Abstract
OBJECTIVE
Sex differences in cerebral ischemic injury are in part due to differences in cerebrovascular perfusion. We determined if brain microvascular endothelial cells (ECs) isolated from female (F) brain are more resistant to ischemic injury compared to male (M) ECs, and if the difference is due to lower expression of soluble epoxide hydrolase (sEH) and higher levels of vasoprotective epoxyeicosatrienoic acids (EETs). We also determined if protection by EETs is linked to inhibition of Rho-kinase (ROCK).
METHODS
EC ischemic damage was measured after oxygen-glucose deprivation (OGD) using propidium iodide (PI) and cleaved caspase-3 labeling. Expression of sEH was determined by quantitative PCR and immunocytochemistry, EETs levels by liquid chromatography-tandem mass spectrometry, and ROCK activity by ELISA.
RESULTS
EC damage was higher in M vs. F ECs, which correlated with higher sEH mRNA, stronger immunoreactivity and lower EETs compared to F ECs. Inhibition of sEH abolished the sex difference in EC damage. ROCK activity was higher in M vs. F ECs after OGD, and sex differences in EC damage and ROCK activity were abolished by 14,15-EET and ROCK inhibition.
CONCLUSION
Sex differences in ischemic brain injury are in part due to differences in EETs-mediated inhibition of EC ROCK activation after ischemia.
Keywords: Soluble epoxide hydrolase (sEH), Endothelial cell (EC), Oxygen-glucose deprivation (OGD), Epoxyeicosatrienoic acids (EETs), Rho-kinase (ROCK)
Premenopausal women have lower stroke risk and mortality compared to age-matched men1. The sex difference in susceptibility to ischemic injury is in part due to differences in vascular regulatory and protective mechanisms, in which the vascular endothelium is a key player2. Endothelial dysfunction has been observed in stroke patients and has been related to stroke pathophysiology, subtypes, clinical severity and outcome3. Furthermore, there is growing appreciation of the role of endothelium in the recovery process after brain injury and as a therapeutic target in stroke4–6. Clinical studies suggested that endothelial function is improved in young women compared to age-matched men7, 8, and experimental studies demonstrated that sex differences in endothelial cell (EC) function contribute to differences in outcome after ischemic brain injury9. The mechanisms underlying the sex difference in EC function and susceptibility to ischemic injury are not fully understood.
Epoxyeicosatrienoic acids (EETs) are endothelium-derived P450 epoxygenase metabolites of arachidonic acid that exhibit multiple vasoprotective actions during cerebral ischemia, including vasodilation and suppression of post-ischemic inflammation. EETs have also been shown to protect coronary and pulmonary vascular ECs from hypoxic injury10, 11. The activity of EETs is regulated through their metabolism by soluble epoxide hydrolase (sEH)12, an enzyme with greater expression in male compared to female cerebral vessels13. Lower levels of sEH leads to higher levels of circulating EETs and greater EETs-mediated protection in females compared to males14. However, it is not clear if sEH is lower in female than male cerebrovascular ECs, and if the difference contributes to differences in EC sensitivity to ischemic injury. Therefore, in the current study, we hypothesized that primary microvascular ECs isolated from adult female mouse brain are more resistant to ischemic injury induced in-vitro by oxygen-glucose deprivation (OGD) compared to male cells, and that greater resistance to ischemic injury in female cerebrovascular ECs results from lower expression of sEH and consequently higher levels of vasoprotective EETs in female compared to male ECs.
Recent in-vivo studies have also shown that after focal cerebral ischemia, activation of Rho-kinase (ROCK) in ECs contributes to endothelial dysfunction and microcirculatory disturbances15. In-vitro data also supports activation of ROCK in ECs during experimental ischemia contributing to EC injury and death16. Interestingly, EETs have been shown to suppress ROCK activation in pulmonary ECs17. Therefore, in our current study we further hypothesized that the protective effect of EETs on cerebrovascular EC survival is mediated by inhibition of ROCK activation after OGD.
Materials and Methods
Studies were performed according to the NIH Guidelines for the use and care of laboratory animals, and the protocols were approved by the OHSU Institutional Animal Care and Use Committee.
