Abstract
Background.
Continuous and efficient error management, including procedures from error detection to their resolution and prevention, is an important part of quality management in blood establishments. At the Croatian Institute of Transfusion Medicine (CITM), error management has been systematically performed since 2003.
Materials and methods.
Data derived from error management at the CITM during an 8-year period (2003–2010) formed the basis of this study. Throughout the study period, errors were reported to the Department of Quality Assurance. In addition to surveys and the necessary corrective activities, errors were analysed and classified according to the Medical Event Reporting System for Transfusion Medicine (MERS-TM).
Results.
During the study period, a total of 2,068 errors were recorded, including 1,778 (86.0%) in blood bank activities and 290 (14.0%) in blood transfusion services. As many as 1,744 (84.3%) errors were detected before issue of the product or service. Among the 324 errors identified upon release from the CITM, 163 (50.3%) errors were detected by customers and reported as complaints. In only five cases was an error detected after blood product transfusion however without any harmful consequences for the patients. All errors were, therefore, evaluated as “near miss” and “no harm” events. Fifty-two (2.5%) errors were evaluated as high-risk events. With regards to blood bank activities, the highest proportion of errors occurred in the processes of labelling (27.1%) and blood collection (23.7%). With regards to blood transfusion services, errors related to blood product issuing prevailed (24.5%).
Conclusion.
This study shows that comprehensive management of errors, including near miss errors, can generate data on the functioning of transfusion services, which is a precondition for implementation of efficient corrective and preventive actions that will ensure further improvement of the quality and safety of transfusion treatment.
Keywords: errors, quality management, near miss
Introduction
Errors in medicine may have fatal consequences for patients. Despite this, the need for systematic error management (detection, recording, error analysis and resolution, and error prevention by analysis of root causes and implementation of appropriate corrective measures) was recognised relatively late in medicine in comparison with high-risk industries. For many years, reports on treatment errors were anecdotal, while the analysis of causes of error was focused mainly on individual factors and only to a lesser extent on organisational and other system factors. Public demands for quality measurement and continuous improvement increased in parallel with the rise in healthcare costs1. These trends significantly influenced faster and wider implementation of quality management systems (QMS) in healthcare institutions, with error prevention by their systematic analysis and use of appropriate corrective actions recognised as important instruments for meeting customer demands for high quality and safer treatment.
Based on the awareness of numerous risks of transfusion treatment and their potentially disastrous consequences, quality has always had a prominent place in transfusion medicine. The emergence of the acquired immunodeficiency syndrome (AIDS) in the 1980s and consequential modifications in the public’s perception of transfusion treatment safety further contributed to QMS implementation in blood establishments all over the world.
Contemporaneously, an array of other measures to improve the product and service’s efficiency, quality and safety in transfusion medicine have been undertaken. With the reduction in other risks, in particular transmission of infectious diseases, errors have been recognised as the leading cause of morbidity and mortality associated with transfusion treatment. Although many reports on errors in transfusion medicine have been published over the years, the majority of them referred to errors with serious or fatal consequences for patients. Recently, however, there has been increasing awareness that such errors represent the tip of an iceberg; successful error management systems should, therefore, also include near miss errors2.
With about 50% of all donations collected at the national level, the Croatian Institute of Transfusion Medicine (CITM) in Zagreb is the largest transfusion institution in the Republic of Croatia. Along with blood bank activities and laboratory testing of patients and pregnant women, the CITM performs a whole array of activities as the Reference Centre for Transfusion Medicine of the Ministry of Health and Social Welfare. In 1997, the commitment of the CITM’s management and staff members to quality resulted in a decision to implement a QMS according to the ISO 9000 set of standards with the integration of the principles of Good Manufacturing Practice (GMP). The quality system was first certified in 2001. Along with implementation of the QMS, the CITM started systematic management of non-conformities in all segments of its activities3, including error management from the very beginning. Given the continuous education and motivation of CITM personnel while fostering the culture of non-punishment, the period of underreporting was relatively short and a considerable body of data on errors in transfusion medicine was collected in several years. In view of the predominance of error reports related to clinical transfusion medicine, these data are very valuable because they mostly refer to blood bank activity and are almost exclusively of the near miss type.
