Abstract
Confluent cell monolayers in tissue culture are fragile and can easily be mechanically disrupted, often leaving an area devoid of cells. This opening in the cell sheet is then repopulated, because the cells on the fringe of the damage, which are no longer contact-inhibited, move into the available space. This mechanical disruption is often done deliberately in a “wound-healing” assay as a means to assess the migration of the cells. In such assays, a scrape is made in the cell layer followed by microscopy to monitor the advance of the cells into the wound. We have found that these types of assays can also be accomplished electrically. In this approach, cells growing on small electrodes and monitored by using electric cell-substrate impedance sensing are subjected to currents, resulting in severe electroporation and subsequent cell death. After this invasive treatment, the electrode's impedance is again monitored to chart the migration and ultimate healing of the wound. We report here that this procedure to study cell behavior is both highly reproducible, quantitative, and provides data similar to that acquired with traditional measurements.
The dynamic behavior of cells in culture has been the subject of research over several decades. When cells in culture encounter appropriate surfaces, they commonly form adhesive contacts to the surface and alter their morphology by forming a flattened or spread cell shape. In this morphology, many cells begin to crawl about by means of the various components of their cytoskeleton. These migration activities of cells in vitro are thought to be related to many in vivo cellular behaviors, including the directed migration of cells during differentiation and the aberrant invasive activities of metastatic tumor cells.
Wound-healing assays have been carried out in tissue culture for many years to monitor cell behavior, including appraising the migration and proliferative capacities of different cells under various culture conditions. These assays generally involve first growing cells to form a confluent monolayer. The layer is then disrupted (wounded) by destroying or displacing a group of cells, often by scratching the layer with a needle or micropipette tip (1). Once a wound is achieved, the open area is then microscopically observed over time to assess the rate at which the neighboring cells are filling in the damaged area (healing). This repair can take from several hours to >1 day, depending on the cell type, medium conditions, and, of course, the extent of the wounded region. The results may be conveyed by a series of photomicrographs, or in more sophisticated measurements, the microscopic views may be subjected to image processing such that data can be expressed in more quantitative terms (2). From these data, one can calculate a migration or repopulation rate. Traditional assays of this sort require extensive manipulation of the cultured cells, both in carrying out the wound and in after the subsequent repair. As might be expected, when the wounded area is not precisely controlled, the method is encumbered with problems of quantification and reproducibility.
In this article, we describe an alternative method to obtain these types of cell migration data by using an electrical means to both wound and follow the subsequent healing process. The method is largely based on an established process to monitor cell behavior referred to as electric cell-substrate impedance sensing (ECIS). In ECIS, cells are grown on a small gold film electrode (5 × 10-4 cm2) deposited on the bottom of a tissue culture well; a much larger counter electrode completes the circuit by using standard tissue culture medium as an electrolyte. A weak (<1μA) ac signal (usually in the frequency range from 1 to 40 kHz) is applied to the system. This small current results in a voltage drop across the small electrode of only a few millivolts. The electrical current and resulting voltage drop has no detectable effect on the cells; the measurement is noninvasive. The cells, however, cause substantial changes in the system's impedance. Impedance changes have been used to monitor cell attachment and spreading (3), cell locomotion and micromotion (4), and changes in cell morphology in response to physical and biochemical changes (5, 6). Furthermore, these measurements can be coupled with assays as a means to measure the toxicity of compounds (7), signal transduction (8), and the invasive activities of transformed cells in culture (9).
