Abstract
Background.
The various contributors to sport-related anaemia include increased plasma volume, exercise-induced oxidative stress, increased body temperature, acidosis, gastrointestinal bleeding, acute and chronic inflammation as well as compression and damage of red blood cells (RBC) in the capillaries within the contracting muscles. The effective contribution of foot-strike haemolysis is unclear.
Materials and methods.
We studied 18 Caucasian male athletes (mean age, 42 years; range, 34–52 years) before and immediately after a 60-km ultramarathon. Laboratory investigations included the haematological profile along with haptoglobin, potassium, aspartate aminotransferase (AST), creatine kinase (CK), lactate dehydrogenase (LDH) and albumin concentrations and a haemolysis index (HI).
Results.
No significant variations were found in post-exercise values of haemoglobin, RBC count and haematocrit. Mean corpuscular volume and haptoglobin were significantly decreased, whereas RBC distribution width was increased. The concentration of haptoglobin was reduced by approximately 50%, whereas enzyme concentrations were all remarkably increased. The HI remained below 0.5 g/L. After adjusting for plasma volume change, the increases were 1.7% for potassium (P=0.17), 30% for AST (P<0.01), 49% for LDH (P<0.01) and 2.39-fold for CK (P<0.01). A statistically significant association was found between haemoconcentration-adjusted variations of CK and those of AST (r=0.803; P<0.01) and LDH (r=0.551; P=0.02).
Discussion.
This is the first study demonstrating that long-distance running does not induce clinically significant changes in haemoglobin, haematocrit, RBC count or potassium concentration. The significant post-exercise decrease of haptoglobin reflects a certain degree of haemolysis, but the concentration of cell-free haemoglobin remaining below 0.5 g/L and the non-significant variation in RBC count both indicate that the foot-strike haemolysis is very modest or even clinically negligible.
Keywords: sport, physical exercise, marathon, haemolysis
Introduction
There is a bidirectional relationship between erythropoiesis and sport performance, since the number of red blood cells (RBC) is the main determinant of aerobic athletic capacity whereas regular physical exercise has been directly associated with a reduction of the RBC count, in the so-called “sports anaemia”1. The need to supplement athletes with iron is supported by the evidence that RBC count and iron-linked parameters vary consistently during periods of intense training, especially in endurance athletes2,3. Sports anaemia is typically a transitory condition and a minority of elite athletes (less than 8%) develop frank anaemia (haemoglobin concentration below 140 g/L in males and 120 g/L in females)4. Sports anaemia is usually considered to have a variety of root causes and several physiopathological mechanisms have been advocated to explain this intriguing phenomenon. The leading cause is a training-dependent increase of plasma volume, which is not balanced by an increased production of RBC, eventually resulting in a net decrease in haematocrit4. Nevertheless, exercise-induced variations in several parameters of haematopoiesis cannot be ascribed completely to haemodilution. Exercise-induced oxidative stress is another important mechanism causing injury to erythrocytes during and after strenuous physical exercise, as confirmed by the evidence that non-traumatic sports such as swimming, cycling, rowing, and weight-lifting are also associated with increased RBC turnover5. Additional important mechanisms include increased body temperature, acidosis, gastrointestinal bleeding, increased production of catecholamines, acute and chronic inflammation as well as compression and damage of RBC in the capillaries within the contracting muscles6–9.
Intravascular haemolysis has also been implicated in the injury and breakdown of RBC during exercise, especially in long-distance running. The most common form of exercise-induced intravascular haemolysis is conventionally known as “foot-strike” haemolysis, whereby the repeated and forceful impact of the feet with the ground is thought to cause direct injury to the erythrocytes within the capillaries6–8. As a result, the haemoglobin and associated iron held within the RBC is released into the surrounding plasma. The effective contribution of this mechanism to sports anaemia has been the object of several studies, which provided rather contradictory results. Nevertheless, its precise definition is essential for both haematology and sports medicine. In fact, although a single haemolytic episode is unlikely to cause iron loss of clinical significance, repeated episodes during hard and continuous training may determine a cumulative effect that might contribute substantially to the pathogenesis of sports anaemia. The aim of this study was, therefore, to assess whether a significant degree of haemolysis occurs during strenuous aerobic exercise, in the form of a 60-km ultramarathon.
