Abstract
Background.
Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion-associated mortality. Antibodies against human leucocyte antigens (HLA) and human neutrophil antigens (HNA) are often detected in the implicated donors. We investigated the incidence and aetiology of TRALI in Lombardy. Moreover, we determined the rate of HLA and HNA alloimmunisation and the HNA genotype in a cohort of local blood donors.
Materials and methods.
During a 2-year observational study in eight blood transfusion services, suspected TRALI cases were collected and characterised by means of HLA and HNA antibody screening of implicated donors, donor/recipient cross-matching and HLA/HNA molecular typing. In addition, 406 Italian donors were evaluated for alloimmunisation and in 102 of them HNA gene frequencies were determined.
Results.
Eleven cases were referred to the central laboratory, of whom three were diagnosed as having TRALI, seven as having possible TRALI and one as having transfusion-associated circulatory overload. Seven TRALI cases were immune-mediated whereas in three we did not find either alloantibodies in implicated donors or a positive reaction in the cross-match. The most frequently implicated blood component was red blood cells (in 5 males and in 1 female), whereas four cases of TRALI were associated with transfusion of fresh-frozen plasma (in 3 females and in 1 male). The frequency of reported TRALI/possible TRALI cases was 1:82,000 for red blood cells and 1:22,500 for fresh-frozen plasma. No cases were observed for platelets. Overall, the frequency of HLA or HNA alloimmunisation in blood donors was 29% for females and 7% for males. The latter could be related, at least in part, to natural antibodies. HNA gene frequencies showed that HNA-1b is more frequent than HNA-1a in our sample of donors.
Discussion.
The recently adopted national policy to prevent TRALI, i.e. using only plasma donated by males, would have had a positive impact in our setting.
Keywords: transfusion-related acute lung injury, HLA and HNA alloimmunisation, HNA frequencies
Introduction
Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion-associated death in many countries1. It is a syndrome consisting of non-cardiogenic pulmonary oedema with hypoxia occurring during or within 6 hours of transfusion. The incidence of TRALI has been estimated as 1/5,000 for all blood components, and current mortality rates are in the range of 5 to 25%2. However, the overall morbidity associated with TRALI is likely to exceed that suggested by the reports of fatality, because the majority of TRALI cases are not fatal and even fatalities may be underreported in many countries. Furthermore, both the difficulty in diagnosing TRALI in the presence of other causes of acute lung injury and the lack of a definition of mild TRALI suggest that TRALI may have a greater impact on patients’ safety than is currently recognised3,4.
All blood components have been implicated in TRALI, but those containing large amounts of plasma are mainly responsible. According to a recent review, white blood cell antibodies, including class I and II human leucocyte antigens (HLA) and specific human neutrophil antigens (HNA), can be identified in the blood donors implicated in 65–90% of cases of TRALI, with the donors most frequently involved being women with a history of pregnancy5. Many blood services are currently implementing interventions to prevent the occurrence of TRALI.
AABB standard 5.4.2.1 requires that blood centres and transfusion services evaluate donors implicated in TRALI or associated with multiple events of TRALI with regard to their continued eligibility to donate. In addition, more proactive measures, including the preferential use of plasma from male donors, have been introduced in some countries6.
When the present study was started, no specific preventive measures had been adopted in Italy. A prospective study was, therefore, conducted under the auspices of the health authorities of the Region of Lombardy, in order to collect evidence supporting health policy decisions.
After initial informative courses aimed at improving the identification of TRALI in several secondary and tertiary care hospitals, in December 2008 we started an active surveillance programme, and organised a centralised database in a dedicated laboratory facility. In this article we report the incidence of certain and possible cases of TRALI in our region during 2009–2010, the antibody specificities in serologically confirmed cases, and the prevalence of HLA and HNA alloimmunisation among the donor population of our area.
Materials and methods
Regional programme of TRALI monitoring
We started the programme with eight training courses offered to public hospital medical and nursing staff in Lombardy. The main aim of the courses, whose faculty included blood transfusion, internal medicine and anaesthesiology specialists, was to share recent TRALI diagnostic and treatment criteria in our region. Soon after completion of the courses, we started the prospective case collection programme, which lasted from December 2008 to December 2010. For this purpose we used the Canadian Consensus Panel criteria for defining TRALI and possible TRALI cases3.
