Abstract
This study examined whether regular exercise training, at a level that would be recommended for middle-aged people interested in improving fitness could lead to improved cognitive performance and increased blood flow to the brain in another primate species. Adult female cynomolgus monkeys were trained to run on treadmills for one hour a day, 5 days a week, for a 5 month period (n=16; 1.9±0.4 miles/day). A sedentary control group sat daily on immobile treadmills (n=8). Half of the runners had an additional sedentary period for 3 months at the end of the exercise period (n=8). In all groups, half of the monkeys were middle-aged (10–12 years old) and half were more mature (15–17 years old). Starting the fifth week of exercise training, monkeys underwent cognitive testing using the Wisconsin General Testing Apparatus (WGTA). Regardless of age, the exercising group learned to use the WGTA significantly faster (4.6±3.4 days) compared to controls (8.3±4.8 days; p=0.05). At the end of 5 months of running monkeys showed increased fitness, and the vascular volume fraction in the motor cortex in mature adult running monkeys was increased significantly compared to controls (p=0.029). However, increased vascular volume did not remain apparent after a three-month sedentary period. These findings indicate that the level of exercise associated with improved fitness in middle-aged humans is sufficient to increase both the rate of learning and blood flow to the cerebral cortex, at least during the period of regular exercise.
Keywords: spatial cognition, discrimination, learning, blood flow, motor cortex, primate
Introduction
The benefits of physical fitness on human brain function and structure have become more evident over the past decade. A strong positive association has been shown between physical fitness, cognitive performance and brain volume in humans (Gordon et al., 2008, Ruscheweyh et al., 2009). An extensive meta-analysis of controlled randomized exercise studies showed a positive effect of exercise on a broad range of cognitive functions in older human adults (Colcombe and Kramer, 2003), and placing humans on an aerobic exercise program appears to mitigate brain volume declines with aging (Colcombe et al., 2003, 2006). Recent studies have also shown positive effects of exercise on slowing of the progression of neurological disorders and enhancing recovery from brain injury (Sasco et al., 1992; Teri et al., 2003; Chen et al., 2005; Podewils et al., 2005; Rolland et al., 2007; Hamer and Chida, 2009; Scarmeas et al., 2009)
Though the mechanisms underlying the positive effects of exercise on the brain can not easily be tested in humans, the concept that exercise can influence many central neural structures is supported by a wealth of data collected in rodents. Chronic exercise increases the vascular volume fraction in rat cerebellum, and motor, visual, and frontal cortices, and striatum, and increases blood flow to the cerebellum (Black et al., 1990; Kleim et al., 2002; Swain et al., 2003; Ding et al., 2004a,b, 2006). Exercise in rodents also increases hippocampal dentate gyrus neurogenesis (van Praag et al., 1999; Rhodes et al., 2003; van Praag et al., 2005), and neurotrophic factor expression (Neeper et al., 1995; Gomez-Pinella et al., 1997; Klintsova et al., 2005; Griesback et al., 2009) in various brain regions. Exercise has also been shown to increase learning of some cognitive tasks in rodents (Barnes et al., 1991; Anderson et al., 2000; Radak et al., 2001).
However, it has been difficult to judge the equivalence of exercise regimens used in rodents to those recommended for humans (Dishman et al., 2006; Cotman et al., 2007); thus it remains unclear whether the exercise-related changes in the central nervous system which have been demonstrated in rodents would occur in humans undertaking moderate levels of exercise recommended for improving fitness, maintaining body weight, and reducing risk of chronic disease (Haskell et al., 2007). To bridge this gap, we have developed a nonhuman primate model of monkeys running on treadmills at an amount sufficient to improve fitness in humans (Williams et al., 2007), in order to examine whether moderate exercise has positive effects on brain structure and function. Use of a nonhuman primate model allowed us to standardize the exercise regimen (i.e., all monkeys were run at 80% maximal capacity) and control other lifestyle factors such as diet and stress exposure so that they did not change during the exercise period, while examining an exercise regimen comparable to that frequently prescribed for people. We exercised adult female cynomolgus monkeys on treadmills using a regimen recommended by the American College of Sports Medicine and the American Heart Association (Haskell et al., 2007) and examined evidence for two changes in the brain that have been well documented in rodent studies: improved learning of new tasks (Cotman and Berchtold, 2002; Vaynman et al., 2004; Leggio et al., 2005) and increased vascular density (Swain et al., 2003; Ding et al., 2004, 2006) in motor cortex.