Endothelial Cell Culture
Primary mouse cerebral endothelial cells (ECs) were isolated from 8-week old male and female C57BL6 mice (Charles River Laboratory) based on the method described by Deli et al.18, 19. Briefly, cerebral cortices devoid of cerebella, white matter, large vessels, and leptomeninges were prepared by aseptic macroscopic dissection from mouse brain and diced into small pieces. Cortices were then digested in 1 mg/ml collagenase (Worthington) and 0.01 mg/ml DNase I (Sigma) in Dulbecco's Modified Eagle Medium (DMEM) for 1.5 hours at 37°C. Cells were pelleted by centrifugation at 1000 g for 8 minutes, resuspended in DMEM containing 20% bovine serum albumin (BSA) and again pelleted. The microvessels in the pellet were digested with 1mg/ml collagenase/dispase (Roche) and 0.1 mg/ml DNase I in DMEM for 1 hour at 37°C and separated on a 33% Percoll gradient (GE Healthcare) by centrifugation. Isolated cells were washed in DMEM before plating on collagen (Sigma)-treated flasks in DMEM supplemented with 20% fetal bovine serum (FBS), 50 µg/ml gentamycin, 2 mM glutamine, 100 µg/ml heparin, 100 µg/ml ECGS, endothelial mitogen (Biomedical Technologies Inc) and 4 µg/ml puromycin. After 48 hours, growth medium was changed to puromycin-free medium. Once confluent, cells were detached (0.05% trypsin-EDTA, Sigma) and plated on collagen- coated multiwell plates. Cells were passaged no more than once. Purity of the culture was confirmed by immunocytochemistry with antibodies against EC markers vWF (1:50, Santa Cruz), CD31 (1:50, BD Pharmingen), CD102 (1:100, BD Pharmingen), CD34 (1:50, Cedarlane) and GLUT1 (1:1000, Abcam). Cell images were taken on an inverted (Leica DFC 350FX) or confocal (Zeiss 710) microscope as shown in Supplemental figure. Purity of the culture was further confirmed by a negative reaction to vascular smooth muscle α-actin and glial fibrillary acidic protein (GFAP).
Drug Treatment
EC cultures (80–90% confluent) were treated with the following reagents: 14,15-epoxyeicosatrienoic acid (14,15-EET, 100 nmole/L, Cayman Chemicals, Ann Arbor, Michigan), trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (t-AUCB, 1µM, a gift from Dr. Bruce Hammock) and trans-4-[(1R)-1-Aminoethyl]-N-4-pyridinylcyclohexanecar boxamide dihydrochloride (Y-27632, 10 µM, Tocris Cookston Ltd., Bristol, United Kingdom). For all reagents, treatment was started one hour before oxygen-glucose deprivation (OGD) and continued throughout the OGD/ reperfusion period. Corresponding vehicles (PBS, ethanol or DMSO) were used as control.
OGD/ Reperfusion
ECs were subjected to OGD for 12 hours at 37°C in an anaerobic chamber (Coy Laboratory Products) filled with an anoxic gas mixture (5% CO2, 5% H2, and 90% N2). The oxygen concentration was maintained at 0 parts per million (ppm) using a palladium catalyst. Anoxic conditions were monitored continuously with an oxygen monitor (Oxygen-Hydrogen Gas Analyzer, COY Laboratory Products) placed inside the chamber. To initiate OGD, growth medium was removed, cells were washed and replaced with pre-warmed, glucose-free DMEM. Cells were maintained in the OGD chamber for 12 hours. OGD was terminated by replacing OGD medium with pre-warmed growth medium containing glucose and returning cultures to a normoxic incubator under 5% CO2/ 95% air at 37°C for reperfusion. Separate cultures in which growth medium was replaced with fresh medium but not subjected to OGD served as control.
Cell Death Assays
Cell viability was assessed by staining ECs with fluorescent markers for live (Calcein-AM, Invitrogen) and dead (Propidium Iodide, PI, Sigma) cells. Briefly, 1 µl of reconstituted Calcein-AM solution (containing 50 µg of Calcein AM in 250 µl of DMSO) was added to each well of confluent EC culture in a 24 well plate and incubated at 37°C for 30 minutes. 15 minutes into the incubation, 0.75µl of PI was added to each well. The cells were then examined under an inverted microscope, where live cells appeared fluorescent green (Calcein-AM) and dead cells stained red (PI). Cell death was calculated from the ratio of PI-positive to the sum of PI- and Calcein-AM- positive cells counted under a fluorescent microscope (Nikon TE200). Cell death was also assessed by immunolabeling ECs for cleaved caspase- 3 (1:250, Cell Signaling Technology Inc).
Immunocytochemistry
Cells were fixed using 4% paraformaldehyde and blocked for 1 hour at room temperature in 5% normal donkey serum + 1% BSA + 0.1% TritonX-100 solution. Primary antibody, diluted as specified in blocking buffer, was applied and incubated overnight at 4°C. Coverslips were washed with PBS + 0.1% Tween-20 and secondary antibody was applied in blocking buffer for 2 hours at room temperature. Coverslips were washed and mounted on slides using Fluoromount-G mounting agent (Southern Biotech, AL, USA).
