Theoretical considerations
The factor V Leiden (FVL) mutation is the most prevalent inheritable risk factor for venous thromboembolism (VTE)1. Heterozygous carriers of FVL have an approximately 3- to 5-fold increased risk of VTE, whereas the risk in homozygous carriers is estimated to be up to 80-fold higher than that in subjects without FVL. The absolute incidence of VTE in patients with FVL ranges from 0.19% per year to 0.45% per year, compared to 0.10% per year in individuals without the mutation2, and heterozygous FVL can be identified in approximately 15–20% of VTE patients3. FVL also bestows a small, increased risk of recurrent VTE (∼1.4-fold)4. Importantly, the vast majority of FVL carriers do not develop VTE and their absolute thrombotic risk depends on other inherited thrombophilic mutations (rare) or acquired (more frequent) high-risk situations, such as older age, pregnancy, immobilisation, prolonged travel, major or orthopaedic surgery, cancer, use of oral contraceptives and hormone replacement therapy5. For instance, the risk of VTE in a young woman with FVL and no family history of VTE is very low, approximating 6/10,000 carriers per year, but her risk increases 5-fold if she is taking oral contraceptives. In addition, although FVL may increase the risk of recurrent foetal loss and has been associated with an increased risk of severe pre-eclampsia, placental abruption, unexplained intrauterine foetal growth retardation, and stillbirth, these associations are debated6–9. Contrasting with the negative associations of FVL in women, the possibility of FVL providing an evolutionary advantage has also been raised10.
FVL is present in 3–7% of otherwise normal individuals in the Caucasian population, but is rare in Asians and Africans11,12. Within the Caucasian population, there is no difference in the incidence of FVL in males and females11.
According to the American College of Medical Genetics13, testing for FVL should be performed in the following circumstances: first VTE before 50 years of age, first VTE over 50 years of age in the absence of malignancy, venous thrombosis in unusual sites (such as hepatic, mesenteric and cerebral veins), recurrent VTE, first VTE and a strong family history of VTE, VTE during pregnancy, in the post-partum period or in women taking oral contraceptives or under hormone replacement therapy, women with unexplained pregnancy loss and asymptomatic adult family members of relatives with documented FVL. Critically, FVL testing is not recommended as a general screening test, as a routine initial test during pregnancy, before use of oral contraceptives or hormone replacement therapy, or as a routine initial test in patients with arterial thrombosis13,14.
Practical realities
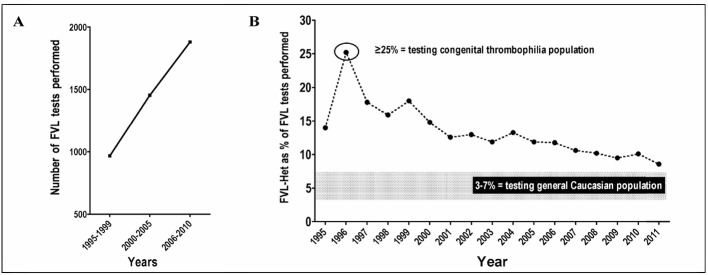
With the above background in mind, it is worthwhile considering how FVL is actually investigated in the real world, in part using data from our own institution as a case study. Our Pathology Institution obtains samples for testing from most of our state, but most notably services Westmead Hospital, a 1,000-bed tertiary level academic teaching hospital with a wide range of services, including emergency, intensive care and maternity. FVL test requests in our institution over the past 15 years appear to be increasing (Figure 1A) while, conversely, the detection rate of FVL heterozygote cases (as a percentage of total tests) is falling sharply (Figure 1B). Thus, it appears that clinical requests were representative of a congenital thrombophilia population in 1997, but thereafter there has been an increasing trend to more general testing of FVL, approaching the expected incidence within the background Caucasian population.
Figure 1.
A: Increasing test requests for FVL at our institution, shown as 5-year averages. B: Decreasing rate of FVL heterozygote detection during the same period.