Materials and methods
This study is based on data from the CITM Department of Quality Assurance and derived from error management during an 8-year period (2003–2010). Only errors made by CITM employees were analysed, i.e. human errors made while performing activities within the scope of the responsibility of the CITM, irrespective of whether the error was detected at the CITM or reported by the customer in the form of complaint. Throughout the study period, errors were recorded in non-conformity management forms and forwarded to the Department of Quality Assurance, where the records regarding errors were separated from those regarding other non-conformities to ensure specificity of further analysis and processing. In addition to the site of occurrence within the CITM (organisational unit), all errors reported were classified using the Medical Event Reporting System for Transfusion Medicine (MERS-TM) encoding the working process in which a particular error occurred4–7. The processes with the greatest proportion of errors identified were additionally analysed to determine the groups of errors and their incidence. The reporting of near miss errors was stimulated from the very introduction of the QMS. For the purpose of this study, near miss errors were defined as those errors recognised and corrected before blood product transfusion or before the service caused harm to the user. The Risk Assessment Index (RAI), as described by the MERS-TM, was used for risk calculations6. The RAI is calculated by taking into account the following factors: the severity (or potential severity) of the event; the probability of recurrence; whether or not a product was issued; and the type of recovery (if there was recovery). According to the adopted recommendations, root cause analysis was performed for all errors with a calculated RAI ≥0.5, or when a high risk for the institution was estimated along with a RAI <0.5. The modified Eindhoven Classification System adjusted to MERS was employed in root cause analysis4,6,7. Table I presents the definitions of codes applicable to the present study.
Table I.
| Code | Category | Definition |
|---|---|---|
| HRI | Intervention | Errors due to erroneous task planning and/or performance |
| HRV | Verification | Errors due to inaccurate or incomplete situation assessment |
| HSS | Slip | Errors in high skill performance |
| HRC | Coordination | Inadequate task coordination within the team |
| OP | Protocols/procedures | Inadequate protocol quality and/or availability at blood establishment (too complicated, inaccurate, unrealistic, lacking or poorly presented) |
| OK | Transfer of knowledge | Inappropriate implementation of measures ensuring transfer of knowledge or information to new or inexperienced personnel |
| TD | Technical design | Inappropriate design of material, equipment or software |
The number of blood products destroyed because of errors and their prevalence are presented according to total number of products manufactured during the study period. The proportion of errors detected before and after issuing the product or test findings from the CITM was also analysed. The prevalence of errors detected by the user and reported as complaints was calculated from the total number of errors detected after the product or test finding had been issued from CITM. The number of products and services for which the procedure of withdrawal was initiated for suspected non-conformities caused by errors, and the level of success achieved by these measures were also assessed.
Results
During the study period (2003–2010), a total of 2,068 errors were recorded and analysed. During the same period, 620,107 donations were collected, 1,748,613 blood products manufactured, and various testing services provided for 424,855 patients. The distribution of errors according to the CITM organisational units in which they occurred is presented in Table II. More than half of all errors (54.4%) detected during the study period were recorded at the Department of Blood Collection.
Table II.
Error distribution according to CITM organisational units.
| Department/Service | n | % |
|---|---|---|
| Promotion of blood donation and blood collection | 1,125 | 54.4 |
| Processing, storage, distribution and issuing of blood products | 447 | 21.6 |
| Immunohaematology testing | 119 | 5.8 |
| Patient service | 82 | 4.0 |
| On-duty service | 81 | 3.9 |
| Information technology department | 54 | 2.6 |
| CITM hospital transfusion department 1* | 42 | 2.0 |
| Serological testing for blood-borne diseases | 36 | 1.7 |
| Quality control and quality assurance | 26 | 1.3 |
| CITM hospital transfusion department 2* | 24 | 1.2 |
| Molecular diagnostics | 11 | 0.5 |
| Technical service | 6 | 0.3 |
| Platelet and leucocyte immunohaematology and haemostasis | 4 | 0.2 |
| Manufacture of test reagents | 3 | 0.1 |
| Shared services | 8 | 0.4 |
| Total | 2,068 | 100.0 |
Hospital transfusion departments under the responsibility of the CITM.
Tables III and IV show the error distribution according to the work processes in which the errors occurred, based on the MERS-TM code system6. Errors recorded in blood bank activities are presented in Table III and those that occurred in providing a transfusion service in Table IV.
Table III.
Error distribution according to blood bank work processes.
| MERS-TM code | Location of event | n | % |
|---|---|---|---|
| LA | Labelling | 482 | 27.1 |
| BC | Collections | 422 | 23.7 |
| DR | Donor record data entry | 221 | 12.4 |
| CP | Component processing | 190 | 10.7 |
| TE | Testing | 93 | 5.2 |
| SD | Storage and distribution | 88 | 4.9 |
| IN | Inventory | 83 | 4.7 |
| PD | Product destruction | 12 | 0.7 |
| DD | Subsequent donor suitability | 6 | 0.3 |
| DS | Initial donor suitability | 5 | 0.3 |
| IS | Information services | 4 | 0.2 |
| CR | Collections review | 3 | 0.2 |
| PQ | Product quarantine | 3 | 0.2 |
| CS | Customer services | 0 | 0.0 |
| MI | Miscellaneous | 166 | 9.3 |
| Total | 1,778 | 100.0 |
Table IV.