TaqMan Real-Time Quantitative PCR
RNA was isolated from ECs using RNAqueous-Micro kit (Ambion) and reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (ABI); the resulting cDNA was amplified using TaqMan Universal PCR amplification (ABI) in the ABI Prism 7000 sequence detection system. Quantitative PCR was performed in a 96 well plate using 50 µl total volume, with each sample run in triplicate. PCR was also run on controls in which template has not been added, in order to determine DNA contamination and primer-dimer formation. RNA that had not been reverse transcribed was also included to discount genomic DNA amplification. 18S was measured as an internal control using the 18S rRNA control kit-FAM-TAMRA (Eurogentec). Commercially available EPHX2 primers (Eurogentec) were used.
EETs Assay
The concentration of EETs in primary ECs was determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Primary ECs cultured in 6 well plates were washed with PBS, scraped and collected in PBS. The cell suspension was centrifuged at 14,000 g for 10 minutes at 4°C, and cell pellets were re-suspended in 200 µl PBS, homogenized on ice with a microultrasonic cell disruptor at a setting of 6.5 (2 mm probe, highest setting 20, Microscan Ultrasonic), hydrolyzed and extracted, then subjected to LC-MS/MS analysis using the 4000 Q-TRAP triple quadrupole mass spectrometer (Applied Biosystems) with electrospray ionization (ESI) in negative mode. The instrument was interfaced with a Prominence High Performance Liquid Chromatography (HPLC) unit (Shimadzu) which allowed pressures to 10,000 psi. Resolution was obtained with a 2.1×250 mm, 5 µ BetaBasic C18 HPLC column with guard and gradient elution with water and acetonitrile; both with 0.002% acetic acid under the following conditions: 45–60% B over 1 minute, linear to 65% B over 15 minutes, linear to 95% B over 0.10 min, isocratic at 95% B for 4 minutes, linear to 45% B over 0.1 minute, isocratic at 45% B for 10 minutes. Flow rate was 0.5 ml/min and column temperature was 40°C. ESI source parameters included curtain gas 50, ion spray voltage −4000, temperature 550 °C with ion source gas 1 and gas 2 at 50 and an entrance potential of −10 V. Samples were infused individually and instrument parameters were optimized for multiple reaction monitoring with selective transitions. The deuterated internal standard 14,15-EET-d8 was spiked into each sample and monitored. The amount of EETs in the sample was calculated by calculating ratio of the compound’s peak area to that of 14,15-EET-d8, then compared to a standard curve generated from blank HBS spiked with known amounts of EETs.
ROCK Assay
ROCK activity in ECs was measured by a commercially available ROCK Activity Assay Kit (Cell Biolabs). Briefly, culture medium was aspirated off and ECs were washed with cold saline buffer, scraped and homogenized in the presence of protease inhibitors (complete MINI, EDTA-free, protease inhibitor cocktail tablets, ROCHE, Germany). Cell homogenate was then applied to a strip well micro-titer plate pre-coated with a recombinant myosin phosphatase target subunit 1 (MYPT1). After incubation, the phosphorylation of MYPT1 at its Thr696 residue by ROCK was detected by an anti-phospho-MYPT1 (Thr696) antibody and HRP-conjugated secondary antibody and read spectrophotometrically.
Hydrolase Activity Assay
Soluble epoxide hydrolase activity was determined using Epoxyfluor 7 (EP7; Cayman Chemical Company; Jones et al.20). ECs were lysed in PBS and immediately used for hydrolase activity quantification. Protein concentration of the lysate was determined using Biorad Protein Assay (Biorad, Hercules, CA). Hydrolase activity was assessed in in 25mM BisTris-HCl solution containing 0.1 mg/ml bovine serum albumin (BSA) and 5µM EP7 substrate incubated for 30 minutes at 37°C in a 96- well flat bottomed plate (Corning, Corning, NY). Fluorescence of hydrolyzed EP7 was determined using an excitation wavelength of 330nm (bandwidth 20nm) and emission 465nm (bandwidth 20nm) on a plate reader (VICTOR, Wallac/ Perkin Elmer, Waltham, MA). Activity was normalized to sample protein concentration and expressed as Relative Fluorescence Units (RFU).
Statistical Analysis
Statistically significant differences between groups were determined by a t-test for two groups and analysis of variance (ANOVA) followed by Holm-Sidak post hoc analysis for multiple groups using the Sigmastat software (Systat software Inc.). All data were expressed as mean ± SEM, with statistical significance set at p<0.05.