The idea of using an electrical means to wound confluent cell layers coupled with ECIS measurements was first reported in 1997 (10). In this work, a DC pulse was used and observed to reduce the impedance of the ECIS microelectrode to that of a cell-free measurement. Normal ECIS measurements were then used to monitor what was assumed to be the repopulation of the wounded area. In this work, the DC signal was essentially uncontrolled, and the fate of the cells receiving the pulse not fully determined. We have since carried out more refined experiments, where the DC signals are controlled by charging a capacitor and discharging it through both the ECIS electrodes and an external series resistance to control the time constant and size of the pulse (C.R.K. and I.G., unpublished work). In these experiments, we have observed problems that differ, depending on the polarity of the wounding current. If the small active ECIS electrode is positive, the extent of the wound is not controlled and cell killing extends beyond the perimeter of the small electrode, presumably due to the electrochemical generation of toxic species such as chlorine. On the other hand, if the polarity is reversed, the small ECIS electrode and/or the insulating film delineating the electrode area is damaged in the process, making measurements uncertain.
We have now circumvented these problems associated with DC currents by using ac wounding currents in the milliampere range at relatively high frequency. Under these conditions, and by using standard ECIS measurements to follow the cell fate, we have found the extent of the wound is restricted to the small 250-μm-diameter electrode without any observable electrode damage, even when the high current is applied for relatively long times. The method carried out under these ac conditions is highly reproducible and returns cell migration data comparable to that obtained with more traditional wound-healing protocols.
Coupling these invasive and noninvasive modalities, the ECIS system comprises an entirely automated means to carry out wound-healing assays. In this article, we characterize the method and address the mechanism of electrical cell wounding and the fate of the killed cells.
Materials and Methods
ECIS. The ECIS Model 100 or Model 1600R (Applied BioPhysics) was used for this work. To study cell behavior with this instrument, cells are grown in culture wells containing gold film surface electrodes; ordinary culture medium serves as the electrolyte. In its normal mode, an approximate constant current source applies an ac signal of 1μA, usually at 4 kHz, between a small measuring electrode (250-μm diameter) and a large counter electrode. The instrument monitors both the voltage across the electrodes and its phase relative to the applied current. In addition to reporting total impedance, these data are converted to resistance and capacitance treating the cell-electrode system as a series RC circuit. As the cells attach and spread on the small electrode, their membranes constrict the current forcing it to flow beneath and between the cells, resulting in large increases in impedance. The microampere current and the resulting voltage drop of a few millivolts have no measurable effect on the cells, and hence, the monitoring of cell behavior is noninvasive (4).
The instrumentation can be also run in a mode that is capable of electroporation of the cells attached to the small electrode (11, 12). To accomplish this invasive activity, the cell-covered electrode is placed in series with a 1,000-Ohm resistor connected to an ac source. When the source provides signals of a few volts, a relatively high current is delivered to the cell-covered electrode (several hundred microamperes). Depending on the ac frequency, this current produces a voltage drop across the plasma membranes of the order of a few volts, which is sufficient for membrane poration. The instrument controls the frequency, voltage, and duration of these high-field applications. For electroporation, the duration is generally a few hundred milliseconds; for the wounding reported here, the elevated field is applied for up to tens of seconds, resulting in cell killing. These current pulses are usually applied at 40 kHz, because this higher frequency results both in higher electric fields across the cell layer and fields that are more uniform over the extent of the cell.
Cell Culture Procedures. BS-C-1 (African green monkey kidney cells; CCL-26), NRK (normal rat kidney cells; CRL-6509) and MDCK (Madin–Darby canine kidney cells; CLL-34) were obtained from the American Type Culture Collection. These were cultured in DMEM (Sigma) containing 10% FBS and 50 μg/ml gentamicin in a 5% CO2 high-humidity atmosphere at 37°C; cell lines were passaged with trypsin/EDTA. For ECIS studies, cells were taken from slightly subconfluent cultures, and a monodisperse cell suspension at 5 × 105 cells per ml was prepared by using standard tissue culture techniques.
Preparation and Inoculation of Electrodes. ECIS electrode arrays (8W1E) were obtained from Applied BioPhysics. Each array slide consists of eight individually addressable wells with surfaces treated for cell culture. On the base of each well is the small active electrode and the large counter electrode; custom arrays with smaller active electrodes were also from Applied BioPhysics. Wells have a maximum volume of ≈600 μl with 0.8 cm2 of substrate area for cell culture.