Materials and Methods
Animals
Twenty four adult female cynomolgus monkeys (Macaca fascicularis), weighing 2.7 to 7.6 kg, which had been born in the wild, imported as young animals and moved to the University of Pittsburgh about 9 years previously were used for this study. Age at the time of importation was determined by dental and bone aging (Clifton and Steiner, 1986). This study utilized two age groups: 12 middle-aged adult females (10–12 years old) and 12 more mature adult females (15–17 years old). For this study, monkeys were housed at the University of Pittsburgh Primate Research Laboratory in individual cages in rooms holding approximately 40–80 monkeys, maintained on a controlled temperature (24±2 C) and lighting schedule (lights on from 0700–1900 h). Monkeys were fed a single daily meal of Purina High Protein monkey chow (12 pellets; approximately 240 Kcal; no. 5045, Ralston Purina Co., St. Louis, MO) and one-quarter piece of fruit (about 20 kcal). Water was available ad libitum. Monkeys also received novel items including non-caloric food treats and toys to play with as part of a psychological enrichment program in accordance with USDA guidelines. Food intake was tabulated every morning between 0800–0900 by subtracting the number of pellets that were not consumed from the total number of pellets provided to the monkeys the preceding day. Body weights before daily feeding were measured weekly. Each monkey had her vaginal area swabbed daily with a cotton-tipped applicator, to detect menses. All experiments were performed in compliance with regulations of the Animal Care and Use Committee of the University of Pittsburgh.
Blood Sampling Procedures
Blood samples for the measurement of plasma lactate were collected from awake animals at time points over the course of the study. For collection of blood samples, each monkey was trained to jump from its cage into a transport box and then enter a specially designed cage that allowed for brief immobilization of the monkey’s leg, using previously published techniques (Williams et al., 2001). Blood samples were taken by femoral venipuncture. Blood was collected in sterile heparinized syringes, transferred into glass tubes that were placed in ice, and immediately centrifuged at 3800 rpm for 12 minutes. Plasma was stored at −20°C until assays were performed.
Heart Rate Monitoring
At intervals throughout the experiment, heart rate was measured using standard electrocardiogram electrodes protected by a nylon jacket. All monkeys had been adapted to wearing jackets as part of a previous behavioral study, in which they wore jackets for 4–7 day intervals several times a year for three years. For EKG electrode placement, monkeys were sedated with 0.1 mg/kg ketamine hydrochloride (Ketaject, Phoenix Pharmaceuticals Inc., St. Joseph, MO) and standard pediatric heart rate electrodes with self-adhesive pads were adhered to the monkey’s chest. The distal ends of the electrodes were attached to a TM8 telemetry transmitter (Life Sensing Instruments, Tullahoma, TN) that was placed in an inside pocket of a jacket that the monkeys wore to prevent them from manipulating the heart rate electrodes and transmitter. The heart rate signal was received by a HST 220 telemetry receiver (Life Sensing Instruments, Tullahoma, TN) and recorded by a computer. Software for heart rate data collection and storage (Samsedate Heart Rate Variability System) was developed by Autrec, Inc. (Winston-Salem, NC).
