but it would be very surprising if it were not, as all other ethanol drinking phenotypes we know of show such influences (Crabbe et al., 2010). A study with the Sardinian Preferring rat selected line offered either 10 or 20% ethanol vs. water, either with CA or IA-MWF, for 3 weeks. The IA groups drank more ethanol than the CA groups, and their intake increased during the first three drinking sessions, but not thereafter (Loi et al., 2010). Simms et al. (2008) also found that MWF access enhanced intake vs. CA in three rat genotypes, Wistar, Long Evans (LE) and the selectively bred P rat. Intakes appeared to increase more in the Wistar and LE rats than in P rats; P rats started at higher intake levels . This study reported increased intake of either 10 or 20% ethanol; however, there were no CA comparison groups for the Wistar and P rats.
The insensitivity of HDID and HS mice appears to be true in both sexes. Because the genetically heterogeneous HS control line for this selection experiment also failed to show escalated drinking with IA, there may be a crucial gene or genes that is absent in this entire population. Although we cannot rule this out, we consider this scenario unlikely for two reasons. First, it implies a simple genetic structure to IA drinking (i.e. only a few genes responsible). Other kinds of ethanol drinking have shown complex genetic structures (multigenic or polygenic, Crabbe et al., 2010). Furthermore, most murine genes are probably segregating in this HS/Npt cross, which was derived from intercrossing eight inbred strains (including C57BL/6J) that represent four distinct mouse lineages (Petkov et al., 2004). The DID phenotype for which HDID-1 and -2 mice were selected is genetically somewhat distinct from standard two-bottle preference drinking (Rhodes et al., 2007; Crabbe et al., 2011), but it will be necessary to test more genotypes for the effects of intermittency, both to establish its sensitivity to the genetic background and to determine its genetic correlation with other traits.
Although entirely speculative, there is the possibility that elevated drinking with IA is only seen in mice of the C57BL/6J or a closely related lineage. This possibility is suggested by the lack of an effect in either male or female HS/Npt mice. Its likelihood is diminished by the fact that multiple rat genotypes have shown IA elevation. On the other hand, the robust effect of cycles of dependence and withdrawal to escalate drinking in mice (Lopez and Becker, 2005; Griffin et al., 2009) has been reported principally in C57BL/6J mice. A recent study (Lopez et al., 2011) showed modest escalation after withdrawal in HAP-2 male mice, but not in HAP-2 females or either LAP-2 males or females (Lopez et al., 2011).
We examined our HS data to see whether a subset of HS mice showed substantial elevation by first creating an index to quantify individual differences in the elevation due to IA. We treated CA and IA groups, and males and females separately. We took each individual's Day 8 g/kg intake (the first day when offered 20% ethanol) and subtracted it from its average intake during Week 4 (see Figs 4 and 5). We then computed the mean and standard deviation increase for each CA group. Using the raw increase score for each IA animal, we then expressed it as a difference from the mean increase score for the appropriate CA group. Finally, we standardized this index by dividing by the standard deviation of the CA group mean. The mean standardized effect of IA in HS females was 1.49, and for males was −0.17. These standardized scores were normally distributed with standard deviations of 1.57 and 0.82, respectively. Of the total population of 17 HS mice, 2 members of the IA groups showed increases of greater than 2 SD. Assuming a prior probability of a frequency of 2%, the presence of 2/17 scores so extreme was significant by the binomial test (P < 0.05). Both animals were females, and the 2 of 8 proportion was significant for the females only as well (P < 0.01). Thus, we tentatively conclude that there was a subset of HS mice (at least of HS females) that was particularly responsive to the effect of IA under the conditions we employed here. To determine whether these putative ‘responders’ were enriched for alleles from the C57 lineage would require detailed genotyping. The main conclusion is that most HS animals did not noticeably escalate intake under IA conditions. There are clearly large individual differences in the degree of escalation in the published rat studies as well.