Results
To assess the sex difference in EC susceptibility to ischemic injury, EC from male (M) and female (F) mouse cerebral vessels were subjected to 12 hours of oxygen glucose deprivation (OGD) followed by 24 hours of reperfusion. Characterization of EC culture is shown in the supplemental figure. Cell death was measured both by propidium iodide (PI) staining and cleaved caspase-3 labeling, and expressed as a percentage of total cells. At baseline, PI-positive cells comprised less than 1% of cells in both F and M cultures. OGD induced a significant increase in cell death, which was significantly lower in F than M ECs (17.0±1.8% vs. 43.3±5.0%, respectively, n=7, p<0.05) (Figure 1A). At baseline, no cleaved caspase-3 positive cells were detected, however following OGD male ECs exhibited significantly higher cell death than female cells (2.9+/− 0.8% vs. 0.48+/− 0.3% respectively, n = 4, p<0.05) (Figure 1B). The absolute levels of cell death vary between these two detection methods, although both show that cell death following OGD is higher in males compared to females. Figure 1B shows that cleaved caspase-3 labeling does not label all cells with condensed nuclei, hence accounting for this difference between PI labeling and cleaved caspase-3 labeling.
Figure 1. Sex difference in endothelial cell (EC) survival following 12 hours of oxygen- glucose deprivation (OGD) and 24 hours of reperfusion.
A. EC viability was assessed using calcein-AM and cell death by propidium iodide (PI). Representative images show calcein-AM (green) and PI (red). Cell death was expressed as percentage of PI positive cells to total number of cells. Female (F) EC sustained significantly lower cell death compared to male (M) EC (n = 7,*P < 0.05 compared to M). B. Cell death was also assessed by immunolabeling for cleaved caspase- 3. Female EC had significantly fewer cells positive for cleaved caspase- 3 compared to male following OGD and reperfusion (n = 4, * P < 0.05). Representative image shows cleaved caspase- 3 (red) and DAPI (blue); arrows point to condensed nuclei positive for cleaved caspase- 3, arrowheads point to condensed nuclei negative for cleaved caspase- 3. Scale bar, 20µm.
We tested the hypothesis that the differential sensitivity to ischemic injury between M and F ECs is linked to differences in EETs and sEH. Immunofluorescent labeling with anti-sEH antibody revealed more intense staining in M vs. F ECs (Figure 2A, n=3). The sex difference was confirmed by measuring mRNA expression of EPHX2, the gene encoding for sEH, using TaqMan real-time quantitative PCR. Figure 2B shows that the level of EPHX2 mRNA was lower in F than M ECs at baseline (0.20±0.08 vs. 0.64±0.18 relative to 18S RNA; n=4, p<0.05). After OGD, EPHX2 mRNA was reduced in both M and F ECs, but the difference remained statistically significant (0.45±0.11 in M vs. 0.26±0.16 in F, n=4, p<0.05).
Figure 2. Sex differences in soluble epoxide hydrolase (sEH) expression and levels of epoxyeicosatrienoic acid (EET) in mouse brain endothelial cells (EC).
A. Confocal images of sEH immunoreactivity (green) in female (F) and male (M) ECs, showing less intensity for sEH in F than M ECs. Cell nuclei were labeled with 4', 6-diamidino-2-phenylindole (DAPI, blue). Scale bar, 20µm. B. EPHX2 mRNA levels, measured by TaqMan quantitative real time PCR, were significantly lower in F ECs compared to M ECs at baseline and following OGD (n = 4, * P < 0.05). C. Levels of total EETs in ECs measured by liquid chromatography-mass spectrometry (LC-MS/MS) showing that levels of EETs were higher in F ECs compared to M ECs at baseline (n = 4, * P < 0.05).
To determine if lower sEH in F ECs results in higher EETs, the concentration of total EETs was measured by LC-MS/MS. Figure 2C demonstrates that F ECs had significantly higher levels of EETs compared to M EC at baseline (856.5 +/− 140.86 vs. 272 +/− 102.33 pg/mL, n=4, p<0.05).