All recipient reactions and adverse events, reported from blood transfusion services in Milan (2 centres), Monza, Lecco, Lodi, Brescia, Pavia and Varese were subsequently independently examined by the study scientific board composed of four physicians from the centres in Milan (EC), Lecco (DP), Monza (MP) and Lodi (GC), to check the presence of the following diagnostic criteria: acute respiratory distress within 6 hours of transfusion, hypoxaemia (PaO2/FiO2 ratio <300), new bilateral infiltrations on chest X-rays and careful exclusion of circulatory overload.
Laboratory investigations for TRALI/possible TRALI
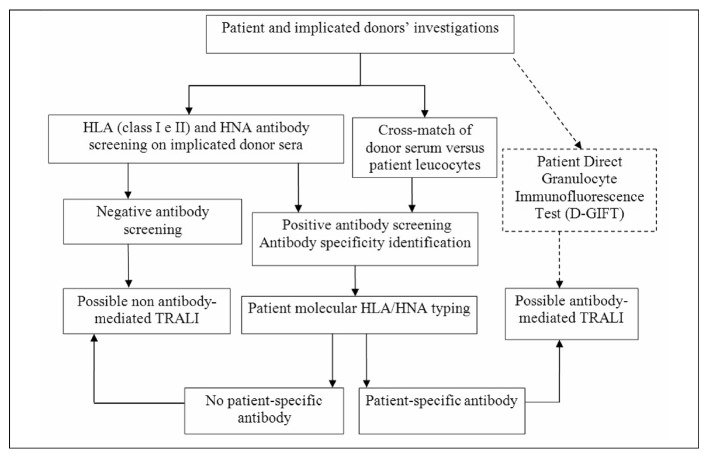
All laboratory investigations were performed in the reference centre, following a previously defined algorithm7 (Figure 1). We tested all donor units transfused within 6 hours of the clinical onset of symptoms. In particular, we performed the indirect granulocyte immunofluorescence test (I-GIFT)8 and the granulocyte-agglutination test (GAT)9,10 on all implicated donor sera against a panel of ten random healthy subjects. Moreover, when a sample of a patient’s neutrophils was available, we also performed the direct GIFT (D-GIFT) (on both pre- and post-transfusion samples) and cross-matching between the patient’s neutrophils and donor’s serum.
Figure 1.
Laboratory diagnostic algorithm for suspected TRALI cases. The D-GIFT provides relevant diagnostic information when a conversion from negative to positive can be documented from pre to post-transfusion patient samples. This portion of the algorithm is reported in dotted lines to indicate that this test, with a potential significance when performed both on pre and post-transfusion reaction samples, is infrequently feasible as it depends on the availability of pre-transfusion patient granulocytes.
HLA class I and II antibodies were detected by Flow-PRA screening (OneLambda, Inc., Canoga Park, CA, USA) both on the implicated donor’s plasma collected immediately after the transfusion reaction and on sera collected after recalling the donor. If HNA or HLA antibodies were present, we proceeded to define their specificity. HNA antibodies were identified performing additional I-GIFT on a panel of granulocytes from typed donors whereas a Flow-PRA High Definition (HD) kit (OneLambda) made up of beads coated with single HLA class I or class II antigens were used to define HLA antibody specificities. To establish whether the cognate antigen was present, the patient’s and donor’s HLA genotype was determined by using a polymerase chain reaction (PCR)-based assay employing sequence-specific primers (SSP). HNA typing for HNA-1a/1b/1c, HNA-3a/3b, HNA-4a/4bw and HNA-5a/5bw antigens was done using HNA-Type extra 3 kit (BAGene, BAG Health Care, Lich, Germany).
HLA and HNA alloimmunisation screening and HNA typing in blood donors
Donor screening and typing studies were approved by the Ethics Committee of our institution, and donors were required to sign a specific informed consent form. For alloimmunisation screening, 406 random donors (131 females, 275 males) were enrolled.
HLA screening was done using the Lambda LABScreen, Luminex technique (OneLambda) following the manufacturer’s instructions. In this study the cut-off used for positive samples was the normalised background ratio (>3) defined by our internal regular quality control. HLA class I or class II positive sera from nulliparous women and from untransfused males were retested by flow cytometry using Flow-PRA screening.