C57BL/6J mice of both sexes show increased intake when offered IA to alcohol; thus, Experiment 5 provided a replication of the main finding of Hwa et al. (2011). We remain puzzled by the difference in absolute intake between the mice in our study and those of Hwa et al. The difference was apparent in both CA and IA groups, of both sexes. While patterns of genotypic influence on behavioral traits can certainly be shown to differ in different laboratory environments (Crabbe et al., 1999; Chesler et al., 2002), we reproduced nearly all the aspects of the Hwa et al. method we could. We purchased mice from the same supplier (though they had to travel far further to reach Portland) and tested them at about the same age (9–10 weeks). The experimental protocol they used was reproduced as faithfully as possible in our laboratory. We could not duplicate their food, bedding, temperature, humidity, air quality or tap water. A reviewer of the current manuscript pointed out that we used 200 proof ethanol vs. 95%, so the dehydrating agents in our alcohol could have reduced our intakes through an effect on taste or through some other mechanism. And, of course, different experimenters performed the study in our laboratory than in theirs. Any of these factors could have affected the results. The only additional, similar study we know of that employed mice also used C57BL/6J males. Adult male mice were offered 15% ethanol vs. water either EOD or daily for 2 weeks. Intake across the 14 drinking sessions of the CA group remained stable at ∼8 g/kg/day, while intake EOD began to increase on the third session and reached a plateau on sessions 4–7 of ∼14 g/kg/day. A second experiment showed the same effect in adolescent mice: at this age EOD intake escalated to reach ∼17 g/kg/day (Melendez, 2011). However, the Melendez procedure did not acclimate the animals to lower concentrations of ethanol at the beginning.
What is the source of the intermittent escalation in drinking? Few studies that have reported on the phenomenon have tried to explain it mechanistically, either biologically or theoretically. Holloway et al. explored several variables that influenced the degree of escalation in rats, which they found to include alcohol concentration, nature of prior experience with alcohol, frequency and regularity of periodic access and individual differences (Holloway et al., 1984). They and all others who have looked also reported that the escalations did not persist if animals were switched from IA to CA—in our studies, we did not examine persistence. Pinel made the interesting suggestion that the effect of IA was not to escalate intake but rather that the effect of CA was to inhibit normal escalation of intake (Pinel and Huang, 1976). He reached this conclusion because he and others showed that intermittency accompanied a higher intake of saccharin and quinine solutions as well as alcohol (Wayner et al., 1972; Pinel and Huang, 1976). Because these compounds did not share reinforcing effects, addictive potential or pharmacological effects, he concluded that the only common factor must be taste-related. He hypothesized that an ‘inhibitory factor’ that dissipated with time must be the explanation for the greater levels of intake in IA groups than CA groups, where dissipation could not be completed between drinking sessions.
Two additional unknowns are the length of the period of access to ethanol (e.g. here 4 vs. 24 h) and the length of the period between sessions of IA to ethanol that show effective increases due to intermittency. One study (Hargreaves et al., 2009) offered beer (increasing to 4.4% ethanol) during two 1-h sessions daily or every third day. In this study, intermittency did not lead to intake greater than seen in a CA group. Other early studies explored the effect of longer periods of IA. Wayner et al. (1972) saw increased intake with once each 3-day IA. Sinclair's group first gave rats 30 days of two-bottle preference testing, and then offered ethanol EOD, or once each 3 or 4 days. All three IA intervals yielded further increases in g/kg/day intake (Sinclair and Bender, 1979). Neither of these studies had a CA comparison group. Holloway tested the effects of offering ethanol every 2nd, 3rd or 5th day, or once a week, and found the longer intervals to be the most effective (Holloway et al., 1984). More recently, in a study in male C57BL/6J mice, adult animals were first given 6 weeks of two-bottle preference testing as a choice between 10% ethanol and water with 24 h CA (Melendez et al., 2006). Thereafter, ethanol was withheld for 6 days in one group, while the other group continued to have an ethanol–water choice. Ethanol was returned for 24 h after the abstinence period, and the cycle of 6 days off, one day on was repeated 10 times. The group offered ethanol only once a week showed escalating intakes beginning in the 2nd week and eventually was consuming 18 g/kg/day, while the CA group maintained a stable intake of ∼10–11 g/kg/day. The author characterized this result as an example of an alcohol deprivation effect (ADE). The ADE was first reported in rats (Sinclair and Senter, 1967) and is a well-known phenomenon that has been replicated in many species. It is taken by many as a model for relapse drinking (for reviews, see Sinclair, 1972; Lê and Shaham, 2002).
Another remaining puzzle is that we do not know how long it takes to develop increased intake under IA conditions. A recent study offering 20% ethanol vs. water on MWF only for 3–4 months included a CA access comparison group. After this initial period, the IA-MWF group was drinking more ethanol than the CA group; however, no data were presented describing their acquisition of drinking during the initial 3–4 months (Hopf et al., 2010). Our data (Experiments 4 and 5), Hwa et al. (2011), and Melendez (2011) suggest that approximately a week of IA is required to see the effect, but this may only be true for paradigms that employ gradually escalated ethanol concentrations. Other studies with or without acclimation have shown the increase either virtually immediately or only after several days (e.g. Wise, 1973; Pinel and Mucha, 1975; Pinel and Huang, 1976; Pinel et al., 1976; Melendez, 2011). With long intervals of intermittency (one week), both immediate and delayed increases have been reported (Holloway et al., 1984; Melendez et al., 2006).