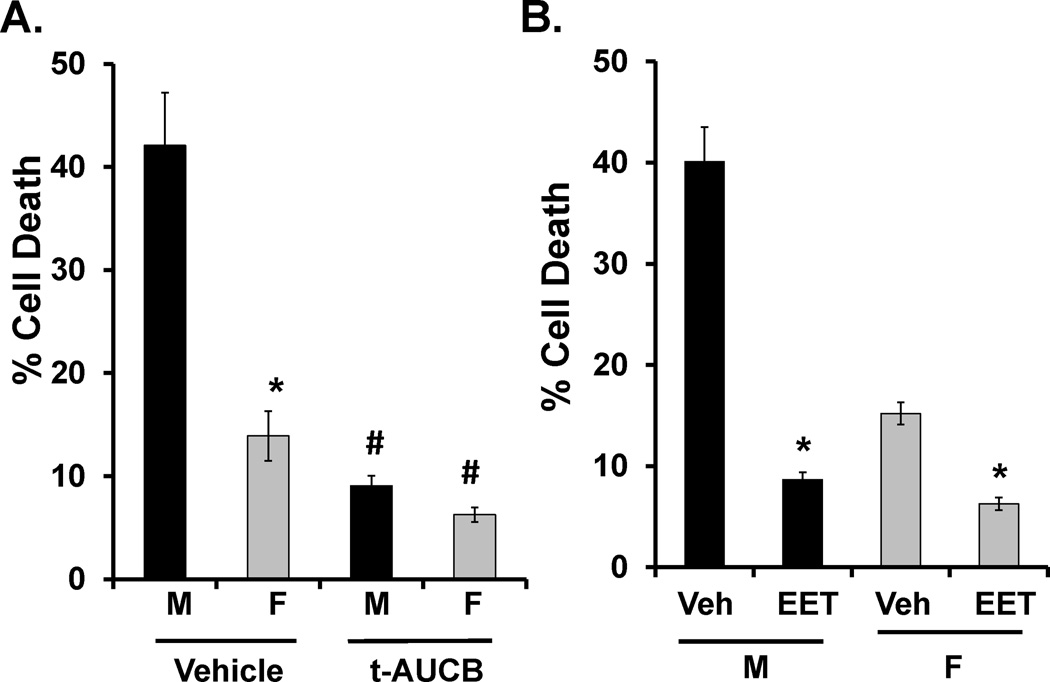
To determine if differences in sEH expression and EETs levels contribute to the sex difference in sensitivity to ischemic injury, we pre-treated M and F EC cultures with sEH inhibitor t-AUCB (1 µM) or vehicle (DMSO). t-AUCB was effective at inhibiting hydrolase activity by 31.15% (not shown). In agreement with Figure 1, Figure 3A shows that vehicle-treated F ECs sustain significantly less damage compared to M ECs (9.0±0.99% vs. 42.1±5.1%, n=7, p<0.05). Inhibition of sEH reduced cell death in both F and M ECs, abolishing the sex difference in cell death (3±0.7% in F vs. 9.1±1.0% in M EC, n=5, p=0.47).
Figure 3. Manipulation of soluble epoxide hydrolase (sEH) and 14,15-epoxyeicosatrienoic acid (EET) levels abolishes the sex difference in endothelial cell (EC) death following OGD.
A. Treatment of ECs with t-AUCB (1µM), prior, during and following OGD, abolished sex difference in OGD- induced cell death. After 12 hours of OGD and 24-hours of reperfusion, female (F) EC had significantly lower cell death, as assessed by calcein-AM and PI labeling, compared to male (M) EC (n = 7, * P < 0.05 compared to M). Treatment with t-AUCB reduced cell death in both sexes (#, P < 0.05, compared to corresponding vehicle group) eliminating the sex difference in EC death (n = 5, P = 0.47). B. Treatment of mouse brain ECs with 14,15-EET (100nM) reduced cell death in both female (F) and male (M) ECs after 12 hours of OGD and 24 hours of reperfusion (n = 7, *P < 0.05 compared to corresponding vehicle).
The results presented above suggest that EETs are protective against OGD-induced ischemic injury in EC damage. To directly test this hypothesis, we pre-treated M and F ECs with 14, 15-EET or vehicle (ethanol) before OGD. Figure 3B shows that 14,15-EET significantly reduced cell death in both F and M ECs (from 15.2±1.1% to 6.27±0.63% in F EC, n=7, p<0.05, and from 40.1±3.4% to 8.65±0.74%, n=7, p<0.05, compared to corresponding vehicle in M ECs).
Finally, we determined if protection by EETs is linked to inhibition of ROCK activation after OGD. Figure 4A shows that ROCK activity was significantly increased after OGD, and that this increase was more pronounced in M compared to F ECs (5.39±0.19 vs. 2.95±0.53 fold increase above baseline, respectively, n=3, p<0.05). Pre-treatment with 14,15 EET inhibited OGD–induced ROCK activation in M (from 5.39±0.19 to 1.32±0.03 folds, n=3, p<0.05) but not in F ECs (2.95±0.53 vs. 3.36±0.19, n=3, p=0.51). To determine if EC protection by ROCK inhibitors is sexually dimorphic, we treated M and F ECs with ROCK inhibitor Y-27632 (10 µM) or vehicle (PBS) before, during and after OGD. Figure 4B shows that ROCK inhibition reduced cell death in M (from 45.4±5.53 to 25.6±3.8%, n=5, p<0.05 compared to corresponding vehicle), but not F EC (16.8±1.8% vs. 10.4±2.4%, n=5, P=0.0613). We also show that treating male cells with both 14,15-EET and Y-27632 does not result in a further decrease in cell death following OGD compared to either treatment alone (Figure 4C), suggesting that the effects are not additive but that the 14,15-EET protection observed is mediated by ROCK inhibition.