HNA antibody screening was performed in two phases. Level 1 testing was an I-GIFT on 10 random group O donor granulocytes performed on all study samples. Level 2 testing consisted of a repeated screening using a panel of cells well characterised for HNA antigens (HNA-1a/1b/1c, HNA-2, HNA-3a/3b, HNA-4a/4bw, and HNA-5a/5bw, Table I), which was performed only on samples resulting positive in Level 1 test. Neutrophils were isolated from freshly drawn whole blood anticoagulated with ethylenediaminetetraacetic acid (EDTA) as previously described11. Assay cut-off values were determined as the mean plus three standard deviations (SD) of the natural log-transformed distribution of fluorescence values in 244 non-transfused male donors (IgG cut-off value=4.7). This approach was chosen based on conventions for establishing normal ranges for laboratory screening assays.
Table I.
General data from the three TRALI (grey background) and seven possible TRALI (white background) cases collected and characterised in this study during December 2008–December 2010.
| Patient age (yr) | Gender | Diagnosis | Donor age and gender | Donor previous immunising events P, T, None | Blood component, type and age (days) | Cross-match | D-GIFT | Flow-PRA screening | Indirect GIFT/GAT | Patient-specific donor antibodies | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | M | B-ALL | 35, M | None | RBC, 13 | neg | pos | neg | neg/neg | No |
|
| |||||||||||
| 2 | 82 | M | Hip prosthesis | 34, F | P (2+1 abortion) | FFP | nd | pos | pos HLA class II | neg/neg | HLA-DR11 |
|
| |||||||||||
| 3 | 32 | M | BCP, parvovirus B19 infection | 54, F | P (2) | RBC, 7 | Pos (T and B-Ly) | pos | pos HLA class I and II | neg/pos | HLA-B51, -DR12 |
| 39, M | None | RBC, 7 | Pos (PMN, Ly) | neg | pos /pos | pan-leucocyte | |||||
|
| |||||||||||
| 4 | 78 | F | AMI | 49, M | None | RBC, 17 | nd | neg | pos HLA class I (anti-A30) | pos/neg | PMN |
|
| |||||||||||
| 5 | 55 | M | Multiple trauma | 27, M | None | RBC, 8 | neg | neg | neg | neg/neg | No |
| 30, M | None | RBC, 9 | neg | neg | neg | neg/neg | No | ||||
|
| |||||||||||
| 6 | 53 | F | Proctorrhagia | 63, M | None | RBC, 29 | neg | neg | neg | neg/neg | No |
| 52, M | None | RBC, 30 | neg | neg | neg | neg/neg | No | ||||
| 41, M | None | RBC, 31 | neg | neg | neg | neg/neg | No | ||||
| 41, M | None | RBC, 33 | neg | neg | neg | neg/neg | No | ||||
|
| |||||||||||
| 7 | 52 | F | Liver Tx | 44, F | P (1) | FFP | weak pos (PMN) | pos | neg | pos/neg | PMN |
|
| |||||||||||
| 8 | 80 | F | Cardiopathy | 46, M | None | RBC, 33 | Pos (PMN) | neg | neg | pos/neg | PMN |
|
| |||||||||||
| 9 | 51 | F | Gastrointestinal cancer | 63, M | None | FFP | neg | pos | pos HLA class I | neg/neg | HLA A30 |
|
| |||||||||||
| 10 | 9 | M | Factor H deficiency | 38, F | P (1) | FFP | pos | nd | pos HLA class I and II | neg/neg | HLA A24, A32, DR11, DR14 |
| 49, M | None | FFP | neg | neg/neg | No | ||||||
Legend: F: female; M: male; BCP: bronchopneumonia; B cell-ALL: B-acute lymphatic leukaemia; AMI: acute myocardial infarction; Tx: transplantation; FFP: fresh-frozen plasma; RBC: red blood cells; Ly: lymphocytes; PMN: polymorphonuclear cells; P: pregnancy; T: transfusion; GIFT: granulocyte immunofluorescence test; GAT: granulocyte agglutination test; nd: not determined.
With regards to HNA typing, we studied 102 randomly selected donors using molecular biology and flow cytometry techniques. In more detail, genomic DNA was prepared from 200 μL of whole blood using the automatic MagNA Pure Compact DNA extraction device (Roche, Basel, Switzerland) according to the manufacturer’s instructions. HNA was genotyped as mentioned above. Although the molecular basis of HNA-2a has been described, genotyping methods are not yet available. We, therefore, evaluated the expression of HNA-2a by typing donor neutrophils with CD177 monoclonal antibodies (clone MEM-166, BD, San Josè, CA, USA). After washing with phosphate-buffered saline, labelled neutrophils were analysed on a flow cytometer (BD FACS-Canto II). Subjects were considered negative for HNA-2a if less than 5% of their neutrophils reacted with anti-CD17712.