Experimental Design
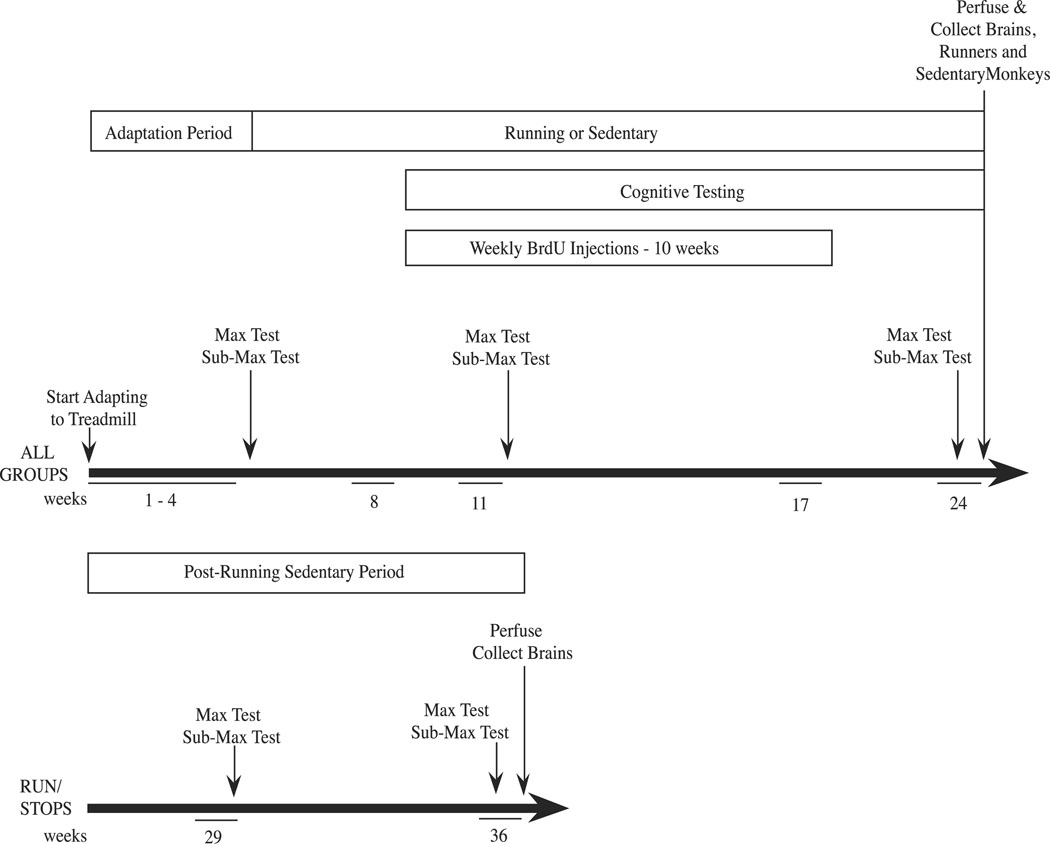
Within each age group (middle-aged adults and more mature adults), monkeys were divided into 3 experimental groups: runners (ran on a treadmill 5 days a week for 5 months, n=4), sedentary controls (sat on the treadmill 5 days a week for 5 months, n=4), and a run-stop group (ran on a treadmill 5 days a week for 5 months and then were sedentary for 3 additional months, n=4). The overall experimental design is shown in Fig. 1.
Figure 1.
Schematic diagram of the experimental design. Maximal exercise test abbreviated as ‘max test’; submaximal exercise test abbreviated as ‘submax test’.