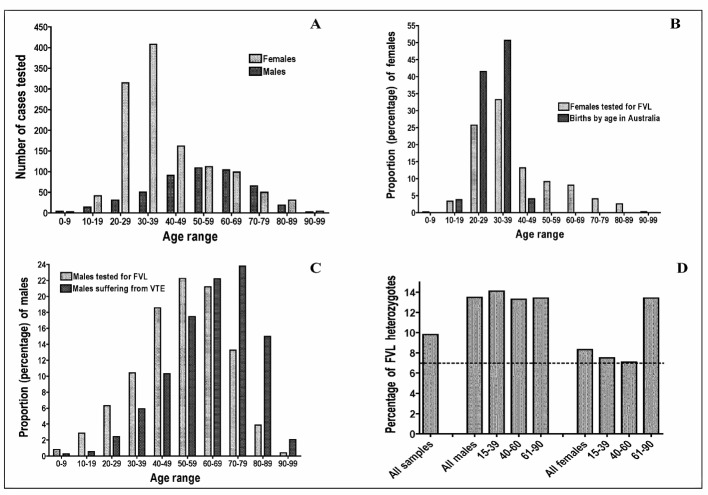
An audit of testing over the past year has identified other interesting trends. Most of the current test requests are for females (71.5% of current requests) and, according to age, predominantly for younger females (Figure 2A); interestingly, test requests for men are, instead, for older males (Figure 2A). Thus, according to clinical suspicion, FVL appears to be primarily a “curse” for young women and for old men. However, these FVL investigation trends are similar to other trends that can be identified, namely births by age (for women; Figure 2B)15, and age-related VTE rates (for males; Figure 2C)16. Thus, the current clinical ordering pattern for FVL appears simply to be following pregnancy trends in women and VTE occurrence trends in males. Finally, identification of FVL detection according to gender and age in this recently tested cohort (Figure 2D) identifies peaks of FVL detection in young men and in old women. Accordingly, contrary to previously noted clinical perceptions of FVL being a “curse” for young women and for old men, FVL appears instead to be a “curse” for old women and for young men.
Figure 2.
A: Age- and gender-related trends for investigation of FVL at our institution; peak age ranges for are 20–39 for females and 50–69 for males. B: Age-related peaks of testing for FVL in females closely follow peak trends for age-related births. C: Age-related peaks of testing for FVL in males closely follow peak trends for age related thrombosis. D: FVL heterozygote detection rate according to age and gender; peak age ranges for detection of FVL are >60 years in females and <40 years in males.
In summary, although there are several theoretical reasons to support selective FVL testing in thrombophilic populations, clinicians are simply failing to follow appropriate guidelines, and are instead requesting FVL testing according to their own targets - fertile women and older males. That thrombophilia testing, including FVL, is broadly and inappropriately requested has been previously highlighted by our laboratory as well as others17–21. There is no doubt that the large majority of test requests are inappropriate, with 91% of thrombophilia requests being identified as inappropriate in one study20. It is important to recognise that identification of FVL in otherwise healthy (non-thrombotic risk patients) will lead to significant adverse psychological and insurance/employment risk issues17,22. Alternatively, negative FVL test results in symptomatic patients may lead to clinicians incorrectly “refraining” from providing appropriate advice regarding antithrombotic measures21.
Factor V Leiden and blood donors
Another interesting field of potential application for FVL testing, of particular interest to this journal’s readership, is the selection of blood donors23, since knowledge of FVL status could in theory influence suitability for blood donation. For example, individuals homozygous for FVL or with double heterozygosity for FVL and another inherited thrombophilic risk factor and a positive history for VTE cannot donate whole blood or an apheresis sample, while those with a negative history for VTE are allowed to donate whole blood (with elimination of the plasma component) but not apheresis24. This practice is in part aimed to safeguard the health of blood donors because a few case reports have described thrombotic complications in thrombophilic donors. However, this practice represents a theoretical risk for FVL status, and to our knowledge there has never been an actual reported incident of any thrombotic complication arising from any blood donation or apheresis collection that could be conclusively linked to FVL status.