Results
TRALI/possible TRALI cases
Between December 2008 and December 2010, 11 suspected TRALI cases were reported, three of which were identified as TRALI, seven as possible TRALI and one as transfusion-associated circulatory overload (TACO). Table I shows the age and gender of the donors and recipients, the implicated blood components (transfused within 6 hours of the clinical onset of symptoms) and main serological findings. Seven cases were diagnosed as possible TRALI because of the following concomitant conditions4: case n. 1 pneumonia and sepsis; n. 3 pneumonia; n. 4 pneumonia, sepsis and shock; n. 5 multiple trauma; n. 6 aspiration and shock; n. 9 pneumonia and shock, or in absence of certain evidence: case n. 7 no chest X-ray. All cases required mechanical ventilation. While two patients died within a few days of the adverse event, the acute respiratory distress resolved in all the others within a few days. Four cases (40%) were associated with the use of fresh-frozen plasma (FFP), whereas in six cases (60%) the implicated blood component was red blood cells (RBC). Taking into account the total number of blood components transfused in the study period, we determined that the frequencies of TRALI/possible TRALI cases associated with RBC and FFP were 1:82,000 and 1:22,500, respectively. No cases were associated with platelets. The risk was 3.6 times higher for FFP than for RBC (95% CI 1.04–13.05).
Antibody mediated-TRALI/possible TRALI was diagnosed in seven cases (70%, 4 female and 3 male implicated blood donors). Antibodies directed against HLA or HNA were detected in the donors and the patients typed positive for the recognised leucocyte antigen, as well as when neutrophil cross-matching between donors’ sera and patients’ leucocytes was positive. As reported in Table I, the most common antibody specificity was against HLA class II DR5 antigen (alleles 11 and 12, cases n. 2, 3 and 10).
In four donors implicated in cases of immune-mediated TRALI/possible TRALI, no patient-specific anti-HLA antibodies were found. Moreover, in one case (n.3) pan-reactive anti-leucocyte antibodies (as detected by a positive polymorphonuclear cell/lymphocyte cross-match and 80% serum reactivity) were present. In the other three cases (n. 4, 7 and 8) the antibodies reacted only with polymorphonuclear cells of a minority of tested donors (20–30%). Interestingly, in the latter three cases we could identify the same donor/patient HNA mismatch (HNA-5bw). Moreover, the implicated component in case 7 was FFP donated by a parous woman, while RBC more than 2 weeks old from male donors were implicated in cases 4 and 8. In these two latter cases, the serological findings were confirmed on fresh serum from the donors.
HLA and HNA alloimmunisation screening and HNA typing in blood donors
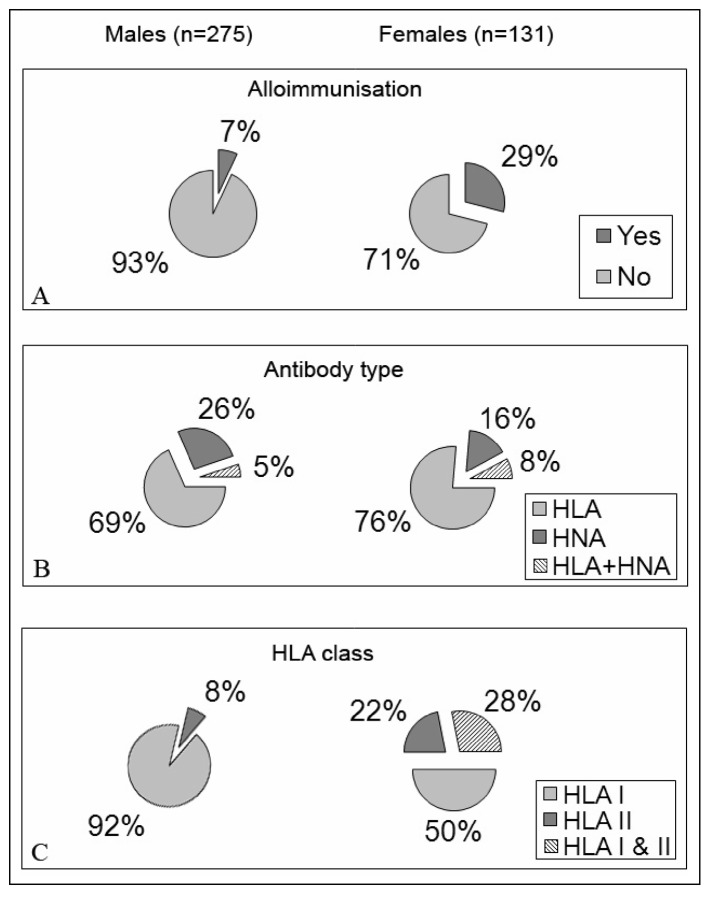
Screening results are shown in Figure 2. Twenty-nine percent of the 131 female donors were alloimmunised compared to 7% of the 275 males. HLA class I and/or II antibodies were shown by 84% and 74% of immunised female and male donors, respectively. Moreover, in 64% of the HLA alloimmunised males we found the same antibody specificities (A1, A2, A25, A30, A34, B8 and B44/45) recently described by Morales-Buenrostro et al.13 as “natural antibodies”. With regard to HNA alloimmunisation, 6.1% and 2.5% of all screened female and male donors had a positive I-GIFT. When these sera were retested against a panel of HNA typed neutrophils from healthy subjects, four male sera showed anti-HNA-1a antibody specificity. In the other 11 alloimmunised donors (3 males and 8 females) pan-reactive alloantibodies were detected. About 75% of the alloimmunised female donors had a previous history of pregnancies. Alloimmunised males did not report previous transfusions.