Before starting ECIS measurements, 200 μl of complete medium was placed in each well and was allowed to equilibrate in the incubator for ≈30 min. To inoculate the wells, 200 μl of cell suspension (5 × 105 cells per ml) were added to each well, resulting in a final surface concentration of 1.25 × 105 cells per cm2 and a volume of 400 μl of medium.
After cell inoculation, the wells were incubated overnight, while attachment and spreading were followed by means of impedance. Impedance levels were used to verify that confluence was achieved and maintained and that the cells exhibited normal levels of impedance fluctuations, which is indicative of healthy cell layers (7). The confluent cell layers were generally incubated for 1 day before wounding.
Vital Staining. The live/dead viability/cytotoxicity kit was from Molecular Probes. Samples were prepared 30 min after the application of the elevated field pulse by incubating cells in Earle's balanced salt solution (EBSS) supplemented with 4 μM ethidium homodimer and 2 μM calcein acetoxymethyl ester for 45 min at 37°C. The dye solution was then replaced with EBSS for microscopic inspections.
Results and Discussion
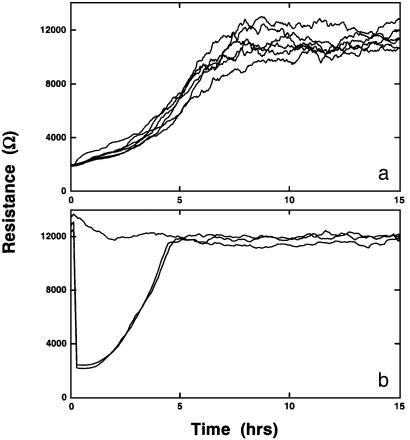
Fig. 1a shows the resistive portion of the impedance measured at 4 kHz for identical inoculations of BS-C-1 cells in six different wells. Cells are added at time 0, and as the cells attach and spread, there is a large increase in the resistance as current from the small active electrode is constrained by the insulating membranes of the cells. Once the confluent monolayer is established (≈8 h), the resistance is observed to level and fluctuate with time, due to movements in the cell monolayer (4). These fluctuations are observed with all cell types that have been monitored with ECIS and are indicative of living cells. Note that the initial resistance (time 0) at 4 kHz of an open electrode is ≈2,000 Ohms.
Fig. 1.
(a) The resistive portion of the impedance at 4 kHz during inoculation of BS-C-1 cells in six identical wells. Cells were added at time 0 to yield an initial surface concentration of 1.25 × 105 cells per cm2. (b) Typical electrical wound-healing data. BS-C-1 cells were grown to confluence in three individual wells before the measurement was begun. Approximately 10 min into the run, data acquisition was briefly suspended, and two of the wells received an elevated field pulse (see text for details), one for 10 sec and the other for 30 sec; data acquisition was then restarted. The resistive portion of the impedance at 4 kHz is shown for the two experimental wells with the other portion serving as a control.
Fig. 1b shows the basic wound-healing measurement. Here, the resistive portion of the impedance of three different wells with established confluent BS-C-1 cell layers is monitored over time. Shortly into this run, the acquisition of data is paused, and two of the wells receive the elevated current pulse. In this case, a 3-V signal at 40 kHz is applied for a duration of 10 sec in one well and 30 sec in the other. The application of this field results in a very rapid drop in the resistance of cell layers to that associated with the cell-free electrode. In the next few hours, this resistance is observed to increase back to the levels of the cell-covered electrode due to the migration of cells from the perimeter of the electrode inward to replace the killed cells. Note that the curves for both the 10- and 30-sec elevated field pulse are essentially identical.