Naturally, one could also in theory consider the potential advantage of purposely selecting FVL positive plasma as a kind of “super-prohaemostatic” agent in selected applications. One wonders if a randomised trial of a head-to-head comparison of the haemostatic efficacy of FVL-negative versus FVL-positive plasma replacement therapy in selected cohorts at risk of bleeding has ever been considered?
Conclusion
The discovery of FVL has provided a great advance in the understanding of the molecular mechanisms leading to VTE. However, for clinical utility, the diagnostic thrombophilic work-up including FVL testing has to be tailored to individual patient’s characteristics. Unfortunately, evidence indicates that FVL testing is requested inappropriately more often than it is not. Apart from the adverse psychological and insurance/employment issues attributable to identification of FVL in otherwise healthy (non-thrombotic risk patients), one also wonders if clinicians are then inappropriately managing patients thus identified, and “treating” their FVL using anticoagulant therapy. Given that FVL is present in 3–7% of otherwise normal Caucasian individuals, most of whom will never suffer a VTE, this indicates a high potential for adverse outcomes, notably serious bleeding, related to anticoagulant therapy use.
With regards to blood donors, given that the risk of thrombotic complications according to FVL status is purely theoretical, we cannot see how the practice of enforced FVL screening can be justified. Apart from the cost, enforced FVL screening actually provides for added risks for a large number of donors - those who are asymptomatic but then found to be FVL positive: are donors advised of their FVL positive status, and if so, what consideration is given to the adverse psychological and insurance/employment issues arising?
In conclusion, FVL testing is more likely to have a negative impact on the health care of the people being tested, as well as the health care of their family members, rather than the positive impact that is promised by theoretical considerations, thereby leading us to ponder on the general futility of FVL testing.
Footnotes
The Author declares no conflicts of interest.
References
- 1.Coppola A, Tufano A, Cerbone AM, Di Minno G. Inherited thrombophilia: implications for prevention and treatment of venous thromboembolism. Semin Thromb Hemost. 2009;35:683–94. doi: 10.1055/s-0029-1242722. [DOI] [PubMed] [Google Scholar]
- 2.Rosendaal FR, Reitsma PH. Genetics of venous thrombosis. J Thromb Haemost. 2009;7(Suppl. 1):301–4. doi: 10.1111/j.1538-7836.2009.03394.x. [DOI] [PubMed] [Google Scholar]
- 3.Rees DC. The population genetics of factor V Leiden. Br J Haematol. 1996;95:579–86. doi: 10.1046/j.1365-2141.1996.d01-1954.x. [DOI] [PubMed] [Google Scholar]
- 4.Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166:729–36. doi: 10.1001/archinte.166.7.729. [DOI] [PubMed] [Google Scholar]
- 5.Franchini M, Veneri D, Salvagno GL, et al. Inherited thrombophilia. Crit Rev Clin Lab Sci. 2006;43:249–90. doi: 10.1080/10408360600552678. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Miletich JP, Buring JE, et al. Factor V Leiden mutation as a risk factor for recurrent pregnancy loss. Ann Intern Med. 1998;128:1000–3. doi: 10.7326/0003-4819-128-12_part_1-199806150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Kupferminc MJ, Eldor A, Steinman N, et al. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med. 1999;340:9–13. doi: 10.1056/NEJM199901073400102. [DOI] [PubMed] [Google Scholar]
- 8.Rath W. Pre-eclampsia and inherited thrombophilia: a reappraisal. Semin Thromb Hemost. 2011;37:118–24. doi: 10.1055/s-0030-1270337. [DOI] [PubMed] [Google Scholar]
- 9.de Maat MP, de Groot CJ. Thrombophilia and preeclampsia. Semin Thromb Hemost. 2011;37:106–10. doi: 10.1055/s-0030-1270335. [DOI] [PubMed] [Google Scholar]
- 10.Franchini M, Lippi G. Factor V leiden in women: a thrombotic risk factor or an evolutionary advantage? Semin Thromb Hemost. 2011;37:275–9. doi: 10.1055/s-0031-1273091. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA. 1997;277:1305–7. [PubMed] [Google Scholar]
- 12.Montagnana M, Favaloro EJ, Franchini M, et al. The role of ethnicity, age and gender in venous thromboembolism. J Thromb Thrombolysis. 2010;29:489–96. doi: 10.1007/s11239-009-0365-8. [DOI] [PubMed] [Google Scholar]
- 13.Grody WW, Griffin JH, Taylor AK, et al. ACMG Factor V. Leiden Working Group American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med. 2001;3:139–48. doi: 10.1097/00125817-200103000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitlatch NL, Ortel TL. Thrombophilias: when should we test and how does it help? Semin Respir Crit Care. 2008;29:25–39. doi: 10.1055/s-2008-1047560. [DOI] [PubMed] [Google Scholar]
- 15.Australian Bureau of Statistics. 33010DO007_2010 Births, Australia, 2010. Available at: http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3301.02010?OpenDocument. Accessed on 18 May 2012.
