Abstract
Although conditioned inhibition of fear (or learned safety) is a learning process critical for preventing chronic stress, a predisposing factor for depression and other psychopathologies, little is known about its functional purposes or molecular mechanisms. To obtain better insight into learned safety, we investigated its behavioral and molecular characteristics and found that it acts as a behavioral antidepressant in two animal models. Learned safety promotes the survival of newborn cells in the dentate gyrus of the hippocampus, while its antidepressant effect is abolished in mice with ablated hippocampal neurogenesis. Learned safety also increases the expression of BDNF in the hippocampus and leads to down-regulation of genes involved in the dopaminergic and neuropeptidergic but not the serotonergic system, in the basolateral amygdala. These data suggest that learned safety is an animal model of a behavioral antidepressant that shares some neuronal hallmarks of pharmacological antidepressants, but is mediated by different molecular pathways.
Introduction
Instinctive and learned fear are essential for survival and are evolutionarily conserved in organisms ranging from simple invertebrates to mammals. In humans, pathological forms of learned fear are hallmarks of severe psychopathologies such as anxiety disorders, post-traumatic stress disorders, and depression. The fact that fear can be enhanced through learning and can become a symptom of psychopathology in humans suggests that this form of learning may not always be appropriate and might, in certain situations, lead to unfavorable consequences. Therefore it seems likely that effective inhibitory constraints exist that prevent the inappropriate expression of learned fear.
In search for such a mechanism, Robert Rescorla extended the early work of Ivan Pavlov and delineated “conditioned inhibition” as a learning paradigm whereby a neutral CS develops the ability to inhibit responses to learned predictors of aversive or rewarding stimuli (Pavlov, 1927; Rescorla, 1969). Fear conditioning results from a positive correlation (pairing) of a previously neutral CS and an aversive US. During conditioned inhibition, by contrast, a CS that is negatively correlated (explicitly unpaired) with an aversive US becomes a positive signal (predictor) for safety and reduces the expression of conditioned fear. Since the animal associates the target signal with protection from an impending aversive event, conditioned inhibition has been thought to represent a form of learned safety, a process by which the animal learns to take advantage of sources of safety and security in the environment (Candido et al., 2004; Dinsmoor, 2001; Wiertelak et al., 1992). The term “safety signal” generally refers to a stimulus that is inversely or negatively correlated to an aversive event (Candido et al., 2004). In our previous study we referred to learned safety as the learning and memory resulting from a conditioned inhibition training procedure (Rogan et al., 2005). We here attempt to first characterize some of the behavioral consequences of learned safety and then to go on to explore it at the molecular level.
The ability to identify events that afford relief from ongoing strain is thought to be crucial for the prevention of chronic stress, a precipitating factor for the development of anxiety disorders and depression (Chan et al., 2001; Davis and Shi, 1999; LeDoux, 1993; Rogan et al., 2001). This led us to investigate whether learned safety, as a predictor of a break from continuously imminent, stress-producing danger, may have antidepressant effects. We tested this idea in mice using the Forced-swim test and the Unpredictable Chronic Mild Stress (UCMS) paradigm. We then assessed if learned safety could also share some major neuronal characteristics of pharmacological antidepressant treatments, specifically modulation of neurogenesis and the expression of BDNF in the dentate gyrus of the hippocampus (Warner-Schmidt and Duman 2006; Dranovsky and Hen 2006; Malberg and Duman 2003).
The amygdala is a key structure for the pathogenesis of the dominant emotional symptoms in major depression. To examine the molecular mechanisms contributing to learned safety using Affymetrix high-density oligonucleotide arrays, we focussed on the basolateral nucleus, the sub-region of the amygdala where we have previously described distinct electrophysiological features of learned safety (Rogan et al., 2005).
Results
The safety signal is a conditioned inhibitor that leads to reduction of conditioned contextual fear and retards subsequent fear conditioning
Safety conditioning is carried out over three days, one session per day and comprises a simple conditioned inhibition of fear paradigm consisting of several explicitly unpaired presentations of the aversive US and the tone CS (see Figure 1A). After safety training, freezing (the endogenous defense response of rodents) to the experimental context in the presence of the CS, is significantly reduced in safety trained mice and significantly increased in fear conditioned mice, while remaining unchanged in tone controls (Figure 1B). This observation provides evidence for summation, one of the two defining criteria of a conditioned inhibitor (Rescorla, 1971). The second test that a true conditioned inhibitor needs to pass is retardation. Indeed, we found that when mice were fear conditioned to the same CS beforehand used in safety training, they do not show freezing to the tone after one day of fear training (Figure 1C). However, with an additional day of fear training, previously safety conditioned animals also learn to freeze to the CS (Figure 1D).
Figure 1. Learned Safety induces reduction of contextual fear and retards subsequent fear conditioning to the same stimulus.
A. Safety conditioning consists of a simple conditioned inhibition of learned fear paradigm in which the delivery of 4 shock US is followed by the presentation of 4 tone CS. In the fear conditioning protocol the number of CS and US presentations is matched to the safety conditioning paradigm (4 paired CS-US). Training is conducted over a period of 3 days, one session per day. A memory recall test, consisting of a single CS presentation is carried out 24 hours after the last training day.
B. Contextual freezing in the in the presence of the CS (n=10–14 per group) (main effect of type of training (i.e. learned safety, learned fear or tone-alone control): F(2,31) = 34.813: p<0.001; effect of interaction between type of training and phase of testing (i.e. preCS or CS): F(2,31) = 24.715: p<0.001; Tukey-Kramer post-hoc test for type of training (CS phase): safety vs. fear and safety vs. tone p < 0.001; fear vs. tone p < 0.001). Separate paired Student's t-test (preCS vs. CS) within each group reveal “CS-effect” (learned safety: p<0.001, learned fear: p<0.01, tone alone: p>0.05). C. CS response after one day of fear conditioning in previously naïve control and previously safety conditioned mice (n=7 per group) (effect of interaction between phase of testing and type of training: F(1,14) = 255.462: p<0.001). “CS-effect” (learned safety: p<0.001, naïve control: p<0.001). Student's t-test between CS phases (p<0.001).
D. CS response after two days of fear conditioning in previously naïve control and previously safety conditioned mice (n=7 per group) (main effect of phase of testing F(1,14) = 47.245: p<0.001). “CS-effect” (learned safety: p<0.01, naïve control: p<0.001). E. Contextual freezing in response to the conditioned CS and an unconditioned tone CS* (n=7 per group) (main effect of phase of testing F(1,14) = 14.763: p<0.01; main effect of type of CS F(1,14) = 6.995: p<0.05; effect of interaction F(1,14) = 21.086: p<0.001). “CS-effect” (CS: p<0.001, CS*: p>0.05). Student's t-test between CS phases (p<0.001). All data are depicted as mean +/− SEM. * p<0.05, **p<0.01, ***p<0.0001, n.s. (not significant) p>0.05.
To further rule out non-specific excitatory effects of the safety signal, we tested whether a different tone (CS*) that has not been explicitly unpaired with the US would reduce freezing to the context in the memory recall test. We found that the CS, but not the CS*, reduced contextual freezing in safety trained mice (Figure 1E).
Learned safety reduces innate fear in the Elevated-Plus Maze
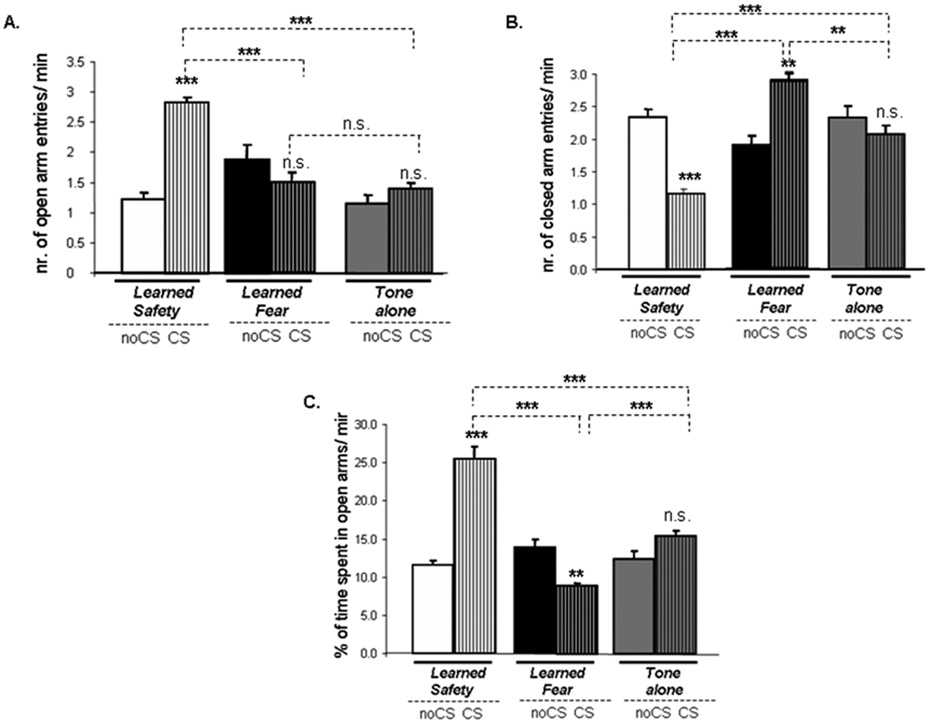
Using a within subject-control design we tested whether the presence of the conditioned stimulus would act to modulate behavioral measures associated with reduced anxiety in the Elevated-Plus maze. We found significantly increased number of open arm entries in safety conditioned mice in the presence of the CS (Figure 2A). Closed arm entries were significantly decreased in safety conditioned mice, and increased in fear conditioned mice, during delivery of the CS (Figure 2B). Moreover, safety conditioned mice spent significantly more time in the open arms in the CS than in the noCS period, whereas the opposite effect was observed in fear conditioned mice (Figure 2C).