Error distribution according to transfusion service work processes.
| MERS-TM code | Location of event | n | % |
|---|---|---|---|
| UI | Unit issue | 71 | 24.5 |
| ST | Sample testing | 68 | 23.4 |
| OE | Order entry | 52 | 17.9 |
| US | Unit storage | 29 | 10.0 |
| SC | Sample collection | 21 | 7.2 |
| SE | Unit selection | 5 | 1.7 |
| SH | Sample handling | 5 | 1.7 |
| PR | Patient request | 3 | 1.0 |
| PC | Product check-in | 3 | 1.0 |
| UM | Unit manipulation | 2 | 0.7 |
| AV | Available for issue | 0 | 0.0 |
| UT | Unit transfusion | 0 | 0.0 |
| MS | Miscellaneous | 31 | 10.7 |
| Total | 290 | 100.0 |
A more detailed classification was applied for the work processes in which the highest proportion of errors was recorded. Particular error categories within each group and their incidences are shown in Table V.
Table V.
Error categories within work processes with the highest proportion of errors recorded.
| MERS-TM code | Error | n | % |
|---|---|---|---|
| BC | Clots (inappropriate blood mixing with anticoagulant) | 251 | 59.5 |
| Blood collected into erroneous type of blood bag | 38 | 9.0 | |
| Excess air in the unit | 37 | 8.8 | |
| Sampling (erroneous or lacking) | 35 | 8.3 | |
| Errors in the procedure of apheresis | 15 | 3.6 | |
| Inappropriate puncture site disinfection | 14 | 3.3 | |
| Errors in handling sterile welding device | 13 | 3.1 | |
| Errors in scale handling | 13 | 3.1 | |
| Other | 6 | 1.4 | |
| Total | 422 | 100.0 | |
|
| |||
| CP | Errors in handling devices for blood component separation | 51 | 26.8 |
| Erroneous choice of units for further processing | 46 | 24.2 | |
| Errors in processing technique | 34 | 17.9 | |
| Computer records of manufacture (erroneous or lacking) | 20 | 10.5 | |
| Errors in welding | 10 | 5.3 | |
| Erroneous entry of blood product volume | 7 | 3.7 | |
| Other | 22 | 11.6 | |
| Total | 190 | 100.0 | |
|
| |||
| DR | Donor file duplicates in computer system | 60 | 27.1 |
| Erroneous entry of donation ID number in computer system | 52 | 23.5 | |
| Erroneous entry of donor personal data in computer system | 50 | 22.6 | |
| Errors on entering puncture failure code in computer system | 18 | 8.1 | |
| Erroneous entry of donor card issuing code | 15 | 6.8 | |
| Other | 26 | 11.8 | |
| Total | 221 | 100.0 | |
|
| |||
| LA | Unlabelled donations from female donors | 216 | 44.8 |
| Erroneous blood group on donation label | 79 | 16.4 | |
| Lack of ID number on blood bag | 47 | 9.8 | |
| Lack of label on ASA use | 41 | 8.5 | |
| Blood collection date erroneous or lacking | 18 | 3.7 | |
| ID number lacking on the sample | 16 | 3.3 | |
| Sample erroneously labelled | 15 | 3.1 | |
| Product name erroneously declared | 11 | 2.3 | |
| Blood product volume erroneously declared | 10 | 2.1 | |
| Other | 29 | 6.0 | |
| Total | 482 | 100.0 | |
|
| |||
| UI, SD, US | Blood product issued without computer record | 53 | 28.2 |
| Erroneous data entry in computer system | 37 | 19.7 | |
| Blood product found on stock after expiry date | 22 | 11.7 | |
| Erroneous blood product issued | 18 | 9.6 | |
| Untimely stock updating from hospital transfusion units | 16 | 8.5 | |
| Inappropriate blood product storage | 12 | 6.4 | |
| Issuing reserved blood product | 7 | 3.7 | |
| Other | 23 | 12.2 | |
| Total | 188 | 100.0 | |
A total of 93 errors were recorded in the category of donor testing (code TE). This category refers exclusively to the errors that occur from donor sample receipt at testing laboratories. Errors occurring in immunohaematology testing predominated over errors in testing for blood-borne diseases (61/93 [65.6%] vs. 24/93 [25.8%], respectively). If errors from the blood collection (BC) and labelling (LA) categories which refer to sampling (35 errors) and sample labelling (31 errors) are added to the errors occurring in the laboratory, then there were 159 donor testing errors in total, 85 (53.5%) of which were recorded in the pre-analytical phase of testing, 15 (9.4%) in the analytical phase and 59 (37.1%) in the post-analytical phase of testing. As a total of 620,107 donations were collected at the CITM during the study period, the overall incidence of errors in donor laboratory testing was 0.026% (1/3,900 donations).