Materials and methods
Eighteen healthy, trained, Caucasian male athletes (mean age, 42 years; range, 34–52 years), who had been engaged in specific endurance training for 3 to 10 years (mean training regimen, 240±32 min/week; maximal oxygen uptake, VO2: 65±2 mL/kg/min) were included in this study. The VO2 max was assessed before the study by cycle ergometric incremental test exercise. The athletes ran a 60 km, ultramarathon equipped with a heart rate (HR) monitor at 80±4% VO2 max. The percentage was calculated on the basis of the VO2/HR relationship determined during the incremental test. None of the athletes had acute or chronic diseases, or reported intake of medications, including antioxidants or nicotine. Strenuous exercise was avoided for 36 to 48 hours before the trial. The run started at 8:00 a.m. on a cloudy and partially rainy day, with a temperature from 6 to 8 °C and humidity from 54% to 87%: the course of the run was hilly and demanding. The athletes were offered beverages ad libitum during the run to prevent excessive dehydration. Blood samples were collected after an overnight fast, 20 minutes before the participants warmed up (“pre-run”), and within 10 min after completion of the run (“post-run”). All subjects gave informed consent to the tests and the study was carried out in accordance with the Declaration of Helsinki and compliant with all relevant local legislation. Blood was collected into two vacuum tubes, one containing no additives and the other K3 EDTA (Becton-Dickinson, Oxford, UK). The contents of the former tube were immediately separated by centrifugation at 3,000 x g and serum was stored at −70 °C. The contents of the latter tube were processed for haematological testing within 1 hour of collection. Serum haptoglobin was assessed on a Behring Nephelometer II Analyzer System (BN II, Siemens Healthcare Diagnostics, Marburg, Germany). Potassium (indirect potentiometry using ion-selective electrode), aspartate aminotransferase (AST, IFCC method with pyridoxal phosphate activation), creatine kinase (CK, N-acetylcysteine activated IFCC method), lactate dehydrogenase (LDH, DGKC method), albumin (bromcresol green colorimetric method) and haemolysis index (HI, direct spectrophotometry) were assessed on a Beckman Coulter AU 5800 (Beckman Coulter Inc., Brea CA, USA). The lowest measurable concentration of cell-free haemoglobin with this test is 0.5 g/L, which roughly corresponds to 0.3% haemolysis in a blood sample with 150 g/L of haemoglobin. It has been previously demonstrated that there is an excellent correlation between the HI and cell-free haemoglobin concentration measured with a reference cyanmethaemoglobin assay10. The haematological parameters (i.e., haemoglobin, haematocrit, RBC count, mean corpuscular volume [MCV], RBC distribution width [RDW], mean haemoglobin content [MCH], mean corpuscular haemoglobin concentration [MCHC]) were assessed on an Advia 2120 (Siemens Healthcare Diagnostics, Tarrytown NY, USA). Identical instruments and lots of reagents were used throughout the study. Total imprecision, as expressed by the coefficient of variation (CV), was less than 2.0%11. Wilcoxon’s signed-rank test was used to evaluate the significance of exercise-induced variations. Statistical analyses were performed using ANALYSE-IT for Excel software (Analyse-It Software, Leeds, United Kingdom) and the level of statistical significance was set at P<0.05. Data are shown as medians and interquartile range (IQR). The post-run biochemical data were also corrected for the plasma volume changes (i.e., haemoconcentration), calculated from post-exercise variation of albumin concentration.
