Abstract
Objectives
Coital use of 1% tenofovir gel was shown to be modestly effective at preventing HIV transmission when applied vaginally in the CAPRISA 004 trial. Because the gel is hyperosmolar, which would reduce the integrity of the epithelium and induce fluid movement into the lumen, rectal use may not be acceptable. This study evaluated the pre-clinical safety and efficacy of a reformulated (reduced osmolality) tenofovir gel product.
Methods
Reduced glycerine (RG)-tenofovir gel was compared with the original tenofovir gel for physiochemical characteristics, product safety and anti-HIV-1 activity.
Results
The formulations were similar in all characteristics except for osmolality and spreadability/firmness. The RG-tenofovir gel had a 73% lower osmolality, a 29.6% increase in spreadability and a 27% decrease in firmness as compared with the original tenofovir gel. When applied to epithelial cell monolayers, tenofovir gel showed a transient reduction in the transepithelial resistance while the RG-tenofovir gel did not. Both gels retained ectocervical and colorectal explant viability. However, tenofovir gel treatment resulted in epithelial stripping that was absent after RG-tenofovir gel treatment of the polarized explants. Anti-HIV-1 activity was confirmed by lack of HIV-1 infection in polarized explants treated with either gel as compared with the control explants.
Conclusions
Reducing the osmolality of the tenofovir gel resulted in improved epithelial integrity, which suggests better safety upon rectal use. The improved gel safety did not compromise drug release or anti-HIV-1 activity. These data support the use of this gel as a dual compartment microbicide.
Keywords: HIV prevention, rectal microbicide, formulation, preclinical testing, safety
Introduction
The CAPRISA 004 trial demonstrated a modest, but significant reduction in HIV-1 acquisition in women using coitally applied tenofovir gel.1 While the MTN-003 (VOICE) trial did not show a benefit to high-risk women using daily vaginal application of tenofovir gel, several new trials are underway to include persons who engage in receptive anal intercourse, which is the highest risk factor for HIV-1 acquisition,2 and will benefit most from an effective product. Although clinically shown to be safe,1,3 tenofovir gel is hyperosmolar and affects epithelial integrity.4,5 These data suggest that rectal use of tenofovir gel may be problematic. Consequently, rectal-specific products are being developed.6 However, products designed for the vagina and rectum (dual compartment) would be beneficial as microbicides.7
To address concerns regarding osmolality, tenofovir gel was reformulated to reduce the glycerine content. The reduced glycerine (RG)-tenofovir gel was evaluated for safety and efficacy using our microbicide-testing algorithm.4 The data presented here show the RG-tenofovir gel was safer (i.e. retained the epithelial integrity) but still effective in vitro and would support the development of a dual compartment microbicide.
Materials and methods
Products
The original tenofovir gel is composed of 1% tenofovir, hydroxyethylcellulose, glycerine, EDTA, citric acid, and methyl and propyl parabens. The RG-tenofovir gel differs from tenofovir gel only in glycerine content (5% versus 20%) and a slight increase in hydroxyethylcellulose to maintain viscosity. CONRAD (Arlington, VA, USA) provided both tenofovir gels. Gynol II (Ortho-McNeil-Janssen Pharmaceutical, Inc. Titusville, NJ, USA), a 2% nonoxynol-9 (N9) gel, was used as a control.
Human tissue
Normal human ectocervical (IRB 0503103) and colorectal (IRB 0602024) tissues were collected by Honest Brokers at the University of Pittsburgh Medical Center.
Physicochemical testing
The drug content was measured using HPLC and the viscosity was determined on a cone/plate Brookfield viscometer.4 The pH was determined with an Accumet AR20 pH meter (Fisher) and the osmolality by using a Vapor Pressure 5520 Osmometer. The spreadability/firmness was measured using a Texture Analyzer (TA.XT.Plus, Texture Technologies Corp.). In vitro release studies were carried out using the Hanson Microette® system.4
Permeability testing
Permeability studies were conducted in a Franz cell maintained at 37°C. Dulbecco's modified Eagle's medium was used in the receptor and the study product was applied to the donor. Aliquots were removed from the receptor and analysed for tenofovir content. Apparent permeability coefficients (Papp) were calculated using:
Safety testing
The transepithelial resistance (TER) was measured in Caco-2 and HEC-1-A epithelial cells (ATCC, Manassas, VA, USA).4 Polarized ectocervical and colorectal tissues were set up as previously described.4 To ensure even spread of the gels and to allow them to be mixed with HIV-1 for the efficacy testing (below), a 1 : 5 dilution of tenofovir gels was applied to the apical side of the explants for 18 h. As controls, explants were untreated or a 1 : 5 dilution of N9 gel was applied apically. The next day, viability was assessed by MTT and histology.4
Efficacy testing
TZM-bl cells (NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) were used to determine the 50% cytotoxic concentration (CC50) and 50% effective dose (ED50) of the gels by GraphPad Prism© (V5.02) software to calculate the therapeutic index. TZM-bl cells were plated and 100 μL of gel serial dilutions was applied. For toxicity testing, 100 μL of medium was added to each well. The next day, 100 μL of medium was removed and replaced with 100 μL of CellTiter-Glo, and the luminescence measured. Viability was determined based on deviations from the cell-only control and presented as the percentage viability ± SEM. For efficacy testing, 100 μL of medium containing HIV-1 was added to each well. After 48 h, 100 μL of medium was removed and replaced with 100 μL of Bright-Glo (Promega Corp.), and the luminescence measured. Inhibition was determined based on deviations from the HIV-1-only control and presented as the percentage inhibition ± SEM.