Figure 2. Learned safety exerts anxiolytic effects in the Elevated-Plus Maze.
A. Open arm entries (main effect of phase of testing F(1,15) = 27.668: p<0.001; main effect of type of training F(2,15) = 18.428: p<0.001); effect of interaction between phase of testing and type of training F(2,15) = 9.354: p<0.01; Tukey-Kramer post-hoc test for type of training (CS phase): safety vs. fear and safety vs. tone p < 0.001; fear vs. tone p > 0.05). “CS-effect” (learned safety: p<0.001, learned fear and tone-alone: p>0.05). B. Closed arm entries (main effect of phase of testing F(1,15) = 102.552: p<0.001; main effect of type of training F(2,15) = 6.665: p<0.01; effect of interaction between phase of testing and type of training F(2,15) = 8.274: p<0.01; Tukey-Kramer post-hoc test for type of training (CS phase): safety vs. fear and vs. tone p < 0.001; fear vs. tone p <0.01). “CS-effect” (learned safety: p<0.001, learned fear: p<0.01 and tone-alone: p>0.05). C. Time in open arms (main effect of type of type of training F(2,15) = 21.635: p<0.001; effect of interaction between phase of testing and type of training F(2,15) = 65.768: p<0.001; Tukey-Kramer post-hoc test for type of training (CS phase): safety vs. tone and vs. fear p < 0.001; fear vs. tone p <0.001). “CS-effect” (learned safety: p<0.001, learned fear: p<0.01 and tone-alone: p>0.05) (n=7–8 per group in each case). All data are depicted as mean +/− SEM. * p<0.05, **p<0.01, ***p<0.0001, n.s. (not significant) p>0.05.
Learned safety can serve as a behavioral antidepressant
Delivery of the safety signal reduces immobility in the Forced-swim test
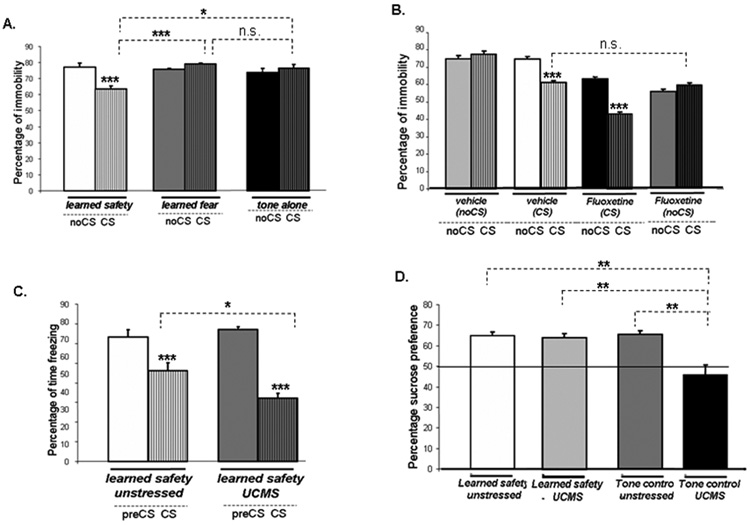
Immobility in the Forced-swim test, interpreted as a form of behavioral despair, was significantly reduced in safety conditioned mice in the presence of the safety signal (Figure 3A). We then evaluated, in safety trained mice, the effect of fluoxetine on immobility in the Forced-swim test, in order to validate the antidepressant activity of the safety signal with respect to a widely used pharmacological antidepressant. Vehicle- and fluoxetine-treated mice were either exposed to the safety signal during the Forced-swim test (CS groups) or served as control (noCS groups). We found that the percentage of time spent immobile in the vehicle treated CS group and the fluoxetine treated noCS group was not different, suggesting that the reduction in immobility induced by the safety signal is comparable to the effect seen with the antidepressant fluoxetine. Immobility was even further decreased in the presence of the safety signal in fluoxetine-treated safety trained mice (Figure 3B).
Figure 3. Learned safety acts to induce antidepressant-like behaviors.
A. Immobility in the presence of the CS (n=10 per group) (main effect of phase of testing F(1,30) = 10.076: p<0.01; interaction between phase of testing and type of training F(1,30) = 27.243: p<0.001). Tukey-Kramer post-hoc test for type of training (CS phase): safety vs. tone p<0.05 and vs. fear p<0.001; fear vs. tone p >0.05). “CS-effect” (learned safety: p<0.001, learned fear and tone-alone: p>0.05). B. Immobility during the CS delivery period and the corresponding time period in the noCS groups (n=8–9 per group) in fluoxetine and vehicle treated groups (main effect of phase of testing F(1,33) = 61.190: p<0.001; main effect of drug F(1,33) = 154.928: p<0.001; main effect of CS delivery F(1,33) = 32.696: p<0.001); effect of interaction between phase of testing and drug F(1,33) = 3.859: p<0.05; effect of interaction between phase of testing and CS delivery F(1,33) = 142.242: p<0.001; effect of interaction between phase of testing and drug and CS delivery F(1,33) = 5.791: p<0.05). “CS-effect” (vehicle CS and fluoxetine CS: p<0.001, vehicle noCS and fluoxetine noCS p>0.05). Student's t-test between CS phases of vehicle CS and fluoxetine noCS p>0.05).
C. Safety response in the memory recall test following 4 weeks of Unpredictable Chronic Mild Stress (UCMS) (n=9–10 per group) (main effect of phase of testing F(1,19) = 135.903: p<0.001; effect of interaction with UCMS exposure F(1,19) = 10.057: p<0.01). “CS-effect” (learned safety unstressed and learned safety UCMS: p<0.001). Student's t-test between CS phases of learned safety unstressed and learned safety UCMS p<0.05).
D. Sucrose preference 24 hrs after the last day of training (values above 50% (horizontal line) indicate sucrose preference above chance) in the presence of the CS (n=7 per group) (main effect of UCMS exposure F(1,28) = 20.085: p<0.001; main effect of type of training F(1,28) = 5.356: p<0.05; effect of interaction between UCMS exposure and type of training F(1,28) = 6.663: p<0.05). Tukey-Kramer post-hoc test: tone control UCMS vs. all other groups: p<0.01). Data are depicted as mean +/− SEM. * p<0.05, **p<0.01, ***p<0.0001, n.s. (not significant) p>0.05.
Learned safety reduces anhedonia brought on by Unpredictable Chronic Mild Stress
We found that a four week exposure to Unpredictable Chronic Mild Stress induced a significantly increased response for learned safety in the memory recall test (Figure 3C). The depressive state induced by UCMS is associated with anhedonic behavior that can be assessed in the sucrose preference test. As expected, all mice showed abolished sucrose preference following UCMS treatment. In UCMS treated safety trained mice sucrose preference was restored to levels of unstressed controls when assessed in the presence of the safety signal. The safety signal had no effect on sucrose preference in tone-alone controls (Figure 3D).
Safety learning promotes the survival of newborn cells in the hippocampal dentate gyrus
To determine whether safety learning has an effect on adult-generated hippocampal neurons, we examined the number and the fate of newborn cells using labeling with the thymidine analog, bromodeoxyuridine (BrdU). We employed two paradigms: The “survival paradigm” and the “proliferation paradigm” (Figure 4A) (Gould et al., 1999; Malberg et al., 2000). We found that learned safety significantly enhanced the number of newborn cells surviving 2 weeks after BrdU administration (Figure 4B–E). Results from the proliferation paradigm indicate that learned safety did not affect the rate of neurogenesis.
Figure 4. Learned safety promotes the survival of newborn cells in the dentate gyrus of the hippocampus.