Results
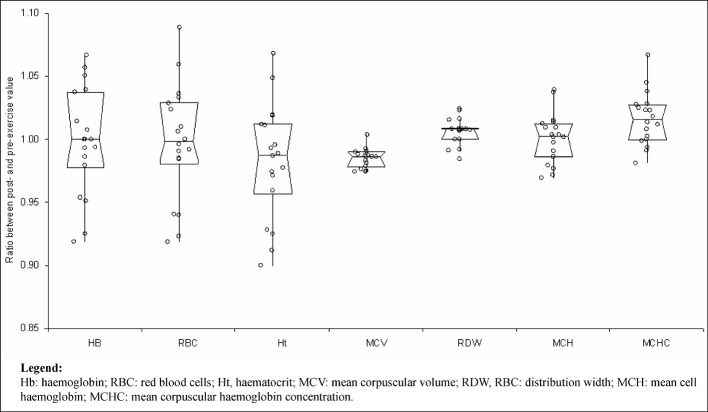
All the 18 athletes completed the ultramarathon successfully and without clinically meaningful symptoms. The main findings of this study are shown in Table I. No statistically significant variations were observed in the post-exercise values of haemoglobin, RBC count and haematocrit. The values of MCV and haptoglobin were significantly decreased, whereas those of RDW were significantly increased (Figures 1 and 2). The concentration of haptoglobin was approximately 50% lower after the end of the ultramarathon, whereas the concentrations of enzymes and potassium were all remarkably increased compared with pre-run values, by nearly 6.4% for albumin (P<0.01), 10% for potassium (P=0.01), 38% for AST (P<0.01), 58% for LDH (P<0.01) and 2.43-fold for CK (P<0.01). The value of the HI remained lower than the limit of detection (i.e. <0.5 g/L) in all the athletes before and after the trial. After adjusting the data for the plasma volume change, the recorded increase was 1.7% for potassium (P=0.17), 30% for AST (P<0.01), 49% for LDH (P<0.01) and 2.39-fold for CK (P<0.01) (Figure 3). Interestingly, a statistically significant association was found between haemoconcentration-adjusted variations of CK and those of AST (r=0.803; P<0.01) and LDH (r=0.551; P=0.02), but not with those of potassium (r= 0.075; P=0.77). Finally, a statistically significant, inverse correlation was found between post-exercise variation of MCV and serum albumin (r=–0.504; P=0.03).
Table I.
Haematological and biochemical variations (median and interquartile range) in 17 trained Caucasian athletes before (pre-run) and after (post-run) a 60 km ultramarathon. Clinical chemistry parameters are shown both adjusted and adjusted for haemoconcentration.
| Pre-run | Post-run | p | |
|---|---|---|---|
| Unadjusted data | |||
| Haemoglobin (g/L) | 142 (138–150) | 142 (137–154) | 0.45 |
| Red blood cells (1012/L) | 4.9 (4.7–5.3) | 4.9 (4.7–5.1) | 0.42 |
| Haematocrit (%) | 44.1 (43.2–46.6) | 44.0 (42.7–45.5) | 0.06 |
| MCV (fL) | 91.7 (88.9–92.7) | 89.8 (87.8–91.4) | <0.001 |
| RDW (%) | 12.5 (12.2–12.9) | 12.6 (12.2–13.0) | 0.01 |
| MCH (pg) | 29.6 (28.3–29.9) | 29.6 (28.3–29.9) | 0.42 |
| MCHC (%) | 32.1 (31.0–33.1) | 32.5 (31.7–33.5) | <0.01 |
| Haptoglobin (mg/dL) | 68 (41–86) | 36 (12–58) | <0.01 |
| Albumin (g/L) | 4.51 (4.31–4.70) | 4.80 (4.63–4.96) | <0.01 |
| Potassium (mmol/L) | 4.09 (3.87–4.48) | 4.49 (4.10–4.77) | 0.01 |
| AST (U/L) | 37 (32–41) | 51 (44–61) | <0.01 |
| LDH (U/L) | 506 (469–592) | 797 (718–900) | <0.01 |
| CK (U/L) | 195 (145–276) | 477 (407–656) | <0.01 |
| Haemolysis Index | 0 (0–0) | 0 (0–0) | - |
| Adjusted data* | |||
| Red blood cells (1012/L) | 4.9 (4.7–5.3) | 4.8 (4.6–5.2) | 0.10 |
| Haematocrit (%) | 44.1 (43.2–46.6) | 43.8 (42.0–45.6) | 0.07 |
| Potassium (mmol/L) | 4.09 (3.87–4.48) | 4.16 (3.90–4.65) | 0.17 |
| AST (U/L) | 37 (32–41) | 48 (41–57) | <0.01 |
| LDH (U/L) | 506 (469–592) | 753 (680–878) | <0.01 |
| CK (U/L) | 195 (145–276) | 466 (376–691) | <0.01 |
Results adjusted for haemoconcentration (i.e., for the plasma volume changes calculated from the difference in albumin concentration pre- and post-exercise).