Figure 2.
Blood donor alloimmunisation. Evaluation of HLA and HNA alloimmunisation in 275 male and 131 female blood donors. Panel A: male and female donor alloimmunisation. Panel B: Antibody specificity in alloimmunised male and female donors. Panel C: HLA antibody class in alloimmunised male and female blood donors.
HNA frequencies determined in 102 donors are reported in Table II: the antigen frequency for HNA-1b (0.84) was about twice that of HNA-1a (0.49). Homozygous HNA-1c was not found, and two HNA-1 null cases were found. The latter suggests that the frequency of FcγRIIIB deficiency in this cohort (2%) may be higher than that reported in other Europeans (0.1%).
Table II.
Frequencies of HNA in Lombardy blood donors.
| Frequencies of human neutrophil antigens (HNA) (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| HNA-1 | HNA-2 | HNA-3 | HNA-4 | HNA-5 | ||||||
| a | b | c | null | a | a | b | a | bw | a | bw |
| 49 | 84 | 7 | 2 | 96 | 95 | 41 | 97 | 25 | 92 | 47 |
Discussion
An important objective of this study was to determine a common approach to the diagnosis and management of TRALI in Lombardy. The programme started with local educational courses, held at the onset of a 24-month period of surveillance of all suspected TRALI cases reported to a central database. The main outcome of the study was the detection and characterisation of ten TRALI/possible TRALI cases reported after the transfusion of approximately 640,000 blood components. Specifically, six, four and no cases of TRALI/possible TRALI cases occurred after the transfusion of approximately 490,000 RBC (frequency=1:82,000), 90,000 FFP (1:22,500) and 60,000 adult platelet doses, respectively. The frequencies of TRALI/possible TRALI cases detected in our region were similar to those reported recently in the literature14,15, except for TRALI associated with platelet transfusion, which was not found in our series. This may be due to the prevalent use of multiple donor platelets suspended in crystalloid solution in the blood centres of our region, which decreases the potential impact of platelets resuspended in plasma from single or multiple donors with high titre HLA and/or HNA antibodies. In this regard, it should be noted that highly variable TRALI frequencies have been reported in retrospective and prospective studies in which different techniques for preparing blood components were used (i.e. single donor versus multiple donor platelets, use of crystalloid storage solutions, universal leucoreduction versus leucoreduction by indication, clinical use of male plasma only, etc.)3. Early studies performed between 1995–2002, before the Canadian Consensus Conference, reported frequencies of TRALI ranging from 1:4,400 to 1:557,000 in association with RBC transfusion, from 1:8,000 to 1:74,000 in association with FFP and from 1:400 to 1:88,000 in association with platelets3. Studies performed after the implementation of the Canadian Conference diagnostic criteria and the introduction of enhanced surveillance systems showed less variable frequencies15. Overall, our data support and complement recent reports, with a frequency of immune-mediated TRALI/possible TRALI (7 out of 10 cases) similar to that reported in the recent literature16.