During the 8-year period, 68 errors were recorded in the category of patient testing (code ST). Like donor testing, this category refers exclusively to the errors occurring from the receipt of patients’ samples at the testing laboratories. Errors occurring in immunohaematology testing prevailed in this category as well (47/68; 69.1%). Adding errors from the OE, SC, PR and SH groups, which are related to patient testing (75 errors in total), to ST errors enables a more precise analysis of errors in patient laboratory testing and yielded 143 errors, including 96 (67.1%) errors recorded in the pre-analytical phase of testing, 13 (9.1%) in the analytical phase and 34 (23.8%) in the post-analytical phase of testing. Considering that the CITM provided testing services for 424,855 patients during the study period, the overall incidence of errors in patient laboratory testing was 0.034% (1/2,971 patients). However, this analysis includes only errors occurring within the CITM, whereas errors made at the requesting institution remain in part unknown (errors resolved before sample referral to the CITM) and in part recorded only through the system of non-conformities but not as errors occurring in the work of CITM personnel (non-conformities of samples and request forms, detected on receipt).
Of the 52 errors classified in group OE, only four concerned erroneous entry of the blood product request. Of the 48 errors in computer entry of requests for laboratory testing, the highest proportion (13; 27.1%) concerned erroneous entry of a patient’s first or last name, followed by erroneous entry of the requesting institution/individual address and erroneous (including lacking) entry of the test required (9 errors each). Considering the total number of patients tested, this type of error appears to be extremely rare. For example, the incidence of erroneous entry of a patient’s name was 1/32,681 patients.
There were 21 errors in the SC category, including nine cases in which not all necessary samples were collected. As 250,966 patients presented for venipuncture at the CITM during the 8-year period, errors in blood sampling were very rare, with an incidence of only 0.008% (1/11,951 patients). Errors in sample manipulation were also extremely rare, with only five cases recorded during the 8-year study period.
A total of 2,094 products (161 whole blood donations and 1,933 blood products) yielding 0.12% of the total manufacturing output during the study period were destroyed as a direct consequence of errors. As many as 1,744 (84.3%) errors were detected before the product or service was issued to the customers. Of the 324 errors detected upon issue from the CITM, more than half (163; 50.3%) were detected by the customers and reported as complaints. In only five cases were errors detected upon blood product transfusion. As none of these errors resulted in a patient having a reaction to transfusion treatment, they were categorised as “no harm” events. During the study period, 55 products (of which 52 returned) and 33 test findings (of which 32 returned) were withdrawn because of errors. Out of 2,068 errors, a RAI ≥0.5 (or RAI <0.5 but with a high risk for the institution) was calculated for only 52 (2.5%) errors. These 52 errors were distributed in several categories, as shown in Table VI.
Table VI.
Errors with RAI ≥0.5 (or high risk for the institution).
| Error | n | % |
|---|---|---|
| Errors in staff communication with donors or patients | 11 | 21.2 |
| Product filtration beyond the allowed time | 10 | 19.2 |
| Issuing erroneous finding of testing for infectious diseases | 6 | 11.5 |
| Non-typed units or erroneous phenotype units issued for immunised patient | 4 | 7.7 |
| Issuing units labelled with erroneous ABO blood group | 3 | 5.8 |
| Issuing product of inadequate quality or type | 3 | 5.8 |
| Issuing erroneous Rh(D) finding | 3 | 5.8 |
| Issuing product suspected to have antierythrocyte or antileucocyte antibodies | 3 | 5.8 |
| Issuing product labelled with erroneous Rh(D) | 2 | 3.8 |
| Erroneous antibody titre finding | 2 | 3.8 |
| Issuing erroneous Rh phenotype unit (typing error) | 2 | 3.8 |
| Error in reagent preparation | 1 | 1.9 |
| Issuing erroneous IAT finding | 1 | 1.9 |
| Issuing autologous unit for another patient | 1 | 1.9 |
| Total | 52 | 100.0 |
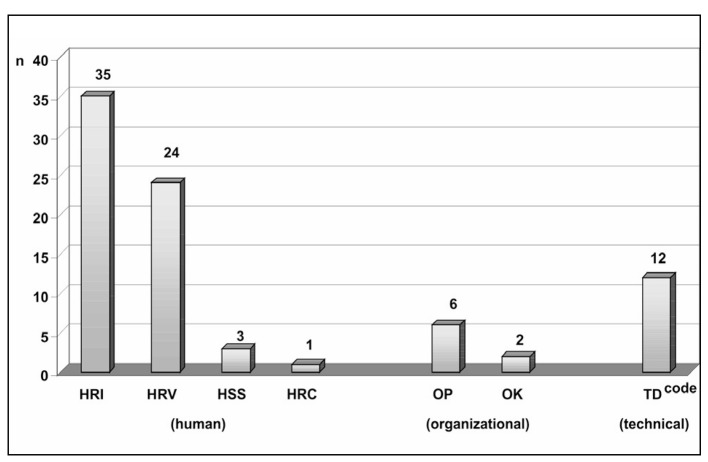
Root cause analysis was performed for the errors shown in Table VI. As these were exclusively active human errors, the causes of the human factors group predominated; however, a number of latent errors related to technical and organisational factors were also identified. The error distribution according to causes is shown in Figure 1, with a note that more than one cause was identified for some errors.