A. In the “survival paradigm”, BrdU was injected on 3 days prior to behavioral training. Mice were sacrificed 14 days after the first BrdU injection. In the “proliferation paradigm” BrdU was injected starting two hours after the last behavioral training and mice were sacrificed 24 hours later. B. Number of BrdU-labeled cells in the dentate gyrus in the survival paradigm. Confocal laser scanning microscopic images (10x) of BrdU labeled cells (light blue) reveal a significant difference in number between C. tone-alone control. D. fear conditioned and E. safety conditioned mice (n=4 per group) (F(2,15) = 5.650: p<0.01, Tukey-Kramer post-hoc test safety vs. tone p<0.01, safety vs. fear p<0.05). F. The vast majority of BrdU labeled cells (red-yellow) were immunoreactive for the neuronal marker NeuN (60x). Data are depicted as mean +/− SEM. * p<0.05, **p<0.01, ***p<0.0001, n.s. (not significant) p>0.05.
Ablation of hippocampal neurogenesis retards safety learning
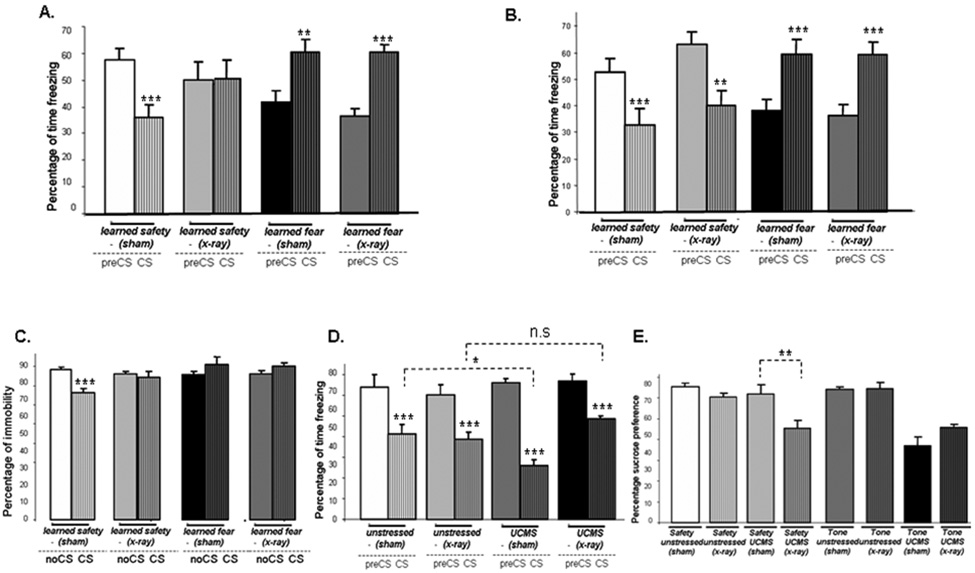
We used x-irradiation of the dentate gyrus to ablate hippocampal neurogenesis in mice and verified the absence of newly generated cells by doublecortin immuno-histochemistry (a marker for neurons younger than one month of age) 6 weeks later (Supplemental Figure S1). We then tested the effect of ablated hippocampal neurogenesis on learned safety and found that x-irradiated mice showed no evidence of safety learning after one day of training, in contrast to sham-irradiated control mice (Figure 6A). However, after additional two days of training, both x-irradiated and sham mice displayed significant reduction of contextual freezing when exposed to the safety signal (Figure 6B). In contrast to learned safety, fear conditioning was not affected by ablation of hippocampal neurogenesis on either day.
Figure 6. Ablation of hippocampal neurogenesis retards safety learning and inactivates the antidepressant effect of the safety signal.
A. Safety response in x–irradiated mice and sham-irradiated controls after one day of training (n=7 per group) (main effect of phase of testing F(1,32) = 5.477: p<0.05; effect of interaction between phase of testing and type of training F(1,32) = 58.152: p<0.001 and phase of testing and x-irradiation F(1,32) = 8.863: p<0.01) and phase of testing and of type of training and x-irradiation F(1,32) = 4.957: p<0.05). “CS-effect” (learned safety sham and learned fear x-ray: p<0.001; learned fear sham: p<0.01; learned safety x-ray: p>0.05). B. Safety response in x–irradiated mice and sham-irradiated controls after three days of training (n=7 per group) (effect of interaction between phase of testing and type of training F(1,32) = 58.152: p<0.001 and phase of testing and x-irradiation F(1,28) = 59.917: p<0.001). “CS-effect” (all groups: p<0.001). C. Immobility during the presentation of the CS compared to the time period without the CS (noCS) (n=8–9 per group) (main effect of type of training F(1,35) = 5.877: p<0.05; effect of interaction between phase of testing and type of training F(1,35) = 16.311: p<0.001 and phase of testing and type of training and x-irradiation F(1,35) = 4.220: p<0.05). “CS-effect” (learned safety sham: p<0.001: all other groups p>0.05). D. Safety response in x-irradiated and sham-irradiated control mice after 4 weeks of UCMS (n=7–8 per group) (main effect of phase of testing F(1,22) = 149.976: p<0.001; interaction between phase of testing and x-irradiation F(1,22) = 6.469: p<0.05; interaction between phase of testing and x-irradiation and UCMS exposure F(1,22) = 7.707: p<0.05; interaction between UCMS exposure and x-irradiation F(1,22) = 5.257: p<0.05). “CS-effect” (all groups: p<0.001). Student's t-test between CS phases of unstressed sham and UCMS sham: p<0.05; CS phases of unstressed x-ray and UCMS x-ray: p>0.05. E. Sucrose preference in the presence of the CS in x-irradiated and sham-controls after UCMS or no-stress control (n=5–7 per group) (main effect of UCMS treatment F(1,43) = 33.988: p<0.01; interaction between type of training and UCMS treatment F(1,43) = 12.088: p<0.01; interaction between type of training and UCMS treatment and x-irradiation F(1,43) = 6.027: p<0.05). Student's t-test between CS phases of safety UCMS sham and safety UCMS x-ray: p<0.01). Data are depicted as mean +/− SEM. * p<0.05, **p<0.01, ***p<0.0001, n.s. (not significant) p>0.05.
Ablation of neurogenesis inhibits the antidepressant action of learned safety
When evaluating the potential of learned safety to reduce depression-like behaviors, we found that safety trained x-irradiated mice did not show reduced immobility in the presence of the safety signal, which was observed in the controls (Figure 6C). Moreover, UCMS induced enhancement of the safety response was absent (Figure 6D) and the ability of learned safety to rescue the UCMS induced reduction of sucrose preference was abolished in x-irradiated safety trained mice (Figure 6E).
Safety learning increases expression of BDNF in the dentate gyrus of the hippocampus
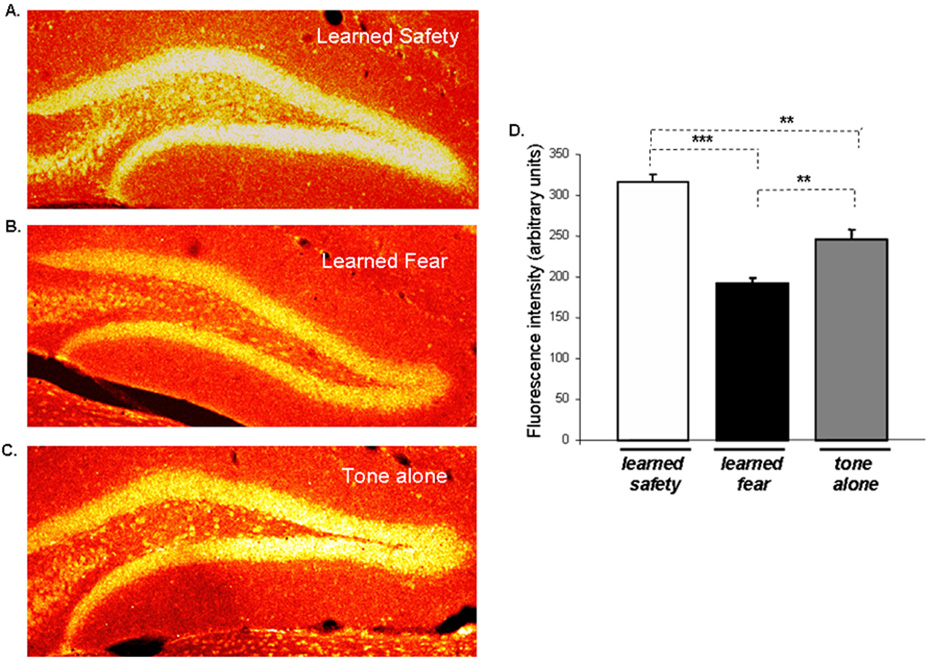
BDNF is known to be induced by antidepressant treatment in the hippocampus, particularly in the dentate gyrus (Nibuya et al., 1995; Russo-Neustadt et al., 2004) and could be responsible for the increase in and survival of hippocampal neurons following antidepressant drug treatment (Duman, 2004a, b). This led us to analyze the expression of BDNF in the dentate gyrus in mice following safety and fear training together with tone-alone controls. Using immuno-histochemical analysis, we found increased BDNF expression in safety conditioned mice, whereas the expression of BDNF in fear conditioned mice was reduced as compared to tone-alone controls (Figure 5).
Figure 5. Learned safety leads to increased expression of BDNF in the dentate gyrus of the hippocampus.