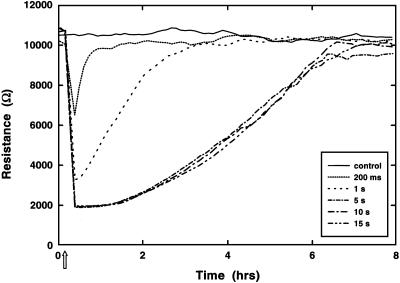
In Fig. 2, a similar experiment was conducted, where the duration of the high-field pulse was varied from 200 msec to 15 sec to appraise the threshold conditions required to achieve complete wounding in BS-C-1 cell layers. By using a 2.5-V pulse at 40 kHz, a 200-msec application has been previously shown to result in electroporation of the cells on the active electrode (12). Here, this pulse results in a noticeable drop in the resistive portion of the impedance but only to ≈6,500 Ohms, which is far from the 2,000-Ohm value commonly associated with the open electrode at this frequency. The transient drop in resistance is assumed to be the response of the cells to membrane poration, and subsequent repair of the membranes and recovery of the injured cells returns the impedance to the control level. As the duration of the high-field application is increased, the drop in impedance becomes more severe. With an application for 1.0 sec, the resistance drops nearly 3,000 Ohms and returns to control levels in ≈2 h. These curves may reflect a partial killing of the cells followed by some cell migration to repopulate the electrode or they may still essentially be reporting cell injury and recovery over time. When the high-field application reaches 5 sec, the damage to the cell layer seems complete, as resistance values drop to that of the open electrodes. Recovery now requires slightly >5 h, which is approximately the same time as observed in Fig. 1b for these cells under similar conditions. If the high field is applied for 10 or 15 sec, the curves are identical to that recorded for the 5-sec pulse. We believe cell death and subsequent repopulating of the electrode area by means of cell migration accounts for these results. As also suggested in Fig. 1b, once the threshold pulse duration is reached, it appears that wounding and repopulating of the electrode is independent of the high-field duration. This finding indicates that the wounded area is not enlarged with extended application of the field, but rather remains confined to the small ECIS electrode. Hence, this approach provides a highly reproducible means to wound an area of defined extent without the need for precise monitoring.
Fig. 2.
The response of cells to different time exposures of an elevated field. BS-C-1 cells were grown to confluence in six wells before measurements began. The cells in different wells received an elevated field pulse (2.5 V at 40 kHz) for times of 0.20, 1.0, 5.0, 10, and 15 sec; the sixth well served as a control. The resistive portion of the impedance at 4 kHz is shown. The arrow marks the application of the elevated field pulses.
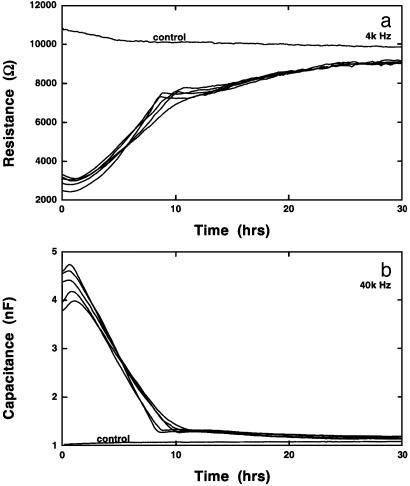
These wound-healing curves are highly dependent on the type of cell being cultured. In the data presented in Fig. 3, NRK cells were grown to confluence, and five identical cultures were wounded with elevated electric fields for various times. The healing/migration were subsequently followed; one culture well remained as a control. The conditions for wounding were similar to those used for BS-C-1 cells; the oscillator signal was set at 2.5 V at 40 kHz, with pulse duration ranging from 10 sec to 3 min. Fig. 3 shows the time course changes in the resistance measured at 4 kHz (Fig. 3a) and the capacitance measured at 40 kHz (Fig. 3b). Several things are apparent from these curves. The time required for the resistance to reach the starting values of the confluent layer is considerably longer than that for BS-C-1 cells. Wounding occurs just before the start of data acquisition, and the resistance curves require >20 h to return to the level of the control that received no elevated field pulse. The shape of these curves is essentially identical and thus the wounding is independent of the pulse duration, because even the lowest time is considerably above the threshold values for complete cell wounding. At ≈10 h after