Exercise Training
Monkeys in the run and run-stop groups were trained to run on standard human size treadmills (Model 910e, Precor, Inc., Bothell, WA), using previously published techniques (Williams et al., 2001, 2002, 2007). Each treadmill was covered by a Plexiglas box that had numerous air holes in the front and back panels for adequate ventilation. Initially, for several days, all of the monkeys were acclimated to the treadmill by sitting on it and being allowed to explore the treadmill belt and the Plexiglas box. Monkeys then learned to walk on the treadmill and the speed and duration of each running session was slowly increased to 1.6 mph for 20 min/day. This treadmill adaptation period lasted 4 weeks. Subsequently, at the beginning of the 5th week of the study each monkey underwent maximal exercise testing (described below), and their prescribed exercise regimen was individually determined as 1 hour of running, 5 days a week, at a speed equivalent to 80% of maximal aerobic power as determined by maximal exercise testing. Monkeys gradually increased speed and duration at each running session, until they reached their individual target speed. Each monkey’s target amount of running was adjusted after a second maximal exercise test, performed at week 12 of the study, so that they continued to train at 80% maximal aerobic power. Sedentary control monkeys sat on the immobile treadmill 5 days a week throughout the entire study.
Maximal Exercise Test Procedures
At intervals throughout the experiment (5th, 12th and 24th weeks for all monkeys and at the 30th and 36th weeks for the run-stops), each monkey in the exercise group completed maximal aerobic power tests performed on a treadmill. This procedure follows the well-accepted practice of performing maximal exercise testing to determine peak oxygen consumption and thus cardiorespiratory fitness in humans (Balady and Weiner, 1987). Our index of maximal aerobic power was the maximal speed obtained prior to signs of fatigue during the maximal exercise test as described below. Prior to the maximal exercise test, heart rate was recorded in the monkey’s home cage. The monkey was then transported to the treadmill and heart rate was recorded while the monkey was sedentary on the treadmill. Running was then initiated at a speed of 0.8 miles per hour (mph; 1.28 km/h) and speed was increased by 0.2 mph every 2 minutes until the monkey was no longer able to keep pace with the treadmill. Heart rate was recorded for six seconds at the end of each speed interval. Once the monkey reached the maximum speed it could run the treadmill was stopped briefly and then running was reinitiated at 0.8 mph for a five-minute recovery period. Heart rate was recorded at 1, 3 and 5 minutes during the recovery period.
Submaximal Exercise Test Procedures
To assess biochemical adaptations to the endurance training, monkeys were run at a submaximal speed (10 min at 1.8 mph) and blood samples for measurement of blood lactate levels (3 ml/sample) were collected at rest and after the 10 min run. Training-induced reductions in blood lactate indicate improved muscle metabolism (Brooks 1986). For monkeys in the exercise groups, submaximal exercise tests were performed the day after each maximal exercise test. Blood lactate levels were also measured in control monkeys, which sat on the treadmill instead of running. Heart rate was recorded during the submaximal tests in the monkey’s home cage, while sedentary on the treadmill, and every 2 min during the 10 min exercise period.
Cognitive Testing Procedures
Cognitive testing was initiated with all animals during the 9th week of the study (the 5th week of exercise training), and was performed 5 days/week until week 24. All cognitive testing was carried out using a standard Wisconsin General Testing Apparatus (WGTA; Meunier et al., 1997) located in a darkened, sound-attenuated room. Monkeys were placed in a testing cage with a solid sliding access door that separated the monkey from the test tray. Opposite the monkey’s access door was a darkened one-way vision screen behind which the experimenter sat. When the monkey’s access door was raised the experimenter was concealed from the monkey. This was the practice for all testing, with the exception of the direct baiting that occurred in the spatial delayed response task, when both the access door and the screen were raised. The test tray contained three food-wells spaced 11.4 cm apart and aligned 9 cm in front of the testing cage. Training, followed by two types of cognitive tasks, a spatial delayed response task and an object discrimination reversal task, were performed with all monkeys from week 8 to week 20 of the study.