Figure 4. Sex differences in Rho-kinase (ROCK) activity and effect of 14,15- EET and ROCK on mouse brain ECs death.
A. ROCK activity measured by ELISA was similar at baseline in male (M) and female (F) ECs (n = 3, P = not significant). After 12 hours of oxygen-glucose deprivation (OGD), there was greater activation of ROCK in M ECs compared to F ECs (n = 3, * P < 0.05). Treatment with 14,15-EET (100nM) reduced ROCK activation after OGD in M ECs but not F ECs (n = 3, # compared to OGD alone). ROCK activity was normalized to protein content and expressed relative to corresponding baseline. B. Treatment with ROCK inhibitor trans-4-[(1R)-1-Aminoethyl]-N-4-pyridinylcyclohexanecar boxamide dihydrochloride (Y-27632, 10µM) resulted in reduction in OGD-induced cell death in male (M) but not female (F) ECs (n = 5, * P < 0.05 compared to corresponding vehicle). Cell death was assessed by calcein-AM and PI labeling after 12 hours of OGD and 24 hours of reperfusion. C. Treatment of male ECs with either 14,15-EET (100nM) or Y-27632 (10µM), or a combination of both, reduced cell death as measured by cleaved caspase-3 immunolabeling, following 12 hours of OGD and 24 hours reperfusion. The protective effects of 14,15-EET and Y-27632 are not additive (n = 5, * P < 0.05 compared to vehicle; P = not significant between treatment groups).
Discussion
We report three new findings: 1) Female mouse brain endothelial cells (ECs) are more resistant to ischemic injury induced by oxygen-glucose deprivation than male ECs, 2) Reduced susceptibility of female ECs to ischemic injury is, in part, due to lower levels of expression of sEH and higher EETs, and 3) Higher EETs in female ECs protects ECs in part by inhibiting OGD-induced ROCK activation.
Sex differences in ischemic brain injury and cerebrovascular regulation are observed in clinical and experimental studies1,21–24. An important determinant of brain tissue perfusion after cerebral ischemia is the integrity of ECs. Indeed, sex differences in endothelial cell function have previously been described between men and women25, and in animals using isolated vessels and in-vivo using preparations such as the cranial window22, 23. However, it is not clear from these studies if sex differences are inherent to the endothelium or affected by surrounding cells and hormones. Furthermore, previous studies assessed baseline endothelium-dependent responses, and did not evaluate sex differences in sensitivity to ischemic injury. Our study demonstrates for the first time that endothelial cells from adult male and female mice exhibit an inherently different response to ischemic injury, with female endothelial cells exhibiting higher resistance to ischemic damage than male cells. Given the importance of endothelial cells in vascular reactivity, inflammation, the blood-brain barrier (BBB) and in angiogenesis, our findings suggest that inherent differences in EC susceptibility to ischemia may in part underlie the differences in acute vascular responses and recovery from injury observed between males and females.
The mechanism underlying the differential sensitivity of male and female ECs to ischemic injury is unknown. We tested the hypothesis that the difference is related to differences in epoxyeicosanoid signaling. Epoxyeicosatrienoic acids (EETs) are arachidonic acid metabolites of cytochrome P450 (CYP) epoxygenases that are produced in the brain by astrocytes and vascular endothelium13, 26. EETs have been shown to protect the brain from ischemic injury by multiple mechanisms, including vasodilatation, cytoprotection and suppression of post-ischemic inflammation27. The cytoprotective effect of EETs following ischemia has been shown in cortical neurons and glial cells in-vitro28. Whether this cytoprotection extends to cerebrovascular ECs had not been studied previously. In the peripheral vasculature, EETs in general, and 14,15-EETs in particular, are protective against endothelial cell apoptosis10. In our study we make the novel observation that 14,15-EETs protect both female and male ECs from OGD induced cell death.
The biological activity of EETs is terminated in-vivo via metabolism by soluble epoxide hydrolase, (sEH)12. Consistent with the protective function of EETs in cerebral ischemia and the role of sEH in regulating cellular EETs levels24, pharmacological inhibition or gene deletion of sEH reduces infarct size after experimental stroke in mice29,30. Interestingly, earlier work from our group has shown that the cerebrovascular expression and activity of sEH in intact cerebral vessels are higher in male compared to female mice13. Whether this difference in sEH expression is attributable to the endothelium or vascular smooth muscle had not been determined. In this study, we show that differences in sEH expression and EETs levels are present in cerebral endothelial cells and that these differences may underlie the observed differences in ischemia induced cell death.