pulsing, the curves show a change in character, and the final return to control levels is now at a slower rate. This sort of behavior was not observed for other cell lines tested and we can only speculate at this time as to its origin. Additional information, however, is provided in the capacitance trace measured at 40 kHz (Fig. 3b). We have previously pointed out that capacitance measurements at this relatively high frequency is a good indication of the amount of open (cell-free) electrode area (3). On wounding, as the cells lift from the gold electrode, the capacitance increases from ≈1 nF to 4 or 5 nF, which is the capacitance associated with the cell-free electrode. The capacitance then rapidly drops during the migration/healing phase, as the electrode regains its cell cover. By 10 h, this process is nearly complete, with only a very small drop to reach control levels. Together, these curves suggest that NRK cells migrate inward and repopulate the electrode in ≈10 h after wounding, but then subtle alterations take place in the morphology of the cell sheet that are more apparent in the resistance traces. Based on modeling studies, we believe this result is due to the formation of more developed intercellular junctions. Hence, in this particular experiment, the data seem to reveal a two-part healing process. First, the migration of the cells to fill the wounded area followed by a modification of the cell layer resulting in increased barrier function. It should be noted that this behavior was not observed in all experiments with NRK cells and therefore is presumably dependent on the condition and density of the cell layer.
Fig. 3.
Wound-healing assay with NRK cells. NRK cells were grown to confluence in six different wells. Just before the acquisition of data, the cells in five of the wells received an elevated field pulse (2.5 V at 40 kHz) for 10, 30, 60, 120, and 180 sec. The resistive portion of the impedance measured at 4 kHz (a) and the capacitance of the system measured at 40 kHz (b) as a function of time after wounding.
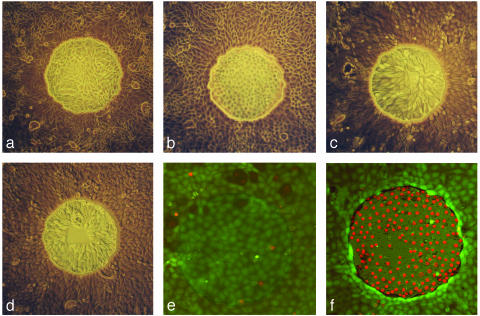
To understand the fate of the wounded cells, microscopic observations of the cells on the active electrode were made before and immediately after wounding. We also observed the cells several hours later after impedance measurements indicated that the healing process was completed. Phase contrast micrographs of the MDCK Type II cell line are shown in Fig. 4 a–d. Fig. 4a shows cells before the high-field application. On wounding, there is a subtle change in the appearance of the cells as they show reduced contrast, with less clearly defined cell boundaries (Fig. 4b). Despite the presence of these cell-like structures, measurements give impedance values associated with totally cell-free electrodes. The explanation for these initially seemingly incongruous results is that the cells have been killed by the elevated field application, resulting in either (i) the lifting of the cell monolayer slightly from substratum or (ii) severe damage of the plasma membranes. In both scenarios, the electrically current would flow freely under or through the dead cells to all regions of the gold electrode without measurable resistance due to the altered cells. To restore the impedance during healing, the migrating cells then either move beneath the lifted cell layer or displace the wounded cells in a snowplow-like fashion.
Fig. 4.
Photomicrographs of cells before and after wounding. (a–d) Phase contrast views of the ECIS electrode (250-μm diameter) with MDCK cells before wounding (a), immediately after wounding pulse (b), and 20 h after wounding (c and d). The wounding pulse was 2.5 V at 40 kHz applied for 30 sec (see text for details). (e and f) Vital staining of NRK cells on the ECIS electrode (250-μm diameter) before and after the elevated field pulse, respectively. The wounding pulse was 3 V at 40 kHz applied for 10 sec (see text for details).