Dentate gyrus BDNF immuno-histochemistry in mice sacrificed 4 hours after the last day of behavioral training. Representative sections (10x) are shown for each group. A. Learned safety B. Learned fear C. Tone alone. D. Statistical analysis revealed increased levels of BDNF in safety conditioned and decreased levels in fear conditioned mice (n=4 per group) (F(2,15) = 42.423 : p < 0.001; Tukey-Kramer post-hoc test - safety vs. fear and tone p < 0.001; fear vs. tone p < 0.01). Data are depicted as mean +/− SEM. * p<0.05, **p<0.01, ***p<0.0001, n.s. (not significant) p>0.05.
Genes differentially expressed in the basolateral amygdala of safety conditioned and fear conditioned mice
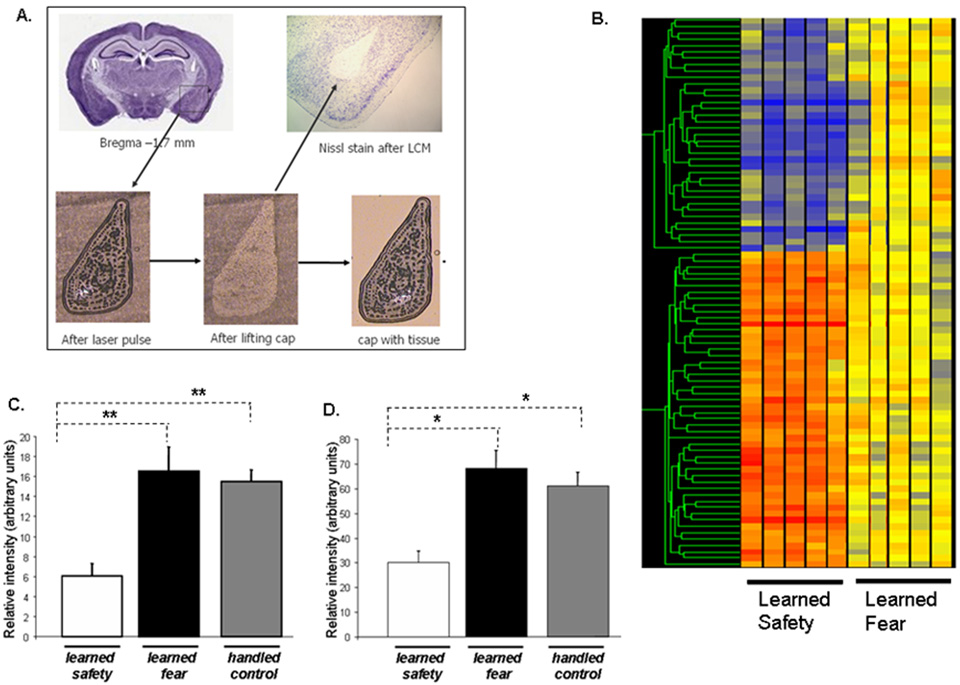
To characterize the molecular mechanisms involved in safety learning we searched for genes whose mRNAs were differentially regulated in safety and fear conditioned mice in the basolateral nucleus of the amygdala. We isolated the basolateral nucleus of the amygdala using Laser-Capture Microdissection (LCM), which permits for rigorously controlled and precise isolation of the target nucleus without contamination from surrounding areas (Figure 7A). Using a combination of hypothesis-free and hypothesis-driven approaches, we examined all significant changes (80 specific probe sets) (Figure 5B and Supplemental Table T1) but thereafter also focused on certain candidate genes which have been implicated in the literature to be involved in stress, anxiety and depression (Supplemental Table T2).
Figure 7. Laser-capture microdissection enables precise isolation of the mouse basolateral amygdala for subsequent gene expression analysis.
A. Firing an infrared laser through a thermoplastic cap leads to the melting of the target tissue with the cap. When the cap is lifted the tissue is removed from the tissue section and remains attached to the cap from where RNA can be isolated and used for molecular analysis. B. Hierarchical clusters of genes regulated by learned safety in the mouse basolateral amygdala (in comparison to fear conditioning controls). Relative expressional changes are colored-coded using a heat map (with red to blue gradient depicting an up to down regulation (≥2-fold increase → ≤2-fold decrease). mRNA expression of C. D2R, D. SP in basolateral amygdala samples of safety trained, fear trained and handled only (naïve control) mice evaluated by RT- PCR. Values normalized to the expression of GAPDH are displayed (n = 5–6 per group). (D2R: F(2,16) = 7.623: p<0.01, Tukey-Kramer post-hoc test safety vs. handled only and safety vs. fear p<0.01; SP: F(2,16) = 5.088: p<0.01, Tukey-Kramer post-hoc test safety vs. handled only and safety vs. fear p<0.05). All data are depicted as mean +/− SEM. * p<0.05, **p<0.01, ***p<0.0001, n.s. (not significant) p>0.05.
We found differential regulation of four genes (dopamine D2 receptor, substance P, prodynorphin and preproenkephalin 1) that have been highly implicated in the response to endogenous and exogenous stressors and depression (McLaughlin et al., 2003; Sinchak et al., 2000). To independently verify the observed changes and to relate them to benchmark values, we carried out RT-PCR analyses for two of these genes dopamine D2 receptor (D2R) and substance P (SP), those on which we focused more in subsequent experiments. In addition, RNA isolated from LCM samples of handled only mice as naïve baseline controls was included (Figure 7C and 7D)..
Blocking D2 Receptors facilitates the memoy for learned safety
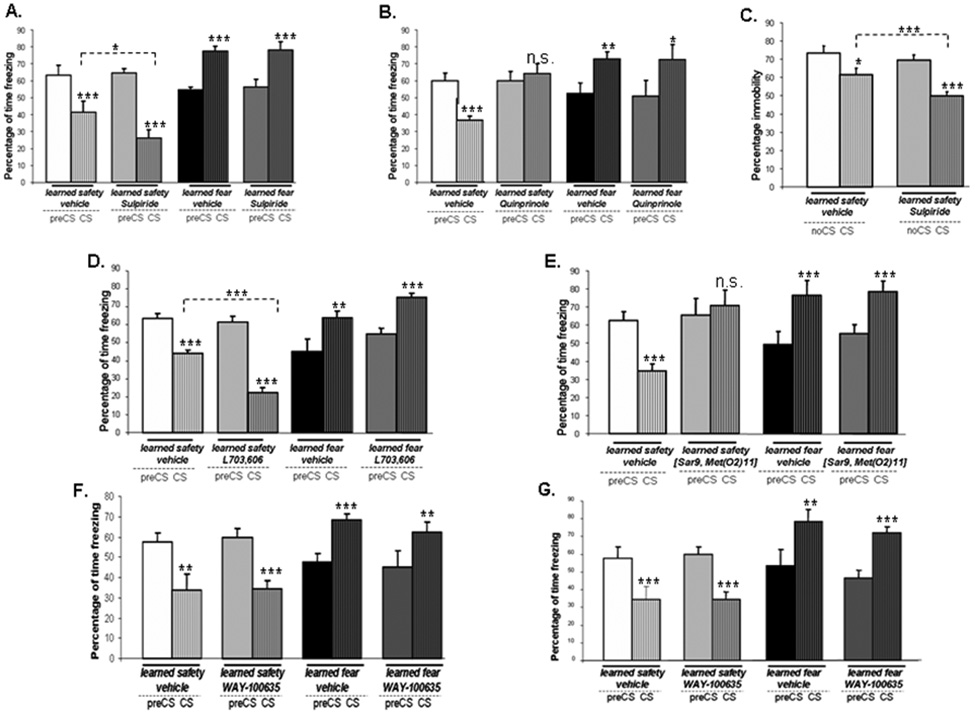
To evaluate the importance of the gene expression findings in vivo we examined the effects of blockade of D2R on the memory for learned safety. We found that the treatment with the D2R antagonist sulpiride before the memory recall test led to a significant enhancement of the safety response (Figure 8A). When sulpiride was administered before the training sessions and mice were tested-drug free, no effect in either safety or fear trained mice was observed (Supplemental Figure S2A)
Figure 8. Dopamine D2 receptor and Substance P but not 5-HT1A receptors are critically involved in learned safety.