Legend
RDW: red blood cell distribution width; MCV: mean corpuscular volume; MCH: mean haemoglobin content; MCHC: mean corpuscular haemoglobin concentration; AST: aspartate aminotransferase; LDH: lactate dehydrogenase; CK: creatine kinase.
Figure 1.
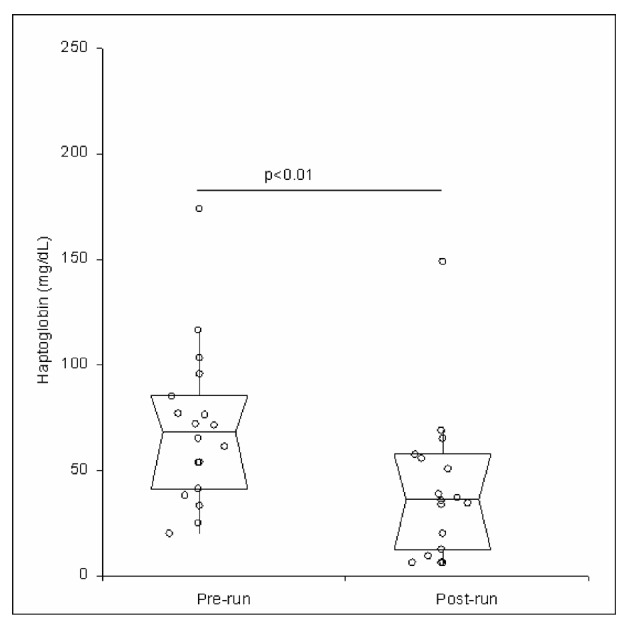
Post-exercise variation of serum hapotoglobin. Values are shown as medians with interquartile ranges.
Figure 2.
Post-exercise variation of haematological parameters. Results are expressed as ratios between the post- and pre-exercise values. Values are shown as medians with interquartile ranges.
Figure 3.
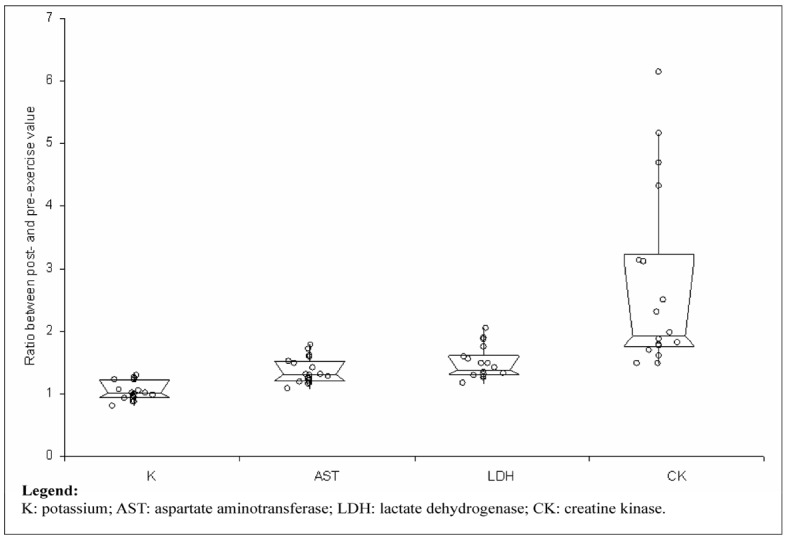
Post-exercise variation of biochemical parameters after adjustment for haemoconcentration. Results are expressed as ratios between the post- and pre-exercise values. Values are shown as medians with interquartile ranges.
Discussion
Sports anaemia might be observed among athletes of some sports disciplines, especially endurance sports. Haemolysis following mechanical trauma was originally described in 1881 by Fleischer, who used the term “march haemoglobinuria” for the phenomena occurring in a young soldier following a strenuous field march12. Afterwards, terms such as “foot-strike haemolysis” and “runner’s haemoglobinuria” have become commonplace in haematology and sports medicine to explain the transient intravascular haemolysis occurring secondary to long-distance running13. Nevertheless, controversial results have been reported about the burden of foot-strike haemolysis during long-distance running.