A 1 : 5 dilution of product was applied to the apical surface of designated explants and then HIV-1BaL (5 × 104 TCID50 for ectocervical explants; 1 × 104 TCID50 for colorectal explants) was added to the apical surface.4 The explants were washed and fresh medium (without drug) was added to the basolateral compartment. The supernatant was collected and stored at −80°C for HIV-1 ELISA (Perkin-Elmer, Waltham, MA, USA) and fresh medium (without drug) was replenished. Immunohistochemistry for p24gag was performed on ectocervical tissue.
Results
Physicochemical characteristics
The rheological profiles of the RG-tenofovir and tenofovir gels were non-Newtonian, pseudoplastic (shear thinning) (Table 1). The viscosities of the tenofovir and RG-tenofovir gels were 9921 and 9161 centipoise (cps), respectively. Both gels were hyperosmolar, with osmolalities being 3111 and 836 mmol/kg, respectively. The RG-tenofovir gel osmolality was reduced by 73%. Both gels were acidic. The spreadability of the tenofovir gel was 49.732 g · s with a firmness of 71.257 g. In contrast, the spreadability of the RG-tenofovir gel was 34.987 g · s with a decreased firmness of 52.070 g. The tenofovir and RG-tenofovir gels showed similar in vitro release rates (81.0 and 94.8 μg/cm2/min0.5, respectively) (Figure S1, available as Supplementary data at JAC Online).
Table 1.
Physical characteristics of the original tenofovir and RG-tenofovir gels
| Test | Tenofovir gel | RG-tenofovir gel |
|---|---|---|
| Drug content (%, w/w) | 1% | 1.0041% |
| Viscosity (cps, 10 rpm at 25°C) | 9921 | 9161 |
| Rheological profile | shear thinning, pseudoplastic | shear thinning, pseudoplastic |
| pH | 4.5 | 4.6 |
| Osmolality (mmol/kg) | 3111 ± 10 | 836 ± 11 |
| Spreadability (g · s) | 49.732 ± 1.008 | 34.987 ± 2.705 |
| Firmness (g) | 71.257 ± 0.714 | 52.070 ± 1.075 |
| In vitro release rate (μg/cm2/min0.5) | 81.014 | 94.775 |
Permeability
Permeability studies reflect the potential systemic uptake of tenofovir when applied topically to the vagina. Both inter- and intrapatient variability was observed in both tenofovir diffusion profiles. This intrapatient variability was similar to that previously reported for the original tenofovir gel.4 The amount of tenofovir permeating through the excised ectocervical tissue following exposure was 80–257 and 208–425 μg for the tenofovir and RG-tenofovir gels, respectively (Table S1, available as Supplementary data at JAC Online). The Papp values for both gels were of the same order of magnitude, indicating similar permeability profiles between the two gel products.
Safety testing
The gels were diluted 1 : 10 and applied to established Caco-2 and HEC-1-A monolayers. For the Caco-2 cells [Figure S2 (available as Supplementary data at JAC Online), upper panel], the N9-treated wells showed complete loss of the TER. The tenofovir gel reduced the TER over the first 4 h by ∼49% and it then returned to pre-dose levels. The RG-tenofovir gel showed a modest increase in the TER, which returned to baseline. The HEC-1-A cells (Figure S2, lower panel) treated with N9 showed a complete loss of the TER while tenofovir gel did not affect the TER, as previously reported.4 RG-tenofovir gel increased the TER by 40% 30 min after application, which then moderated.