The mechanism of protection by EETs is multifactorial and involves membrane-associated and intracellular targets. In our study we show that there are sex-specific differences in ROCK activation after OGD and link these differences to the inhibitory effect of endogenous EETs. We also demonstrate a novel therapeutic potential role of ROCK inhibitor Y-27632 by promoting EC survival. ROCK is the best-characterized effector of Rho, a member of the small GTPase family of proteins. Hypoxia-induced EC apoptosis has in part been attributed to activation of ROCK16. ROCK activation during apoptosis results in increased myosin activity, bundling of F-actin by activated myosin, actin-myosin contractile force generation, cell contraction and ultimately cell blebbing31, 32. In a mouse model of middle cerebral artery occlusion (MCAO), ROCK was activated in the ischemic region of the brain, and inhibition of ROCK reduced cerebral infarct size and improved neurologic deficit after MCAO33. In culture, inhibition of ROCK prevents ischemia-induced EC apoptosis in part by maintaining pro-survival phosphotidylinositol 3-kinase (PI3-K)/Akt activity and preventing F-actin re-arrangement16.
In conclusion, the present study demonstrates that ECs isolated from female cerebral microvessels are more resistant to ischemic injury, in part due to lower sEH expression and higher EETs levels, leading to stronger inhibition of ROCK activation after OGD in female compared to male ECs. These findings suggest that higher EETs signaling is an endogenous vasoprotective pathway in female EC survival after ischemic injury and identify a novel mechanism for ROCK-mediated vasoprotection. Given the critical role of the endothelium for proper brain function, EETs signaling may represent a therapeutic target against cerebral endothelial dysfunction after ischemic stroke.
Supplementary Material
Acknowledgements
The authors would like to acknowledge support by the OHSU Bioanalytical Shared Resource/Pharmacokinetics Core (BSR/PKCore) in performing the EETs assay.
Sources of Funding: Studies were supported by National Institute of Health (NIH) grants R01NS070837 and NS044313 to Dr. Nabil Alkayed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Authors have no conflicts of interest to disclose.
Contributor Information
Nandita C. Gupta, Email: gupta@ohsu.edu.
Catherine M. Davis, Email: davis@ohsu.edu.
Jonathan W. Nelson, Email: nelsonjo@ohsu.edu.
Jennifer M. Young, Email: youngj@ohsu.edu.
References
- 1.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller VM, Mulvagh SL. Sex steroids and endothelial function: translating basic science to clinical practice. Trends Pharmacol Sci. 2007;28:263–270. doi: 10.1016/j.tips.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Roquer J, Segura T, Serena J, Castillo J. Endothelial dysfunction, vascular disease and stroke: the ARTICO study. Cerebrovasc Dis. 2009;27(Suppl 1):25–37. doi: 10.1159/000200439. [DOI] [PubMed] [Google Scholar]
- 4.Narasimhan P, Liu J, Song YS, Massengale JL, Chan PH. VEGF Stimulates the ERK 1/2 signaling pathway and apoptosis in cerebral endothelial cells after ischemic conditions. Stroke. 2009;40:1467–1473. doi: 10.1161/STROKEAHA.108.534644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 6.Bastide M, Ouk T, Plaisier F, Petrault O, Stolc S, Bordet R. Neurogliovascular unit after cerebral ischemia: is the vascular wall a pharmacological target. Psychoneuroendocrinology. 2007;32(Suppl 1):S36–S39. doi: 10.1016/j.psyneuen.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Kublickiene K, Luksha L. Gender and the endothelium. Pharmacol Rep. 2008;60:49–60. [PubMed] [Google Scholar]
- 8.Villar IC, Hobbs AJ, Ahluwalia A. Sex differences in vascular function: implication of endothelium-derived hyperpolarizing factor. J Endocrinol. 2008;197:447–462. doi: 10.1677/JOE-08-0070. [DOI] [PubMed] [Google Scholar]
- 9.Geary GG, Krause DN, Duckles SP. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am J Physiol. 1998;275:H292–H300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- 10.Dhanasekaran A, Al-Saghir R, Lopez B, Zhu D, Gutterman DD, Jacobs ER, Medhora M. Protective effects of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. Am J Physiol Heart Circ Physiol. 2006;291:H517–H531. doi: 10.1152/ajpheart.00953.2005. [DOI] [PubMed] [Google Scholar]
- 11.Yang B, Graham L, Dikalov S, Mason RP, Falck JR, Liao JK, Zeldin DC. Overexpression of cytochrome P450 CYP2J2 protects against hypoxia-reoxygenation injury in cultured bovine aortic endothelial cells. Mol Pharmacol. 2001;60:310–320. doi: 10.1124/mol.60.2.310. [DOI] [PubMed] [Google Scholar]