We critically reviewed our findings in relation to the guidelines implemented in Italy 3 months after the conclusion of our study. As expected, in our series a higher frequency of TRALI/possible TRALI reactions was found in association with plasma-rich as compared with plasma-poor blood components17,18. Moreover, three of four TRALI/possible TRALI reactions were attributed to FFP from female donors. Recent studies clearly showed that exclusion of female donors from plasma donation for transfusion was associated with a 33% reduction in the incidence of TRALI6. Of note, this recommendation, which has also been included in the recent Italian guidelines (www.centronazionalesangue.it/newsbox/linee-guida-trali), would have prevented three of the above reactions.
As far as concerns the antibody specificities detected in our seven cases of immune-mediated TRALI/possible TRALI, we found HLA antibodies in four cases. Interestingly, as reported by the SHOT19 in a long period of haemovigilance, we noted that the principal HLA antibody specificity was related to class II antigens. Moreover, in three cases of immune-mediated TRALI/possible TRALI we detected anti- HLA-DR5 specificity, which represents an antigen of the DR52 broad cluster, thus confirming the important role of anti-HLA class II alloantibodies in the development of TRALI20.
In both cases n. 4 and 9, in which two male donors with no history of transfusion were implicated, HLA-A30 antibodies were detected in donor serum. As previously reported by Morales-Buenrostro et al.13, this HLA specificity is the most frequently detected in healthy male blood donors with no previous immunological events. This suggests their natural development, which could be due to a cross-reaction with epitopes from micro-organisms, ingested proteins or allergens. Since in one case of our series of possible TRALI (n. 4) the cognate antigen was absent in the patient, and in the other case (n. 9) the cross-match results were negative, we cannot conclusively support a causative role for these antibodies. Further studies with a larger number of well characterised TRALI cases will be necessary to elucidate this issue fully.
Although the frequency with which HNA antibodies are implicated in TRALI is not well established, given the limited HNA serological evaluations usually performed21, several studies clearly established HNA antibodies as a serious and life-threatening cause of TRALI4,18,19,22. We observed one case of TRALI and two possible cases of TRALI in which HNA antibodies were implicated, but no particular antigen specificity was identified. The only unexpected finding was the same HNA donor/patient mismatch (HNA-5bw), whose specific antibodies had not yet been detected in TRALI cases18.
In addition to the immunohaematology data collected in our study, the TRALI surveillance programme gave us an opportunity to review and consolidate our standard approach to the laboratory work-up of these cases. More specifically, when leucocyte antibody screening on the residual blood transfusion sample was positive (8 donors), we repeated the screening on a fresh sample of serum from the donor. In only one case, which occurred during the early phase of the study, we were unable to confirm the alloimmunisation status of one blood donor originally detected in samples collected from the blood transfusion set when tests were repeated on fresh donor samples. The outcome of additional investigations performed in this case raised the hypothesis of cross-contamination of the sample collected for prompt diagnostic purposes from the transfusion set, with plasma from another blood component concurrently transfused during the index transfusion event.
This case supports the need for confirmatory tests on fresh donor samples before conclusions on donor eligibility are drawn, as erroneous results can be obtained from residual samples from blood bags and transfusion sets. Nonetheless, the latter may provide precious diagnostic information in the early phase after TRALI detection, before fresh donor samples become available.
With regards to our study on donor alloimmunisation, we detected a relatively high rate in male donors (7%), which is approximately three to four times higher than the values of 0, 1.7, 2.5% reported in the literature23. The prevalence of HLA antibodies in blood donors is clearly dependent on the testing method used to detect the antibody (e.g. enzyme-linked immunosorbent assay versus flow cytometry24,25), as well as the cut-off chosen in the Luminex assay13. Moreover, the most recent screening methods, such as that used in this study, allow the detection of the previously mentioned natural HLA antibodies, which are usually present at a low titre. Since no immunological events, such as previous transfusions, were reported by the male donors included in this study, and since the majority of HLA antibody specificities that we found are more frequently associated with natural alloantibodies13, we can hypothesise that this male alloimmunisation could be related, at least in part, to natural alloreactivity, whose clinical role in the development of TRALI and other adverse reactions is not yet clear. Clarification of the clinical relevance of these natural antibodies is of utmost importance in relation to donor exclusion criteria. In line with previous studies22,26, donor alloimmunisation found in our region was primarily related to HLA class I antibodies, whereas HLA class II antibodies were the principal implicated specificities of our and other reported cases of immune-mediated TRALI/possible TRALI17. A less clear specificity definition was found with regards to the HNA system. This could be due to a lower level of development and standardisation of HNA serological techniques.