During training, monkeys were acclimated to entering the WGTA testing cage and learning to retrieve a food reward (small pieces of fruit, nuts or candy) from the wells in the testing tray. The treat was then covered by a plaque and the monkeys learned to remove the plaque to retrieve the food reward. Criterion for proceeding to the spatial delay task was completion of 20 trials of retrieving a reward from an uncovered well, 20 trials of retrieving a reward with identical plaques covering the wells, and 20 trials of retrieving a reward with plaques covering the wells and lowering the monkey’s access door between well-baiting and reward retrieval. During the spatial delayed response testing monkeys were given 30 trials per test session. In this task, one of two lateral food wells was baited with a food treat in full view of the monkey, and then both wells were simultaneously covered with identical plaques. The vision screen and the monkey’s access door were lowered, and after a brief delay (1–2 sec) the access door was raised and the monkey was allowed to displace one of the plaques. If the displaced plaque was the correct plaque, the monkey obtained a food reward. The position of the reward in the left-right position was varied from trial to trial in a random order. Monkeys were tested until they reached a criterion of 90% correct = 27/30 correct. Once monkeys reached criterion they proceeded to the object discrimination reversal task. In the object discrimination reversal task two easily discernable objects were placed over the lateral wells of the testing tray, with the position of the objects varied from trial to trial according to a random sequence. The monkey’s access door was lowered, and the experimenter placed a treat under a designated object. The access door was raised and the monkey could retrieve the treat by displacing the designated object. Once monkeys reached 90% criterion (18/20 trials correct object displacement in a single testing session) with the designated object, the location of the treat was switched to under the other object, until criterion was again met. Ten reversals were planned, but most monkeys did not complete all 10 reversals by the end of the study (week 24).
Collection of Brain Tissue
Monkeys were sedated with Ketamine HCl (10 mg/kg, i.m.), and then deeply anesthetized with sodium pentobarbital (30 mg/kg, i.v.). The chest was opened and a catheter was placed in the heart so that the tip was in the ascending aorta. The descending aorta was clamped to direct perfusate to the brain. Approximately 1 liter of cold 0.9% NaCL containing 5,000 IU heparin and 2% sodium nitrite was perfused transcardially, followed by 5–6 liters of cold 4% paraformaldehyde in 0.1 M potassium phosphate buffer (pH 7.2). The brain was removed, divided into hemispheres and placed in a post-fix of 4% paraformaldehyde for 2 hours at 4 C. Subsequently the tissue was placed in 10% glycerol for 24 hours and then placed in 20% glycerol, which was replaced every 24 hours, until the tissue sank to the bottom of the container. Tissue was stored at −20 C and shipped from Pittsburgh to Urbana.
Immunohistochemistry procedure
Prior to immunohistochemistry, the brains were transitioned through 7 changes of 30% sucrose in Tris buffered Saline (TBS, 50 mM Tris, 150 mM NaCl pH 7.4). Coronal frozen sections at 40 µm thickness were prepared with a cryostat and collected into a 30% sucrose, 30% Ethylene glycol TBS solution. Anti-human CD31 (PECAM-1) antibody (DAKO) was used for labeling blood vessels in monkey brain. CD31 is a cell-cell adhesion glycoprotein that is expressed on endothelial cells and the surface of platelets (Albelda et al. 1990, 1991). This has been used as a vascular endothelial cell marker in studying brain vascular structures (Uranishi et al. 2001, 2002).