Figure 1.
Root cause classification of serious errors (abbreviations are explained in Table I).
Discussion
Efficient event management in transfusion medicine is of paramount importance for improving the quality of products and services and, thereby, customer satisfaction. A large body of data is being collected through the established systems of quality management and haemovigilance; however, considerable variations are observed in the extent, method of reporting, and even in definitions of reportable events and reactions. Although legal acts generally regulate reporting of serious adverse events and major errors, haemovigilance systems worldwide have recently insisted upon more comprehensive reports and analysis of events in the overall chain of blood transfusion, with special reference to recording near miss events. Studies have shown that near miss events are several times more common than events causing harm. Therefore, significantly more information on process functioning is collected by recording near miss events, the detection of process shortcomings is faster and more accurate, and the strategy also allows for timely introduction of corrective and preventive actions.
In Croatia, the system of haemovigilance was first legally regulated as early as 1999. Since 2007, when the process of European directives regulating the field of transfusion service was transposed to national legislation, the haemovigilance system has been regulated by a by-law deriving from Directive 2005/61/EC8. The by-law regulates compulsory reporting of adverse events and serious adverse reactions to the Ministry of Health and Social Welfare and to the CITM. Along with these regulations, in Croatia there is long experience in data collection through voluntary systems of haemovigilance and quality management. This especially applies to the CITM as the umbrella transfusion medicine institution in Croatia.
In the beginnings of establishing the quality management system (1997–1998) at the CITM, there was continuous discussion as to what data should be collected and how, and how to motivate staff members to participate actively in the continuous quality improvement. The efficiency of error and other event management depends greatly on motivation and active engagement of all personnel, while their non-reporting is mostly due to fear of punishment for the error.
In order to avoid error under-reporting because of workers’ uneasiness and fear of punishment, it was decided at the CITM to record, through the system of non-conformity management, not only deviations in the quality of products, services, input material and equipment, but also all events (including errors) irrespective of the risk level and outcome (incident or near miss). It should be noted that only events occurring in the activities performed at the CITM and those for which the CITM is directly responsible are recorded in this system. Adverse events and reactions recorded in hospitals are included if they develop as a result of a deviation in the quality of a product or service from the CITM. Donor reactions are recorded through the system of non-conformity management only when this reaction influences donation. All records are submitted to the Department of Quality Assurance, where they are analysed, decisions are made on the non-conformities observed, and records are classified into specific categories. Since 2003, records related to errors have been analysed using the MERS-TM system.
During the 8 years studied, 2,068 errors were recorded, the majority of them in blood bank activities. The incidence of errors according to the total number of donations collected and blood products manufactured was found to be 2.9 per 1,000 donations or 1 error per 1,000 blood products, whereas the estimated incidence of errors in patients’ services was 0.7 per 1,000 patients. For comparison, in their study Taswell et al.9 found an overall error incidence of 2–3 per 1,000 procedures carried out at the Mayo Clinic transfusion unit during the period from 1982 to 1992. It should be noted that comparing error incidences calculated in different studies may be quite unreliable because of large differences in error definitions and the extent and type of activity in which the errors were monitored.
In their study of 2005, Kaplan et al.10 found that about 90% of all events belong to the near miss category according to the MERS-TM system, of which 10% are detected after release but before blood product transfusion. Our results are consistent with these observations, i.e. 15.7% of all errors having occurred at our institution were detected after the product or service had been issued to the buyer/user. Of these, the error failed to be recognised in time and resulted in transfusion of a blood product that did not meet the specified request in only five cases. Fortunately, none of these cases was associated with a patient having a reaction to the transfusion treatment.