- 12.Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs) Prostaglandins Other Lipid Mediat. 2007;82:42–49. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iliff JJ, Wang R, Zeldin DC, Alkayed NJ. Epoxyeicosanoids as mediators of neurogenic vasodilation in cerebral vessels. Am J Physiol Heart Circ Physiol. 2009;296:H1352–H1363. doi: 10.1152/ajpheart.00950.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Iliff JJ, Campbell CJ, Wang RK, Hurn PD, Alkayed NJ. Role of soluble epoxide hydrolase in the sex-specific vascular response to cerebral ischemia. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yagita Y, Kitagawa K, Sasaki T, Terasaki Y, Todo K, Omura-Matsuoka E, Kaibuchi K, Hori M. Rho-kinase activation in endothelial cells contributes to expansion of infarction after focal cerebral ischemia. J Neurosci Res. 2007;85:2460–2469. doi: 10.1002/jnr.21375. [DOI] [PubMed] [Google Scholar]
- 16.van der Heijden M, Versteilen AM, Sipkema P, van Nieuw Amerongen GP, Musters RJ, Groeneveld AB. Rho-kinase-dependent F-actin rearrangement is involved in the inhibition of PI3-kinase/Akt during ischemia-reperfusion-induced endothelial cell apoptosis. Apoptosis. 2008;13:404–412. doi: 10.1007/s10495-007-0173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keseru B, Barbosa-Sicard E, Popp R, Fisslthaler B, Dietrich A, Gudermann T, Hammock BD, Falck JR, Weissmann N, Busse R, Fleming I. Epoxyeicosatrienoic acids and the soluble epoxide hydrolase are determinants of pulmonary artery pressure and the acute hypoxic pulmonary vasoconstrictor response. FASEB J. 2008;22:4306–4315. doi: 10.1096/fj.08-112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu C, Pelech S, Zhang H, Bond J, Spach K, Noubade R, Blankenhorn EP, Teuscher C. Pertussis toxin induces angiogenesis in brain microvascular endothelial cells. J Neurosci Res. 2008;86:2624–2640. doi: 10.1002/jnr.21716. [DOI] [PubMed] [Google Scholar]
- 19.Szabo CA, Deli MA, Ngo TK, Joo F. Production of pure primary rat cerebral endothelial cell culture: a comparison of different methods. Neurobiology (Bp) 1997;5:1–16. [PubMed] [Google Scholar]
- 20.Jones PD, Wolf NM, Morisseau C, Whetstone P, Hock B, Hammock BD. Fluorescent substrates for soluble epoxide hydrolase and application to inhibition studies. Anal. Biochem. 2005;343:66–75. doi: 10.1016/j.ab.2005.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med. 2008;6:33. doi: 10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Iliff JJ, Campbell CJ, Wang RK, Hurn PD, Alkayed NJ. Role of soluble epoxide hydrolase in the sex-specific vascular response to cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:1475–1481. doi: 10.1038/jcbfm.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turtzo LC, McCullough LD. Sex differences in stroke. Cerebrovasc Dis. 2008;26:462–474. doi: 10.1159/000155983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinberg HO, Paradisi G, Cronin J, Crowde K, Hempfling A, Hook G, Baron AD. Type II diabetes abrogates sex differences in endothelial function in premenopausal women. Circulation. 2000;101:2040–2046. doi: 10.1161/01.cir.101.17.2040. [DOI] [PubMed] [Google Scholar]
- 26.Medhora M, Narayanan J, Harder D. Dual regulation of the cerebral microvasculature by epoxyeicosatrienoic acids. Trends Cardiovasc Med. 2001;11:38–42. doi: 10.1016/s1050-1738(01)00082-2. [DOI] [PubMed] [Google Scholar]
- 27.Iliff JJ, Jia J, Nelson J, Goyagi T, Klaus J, Alkayed NJ. Epoxyeicosanoid Signaling in CNS Function and Disease. Prostaglandins Other Lipid Mediat. 2009 doi: 10.1016/j.prostaglandins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu M, Alkayed NJ. Hypoxic preconditioning and tolerance via hypoxia inducible factor (HIF) 1alpha-linked induction of P450 2C11 epoxygenase in astrocytes. J Cereb Blood Flow Metab. 2005;25:939–948. doi: 10.1038/sj.jcbfm.9600085. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–2078. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, Hammock BD, Falck JR, Alkayed NJ. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007;27:1931–1940. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Y, Hoang BQ, Chang DD. ROCK-II-induced membrane blebbing and chromatin condensation require actin cytoskeleton. Exp Cell Res. 2002;278:45–52. doi: 10.1006/excr.2002.5565. [DOI] [PubMed] [Google Scholar]
- 32.Coleman ML, Olson MF. Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ. 2002;9:493–504. doi: 10.1038/sj.cdd.4400987. [DOI] [PubMed] [Google Scholar]
- 33.Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–2257. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.