HNA-1a/b/c frequencies determined by PCR-SSP in our donor cohort are in good agreement with the corresponding frequencies determined serologically among Caucasians27. Since we observed two FcγRIIIB-deficient individuals among 102 subjects from northern Italy, this mutation seems to be more frequent in our population as compared to the estimated 1:1,000 frequency in the French population28. Given the small number of donors evaluated, additional donors would have to be tested in order to verify these findings.
In spite of the limited number of TRALI/possible TRALI cases in our series, it is encouraging to note that no case was reported following platelet transfusions. This could be due to the extensive use of multiple-donor platelets and crystalloid solution for platelet storage in our study. Conversely, it is possible that the lack of universal pre-storage leucoreduction of RBC may have caused a relatively high proportion of TRALI cases associated with RBC transfusions in our series. Although we cannot exclude this hypothesis, we do not support it based on very recent findings reported by Silliman et al.29. These investigators showed that the agent responsible for antibody-negative TRALI, namely, non-polar lipids, derive from RBC membrane disruption during storage both in non pre-storage leucoreduced and pre-storage leucoreduced RBC.
In conclusion, our study not only supports and expands other investigations, but also identifies possible immunological causes of TRALI that may be missed by laboratory tests with insufficient sensitivity. Finally, we raise the hypothesis of a causative role of natural anti-HLA antibodies in the donors implicated in TRALI cases.
Acknowledgments
We thank all participating blood services for their professional and supportive co-operation.
Footnotes
Grants
This study was supported by a grant “Caratterizzazione e prevenzione della transfusion related acute lung injury (TRALI) in Lombardia” from V Piano Sangue Regione Lombardia, 2009–2010.
The Authors declare no conflicts of interest.
References
- 1.Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406–17. doi: 10.1182/blood-2008-10-167643. [DOI] [PubMed] [Google Scholar]
- 2.Wallis JP, Sachs UJ. Transfusion-related acute lung injury. In: Simon TL, Snyder EL, Solheim BG, et al., editors. Rossi’s Principles of Transfusion Medicine. Bethesda, MD: American Association of Blood Banks; 2009. pp. 870–84. [Google Scholar]
- 3.Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774–89. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 4.Kopko PM, Marshall MR, Holland PV, et al. Transfusion-related acute lung injury: report of a clinical look-back investigation. JAMA. 2002;287:1968–71. doi: 10.1001/jama.287.15.1968. [DOI] [PubMed] [Google Scholar]
- 5.Eder AF, Herron RM, Jr, Strupp A, et al. Effective reduction of transfusion-related acute lung injury risk with male-predominant plasma strategy in the American Red Cross (2006–2008) Transfusion. 2010;50:1732–42. doi: 10.1111/j.1537-2995.2010.02652.x. [DOI] [PubMed] [Google Scholar]
- 6.Wiersum-Osselton JC, Middelburg RA, Beckers EAM, et al. Male-only fresh-frozen plasma for transfusion-related acute lung injury prevention: before-and-after comparative cohort study. Transfusion. 2011;51:1278–83. doi: 10.1111/j.1537-2995.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- 7.Porretti L, Coluccio E, Prati D, et al. Flow-cytometric approach to the prompt laboratory diagnosis of TRALI: a case report. Eur J Haematol. 2004;73:295–9. doi: 10.1111/j.1600-0609.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- 8.Verheugt FW, von dem Borne AE, van Noord-Bokhorst JC, et al. Autoimmune granulocytopenia: the detection of granulocyte autoantibodies with the immunofluorescence test. Br J Haematol. 1978;39:339–50. doi: 10.1111/j.1365-2141.1978.tb01106.x. [DOI] [PubMed] [Google Scholar]
- 9.McCullough J, Clay M, Press C, Kline W. Granulocyte Serology: a Clinical and Laboratory Guide. American Society of Clinical Pathologists Press; 1988. [Google Scholar]
- 10.Stronceck DF, Fadeyi E, Adams S. Leukocyte antigen and antibody detection assays: tools for assessing and preventing pulmonary transfusion reactions. Transfusion. 2007;21:273–86. doi: 10.1016/j.tmrv.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen XD, Flesch B, Sachs UJ, et al. Rapid screening of granulocyte antibodies with a novel assay: flow cytometric granulocyte immunofluorescence test. Transfusion. 2009;49:2700–8. doi: 10.1111/j.1537-2995.2009.02330.x. [DOI] [PubMed] [Google Scholar]