As presented in Table II, more than half of all errors (54.4%) recorded during the study period occurred in the Department of Blood Collection. We believe that the higher incidence of errors in this department than in other organisational units is due to a number of specificities associated with this working process; in this department, many different activities are performed, including communication with donors, manual entry of a large number of data into the computer system, the process of donor selection, labelling blood bags and sample bags, blood collection, handling apheresis devices, etc. These activities are performed with many people around (donors, personnel, Red Cross representatives), which may have an unfavourable impact on the concentration of technicians. Unlike a laboratory, characterised by a high level of automation and robotics, the processes of blood collection are mainly manual and depend on the technicians’ skill, experience and concentration. Blood is not only collected inside the CITM, but is also collected by mobile teams, implying constantly changing environmental conditions that require frequent adjustments of work organisation. The possibility of errors is also increased when the number of donors presenting for blood donation exceeds the expected or planned number.
During the 8-year study period, 447 errors were recorded at the Department of Blood Product Processing, Storage, Distribution and Issuing. There was one error per 3,912 blood products manufactured on average or approximately one error weekly. Errors in handling devices for blood product separation predominated in the manufacturing process, whereas administrative errors prevailed in the process of blood product issuing and distribution.
The results of the study indicate that errors in immunohaematology testing predominated over errors in testing for blood-borne disease markers. The main reason for this finding may be the lower level of automation and more complex testing algorithms in the field of immunohaematology testing.
Besides distribution according to organisational units, all errors recorded were analysed using the MERS-TM system. Before the introduction of the MERS-TM system, there was no standardised method for the collection and analysis of errors and other events in transfusion medicine. MERS-TM uses descriptive codes and a causative classification7, thus enabling standardisation of error reporting, classification and analysis, and thus also comparison of data among different institutions. Based on the MERS-TM classification of errors according to the site of occurrence, errors in labelling predominated at our institution during the study period (see Table III). Relative to the total number of donations collected during the study period (n=620,107), the overall incidence of these errors was 0.8 per 1,000 donations. This group consisted mainly of errors related to failure of labelling donations from female donors (216/482; 44.8%). In 2004, a decision was made at the CITM that fresh-frozen plasma for clinical use should be manufactured exclusively from blood collected from male donors, obliging blood collection technicians to label donations from female donors (16.6% of donations collected during the study period). Although finalisation and release of fresh-frozen plasma manufactured from female blood donations were prevented by computer blockade, this type of error led to economic losses due to the inability of the plasma manufactured as fresh-frozen plasma to be re-used as plasma for fractionation. This is a consequence of an agreed commitment for the units intended for fractionation to be manufactured with an additional plastic segment. As this type of non-conformity was identified as the leading non-conformity in the category of labelling, it was regulated, from 2009, by a corrective action to lift the commitment to label female donations in the Department of Blood Collection and to introduce incoming control in the Processing Department, in which each unit is checked for donor sex and then the further manufacturing process decided accordingly. This modification in the work structure resulted in eradication of a large group of errors due to failure of labelling female donations in the Department of Blood Collection, while introducing 100% control of the accuracy of sex entry in the donor’s computer file revealed previously undetectable errors.
The 79 errors in manual blood group entry on the labels of bags containing whole blood donations were the second most common errors accounting for 16.4% of errors in the LA category. This procedure was introduced to facilitate the selection of buffy coat units for preparation of platelet concentrate pools in the Processing Department. Although computer verification of blood groups is done at finalisation of the blood product and further procedures of labelling and release to the stock are blocked in the case of any deviation, this type of error results in a non-conforming blood product, and thus in economic loss. The next most frequent errors in this category were 47 cases (9.8%) in which one or more bags were not labelled with the ID number. Interestingly, as many as 44 of these 47 errors were recorded in the last 2 years, a period in which the blood bag configuration had changed (different sequence of blood bag arrangement in the blood collection set): the technicians who until then were used to a particular mode of separation and labelling of transfer bags started to make errors due to the acquired automatism.
Out of 422 errors recorded in the BC category, the majority (251 or 59.5%) were related to the presence of clots in red blood cell products (0.04% of red blood cell products). Although a number of factors may influence the occurrence of this type of product non-conformity, in the experience of the CITM, acquired over years, inappropriate blood mixing with anticoagulants is the major causative factor. It was, therefore, decided to classify the presence of clots as a human error in the conditions of manual blood mixing during donation. The decision proved correct because the incidence of units with clots decreased from 0.23% in 1998 to 0.01%–0.03% in the past few years as the result of continuous implementation of corrective actions based on staff education. The project of introducing automated scale-mixers in our routine is expected to be completed in 2011; however, some time will have to pass before we can evaluate the effect of this intervention on the incidence of donations with clots. Other errors in the BC category were recorded at a much lower incidence, as shown in Table V.