A. Effect of sulpiride administration before the memory recall test (n = 7–9 per group) (main effect of phase of testing F(1,35) = 5.223: p<0.05; main effect of type of training F(1,35) = 17.430: p<0.001; interaction between phase of testing and type of training F(1,35) = 181.482: p<0.001; interaction between phase of testing and drug F(1,35) = 5.018: p<0.05; interaction between phase of testing and type of training and drug F(1,35) = 5.163: p<0.05). “CS-effect” (all groups: p<0.001). Student's t-test between CS phases of learned safety vehicle vs.learned safety sulpiride: p<0.05). B. Effect of quinprinole administration before the memory recall test (n = 7–9 per group) (interaction between phase of testing and type of training F(1,36) = 22.276: p<0.001; interaction between phase of testing and drug F(1,36) = 5.113: p<0.05; interaction between phase of testing and type of training and drug F(1,40) = 8.445: p<0.01). “CS-effect” (learned safety vehicle: p<0.001; learned safety quinprinole: p>0.05; learned fear vehicle: p<0.01, learned fear quinprinole: p<0.05). C. Effect of treatment with sulpiride prior to the Forced-swim test (n = 7–8 per group) (effect of phase of testing F(1,15) = 44.176: p<0.001; interaction between phase of testing and drug F(1,15) = 5.932 : p < 0.05). “CS-effect” (learned safety vehicle: p<0.05; learned safety sulpiride: p<0.001). Student's t-test between CS phases of learned safety vehicle vs. learned safety sulpiride: p<0.001). D. Effect of blockade of NK-1 receptors by L703,606 during safety training on response to the CS in the memory recall test (n = 8–9 per group) (main effect of phase of testing F(1,35) = 5.734: p<0.05; main effect of type of training F(1,35) = 8.899: p<0.01; effect of interaction between phase of testing and type of training F(1,35) = 118.869: p<0.001; effect of interaction between phase of testing and drug F(1,35) = 4.692: p<0.05; effect of interaction between phase of testing and type of training and drug F(1,35) = 5.644: p<0.05). “CS-effect” (learned safety vehicle: learned safety L703,606, learned fear L703,606: p<0.001; learned fear vehicle: p<0.01). Student's t-test between CS phases of learned safety vehicle vs. learned safety L703,606: p<0.001). E. Effect of administration of a NK-1 agonist ([Sar9, Met(O2)11]-Substance P) during training on the CS response in the memory recall test (n =8 per group) (effect of interaction between phase of testing and type of training F(1,32) = 8.357: p<0.01; effect of interaction between phase of testing and type of training and drug F(1,32) = 26.249: p<0.001). “CS-effect” (learned safety vehicle, learned fear vehicle, learned fear ([Sar9, Met(O2)11]: p<0.001; learned safety ([Sar9, Met(O2)11]: p>0.05). E. Effect of blockade of 5-HT1A receptors by WAY-100635 during training on the response to the CS in the memory recall test (n = 8 per group) (main effect of phase of testing F(1,32) = 8.887: p<0.01; effect of interaction between phase of testing and type of training F(1,32) = 18.873: p<0.001). “CS-effect” (learned safety vehicle, learned fear WAY-100635: p<0.01; learned safety WAY-100635, learned fear vehicle: p<0.001). F. Effect of administration of WAY-100635 before the memory recall test (n = 8 per group) (main effect of phase of testing F(1,32) = 10.098: p<0.01; effect of interaction between phase of testing and type of training F(1,32) = 43.337: p<0.001). “CS-effect” (learned safety vehicle, learned safety WAY-100635, learned fear WAY-100635: p<0.001; learned fear vehicle: p<0.01). All data are depicted as mean +/− SEM. * p<0.05, **p<0.01, ***p<0.0001, n.s. (not significant) p>0.05.
Application of the D2R agonist quinprinole before the memory recall test (Figure 8B) but not before the training sessions (Supplemental Figure S2B) abolished the safety response in the memory recall test. Quinprinole treatment did not affect learned fear under either condition.
A significant interaction between the CS delivery and the drug administration was revealed when we trained mice drug-free in the learned safety paradigm and administered sulpiride before the Forced-swim test. This result suggests that dopaminergic transmission is an important, although not the exclusive mediator of learned safety (Figure 8C).
Blocking NK-1 receptors facilitates the acquisition of learned safety
To determine the importance of Substance P in vivo we first tested the effect of blockade of the preferred receptor for Substance P, the NK-1 receptor using L-703,606. We found that the response to the safety CS was not different in L-703,606-treated and vehicle-treated mice in both safety and fear conditioned mice when mice were trained drug-free and NK-1 receptors were blocked only during the memory recall test (Supplemental Figure S2C). However, when we trained mice under the influence of L-703,606 and then tested them drug-free, we observed a significantly enhanced safety response in L-703,606-treated mice whereas the response to the CS was not altered in fear conditioned mice. NK-1 inhibition during training did not affect the CS response in the memory recall test (Figure 8C). Application of the NK-1 agonist ([Sar9, Met(O2)11]-Substance P) before the memory recall test did not affect learned safety (Supplemental Figure S2D). However, when we exposed the animals to the NK-1 agonist before each training session, we observed a reduced safety response during the memory recall test (Figure 8E). The learned fear response was not affected by drug treatment under either condition.
Blocking serotonin (5-HT1A) receptors does not affect safety learning
Several lines of evidence support an important role of 5-HT1A receptors in depressive illness (Bowen et al., 1989; Drevets et al., 1999) and in the response to antidepressants (Li et al., 1998; Singh and Lucki, 1993). However, blockade of 5-HT1A receptors affected learned safety neither in the acquisition nor in the memory recall phase (Figure 8F and 8 G).
Discussion
We find that learned safety reduces depression-like behavior in mice. Consistent with its behavioral antidepressant effects, learned safety enhances the survival of newborn cells and leads to increased expression of BDNF in the hippocampal dentate gyrus. In the amygdala, learned safety strongly modulates the expression of key components of the dopaminergic and neuropeptidergic system while having no effect on elements of the serotonergic transmission. Learned safety thus exerts its antidepressant activity through cell biological steps also recruited by conventional, serotonergically based antidepressants, but triggers these through different molecular pathways.
Learned safety reduces learned contextual and unlearned, innate fear
We produced learned safety by a conditioned inhibition of fear protocol in which the animal learns about a stimulus - the safety signal - that indicates the absence of impending aversive events. We and others have found that the behavioral response triggered by the safety signal can become independent from the context in which it has been acquired and might even be effective to control a different, unconditioned response (Denniston et al., 1998; Rogan et al., 2005). We now find that the safety signal in itself contains an autonomous informational content that can be transferred and lead to reduction in unlearned, innate fear in the Elevated-Plus Maze.
Learned safety reduces depression-like behavior in two animal models
We used two animal models of depression to test the idea that the safety signal may come to indicate a general “relief period” from ongoing stress and thus, may counteract depressive states. We found an antidepressant effect in the Forced-swim test (similar and in magnitude comparable to pharmacological treatment with fluoxetine) and complemented this result by the rescue of chronic mild stress induced reduction in sucrose preference by the safety signal, similar to that obtained with pharmacological antidepressants (Gittos and Papp, 2001; Moreau et al., 1996).
Antidepressant pharmacotherapy is more effective in patients with depressive disorders than in healthy controls. The enhancement of the learned safety response in mice, in which a depressive state has been induced by chronic mild stress, resembles this situation in humans and supports learned safety as an animal model of behavioral antidepressant treatment with good face- and content validity.
Learned safety shares neurobiological hallmarks of pharmacological antidepressants
Many pharmacological antidepressants and other interventions achieving antidepressant effects increase neurogenesis, whereas, conversely, stress typically reduces neurogenesis (Warner-Schmidt and Duman 2006; Dranovsky and Hen 2006; Malberg and Duman 2003). We found that in the “survival paradigm” learned safety enhances the number of BrdU-positive cells in the dentate gyrus 14 days after BrdU labeling. The number of new cells in the dentate gyrus increases between 2 hours and 1 week after DNA synthesis and then declines rapidly by the two week time point (Cameron et al., 1993). The ability of learned safety to rescue cells that were generated shortly before the training procedure provides a direct link between newborn cells in the adult hippocampus and this behavioral paradigm. These results on neurogenesis add further significance to the behavioral results suggestive of an antidepressant activity of learned safety. The fact that we observed an enhanced survival of those cells that were generated before training but did on observe an effect when BrdU was injected after training, suggests that the effect of learned safety on newborn cells may occur only during a specific 'sensitive period' following the generation of these cells. Interestingly it is precisely within this time frame (between 1 and 2 weeks after mitosis) that adult-generated granule cells of the dentate gyrus appear to be forming connections with the CA3 region (Gould et al. 1999). Learned safety, thus, may facilitate the integration of these cells into an established circuitry and promote their survival.
Another factor potentially contributing to enhanced cell survival following learned safety may be the increased neurotrophic support by BDNF. BDNF has been shown to be regulated by antidepressant and is thought to oppose the effects of stress on neuronal cells (e.g. inhibiting excitotoxic damage, blocking neuronal atrophy etc.) by helping to make neurons more resilient to stress and by maintaining basal levels of hippocampal neurogenesis (Nibuya et al., 1995).
The delayed acquisition of learned safety in mice with ablated hippocampal neurogenesis shows that neurogenesis is importantly involved, although not the only process required for the acquisition of learned safety. For the behavioral antidepressant effects, however, neurogenesis seems to be more essential since the response to the antidepressant activity of the safety signal in the Forced-swim test and the sucrose preference test was blunted in mice with ablated hippocampal neurogenesis. This result is also in agreement with other studies demonstrating ineffectiveness of pharmacological antidepressants in mice with ablated hippocampal neurogenesis (Manev et al., 2001; Santarelli et al., 2003).