Before these microscopic observations, we had speculated that the wounded cells were rounding and lifting from the electrode, but this occurrence has not been observed for any cell type in this assay. We have carried out other experiments to verify that the cells are in fact killed and replaced in the assay, rather than being transiently damaged, releasing from the substratum and then recovering to establishing a tight monolayer over time. Two very different experimental approaches have been as outlined below.
The results of vital staining experiments of control and electrically wounded NRK cells are shown in Fig. 4 e and f, respectively. The assay is based on double staining with the DNA-intercalating dye, ethidium homodimer (red fluorescence) as a marker for loss of membrane integrity, and the green fluorophore, calcein acetoxymethyl ester, that probes intracellular esterase activity as a measure of cell viability. The staining solution was added 30 min after the application of the wounding pulse (40 kHz for 10 sec at 3 V) to prevent entry of the ethidium through transient pores formed by the high field. Other data (not shown) demonstrated the uptake of ethidium through electroporation pulses (<200 msec) when the dye was added immediately before the pulse. If, however, the dye was added as little as 1 min after the pulse, there was no observable uptake of the dye because the pores had presumably sealed. Accordingly, the use of a 30-min delay is more than adequate to assure entry of ethidium to the nucleus results from permanently compromised plasma membranes. The vital staining again shows that the cell killing is restricted to those cells on or mainly on the small electrode.
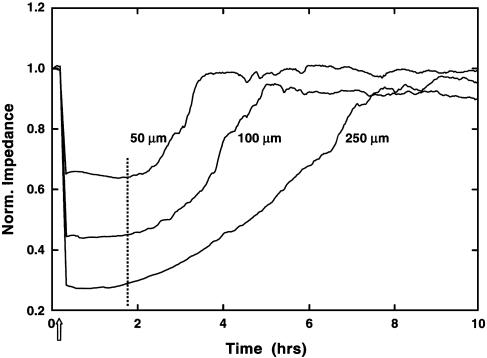
Another experiment eliminating cell recovery as an explanation is shown in Fig. 5. BS-C-1 cells were inoculated into wells having active electrodes of different sizes, namely 50, 100, and 250 μm in diameter. Wounding was accomplished by using a 10-sec pulse with the same oscillator output voltage (3 V at 40 kHz, which is clearly above the wounding threshold level for these cells) for all sized electrodes. Because the interface impedance of the active electrode (for the standard electrode, ≈10 times that of the 1,000-Ohm series resistance) is inversely related to the electrode area, the current will be proportionally reduced causing approximately the same voltage drop across the cell layer. To allow ready comparison of the results for the three different size electrodes, the total impedance is normalized (divided by the value at time 0) for these data. Looking at the data, we see all traces following wounding exhibit a lag period of ≈1.5 h. After this time (at the broken vertical line), impedance begins to increase and plateaus at the starting level of the confluent monolayer. The overall percentage drop for each electrode size results from different levels of constriction resistance (solution resistance above the electrode) that remain unaffected by the presence or absence of the cells. The important point is that the time required for cell healing depends on the size of the active ECIS electrode, whereas were the healing process a recovery of damaged cells, the rates should be identical, which is independent of electrode area. Under the assumption that the cells migrate inward at a fixed change in radial distance per unit time, the curves give an average value for the migration rate of 18 μm per hour with good agreement between the three situations. This rate is also in good agreement with independent microscopic measurements of cell migration in BS-C-1 monolayers after traditional scrape wounding by using a micropipette tip (data not shown).
Fig. 5.
Wound-healing assays on different size electrodes. Electrode arrays were supplied with active electrodes that were 250 (standard ECIS arrays), 100, and 50 μm in diameter. BS-C-1 cells grown to confluence were wounded at the arrow as described in the text. The impedance measured at 4 kHz and normalized to its value at the start of data acquisition is plotted as a function of time.