Our study results revealed that errors in blood product manufacture are extremely rare. During the study period, the incidence of such errors at the CITM was 0.011% or 1/9,203 blood products manufactured. The greatest proportion of these errors concerned inappropriate handling of devices for blood product separation. Among the group of administrative errors, those concerning a lack or erroneous entry of manufacturing records in the computer system prevailed. In this group, errors in the selection of units for further processing (in particular expiry date for filtration) and inappropriate handling of devices for sterile welding of plastic tubes should be noted as potentially high-risk errors.
The highest proportion (n=60; 27.1%) of 221 errors recorded in the donor record (DR) group concerned duplicate files for the same donors in the blood bank information system. Although active error implies failure of appropriate administrative control, a significant latent cause was a decision to suspend use of a unique identification number (JMBG), which had been used for identification for years, and to introduce a new personal number (OIB) which, unlike the JMBG, is not printed on personal documents with a photograph (ID card, passport or driving license). The next most common errors in this group were those of an erroneous or lacking entry of donation in the donor’s computer file (n=52; 23.5%). These errors mostly occur due to inattentive reading of line codes of the donor and donation ID number and lack of administrative entry control. Thus, these errors result in entering two donations in the same donor file, donation entry in a wrong donor file, or failure of donation entry. Errors occurring on entering a donor’s personal data into the computer software were recorded in 50 (22.6%) cases and mostly involved erroneous sex entry. Managing this type of non-conformity was a priority because of the decision to use exclusively plasma from blood from male donors for clinical usage. During the implementation of new computer software, double and independent entry of donor sex in the computer software at the time of donor registration was required. The software compares these two records and blocks further procedures in the case of discrepancy. The incidence of other errors in this classification group was very low. Considering the total number of donations collected during the study period, the incidence of errors of this type was 0.04%.
The efficiency and safety of transfusion therapy depend greatly on ensuring appropriate storage conditions for blood products. Deviation from the regulated storage and transport temperature may directly influence cell and protein stability and function. Erroneous storage placement of blood products may also result in issuing errors and a threat for the patient. It is, therefore, of utmost importance for the non-conforming, returned or quarantined products to be separated spatially from the products suitable for release. In addition to permanent surveillance of storage conditions, admittance to the storage area should be restricted exclusively to authorised personnel. Shulman and Kent11 reported a 0.12% prevalence of errors related to placement of blood products in a refrigerator, with one-third of these associated with the potential for ABO incompatible transfusion in the case of double control failure on release. In our study, storage errors were extremely rare, with only 22 cases of expired products found on stock during the 8-year period. Human errors resulting in inappropriate storage conditions were also very rare. During the study period, only 12 such errors were recorded, which resulted in 107 non-conforming blood product units. Two errors involved damage to units in storage due to inappropriate manipulation, while the other 10 errors were related to inappropriate temperature conditions (mostly products left outside the refrigerator by mistake). There were 16 cases of expired products because the products had not been returned from the hospital transfusion unit to the CITM on time (stock updating is regulated for all units that have not been used at hospital transfusion units within 3 weeks).
Errors in blood product issuing have a high potential to cause serious adverse events and reactions associated with transfusion therapy, as demonstrated by a large body of literature data. Sazama12 reported that 6% of the total number of errors identified as causes of fatal reactions consequent to an ABO-incompatible transfusion were related to the release of an erroneous blood product. In a study by Linden et al.13, 11% of errors responsible for transfusion of erroneous blood products were related to blood product issuing.
At the CITM, a total of 188 errors were recorded in the storage, distribution and issuing of blood products during the study period. Almost half of these errors occurred due to failure of administrative control of blood product distribution/issuing. Errors related to blood product distribution/issuing without computer records were most common (n=53; 28.2%). In the IN (Inventory) group, 50 more errors were recorded which had most probably been due to distribution/issuing without computer records as well, although this could not be demonstrated by investigation. In the category of blood product distribution/issuing, 37 errors in data entry into the computer system were recorded, most involving entry of the requesting institution’s code.
Reliable and accurate results of laboratory testing are prerequisites for safe transfusion treatment. Prevention of errors and continuous surveillance of this part of the service is, therefore, important. Automation of laboratory testing and continuous upgrading of test quality have resulted in high analytical process dependability, so errors generally occur in the pre-analytical and post-analytical phases of laboratory testing. In the study by Boone et al.14, 41% of 88,000 reported errors occurred in the pre-analytical phase, 55% in the post-analytical phase, and only 4% in the analytical phase of testing. Our results confirm earlier observations of rare analytical errors, while more errors were recorded in the pre-analytical phase than in the post-analytical phase in both donor and patient testing. In the present study, significantly more errors were recorded in the area of immunohaematology testing than in testing for blood-borne diseases. The possible reasons for this include a lower level of automation and computerisation in particular segments of immunohaematology testing, along with more complex test algorithms and a significantly greater use of manually kept records as compared with testing for blood-borne diseases.