Learned safety acts through molecular mechanisms distinct from conventional pharmacological antidepressants
In the search for the molecular basis for the behavioral characteristics and functional properties of learned safety, we turned towards the amygdala, where we have previously identified distinct neural changes in safety trained mice (Rogan et al., 2005) We focused on two candidate systems: the dopaminergic and neuropeptidergic Substance P system. The amygdala are modulated by dopaminergic inputs, which are central for mediating physiological and pathological responses to positive and negative stimuli. Moreover, in animal models of depression, stress has been found to activate the midbrain dopaminergic system by stimulating dopaminergic transmission from the ventral tegmental area (VTA) to its limbic targets, including the amygdala (Di Chiara et al., 1999; Horger and Roth, 1996). Our findings that sulpiride reduces behavioral despair in the Forced-Swim test is in agreement with reports on the antidepressant effects of amisulpirde in rats (Papp and Wieronska, 2000) and in humans (see for review (Jarema, 2007)). Down-regulation of D2R in the course of safety training may act to reduce or to relieve the experience of stress and thereby contribute to the antidepressant potential of learned safety.
The parallel regulation of Substance P together with D2R is consistent with a bidirectional feedback mechanism between the two neurotransmitter systems that is thought to be important in mediating associative learning and emotional responses has been recently been demonstrated (Kovacs et al., 2006).
We propose a model in which the stress-reducing and antidepressant effects of learned safety are mediated through the interaction of (at least) two different transmitter systems, leading to neuronal modifications typical of pharmacological antidepressant treatment: Stress relief, as a consequence of learned safety may reduce the firing of dopaminergic cells of the VTA which in turn leads to down-regulation of D2R in the basolateral amygdala. Reduction of dopamine may then inhibit the expression of Substance P mRNA (Kovacs et al., 2006). Reduced levels of Substance P may then feed back to further reduce the activation of the midbrain dopaminergic system ((Renoldi and Invernizzi, 2006) and in part, indirectly through increased expression of BDNF, provide enhanced neurotrophic support to promote the survival of newborn cells in the hippocampus (Morcuende et al., 2003).
In summary, our findings make three main points. One, learned safety represents a novel animal model of a behavioral intervention for depression that leads to behavioral outcomes similar to pharmacological interventions. Two, learned safety induces cell biological changes known to result from antidepressant pharmacotherapy but is mediated through different molecular pathways. Three, learned safety may provide a novel paradigm for the screening of pharmacological targets for the treatment of depressive disorders and their interaction with behavioral antidepressant strategies.
Experimental procedures
Animals
Male C57BL6/N mice (10- to 12-weeks-old) (Charles River Laboratories, Willington, MA) were used for all experiments. Mice were kept in clear plastic cages with ad libitum food and water, unless otherwise described. All animal procedures described were executed in accordance with National Institute of Health regulations and approved by the institutional Animal Care and Use Committees of Columbia University and the New York State Psychiatric Institute.
Behavioral training and testing
Animals were handled daily for three days prior to the safety or fear training procedure. In case of the pharmacology experiments, mice were injected i.p. with saline in the course of each of these handling sessions. All behavioral conditioning paradigms and control protocols were carried out over three days followed by a test day 24 hours after the last training day. Training for all animals occurred in behavioral chambers (MED Associates, Vermont, USA) housed within a sound-proof box.
Safety conditioning consisted of four explicitly unpaired US and CS presentations (one session per day for 3 days). The fear conditioning protocol was matched to the number of auditory CS and shock US presentations of the safety conditioning paradigm and thus constituted 4 paired CS-US presentations per day (see Figure 1A for details). In tone-alone controls 4 CS were delivered at the same time points as in the safety conditioning protocol. The precise timing of stimuli varied within session and across days. A memory recall test, consisting of the solely presentation of 1 CS (20 sec), was carried out 24 hours after the last training day. In any instance, the behavior during the CS period (20 sec) was compared to the corresponding length of time (20 sec) prior to the onset of the CS (preCS period). For the fear conditioning retardation test, previously safety conditioned mice were trained a simple fear conditioning paradigm (consisting of 3 CS-US pairings) starting 24 hours after the last day of safety training. Control mice were naïve (handled only) prior to fear conditioning. Freezing to the tone was evaluated on the following day in a novel context. In each instance, the US was a scrambled footshock (0.6 mA) delivered for 2 sec through the bars of the conditioning chamber. The CS was a software-generated 350 Hz pulsed tone (2 ms rise time, 2 % duty cycle) at 72dB with a total duration of 20 sec. For the differential tone testing, the second, unconditioned tone was a software-generated 50 Hz steady tone at 72dB with a total duration of 20 sec. For this experiment mice were exposed to 4 presentations of the CS* after the handling period (day 4). All freezing behavior was evaluated by digital video recordings analyzed with FreezeFrame software (Actimetrics, Evanston, IL).
Forced- swim test
The Forced- swim test was carried out as described elsewhere (Dulawa et al., 2004). The last 4 minutes of the 6 minutes test were scored by analysis of videotapes for immobility. To evaluate the effects of the delivery of the auditory CS on immobility in conditioned mice, the CS was presented during minutes four and five. The time spent immobile of these two minutes was averaged and compared to the average of the time spent immobile during minutes three and six.
Unpredictable Chronic Mild Stress (UCMS)
The protocol to induce UCMS was adapted from (Goshen et al., 2007). X-irradiated and sham control mice were allowed a five weeks recovery period before being subjected to the UCMS protocol.
Sucrose preference test
The sucrose preference test was modified from (Yu et al., 2007). 12 hrs after exposure to the last stressor of the UCMS regime, animals were deprived of food and water and tested for sucrose preference 23 hours later. Testing was carried out in the home cage in the form of a two bottle choice paradigm (2% sucrose versus water) in the presence of the CS for 1 hour. Sucrose preference rate was calculated according to the formula: % preference= [(sucrose intake/total intake) × 100%].
Elevated Plus Maze
The Elevated plus maze test was carried out as described (Shumyatsky et al., 2002). During the five minutes of testing the auditory CS was delivered during minutes three and four and the time spent (in seconds) and entries in the different compartments (closed and open arms), was averaged and compared to the average of the noCS period (minutes two and five).
Pharmacology
Chemicals
All drugs were purchased from Sigma Aldrich (Sigma, St. Louis, MO). Sulpiride was dissolved in 0.9% physiological saline and was adjusted to a neutral pH. L703,606 was dissolved in 0.9% physiological saline and 0.5% v/v DMSO. Fluoxetine was dissolved in distilled water. WAY100635, Quinprinole and [Sar9, Met(O2)11]-Substance P were dissolved in 0.9% physiological saline.
Drug administration
Sulpiride (20mg/kg) was injected 45 min, L703,606 (1mg/kg), WAY100635 (1mg/kg) and Quinprinole (0.1mg/kg) were injected 30 min and [Sar9, Met(O2)11]-Substance P (0.5mg/kg) was injected 20 min prior behavioral experiments. For the Forced-swim test, Fluoxetine (15mg/kg) was injected in a repeated injection schedule at 24 hrs, 5hrs and 1 hr prior to the Forced-swim test. All drugs were injected i.p. and the final injection volume was 5ml/kg in each case.
X-ray irraditation procedure
Mice received fractionated low dose x-irradiation to the head as previously described (Santarelli et al., 2003).
Doublecortin Immuno-histochemistry
Doublecortin immuno-histochemistry was performed as previously described (Holick et al., 2008).
Analysis of neurogenesis
Mice were administered BrdU (50 mg/kg i.p.) ((+)-5' Bromo-2'-deoxyuridine; 97%; Sigma, St. Louis, MO) twice per day (8 hrs interval) for 3 days prior to safety or fear conditioning and sacrificed 14 days after the first BrdU injection for assessment of survival of the newborn cells (“survival paradigm”). For assessment of stimulation of neurogenesis, BrdU was administered 4 times (every 2 hrs) starting 2 hrs after termination of safety or fear conditioning and mice were sacrificed 24 hours after the last BrdU injection (“proliferation paradigm”). After anesthesia with ketamine/xylazine, mice were transcardially perfused with 4% paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline (PBS; pH 7.4). Brains were collected, post-fixed overnight in 4% PFA at 4°C. The following day serial coronal sections (30 µm) along the entire rostro-caudal extension of the hippocampus were cut on a vibratome and stored in a cryoprotective solution (30% ethylene glycol, 30% glycerol in 0.1 M PBS) at −20 °C until further processed. BrdU immuno-histochemistry was performed on every 10th free-floating section (n=4 per group) essentially as described elsewhere(Wojtowicz and Kee, 2006).