Miller et al. assessed the relationship between foot impact force and the intravascular haemolysis during distance running in 14 male athletes who completed two treadmill runs based on running for 10,000 foot-strikes at elevations of either +6% or −6%14. Repeated measures ANOVA revealed that plasma free haemoglobin was significantly increased after treadmill running and that these changes were significantly greater with downhill than uphill running. Hardin and Hamill assessed the haematological response after a 30-minute downhill run (gradient of −12%) in 24 athletes who wore soft, medium, or hard midsole shoes15. Although downhill running was characterised by increased haemolysis and significant muscle damage, the intravascular haemolysis could not be related to the softness of the midsole cushioning, thus questioning the impact of foot-strike on acute injury of RBC in vivo. Telford et al. assessed the degree of haemolysis in 10 male triathletes after either 1 hour of running or 1 hour cycling at an equivalent 75% peak oxygen uptake16. Haemolysis was assessed by plasma free haemoglobin and serum haptoglobin concentrations. In agreement with our study, there were no significant differences in haematocrit, RBC count and haemoglobin concentration before and after each of the two exercise trials. One hour after exercise the mean cell-free haemoglobin was not significantly different from the pre-exercise values in both the cycling and running trials. A significant increase of plasma free haemoglobin was only noted 24 hours after both types of exercise, which could hardly be attributed to foot-strike haemolysis occurring during the run.
Banfi et al. studied a large number of athletes performing marathons and ultramarathons at altitude and observed that the pre- and post-race values of RBC count, haemoglobin, packed cell volume, MCV and RDW were not significantly modified by the strenuous effort, thus indicating that frank anaemia did not occur in their study population17. Robison et al. investigated the effect of running on a hard floor with mechanical foot-strike trauma in two groups of athletes who ran on a hard or not hard floor. Interestingly, the athletes running on a hard floor exhibited lower erythrocyte AST activity and an older RBC population than those not running on a hard floor18. These findings led the authors to conclude that mean RBC age is not reduced in runners and, therefore, foot-strike haemolysis should not be considered a major determinant of sports anaemia. Peeling et al. studied ten male athletes who completed two experimental trials involving a one-running-session trial based on 10×1 km interval repeats (90% peak VO2 velocity), follows 12 hours later by a two-running-session trial based on a continuous 10-km run (70% peak VO2 velocity), and a 10x1 km interval run (90% peak VO2 velocity)19. Plasma free haemoglobin, as measured by a photometric technique, marginally increased from 0.03 to 0.05 g/L after the first trial and, analogously, from 0.03 to 0.05 g/L after the second trial, which is still below the upper limit of the reference range of cell-free haemoglobin in blood (i.e., 0.05–0.10 g/L)20. Finally, Yusof et al. demonstrated the occurrence of exercise-induced haemolysis in six athletes who performed a 216-Km, ultra-endurance race. Although the very low number of athletes included in this study prevents definitive conclusions from being drawn, the authors observed an approximately 50% decrease in plasma haptoglobin after 84 km (43±18 versus 87±24; P<0.01), which is almost identical to that observed in our study (36 versus 68 mg/dL; P<0.01), but also described a statistically significant loss of RBC during the initial 84 km, which did not occur in our study, either considering the unadjusted data for the RBC count (4.9×1012 post-exercise versus 4.9×1012 pre-exercise; P=0.42) or after adjustment for plasma volume changes (4.8×1012 post-exercise versus 4.9×1012 pre-exercise; P=0.10)21.