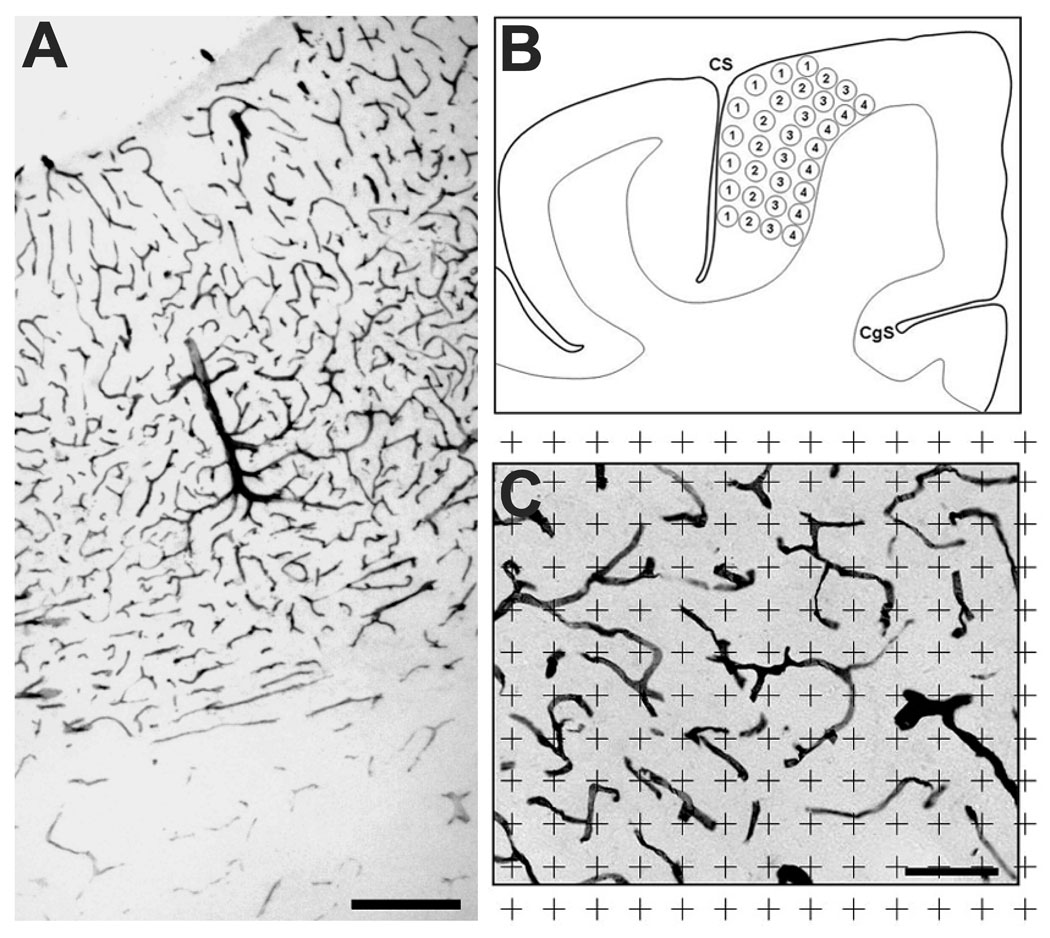
We selected sections through the precentral gyrus containing the primary motor cortex (−9 mm to approximately −13 mm posterior to the anterior commissure; Martin and Bowden, 2000). Free-floating sections were rinsed in water to remove the 30% sucrose, 30% ethylene glycol cryoprotectant. After sequential treatment with 0.5% Triton X-100 in TBS/2% normal goat serum (NGS) and 0.3% H2O2 TBS/ 2% NGS, the sections were incubated with anti-human CD31 antibody diluted 1:100 in TBS/2% NGS for two days. On the third day, the sections were washed (2% NGS in TBS) and transferred to a secondary antibody solution (biotinylated anti-mouse IgG diluted 1:200 in 2% NGS in TBS) for 2 hours at room temperature. The tissue was then washed 3 times 2% NGS in TBS and incubated in Avidin Biotin Complex solution (Vector Laboratories) for 60 min at room temperature. The sections were then washed in phosphate-buffered saline (PBS) followed by Tris buffer. Sections were transferred to a diaminobenzidine (DAB) solution (100 mg DAB, 1.39 g nickel ammonium sulfate) for 10 minutes. The sections were washed and mounted on slides. Blood vessels stained with this procedure were clearly delineated with virtually no background immunoreactivity (Fig. 2A, C).
Figure 2.
CD31 immunostaining of vasculature and sampling region. A, Immunostaining across the full depth of the precentral gyrus, showing a clear transition to reduced vascularization in white matter, demarcated by arrows (scale bar = 200 µm). B, Location of 8 sample regions in 4 equal zones through the primary motor cortex (CS = central sulcus; CgS = cingulate sulcus). C, Sampling grid superimposed on immunostained tissue. A blood vessel was counted if it was under the center of a grid point (+). The calculated volume fraction was equal to the total number of grid points over vessels divided by the total number of grid points in the reference area/volume.