BDNF immunofluorescence
Animals were sacrificed 4 hours after the last day of training and brains were processed as described above for BrdU analysis. BDNF immuno-histochemistry was performed on every sixth section from each animal (n=4 per group). Sections were rinsed in 0.1 M PBS (at pH 7.4) before incubating for 1 hr in blocking solution (0.1 M PBS, 0.3% Triton X-100, 10 % fetal bovine serum). Sections were then incubated with a monoclonal anti-human BDNF antibody (Promega, Madison, WI, USA) overnight at 4 °C on a shaker. The following day, sections were rinsed in 0.1 M PBS (at pH 7.4) and incubated for 1 hr at with a secondary antibody (Alexa Fluor 647 goat anti-human IgG (H+L); Molecular Probes, Invitrogen Corporation, Carslbad, CA). After rinsing in 0.1 M PBS sections were dried and coverslipped (Fluorosave; Calbiochem, La Jolla, CA).
Imaging and quantification of BrdU, BrdU/NeuN and BDNF immunofluorescence
Hippocampal BrdU labeling was quantified according to a modified unbiased stereology protocol by an experimenter blind to the experimental condition (Gould et al., 1999; West et al., 1991). Confocal images were acquired using an Olympus Fluoview FV1000 scanning module with an Olympus IX81 microscope (Olympus America, Center Valley, PA, USA). BrdU/NeuN double labeling was carried out essentially as described elsewhere (Meshi et al., 2006). Quantification of BDNF immunofluorescence intensity was performed according to a published method (Gazzaley et al., 1996).
Gene expression analysis
Brain dissection
Animals were sacrificed by cervical dislocation 4 hours after the last training day. Brains were rapidly dissected out, snap-frozen and stored at −80°C until needed. 8µm brain sections were sliced with a cryostat and immediately stored at −80°C in a dry container.
Laser Capture Microdissection (LCM)
A total of three coronal sections of the amygdala per animal (n=5 per group) were subjected to LCM. The left and right basolateral nucleus of the amygdala of sections from the rostral (Bregma −0.82 mm), the medial (Bregma −1.34 mm) and the caudal (Bregma −1.82 mm) amygdala were used for LCM. Brain sections were removed from −80°C and immediately dehydrated in a gradient alcohol series and a final incubation in xylene for clearance. Sections were then air-dried under a laminar flow and immediately used for LCM. LCM was carried out using a PixCell II system (Arcturus Bioscience Inc., Mountain View, CA). Selected regions were lifted onto CapSure LCM plastic caps (Arcturus Bioscience Inc., Mountain View, CA) using a spot size of 15µm, a laser power of 50µV and a duration of 2ms. Caps with transfer films with the microdissected tissue were immediately placed into Eppendorf tubes containing lysis buffer, incubated at 37°C for 30 min and stored in −80°C before RNA isolation. Total RNA was extracted from samples collected by LCM caps using RNAqueous®-Micro Kit (Ambion Inc., Austin TX) including DNAse treatment to remove potential genomic DNA contamination according to the manufacturer's instructions.
Microarray experiments
Two rounds of linear amplification were carried out using the GeneChip® Two-Cycle Target Labeling kit (Affymetrix Inc., Santa Clara, CA) according to the supplier’s instruction. cRNA samples derived from single animals were hybridized in recommended buffers to microarrays (Affymetrix GeneChip® Mouse Genome 430A 2.0 Array). The samples were stained and washed according to the manufacturer's protocol on a Fluidics Station 400 (Affymetrix Inc.) and scanned on a GeneArray Scanner (Affymetrix Inc., Santa Clara, CA). Primary data extraction was performed with Microarray Suite 5.0 (Affymetrix Inc., Santa Clara, CA). Data were filtered and sorted the by a sequential analysis using the GeneSpring GX 7.3. software (Affymetrix Inc., Santa Clara, CA). Briefly, raw values were normalized using the GCRMA algorithm (Lim et al., 2007), filtered for two-fold expressional changes and subjected to statistical analysis using a non-parametric using two-way analysis of variance. Genes with significant effects were selected by adjusting the resulting p-values for multiple testing by means of the False Discovery Rate using the linear step-up procedure (BH) of Benjamini and Hochberg (Reiner et al., 2003).
Reverse Transcriptase-PCR
Total RNA was diluted to a final concentration of 100 ng/µl and reverse transcribed using the SuperScript™ First-Strand synthesis system for RT-PCR (Invitrogen Corporation, Carslbad, CA) following the suppliers manual. 2 µl of the RT-reaction was subjected to PCR amplification using the AccuPrime™ DNA polymerase system (Invitrogen Corporation, Carslbad, CA) following the suppliers manual. The primer pairs for the dopamine D2 receptor, substance P and GAPDH and respective amplification conditions were based upon published protocols (Ding et al., 2007; Mutiara et al., 2006; Silva et al., 2006). PCR products were separated by electrophoresis on a 1.5% agarose gel and stained with ethidium bromide. Band signals were analyzed and quantified by densitometry analysis using the Kodak Gel Logic 100 imaging system and software. Relative intensities were calculated by normalization to the band intensity levels of GAPDH.
Data analysis
For analyses of all behavioral experiments involving comparisons between the CS and the pre/noCS period repeated measures ANOVAs were used, with phase of testing as the repeated measure (within-subject factor). Type of training, drug or UCMS were between-subject factors. Significant main effects or interactions were followed by Tukey-Kramer post-hoc tests or paired or unpaired two-tailed Student's t-tests where appropriate. For the sucrose preference test 2 (x-ray: sham or x-irradiated) × 2 (type of training: safety or control) × 2 (stress: UCMS or control; for the experiment involving x-irradiated mice) ANOVAs were performed. Histological and RT-PCR data were analyzed using one-way ANOVA followed by Tukey-Kramer post-hoc tests for pair-wise comparisons for significant ANOVA results. A α-level of 0.05 was adopted in all instances. All analyses were carried out using StatView software (SAS Institute, Cary, NC, USA).
Supplementary Material
Supplemental Figure S1. Doublecortin – a marker for immature neurons is absent in the dentate gyrus of x-irradiated mice.
Doublecortin immunohistochemistry in the dentate gyrus 6 weeks after treatment with either A. sham or B. hippocampal x-irradiation (40X). Absence of doublecortin (a marker for immature neurons) immunostaining is observed in x-irradiated mice.
Supplemental Figure S2. Dopamine D2 receptor are not involved in the acquisition and Substance P is not involved in the expression of learned safety.
A. Effect of sulpiride administration during training on the CS response in the memory recall test (n = 7–9 per group) (interaction between phase of testing and type of training F(1,33) = 42.523: p<0.001). “CS-effect” (learned safety vehicle, learned safety Sulpiride, learned fear vehicle: p<0.001; learned fear sulpiride: p<0.01). B. Effect of application of quinprinole during training on the CS response in the memory recall test (n = 7 per group) (interaction between phase of testing and type of training F(1,28) = 37.373: p<0.001). “CS-effect” (learned safety vehicle, learned fear vehicle, learned fear quinprinole: p<0.01; learned safety quinprinole: p<0.001). C. Effect of L703,606 application before the memory recall test (n = 7 per group) (effect of interaction between phase of testing and type of training F(1,28) = 39.597: p<0.001). “CS-effect” (learned safety vehicle, learned safety L703,606; learned fear L703,606: p<0.001, learned fear vehicle: p<0.01). D. Effect of [Sar9, Met(O2)11]-Substance P application before the memory recall test (n = 7–9 per group) (effect of interaction between phase of testing and type of training F(1,28) = 59.913: p<0.00). “CS-effect” (learned safety vehicle, learned safety [Sar9, Met(O2)11]: p<0.001; learned fear [Sar9, Met(O2)11], learned fear vehicle: p<0.01). All data are depicted as mean +/− SEM. * p<0.05, **p<0.01, ***p<0.0001, n.s. (not significant) p>0.05.
Acknowledgements
This research project was supported by the Hope for Depression Research Foundation. Daniela D. Pollak was supported by the Austrian Academy of Science (Max-Kade-Fellowship) and the Austrian Science Fund (Erwin-Schrödinger-Fellowship). Eric R. Kandel was supported by the Howard Hughes Medical Institute.
We thank Rae Silver, Department of Psychology, Columbia University, for enabling the use of the laser-capture microdissection facility and Yonghui Zhang, Institute for Cancer Genetics, Columbia University, for bioinformatic assistance with the analysis of microarray experiments.