- 12.Xia W, Bayat B, Sachs U, et al. The frequencies of human neutrophil alloantigens in the Chinese Han population of Guangzhou. Transfusion. 2011;51:1271–7. doi: 10.1111/j.1537-2995.2010.02979.x. [DOI] [PubMed] [Google Scholar]
- 13.Morales-Buenrostro LE, Terasaki PI, Marino-Vázquez LA, et al. Natural human leukocyte antigen antibodies found in nonalloimmunized healthy males. Transplantation. 2008;86:1111–5. doi: 10.1097/TP.0b013e318186d87b. [DOI] [PubMed] [Google Scholar]
- 14.Shaz BH, Stowell SR, Hillyer CD. Transfusion-related acute lung injury: from bedside to bench and back. Blood. 2011;117:1463–71. doi: 10.1182/blood-2010-04-278135. [DOI] [PubMed] [Google Scholar]
- 15.Keller-Stanislawski B, Reil A, Günay S, et al. Frequency and severity of transfusion-related acute lung injury - German haemovigilance data (2006–2007) Vox Sang. 2010;98:70–7. doi: 10.1111/j.1423-0410.2009.01232.x. [DOI] [PubMed] [Google Scholar]
- 16.Goldman M, Webert KE, Arnold DM, et al. Proceedings of a consensus conference: towards an understanding of TRALI. Transfus Med Rev. 2005;19:2–31. doi: 10.1016/j.tmrv.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Win N, Chapman CE, Bowles KM, et al. How much residual plasma may cause TRALI? Transfus Med. 2008;8:276–80. doi: 10.1111/j.1365-3148.2008.00885.x. [DOI] [PubMed] [Google Scholar]
- 18.Bux J. Antibody-mediated (immune) transfusion-related acute lung injury. Vox Sang. 2011;100:122–8. doi: 10.1111/j.1423-0410.2010.01392.x. [DOI] [PubMed] [Google Scholar]
- 19.Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49:440–52. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura M, Hashimoto S, Takanashi M, et al. Role of anti-human leukocyte antigen class II alloantibody and monocytes in development of transfusion-related acute lung injury. Transfus Med. 2007;17:129–34. doi: 10.1111/j.1365-3148.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- 21.Gottschall JL, Triulzi DJ, Curtis B, et al. for the NHLBI Retrovirus Epidemiology Donor Study II The frequency and specificity of human neutrophil antigen antibodies in blood donor population. Transfusion. 2011;51:820–7. doi: 10.1111/j.1537-2995.2010.02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reil A, Keller-Stanislawski B, Günay S, et al. Specificities of leucocyte alloantibodies in transfusion-related acute lung injury and results of leucocyte antibody screening of blood donors. Vox Sang. 2008;95:313–7. doi: 10.1111/j.1423-0410.2008.01092.x. [DOI] [PubMed] [Google Scholar]
- 23.Triulzi DJ, Kleinman S, Kakaiya RM, et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009;49:1825–35. doi: 10.1111/j.1537-2995.2009.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadeyi E, Adams S, Peterson B, et al. Analysis of high-throughput HLA antibody screening assay for use with platelet donors. Transfusion. 2008;48:1174–9. doi: 10.1111/j.1537-2995.2008.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopes LB, Fabron A, Jr, Chiba AK, et al. Impact of using different laboratory assays to detect human leukocyte antigen antibodies in female donors. Transfusion. 2010;50:902–7. doi: 10.1111/j.1537-2995.2009.02523.x. [DOI] [PubMed] [Google Scholar]
- 26.Maślanka K, Michur H, Zupanska B, et al. Leukocyte antibodies in blood donors and a look back on recipients of their blood components. Vox Sang. 2007;92:247–9. doi: 10.1111/j.1423-0410.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- 27.Bux J, Stein EL, Santoso S, Mueller-Eckhardt C. NA gene frequencies in the German population, determined by polymerase chain reaction with sequence-specific primers. Transfusion. 1995;35:54–7. doi: 10.1046/j.1537-2995.1995.35195090663.x. [DOI] [PubMed] [Google Scholar]
- 28.Fromont P, Bettaieb A, Skouri H, et al. Frequency of the polymorphonuclear neutrophil Fc gamma receptor III deficiency in the French population and its involvement in the development of neonatal alloimmune neutropenia. Blood. 1992;79:2131–4. [PubMed] [Google Scholar]
- 29.Silliman CC, Moore EE, Kelher MR, et al. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2001;51:2549–54. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]