(Scale bar = 100 µm).
Measurement of vascular volume fraction
All slides were coded and analyzed blindly without coder knowledge of individual subject treatment conditions. Precentral motor areas in four sections from each animal were observed at 250X and marked with a marker pen using a camera lucida. Gray matter/white matter boundaries were easily demarcated by the higher vascular density than white matter (Fig. 2A). The gray matter was divided into 4 equal, arbitrary zones, (superficial to deep: Zones A–D), and the vascular volume fractions of 8 sampling regions from each zone were collected based on an unbiased point counting method (Fig. 2 B). The image from each region was analyzed using a camera lucida projection of a 40 µm equivalent grid and the number of grid points coincident with labeled vessels was marked (Fig. 2C). The vascular volume fraction was calculated based on the ratio of the number of coincident grid points to the total number of points in the reference area/volume according to the stereological procedures of Gundersen et. al (Gundersen et al., 1988; Swain et al., 2003; Ding et al., 2006). As the vessels were observable throughout the section thickness and blood vessels never obscured others in that volume, the reference volume was not adjusted for section thickness. Thus any underestimate of true reference volume was constant across groups.
Statistical Analyses
For each physiological variable an initial analysis was performed to determine if there were significant differences between middle-aged and mature animals, using a Student’s t-test, or two-way analysis of variance (ANOVA) for repeated measures, as appropriate. If significant differences were detected all further analyses were performed separately in the middle-aged and mature animals, but if no significant age differences were detected the age groups were combined for further analyses to test the effects of exercise. Changes in fitness parameters, including maximum speed and maximum heart rate achieved during maximal exercise tests and lactate levels during submaximal exercise tests were analyzed using repeated measures ANOVAs, followed by least squares means post-hoc tests where appropriate, with a Bonferroni correction used when multiple comparisons were made. Paired t-tests were used to examine parameters measured at the beginning and the end of the training period, including calorie consumption and body weight. All other differences between runners and controls were assessed using Student’s t-tests.
Analysis of vascular volume fraction initially examined the interaction between age (middle aged and more mature adults) and treatments with the Friedman test within the SAS program (ver 9.13). Because there was an interaction between age and treatment, the two ages were examined separately. The effects of exercise were analyzed with the Kruskall-Wallis test using multiple comparisons (Miller, 1981).
Results
Changes in Fitness
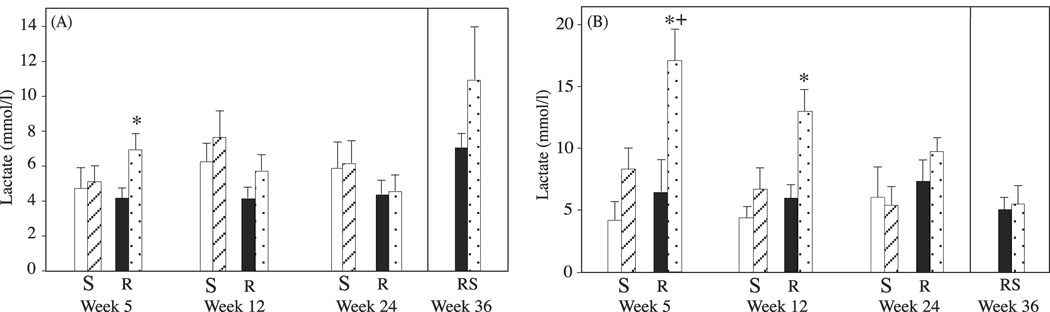
At the start of exercise training, the middle-aged animals were more fit than the more mature animals, as indicated by significantly lower plasma levels of lactate after 10 minutes of submaximal exercise (Fig. 3A vs 3B, week 5; p=0.005), as well as their ability to reach a significantly higher maximal speed in the initial test (Fig. 4A1 vs 4B1: p=0.047).