- 16.Access Economics. The burden of venous thromboembolism in Australia. May, 2008. Report for the Australia and New Zealand Working Party on the Management and Prevention of Venous Thromboembolism. Available at: http://www.mpdgp.com.au/files/docs/laos%20recommendations/prevention%20of%20venus%20thromboembolism/the%20burden%20of%20venous%20thromboembolism%20in%20australia.%20access%20economics.pdf. Accessed on 18 May 2012)
- 17.Favaloro EJ, McDonald D, Lippi G. Laboratory investigation of thrombophilia: the good, the bad, and the ugly. Semin Thromb Hemost. 2009;35:695–710. doi: 10.1055/s-0029-1242723. [DOI] [PubMed] [Google Scholar]
- 18.Favaloro EJ, Mohammed S, Pati N, et al. A clinical audit of congenital thrombophilia investigation in tertiary practice. Pathology. 2011;43:266–72. doi: 10.1097/PAT.0b013e328344e5fc. [DOI] [PubMed] [Google Scholar]
- 19.Favaloro EJ, Reben R, Mohammed S, Koutts J. A clinical audit of antiphospholipid antibody testing in tertiary practice. Towards improved relevance in thrombophilia investigations? Internal Med J. 2012;42:427–34. doi: 10.1111/j.1445-5994.2010.02329.x. [DOI] [PubMed] [Google Scholar]
- 20.Tientadakul P, Chinthammitr Y, Sanpakit K, et al. Inappropriate use of protein C, protein S, and antithrombin testing for hereditary thrombophilia screening: an experience from a large university hospital. Int J Lab Hematol. 2011;33:593–600. doi: 10.1111/j.1751-553X.2011.01332.x. [DOI] [PubMed] [Google Scholar]
- 21.Blinkenberg EØ, Kristoffersen AH, Sandberg S, et al. Usefulness of factor V Leiden mutation testing in clinical practice. Eur J Hum Genet. 2010;18:862–6. doi: 10.1038/ejhg.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varga EA, Kerlin BA, Wurster MW. Social and ethical controversies in thrombophilia testing and update on genetic risk factors for venous thromboembolism. Semin Thromb Hemost. 2008;34:549–61. doi: 10.1055/s-0028-1103366. [DOI] [PubMed] [Google Scholar]
- 23.Gessoni G, Valverde S, Canistro R, Manoni F. GeneXpert in the diagnosis of risk factors for thrombophilia: evaluation of its use in a small laboratory. Blood Transfus. 2012;10:228–9. doi: 10.2450/2011.0077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Consulta Tecnica Permanenet per il Sistema Trasfusionale Regionale. Protocollo per la selezione del donatore di sangue ed emocomponenti. Regione Emilia-Romagna 1 ottobre 2009. Available at: http://www.avis.it/repository/cont_schedemm/4591_documento.pdf