In addition to these two approaches, there are general microscopic observations supporting the cell killing and migratory activities of the cells from the perimeter of the wound. Often, on the completion of the wounding/healing process, a small aggregate or flap of tissue can be seen near or on the cells on the small electrode. We believe this tissue is composed of dead cells released from the area during the healing process. With most cell types, we commonly see radial like patterns in the cells near the electrode periphery, suggesting that they have aligned during the migration process (Fig. 4c). Finally, after wounding it is sometimes possible to selectively remove the dead cells from the electrode by subjecting the entire cell layer to a forceful stream of medium from a pipette. After this removal, we can now observe more traditional wound-healing migration because this open area is gradually covered by cell migration. A well treated in this manner is shown in Fig. 4d a few hours after wounding.
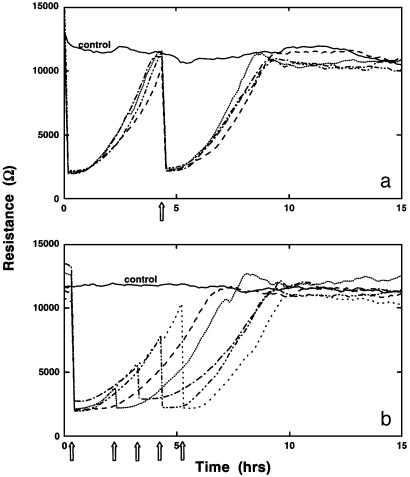
We have investigated the reproducibility of these cell migration assays by using the BS-C-1 cell line that has been the archetypical culture for these assays. In Fig. 6a, cells grown to confluence in four wells are wounded, and on return to control levels, are wounded for a second time, producing essentially identical traces. In Fig. 6b, the same protocol is followed, except the wounding pulses are applied before cell migration is completed, producing the family of very similar curves. In these experimental runs with repeated wounding, the radial patterns described above become even more prominent.
Fig. 6.
Multiple wounding data. BS-C-1 cells were grown to confluence and were wounded at the times indicated by the arrows, using an elevated field pulse of 2.5 V at 40 kHz applied for 15 sec. The resistive portion of the impedance measured at 4 kHz is presented as a function of time.
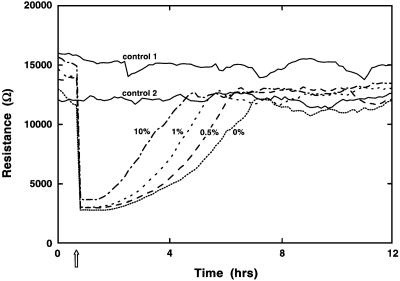
As would be anticipated, the rate at which the impedance returns to control levels after electrical wounding depends on the culture conditions. This conclusion is evident in the healing traces seen in Fig. 7. In this case, BS-C-1 cells were first grown to confluence and were then incubated for ≈20 h in medium containing different levels of FBS. An identical wounding pulse (40 kHz at 3.0 V for 15 sec) is applied to all but the two controls. The resulting curves clearly show that the time required for the healing phase is reduced at higher serum concentrations, presumably due to alterations in cell migration rates.
Fig. 7.
The effect of serum concentration on healing (cell migration). BS-C-1 cells were grown to confluence in complete medium with 10% FBS. Approximately 20 h before wounding, the cells were washed and the medium was replaced with the percentages of serum shown. Wounding was accomplished at the arrow as described in the text. Control 1 and control 2 with 10% and 0% serum, respectively, were not wounded. The resistive portion of the impedance at 4 kHz is shown.