Although improvement of the quality of products and services, and thus of customer satisfaction, is the main objective of error management, the economic aspect of error management should not be neglected. In 1996, the expenses related to preventable side effects in the USA accounted for 2% of overall healthcare expenditure15. Obviously, prevention and reduction in the rate of errors can entail savings and rationalise healthcare expenditure. It should be noted that errors do not only result in directly measurable costs but also in damage due to the loss of customer confidence in the quality of the service provided. Although the economic loss caused by the destruction of 2,094 products can be easily calculated from data presented herewith, more in-depth analysis should also include an array of other variables (working hours spent in error management, repeat testing, administrative costs, etc.). This is considered one of the priorities in upgrading the procedure of error management at our institution. Improvement should also involve more comprehensive risk assessment for recorded errors, while applicable corrective and preventive actions should be taken to further reduce the rate of errors and to improve the level of satisfaction of our customers and their safety.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Vuk T. Quality indicators in blood establishments: CITM experience. Blood Transfusion. 2010;8(Suppl. 1):20–4. [Google Scholar]
- 2.Van der Schaaf TW. Near miss analysis. In: Aspden P, Corrisan JW, Erickson SM, editors. Patient Safety: Achieving a New Standard for Care. Washington, DC: National Academic Press; 2004. pp. 226–46. [PubMed] [Google Scholar]
- 3.Vuk T, Balija M, Jukić I. Quality system in reducing the rate of blood collection nonconformities - CITM experience 1998–2001. Blood Banking and Transfusion Medicine. 2004;2:27–31. [Google Scholar]
- 4.Battles JB, Kaplan HS, Van der Schaaf TW, Shea CE. The attributes of Medical Event-Reporting Systems: experience with a prototype medical event - reporting system for transfusion medicine. Arch Pathol Lab Med. 1998;122:231–8. [PubMed] [Google Scholar]
- 5.Kaplan HS, Callum JL, Rabin Fastman B, Merkley LL. The Medical Event Reporting System for Transfusion Medicine: will it help get the right blood to the right patient? Transfus Med Rev. 2002;16:86–102. doi: 10.1053/tmrv.2002.31459. [DOI] [PubMed] [Google Scholar]
- 6.Medical Event Reporting System for Transfusion Medicine Reference Manual Version 30 prepared by Westat for Columbia University under a grant provided by the National Heart, Lung, and Blood Institute. Available at: http://mers-tm.org/. (Accessed on 5/7/2011).
- 7.Kaplan HS, Battles JB, Van der Schaaf TW, et al. Identification and classification of the causes of events in transfusion medicine. Transfusion. 1998;38:1071–81. doi: 10.1046/j.1537-2995.1998.38111299056319.x. [DOI] [PubMed] [Google Scholar]
- 8.Commission Directive 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards traceability, requirements and notification of serious adverse reactions and events. Official Journal of the European Union. 2005. L256/32. Available at: http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:256:0032:0040:EN:PDF. (Accessed on 8/3/2011).
- 9.Taswell HF, Galbreath JL, Harmsen WS. Errors in transfusion medicine: detection, analysis, frequency and prevention. Arch Pathol Lab Med. 1994;118:405–10. [PubMed] [Google Scholar]
- 10.Kaplan HS. Getting the right blood to the right patient: the contribution of near-miss event reporting and barrier analysis. Transfus Clin Biol. 2005;12:380–4. doi: 10.1016/j.tracli.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Shulman IA, Kent D. Unit placement errors: a potential risk factor for ABO and Rh incompatible blood transfusions. Lab Med. 1991;22:194–6. [Google Scholar]
- 12.Sazama K. Reports of 355 transfusion-associated deaths: 1976 through 1985. Transfusion. 1990;30:583–90. doi: 10.1046/j.1537-2995.1990.30790385515.x. [DOI] [PubMed] [Google Scholar]
- 13.Linden JV, Paul B, Dressler KP. A report of 104 transfusion errors in New York State. Transfusion. 1992;32:601–6. doi: 10.1046/j.1537-2995.1992.32792391030.x. [DOI] [PubMed] [Google Scholar]
- 14.Boone DJ, Steindel SJ, Herron R, et al. Transfusion medicine monitoring practices. A study of the College of American Pathologists/Centers for Disease Control and Prevention Outcomes Working Group. Arch Pathol Lab Med. 1995;119:999–1006. [PubMed] [Google Scholar]
- 15.Thomas EJ, Studdert D, Newhouse JP, et al. Costs of medical injuries in Utah and Colorado. Inquiry. 1999;36:255–64. [PubMed] [Google Scholar]