To the best of our knowledge, this is the first study to demonstrate that although running an ultramarathon causes significant muscle injury, it is not associated with clinically significant variations in haemoglobin, haematocrit, RBC count and potassium, so we can conclude that the practice of aerobic exercise, even very exhausting, is not associated with clinically threatening acute anaemia. We have also shown that a significant and acute post-exercise decrease of serum haptoglobin occurred, reflecting a certain degree of haemolysis during the long distance run. However, the concentration of cell-free haemoglobin always remained below 0.5 g/L (considering the formation of free haemoglobin-haptoglobin complexes), thereby indicated that the degree of RBC injury might be considered very modest or even clinically negligible. The statistically significant correlation observed between CK (which is the most peculiar and specific muscle enzyme)22 and both LDH and AST confirms that the post-exercise increase of these enzymes should be mainly attributed to muscle injury rather than RBC damage. The significant reduction of MCV, previously observed by Banfi et al. in rugby players23, was accompanied by an increase of the MCHC and can be reasonably attributed to a compensatory mechanism involving a shift of intracellular water out of the RBC to counterbalance the loss of fluid which is commonplace during strenuous sports such as running marathons and ultramarathons, with the net result of a reduction in the MCV. This hypothesis is strongly supported by the significant inverse correlation found between haemoconcentration (i.e., post-exercise increase of serum albumin) and post-exercise decrease of MCV (P=0.03). In very long ultra-endurance races loss of fluids and haemoconcentration typically occur during the first part of the run, whereas haemodilution may occur in the final part of the run24. The post-exercise increase of RDW further reflects a greater heterogeneity of RBC volumes and mirrors the presence of reactive anisocytosis due to acute variations in the MCV. We observed stable MCH values, whereas the parameter increased when more than 100 km were run. This can be explained by the fact that the release of new, hyperchromic erythrocytes requires more strenuous exercise24.
There are some potential explanations for the findings that an ultramarathon run causes only a modest degree of in vivo haemolysis. First, we cannot rule out that our trained athletes had already reached a stable erythropoietic response, such that the effects on RBC metabolism were only marginally detectable. The significant decrease of haptoglobin, which cannot be influenced by inflammation during the relatively short time course of a race, testifies to the correct and valid response against haemolysis, which is not sufficient to influence the haematological values negatively and, finally, the oxygen delivery to muscles. It is also conceivable that the remarkable improvements made in the materials used for running shoes materials might have consistently attenuated direct plantar injury, thus reducing the severity of foot-strike haemolysis as compared with that occurring in past decades. Taken together these data indicate that acute foot-strike RBC injury might no longer be considered a major determinant of sports anaemia in long-distance runners. One inherent limitation of our study is that the 0.5 g/L threshold of cell-free haemoglobin that was used in this study is relatively high, being three to five times higher than the normal serum concentration. This value does, however, reflect very modest RBC lysis, up to 0.3% in a patient with 150 g/L of haemoglobin. Therefore, although there was a minor degree of haemolysis immediately after the ultramarathon, as demonstrated by the decrease of serum haptoglobin, this involved much less than 1% of the total RBC mass and can be considered of little of even no clinical relevance.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Sawka MN, Convertino VA, Eichner ER, et al. Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sports Exerc. 2000;32:332–48. doi: 10.1097/00005768-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Reinke S, Taylor WR, Duda GN, et al. Absolute and functional iron deficiency in professional athletes during training and recovery. Int J Cardiol. 2010;156:186–91. doi: 10.1016/j.ijcard.2010.10.139. [DOI] [PubMed] [Google Scholar]