We want to thank Pierre Trifilieff and Joe Rayman for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bowen DM, Najlerahim A, Procter AW, Francis PT, Murphy E. Circumscribed changes of the cerebral cortex in neuropsychiatric disorders of later life. Proc Natl Acad Sci U S A. 1989;86:9504–9508. doi: 10.1073/pnas.86.23.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Candido A, Gonzalez F, de Brugada I. Safety signals from avoidance learning but not from yoked classical conditioning training pass both summation and retardation tests for inhibition. Behav Processes. 2004;66:153–160. doi: 10.1016/j.beproc.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Chan KH, Morell JR, Jarrard LE, Davidson TL. Reconsideration of the role of the hippocampus in learned inhibition. Behav Brain Res. 2001;119:111–130. doi: 10.1016/s0166-4328(00)00363-6. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann N Y Acad Sci. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Denniston JC, Cole RP, Miller RR. The role of temporal relationships in the transfer of conditioned inhibition. J Exp Psychol Anim Behav Process. 1998;24:200–214. doi: 10.1037//0097-7403.24.2.200. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Loddo P, Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatry. 1999;46:1624–1633. doi: 10.1016/s0006-3223(99)00236-x. [DOI] [PubMed] [Google Scholar]
- Ding X, Mountain DJ, Subramanian V, Singh K, Williams CA. The effect of high cervical spinal cord stimulation on the expression of SP, NK-1 and TRPV1 mRNAs during cardiac ischemia in rat. Neurosci Lett. 2007;424:139–144. doi: 10.1016/j.neulet.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Dinsmoor JA. Stimuli inevitably generated by behavior that avoids electric shock are inherently reinforcing. J Exp Anal Behav. 2001;75:311–333. doi: 10.1901/jeab.2001.75-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. 2004a;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004b;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittos MW, Papp M. Antidepressant-like action of AGN 2979, a tryptophan hydroxylase activation inhibitor, in a chronic mild stress model of depression in rats. Eur Neuropsychopharmacol. 2001;11:351–357. doi: 10.1016/s0924-977x(01)00103-1. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Horger BA, Roth RH. The role of mesoprefrontal dopamine neurons in stress. Crit Rev Neurobiol. 1996;10:395–418. doi: 10.1615/critrevneurobiol.v10.i3-4.60. [DOI] [PubMed] [Google Scholar]
- Jarema M. Atypical antipsychotics in the treatment of mood disorders. Curr Opin Psychiatry. 2007;20:23–29. doi: 10.1097/YCO.0b013e328010e29b. [DOI] [PubMed] [Google Scholar]
- Kovacs KA, Steinmann M, Magistretti PJ, Halfon O, Cardinaux JR. C/EBPbeta couples dopamine signalling to substance P precursor gene expression in striatal neurones. J Neurochem. 2006;98:1390–1399. doi: 10.1111/j.1471-4159.2006.03957.x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory: in search of systems and synapses. Ann N Y Acad Sci. 1993;702:149–157. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
- Li DL, Simmons RM, Iyengar S. 5HT1A receptor antagonists enhance the functional activity of fluoxetine in a mouse model of feeding. Brain Res. 1998;781:119–126. doi: 10.1016/s0006-8993(97)01221-3. [DOI] [PubMed] [Google Scholar]
- Lim WK, Wang K, Lefebvre C, Califano A. Comparative analysis of microarray normalization procedures: effects on reverse engineering gene networks. Bioinformatics. 2007;23:i282–i288. doi: 10.1093/bioinformatics/btm201. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev R, Uz T, Manev H. Fluoxetine increases the content of neurotrophic protein S100beta in the rat hippocampus. Eur J Pharmacol. 2001;420:R1–R2. doi: 10.1016/s0014-2999(01)00989-x. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Morcuende S, Gadd CA, Peters M, Moss A, Harris EA, Sheasby A, Fisher AS, De Felipe C, Mantyh PW, Rupniak NM, et al. Increased neurogenesis and brain-derived neurotrophic factor in neurokinin-1 receptor gene knockout mice. Eur J Neurosci. 2003;18:1828–1836. doi: 10.1046/j.1460-9568.2003.02911.x. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Bos M, Jenck F, Martin JR, Mortas P, Wichmann J. 5HT2C receptor agonists exhibit antidepressant-like properties in the anhedonia model of depression in rats. Eur Neuropsychopharmacol. 1996;6:169–175. doi: 10.1016/0924-977x(96)00015-6. [DOI] [PubMed] [Google Scholar]
- Mutiara S, Kanasaki H, Harada T, Miyazaki K. Dopamine D(2) receptor expression and regulation of gonadotropin alpha-subunit gene in clonal gonadotroph LbetaT2 cells. Mol Cell Endocrinol. 2006;259:22–29. doi: 10.1016/j.mce.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M, Wieronska J. Antidepressant-like activity of amisulpride in two animal models of depression. J Psychopharmacol. 2000;14:46–52. doi: 10.1177/026988110001400106. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. New York: Dover; 1927. [Google Scholar]
- Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19:368–375. doi: 10.1093/bioinformatics/btf877. [DOI] [PubMed] [Google Scholar]
- Renoldi G, Invernizzi RW. Blockade of tachykinin NK1 receptors attenuates stress-induced rise of extracellular noradrenaline and dopamine in the rat and gerbil medial prefrontal cortex. J Neurosci Res. 2006;84:961–968. doi: 10.1002/jnr.20997. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Conditioned inhibition of fear resulting from negative CS-US contingencies. J Comp Physiol Psychol. 1969;67:504–509. doi: 10.1037/h0027313. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Summation and retardation tests of latent inhibition. J Comp Physiol Psychol. 1971;75:77–81. doi: 10.1037/h0030694. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Leon KS, Perez DL, Kandel ER. Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron. 2005;46:309–320. doi: 10.1016/j.neuron.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Weisskopf MG, Huang YY, Kandel ER, Le Doux JE. Long-term potentiation in the amygdala: Implications for memory. In: Holscher C, editor. Neural Mechanisms of Memory Formation: concepts of long-term potention and beyond. Cambridge: Cambridge University Press; 2001. pp. 58–76. [Google Scholar]
- Russo-Neustadt AA, Alejandre H, Garcia C, Ivy AS, Chen MJ. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology. 2004;29:2189–2199. doi: 10.1038/sj.npp.1300514. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hampton L, Battey JF, Dulac C, Kandel ER, Bolshakov VY. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002;111:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- Silva AB, Aw D, Palmer DB. Evolutionary conservation of neuropeptide expression in the thymus of different species. Immunology. 2006;118:131–140. doi: 10.1111/j.1365-2567.2006.02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Eckersell C, Quezada V, Norell A, Micevych P. Preproenkephalin mRNA levels are regulated by acute stress and estrogen stimulation. Physiol Behav. 2000;69:425–432. doi: 10.1016/s0031-9384(99)00261-9. [DOI] [PubMed] [Google Scholar]
- Singh A, Lucki I. Antidepressant-like activity of compounds with varying efficacy at 5-HT1A receptors. Neuropharmacology. 1993;32:331–340. doi: 10.1016/0028-3908(93)90153-t. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wiertelak EP, Maier SF, Watkins LR. Cholecystokinin antianalgesia: safety cues abolish morphine analgesia. Science. 1992;256:830–833. doi: 10.1126/science.1589765. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Kee N. BrdU assay for neurogenesis in rodents. Nat Protoc. 2006;1:1399–1405. doi: 10.1038/nprot.2006.224. [DOI] [PubMed] [Google Scholar]
- Yu J, Liu Q, Wang YQ, Wang J, Li XY, Cao XD, Wu GC. Electroacupuncture combined with clomipramine enhances antidepressant effect in rodents. Neurosci Lett. 2007;421:5–9. doi: 10.1016/j.neulet.2007.02.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Doublecortin – a marker for immature neurons is absent in the dentate gyrus of x-irradiated mice.
Doublecortin immunohistochemistry in the dentate gyrus 6 weeks after treatment with either A. sham or B. hippocampal x-irradiation (40X). Absence of doublecortin (a marker for immature neurons) immunostaining is observed in x-irradiated mice.
Supplemental Figure S2. Dopamine D2 receptor are not involved in the acquisition and Substance P is not involved in the expression of learned safety.
A. Effect of sulpiride administration during training on the CS response in the memory recall test (n = 7–9 per group) (interaction between phase of testing and type of training F(1,33) = 42.523: p<0.001). “CS-effect” (learned safety vehicle, learned safety Sulpiride, learned fear vehicle: p<0.001; learned fear sulpiride: p<0.01). B. Effect of application of quinprinole during training on the CS response in the memory recall test (n = 7 per group) (interaction between phase of testing and type of training F(1,28) = 37.373: p<0.001). “CS-effect” (learned safety vehicle, learned fear vehicle, learned fear quinprinole: p<0.01; learned safety quinprinole: p<0.001). C. Effect of L703,606 application before the memory recall test (n = 7 per group) (effect of interaction between phase of testing and type of training F(1,28) = 39.597: p<0.001). “CS-effect” (learned safety vehicle, learned safety L703,606; learned fear L703,606: p<0.001, learned fear vehicle: p<0.01). D. Effect of [Sar9, Met(O2)11]-Substance P application before the memory recall test (n = 7–9 per group) (effect of interaction between phase of testing and type of training F(1,28) = 59.913: p<0.00). “CS-effect” (learned safety vehicle, learned safety [Sar9, Met(O2)11]: p<0.001; learned fear [Sar9, Met(O2)11], learned fear vehicle: p<0.01). All data are depicted as mean +/− SEM. * p<0.05, **p<0.01, ***p<0.0001, n.s. (not significant) p>0.05.