For polarized colorectal and ectocervical tissue, the viability was not affected after exposure to either tenofovir gel (Figure 1a). However, the N9-treated tissue showed a significant (P < 0.05) decrease in viability. Histologically, the colorectal and ectocervical epithelium was intact after exposure to the RG-tenofovir gel while the epithelium was fractured or sloughed off after exposure to the original tenofovir gel (Figure S3a, available as Supplementary data at JAC Online).
Figure 1.
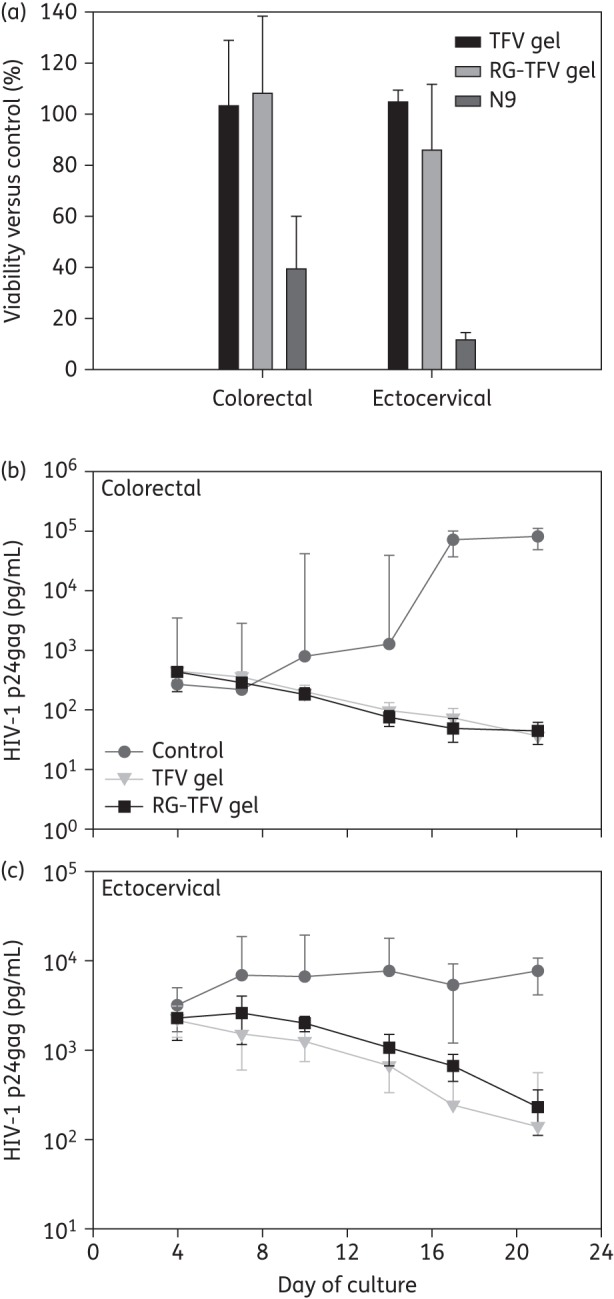
RG-tenofovir (TFV) gel and the original TFV gel effects on tissue viability and protection from HIV-1 challenge. (a) The effect of RG-TFV gel and original TFV gel on viability of polarized colorectal and ectocervical explant cultures. Duplicate polarized colorectal and ectocervical explant cultures were treated overnight with 1 : 5 dilutions of RG-TFV, original TFV or Gynol II (2% N9-containing) gels. Untreated explants were used as the reference control for each tissue. After product exposure, explants were washed and placed in medium containing MTT to evaluate mitochondrial activity. The data shown are the mean ± SD from a minimum of three independent tissues. (b) Anti-HIV-1 activity of the RG-TFV and original TFV gels were determined using colorectal and ectocervical (c) explant cultures. After overnight exposure to HIV-1 with or without either gel, the explant cultures were washed and followed for 21 days. Supernatant was collected and replenished every 3–4 days and used for HIV-1 p24 analysis by ELISA. The data shown are the median ± 95% CI of a minimum of three independent colorectal and ectocervical tissues.
Efficacy testing
The ED50s for the tenofovir (2.9 μM) and RG-tenofovir (2.4 μM) gels were similar. The CC50 for both was >1640 μM, providing therapeutic indices of >565. Colorectal explants showed a 3 log10 decrease and ectocervical explants a ≥1.5 log10 decrease in HIV-1 p24 release after dosing with either tenofovir gel (Figure 1b). Protection was confirmed in the ectocervical explants by the lack of infected cells by immunohistochemistry at study endpoint (Figure S3b, available as Supplementary data at JAC Online).