- 3.Hinton PS, Sinclair LM. Iron supplementation maintains ventilatory threshold and improves energetic efficiency in iron-deficient nonanemic athletes. Eur J Clin Nutr. 2007;61:30–9. doi: 10.1038/sj.ejcn.1602479. [DOI] [PubMed] [Google Scholar]
- 4.Zoller H, Vogel W. Iron supplementation in athletes-first do no harm. Nutrition. 2004;20:615–9. doi: 10.1016/j.nut.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Sentürk UK, Gündüz F, Kuru O, et al. Exercise-induced oxidative stress leads hemolysis in sedentary but not trained humans. J Appl Physiol. 2005;99:1434–41. doi: 10.1152/japplphysiol.01392.2004. [DOI] [PubMed] [Google Scholar]
- 6.Carlson DL, Mawdsley RH. Sports anemia: a review of the literature. Am J Sports Med. 1986;14:109–12. doi: 10.1177/036354658601400202. [DOI] [PubMed] [Google Scholar]
- 7.Balaban EP. Sports anemia. Clin Sports Med. 1992;11:313–25. [PubMed] [Google Scholar]
- 8.Mechrefe A, Wexler B, Feller E. Sports anemia and gastrointestinal bleeding in endurance athletes. Med Health R I. 1997;80:216–8. [PubMed] [Google Scholar]
- 9.Peeling P, Dawson B, Goodman C, et al. Athletic induced iron deficiency: new insights into the role of inflammation, cytokines and hormones. Eur J Appl Physiol. 2008;103:381–91. doi: 10.1007/s00421-008-0726-6. [DOI] [PubMed] [Google Scholar]
- 10.Lippi G, Salvagno GL, Blanckaert N, et al. Multicenter evaluation of the hemolysis index in automated clinical chemistry systems. Clin Chem Lab Med. 2009;47:934–9. doi: 10.1515/CCLM.2009.218. [DOI] [PubMed] [Google Scholar]
- 11.Harris N, Jou JM, Devoto G, et al. Performance evaluation of the ADVIA 2120 hematology analyzer: an international multicenter clinical trial. Lab Hematol. 2005;11:62–70. doi: 10.1532/LH96.04064. [DOI] [PubMed] [Google Scholar]
- 12.Fleischer R. Ueber eine neue Form von Haemoglbinuric beim menschen. Berl Klin Wschr. 1881;18:691. [Google Scholar]
- 13.Vasudev M, Bresnahan BA, Cohen EP, et al. Percussion hemoglobinuria - a novel term for hand trauma-induced mechanical hemolysis: a case report. J Med Case Reports. 2011;5:508. doi: 10.1186/1752-1947-5-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller BJ, Pate RR, Burgess W. Foot impact force and intravascular hemolysis during distance running. Int J Sports Med. 1988;9:56–60. doi: 10.1055/s-2007-1024979. [DOI] [PubMed] [Google Scholar]
- 15.Hardin EC, Hamill J. The influence of midsole cushioning on mechanical and hematological responses during a prolonged downhill run. Res Q Exerc Sport. 2002;73:125–33. doi: 10.1080/02701367.2002.10609001. [DOI] [PubMed] [Google Scholar]
- 16.Telford RD, Sly GJ, Hahn AG, et al. Footstrike is the major cause of hemolysis during running. J Appl Physiol. 2003;94:38–42. doi: 10.1152/japplphysiol.00631.2001. [DOI] [PubMed] [Google Scholar]
- 17.Banfi G, Roi GS, Dolci A, Susta D. Behaviour of haematological parameters in athletes performing marathons and ultramarathons in altitude (‘skyrunners’) Clin Lab Haematol. 2004;26:373–7. doi: 10.1111/j.1365-2257.2004.00642.x. [DOI] [PubMed] [Google Scholar]
- 18.Robinson Y, Cristancho E, Böning D. Intravascular hemolysis and mean red blood cell age in athletes. Med Sci Sports Exerc. 2006;38:480–3. doi: 10.1249/01.mss.0000188448.40218.4c. [DOI] [PubMed] [Google Scholar]
- 19.Peeling P, Dawson B, Goodman C, et al. Cumulative effects of consecutive running sessions on hemolysis, inflammation and hepcidin activity. Eur J Appl Physiol. 2009;106:51–9. doi: 10.1007/s00421-009-0988-7. [DOI] [PubMed] [Google Scholar]
- 20.Lippi G, Blanckaert N, Bonini P, et al. Haemolysis: an overview of the leading cause of unsuitable specimens in clinical laboratories. Clin Chem Lab Med. 2008;46:764–72. doi: 10.1515/CCLM.2008.170. [DOI] [PubMed] [Google Scholar]
- 21.Yusof A, Leithauser RM, Roth HJ, et al. Exercise-induced hemolysis is caused by protein modification and most evident during the early phase of an ultraendurance race. J Appl Physiol. 2007;102:582–6. doi: 10.1152/japplphysiol.00580.2006. [DOI] [PubMed] [Google Scholar]
- 22.Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med. 2010;48:757–67. doi: 10.1515/CCLM.2010.179. [DOI] [PubMed] [Google Scholar]
- 23.Banfi G, Di Gaetano N, Lopez RS, Melegati G. Decreased mean sphered cell volume values in top-level rugby players are related to the intravascular hemolysis induced by exercise. Lab Hematol. 2007;13:103–7. doi: 10.1532/LH96.07012. [DOI] [PubMed] [Google Scholar]
- 24.Fallon KE, Bishop G. Changes in erythropoiesis assessed by reticulocyte parameters during ultralong distance running. Clin J Sport Med. 2002;12:172–8. doi: 10.1097/00042752-200205000-00005. [DOI] [PubMed] [Google Scholar]