These basic types of assays have now been successfully demonstrated with several cell lines including BS-C-1, NRK, MDCK, and endothelial cells of both bovine and human origin. In these cases, the impedance after wounding drops to that of the cell-free electrode with the subsequent return of impedance to control levels after healing. The method, however, does not work for NIH 3T3 cells, and likely, other cell lines we have not evaluated. On wounding of well established confluent NIH 3T3 cell layers, the impedance fails to fully drop to cell-free levels, and in the healing phase, is unable to fully return to control levels (data not shown). We speculate that on wounding, a portion of the killed cells fail to detach from the substrate, and hence, are able to partially constrain the current flow. On migration of healthy cells from the periphery to replace the cells that do detach, these altered dead cells remain in place and prevent full recovery to control impedance levels. In other experiments, NIH 3T3 cells were inoculated at 105 cells per cm2 to produce confluent cells layers in only a few hours. Once impedance levels indicated confluence had been achieved, these cells were immediately wounded. Under these conditions, the wounding and healing curves nearly resembled those of the other cell lines that we have tested with this assay (data not shown). Hopefully, further experimentation and the discovery of other cell lines that mimic this behavior will shed light on the differences we observe. This information may lead to ways in which this assay can be used for cell lines that exhibit this refractory behavior.
In conclusion, we have demonstrated an electrical method to carry out wound-healing assays in tissue culture, which is applicable for some and possibly most cell lines. The approach returns highly quantitative data regarding cell migration in relatively short times and with a minimum of labor and cell culture manipulations. More experimentation will be required to ascertain the type of cellular injury produced by the elevated electrical fields and currents and to further compare results of this assay with traditional mechanical wounding.
Acknowledgments
We thank Mr. William W. Woo for excellent technical work, Mr. George Edick for useful discussions and for providing access to laboratory equipment, and Dr. George Plopper for critical reading of the manuscript. This work was supported in part pursuant to a contract from the National Foundation for Cancer Research.
Abbreviations: ECIS, electric cell-substrate impedance sensing; NRK, normal rat kidney; BS-C-1, African green monkey kidney.
References
- 1.Pinco, K. A., He, W. & Yang, J. T. (2002) Mol. Biol. Cell 13, 3203-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zornyei, Z., Czirok, A, Vicsek, T. & Madarasz, E. (2000) J. Neurosci. Res. 61, 421-429. [DOI] [PubMed] [Google Scholar]
- 3.Wegener, J., Keese, C. R. & Giaever, I. (2000) Exp. Cell Res. 259, 158-166. [DOI] [PubMed] [Google Scholar]
- 4.Giaever, I. & Keese, C. R. (1991) Proc. Natl. Acad. Sci. USA 88, 7896-7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiruppathi, C., Malik, A., Del Vecchio, P., Keese, C. R. & Giaever, I. (1992) Proc. Natl. Acad. Sci. USA 89, 7919-7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy, L., Wang, H. S., Keese, C. R., Giaever, I. & Smith, T. J. (1998) Exp. Cell Res. 245, 360-367. [DOI] [PubMed] [Google Scholar]
- 7.Keese, C. R., Karra, N., Dillon, B., Goldberg, A. & Giaever, I. (1998) In Vitro Toxicol. 11, 183-192. [Google Scholar]
- 8.Wegener, J., Zink, S., Rosen, P. & Galla, H. J. (1999) Eur. J. Physiol. 437, 925-934. [DOI] [PubMed] [Google Scholar]
- 9.Keese, C. R., Bhawe, K., Wegener, J. & Giaever, I. (2002) BioTechniques 33, 842-851. [DOI] [PubMed] [Google Scholar]
- 10.Noiri, E., Hu, Y., Bahou, W. F., Keese, C. R., Giaever, I. & Goligorsky, M. S. (1997) J. Biol. Chem. 272, 1747-1752. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh, P. M., Keese, C. R. & Giaever, I. (1993) Biophys. J. 64, 1602-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wegener, J., Keese, C. R. & Giaever, I. (2002) BioTechniques 33, 348-357. [DOI] [PubMed] [Google Scholar]