Another variant of this paradigm has come to be termed ‘multiple scheduled access’ (MSA). In the initial report (Murphy et al., 1986), male P rats were given access to 10% ethanol and water either continuously (24 h/day, 7 days/week) or during a single 4 h period of access each day, or during 4, 1 h access periods, spaced 2 h apart during the dark cycle. Animals given four separated exposures drank more during their total 4 h of access than animals given all 4 h continuously: both groups drank less (g/kg/day) than animals continuously exposed to ethanol for 24 h/day. The development of the groups’ drinking across days was not reported. A later study offered female rats concurrent access to 15 and 30% ethanol either continuously or MWF—i.e. offered MSA. All animals always had water access. By the third week of MSA, these animals were drinking as much on a g/kg/day basis during their total 3 h access as the CA, 24-h group (Bell et al., 2006a). Subsequent studies using this method have shown similar increases with intermittent MSA (Bell et al., 2006b, 2009). In these studies, ethanol intake remained less on a g/kg/day basis in animals receiving only 3 × 1 h access than in animals offered CA. A recent study compared CA with intermittent MSA in adolescent and adult P rats. Remarkably, in this study, adolescent rats drank more during the 3 h MSA periods than CA adolescents did over the whole 24 h access period (Bell et al., 2011). Also, we only offered a single, uninterrupted access period each day (of 24 h length in most studies), so it is unclear how to compare our results with those studies that saw increased intake in rats offered 2, 3 or 4 × 1 h access to two different ethanol concentrations. These MSA studies led to increased intake vs. a single 2, 3 or 4 h exposure.
Finally, we reiterate several limitations of these results. We do not know whether periods of access longer than 4 h but less than 24 h would lead to greater intake. It may be imprudent to generalize characterizations of the effect across murine and rat species. While EOD and MWF schedules of intermittency seem equivalent in rats, once/week also works in mice, and we have no directly comparable mouse data for such schedules as the MSA procedure. We have explored a limited number of genotypes. Although the effect seems to be present with or without choice of ethanol and water, it is unclear what its parameters might be if multiple ethanol concentrations were to be offered simultaneously (for example, ethanol intake increases as a direct function of the number of ethanol bottles offered in addition to a water bottle) (Tordoff and Bachmanov, 2003). Although the effect seems to appear early during repeated intermittent offerings, we have little data systematically exploring this parameter. We are more comfortable in believing that both sexes of both species show an IA increase in drinking, but this does not necessarily imply that the underlying mechanisms are the same. In conclusion, further work will be needed to determine whether this method can yield substantial and repeated levels of self-intoxication.
Funding
Supported by NIH grants AA010760, AA013519, AA020245 and DA018165, and the US Department of Veterans Affairs. J.H.H. is supported by DA007262. P.M. is supported by L40 AA018640.
Acknowledgments
We thank Andy-Jade Cameron and Mark Rutledge-Gorman for assistance with manuscript preparation.