Discussion
Providing a safe microbicidal product is important for protection against HIV-1 acquisition and for product acceptability. The tenofovir gel currently being tested in clinical trials is hyperosmolar.4 While vaginal use of tenofovir gel was acceptable with minimal safety concerns,1,3 rectal application of tenofovir gel resulted in poor acceptability and safety concerns.8 We show the reformulated RG-tenofovir gel preserves the tissue epithelium and retains anti-HIV-1 activity. These data suggest better acceptability with rectal application of the gel.
Hyperosmolar enemas are used to evacuate the lower gastrointestinal tract. Consequently, product retention in the lumen may be impacted by hyperosmolar gels. Therefore, having a formulation that is isotonic should be the objective of any microbicidal product, to ensure mucosal safety and product retention. The formulation modifications of tenofovir gel resulted in near physiological osmolality, comparable viscosity, similar drug release profiles and tissue permeability in conjunction with improved safety. The formulation change did not affect the in vitro activity of tenofovir. The amount of tenofovir permeating tissue by 6 h exceeded the ED50 by 100- to 500-fold.
Despite the formulation modifications, the gel is acidic (pH 4.5). A study evaluating another vaginal microbicidal product (UC781 gel) rectally found no adverse events associated with gel use, despite the low pH of 5.2.9 Unfortunately, no systematic testing of the safety of the extended use of lower pH products on the distal gastrointestinal tract has been done. Our evaluation of the RG-tenofovir gel was after a modest dilution in medium that raised the pH to near physiological levels.4 While it is unclear whether frequent rectal use of acidic pH products will impact the gastrointestinal tract in terms of epithelial integrity, further work is warranted.
The reduction of glycerine content did not affect drug release or activity, indicating that the two gels were similarly effective. The reduced osmolality of the RG-tenofovir gel should increase acceptance in persons using the product rectally. This will allow the new formulation to be used as a dual compartment gel, the first microbicide with this distinction.
Funding
This work was supported by the Microbicide Trials Network funded by the National Institute of Allergy and Infectious Diseases (5UM1 AI068633), the National Institute of Child Health and Development, and the National Institute of Mental Health, all of the US National Institutes of Health (NIH). Gel preparation was funded by the United States Agency for International Development (USAID) through Cooperative Agreement GPO-00-08-00005-00.
Transparency declarations
D. F. is an employee of CONRAD and provided study product for testing. CONRAD provided no additional resources for the pre-clinical evaluation of the RG-tenofovir gel. All other authors: none to declare.
Disclaimer
The NIH and USAID had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views expressed here do not necessarily reflect those of NIH or USAID.
Supplementary data
Figures S1 to S3 and Table S1 are available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
Acknowledgements
We wish to thank the University of Pittsburgh Medical Center tissue procurement programme and the patients for their willingness to participate in research.
References
- 1.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vittinghoff E, Douglas J, Judson F, et al. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999;150:306–11. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 3.Mayer KH, Maslankowski LA, Gai F, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;20:543–51. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- 4.Rohan LC, Moncla BJ, Kunjara Na Ayudhya RP, et al. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS ONE. 2010;5:e9310. doi: 10.1371/journal.pone.0009310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs EJ, Lee LA, Torbenson MS, et al. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: potential implication for HIV transmission. J Infect Dis. 2007;195:703–10. doi: 10.1086/511279. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Schnaare RL, Dezzutti C, et al. Rectal microbicides: clinically relevant approach to the design of rectal specific placebo formulations. AIDS Res Ther. 2011;8:12. doi: 10.1186/1742-6405-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boily MC, Dimitrov D, Abdool Karim SS, et al. The future role of rectal and vaginal microbicides to prevent HIV infection in heterosexual populations: implications for product development and prevention. Sex Transm Infect. 2011;87:646–53. doi: 10.1136/sextrans-2011-050184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anton P, Cranston R, Carballo-Dieguez A, et al. RMP-02/MTN-006: a Phase 1 placebo-controlled trial of rectally applied 1% vaginal TFV gel with comparison to oral TDF. Abstracts of the Eighteenth Conference on Retroviruses and Opportunistic Infections, Boston, MA, 2011; Alexandria, VA, USA: Abstract #Y-1025. Foundation for Retrovirology and Human Health; [Google Scholar]
- 9.Anton PA, Saunders T, Elliott J, et al. First Phase 1 double-blind, placebo-controlled, randomized rectal microbicide trial using UC781 gel with a novel index of ex vivo efficacy. PLoS ONE. 2011;6:e23243. doi: 10.1371/journal.pone.0023243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