REFERENCES
- Amit Z, Stern MH. A further investigation of alcohol preference in the laboratory rat induced by hypothalamic stimulation. Psychopharmacologia. 1971;21:317–27. doi: 10.1007/BF02419055. [DOI] [PubMed] [Google Scholar]
- Amit Z, Stern MH, Wise RA. Alcohol preference in the laboratory rat induced by hypothalamic stimulation. Psychopharmacologia. 1970;17:367–77. doi: 10.1007/BF00403808. [DOI] [PubMed] [Google Scholar]
- Bell RL, Kimpel MW, Rodd ZA, et al. Protein expression changes in the nucleus accumbens and amygdala of inbred alcohol-preferring rats given either continuous or scheduled access to ethanol. Alcohol. 2006a;40:3–17. doi: 10.1016/j.alcohol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, et al. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006b;11:270–88. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Bell RL, Kimpel MW, McClintick JN, et al. Gene expression changes in the nucleus accumbens of alcohol-preferring rats following chronic ethanol consumption. Pharmacol Biochem Behav. 2009;94:131–47. doi: 10.1016/j.pbb.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Smith RJ, et al. Modeling binge-like ethanol drinking by peri-adolescent and adult P rats. Pharmacol Biochem Behav. 2011;100:90–7. doi: 10.1016/j.pbb.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Wilson SG, Lariviere WR, et al. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci Biobehav Rev. 2002;26:907–23. doi: 10.1016/s0149-7634(02)00103-3. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–2. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Rhodes JS, et al. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol Psychiat. 2009;65:662–70. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Belknap JK. The complexity of alcohol drinking: studies in rodent genetic models. Behav Genet. 2010;40:737–50. doi: 10.1007/s10519-010-9371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Spence SE, Brown LL, et al. Alcohol preference drinking in a mouse line selectively bred for high drinking in the dark. Alcohol. 2011;45:427–40. doi: 10.1016/j.alcohol.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, III, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res. 2009;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves GA, Monds L, Gunasekaran N, et al. Intermittent access to beer promotes binge-like drinking in adolescent but not adult Wistar rats. Alcohol. 2009;43:305–14. doi: 10.1016/j.alcohol.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Holloway FA, Bird DC, Devenport JA. Periodic availability: factors affecting alcohol selection in rats. Alcohol. 1984;1:19–25. doi: 10.1016/0741-8329(84)90031-4. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, et al. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res. 2010;34:1565–73. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, et al. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–47. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–56. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Loi B, Lobina C, Maccioni P, et al. Increase in alcohol intake, reduced flexibility of alcohol drinking, and evidence of signs of alcohol intoxication in Sardinian alcohol-preferring rats exposed to intermittent access to 20% alcohol. Alcohol Clin Exp Res. 2010;34:2147–54. doi: 10.1111/j.1530-0277.2010.01311.x. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–96. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Grahame NJ, Becker HC. Development of ethanol withdrawal-related sensitization and relapse drinking in mice selected for high- or low-ethanol preference. Alcohol Clin Exp Res. 2011;35:953–62. doi: 10.1111/j.1530-0277.2010.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:652–8. doi: 10.1111/j.1530-0277.2010.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW. Development of an alcohol deprivation and escalation effect in C57BL/6J mice. Alcohol Clin Exp Res. 2006;30:2017–25. doi: 10.1111/j.1530-0277.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, Waller MB, et al. Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol. 1986;3:331–6. doi: 10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- Petkov PM, Ding Y, Cassell MA, et al. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004;14:1806–11. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinel JP, Huang E. Effects of periodic withdrawal on ethanol and saccharin selection in rats. Physiol Behav. 1976;16:693–8. doi: 10.1016/0031-9384(76)90238-9. [DOI] [PubMed] [Google Scholar]
- Pinel JPJ, Mucha RF. Suppression of voluntary ethanol consumption in rats by electroconvulsive shock. Physiol Behav. 1975;15:585–91. [Google Scholar]
- Pinel JP, Mucha RF, Rovner LI. Temporary effects of periodic alcohol availability. Behav Biol. 1976;16:227–32. doi: 10.1016/s0091-6773(76)91352-3. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, et al. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu C-H, et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Crabbe JC. Genetic analysis of rapid tolerance to ethanol's incoordinating effects in mice: inbred strains and artificial selection. Behav Genet. 2004;34:441–51. doi: 10.1023/B:BEGE.0000023649.60539.dd. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–23. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD. The alcohol-deprivation effect. Influence of various factors. Q J Stud Alcohol. 1972;33:769–82. [PubMed] [Google Scholar]
- Sinclair JD, Bender DO. Limited increases in alcohol intake by rats produced by infrequent periodic access. J Stud Alcohol. 1979;40:729–31. doi: 10.15288/jsa.1979.40.729. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Increased preference for ethanol in rats following alcohol deprivation. Psychonomic Sci. 1967;8:11–2. [Google Scholar]
- Tordoff MG, Bachmanov AA. Influence of the number of alcohol and water bottles on murine alcohol intake. Alcohol Clin Exp Res. 2003;27:600–606. doi: 10.1097/01.ALC.0000060529.30157.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner MJ, Greenberg I. Effects of hypothalamic stimulation, acclimation and periodic withdrawal on ethanol consumption. Physiol Behav. 1972;9:737–40. doi: 10.1016/0031-9384(72)90043-1. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Greenberg I, Tartaglione R, et al. A new factor affecting the consumption of ethyl alcohol and other sapid fluids. Physiol Behav. 1972;8:345–62. doi: 10.1016/0031-9384(72)90383-6. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–10. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]