Figure 3.
Plasma lactate concentrations in (A) middle-aged and (B) more mature monkeys, measured in runners (R) and run-stops (RS) before and after submaximal exercise testing, or in sedentary controls (S) before and after sitting on the treadmill. Week of testing appears below each pair of data bars. Solid black and open bars are pre-test plasma lactate concentrations, stippled and striped bars are post-test concentrations. Asterisks indicate a significant difference between pre- vs. post- exercise values within a specific experimental group. The plus sign indicates a significant difference between more mature and middle-aged monkeys.
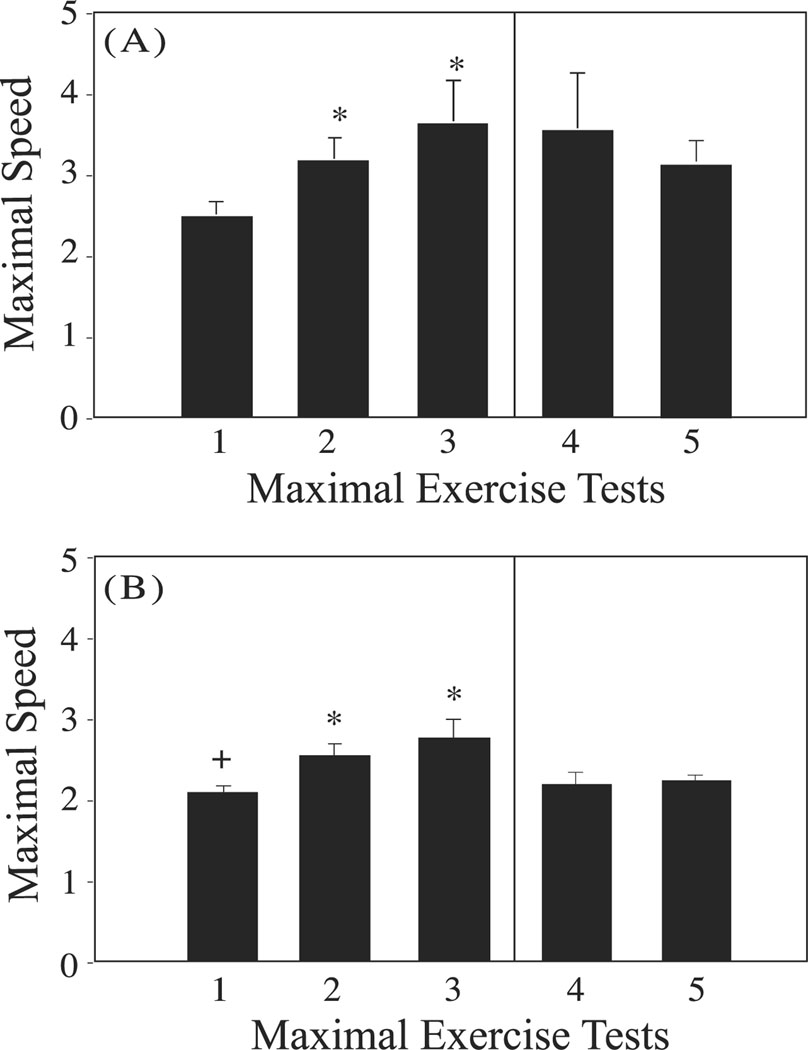
Figure 4.
Maximal speed attained during maximal exercise tests in (A) middle-aged adult and (B) more mature adult runners at the initiation of the training period (week 5, Maximal Exercise Test 1), during the training period (week 12, Maximal Exercise Test 2) and at the end of the training period (week 24, Maximal Exercise Test 3). Asterisks indicate a significant difference from the maximum speed attained at Maximal Exercise Test 1. Plus sign indicates a significant difference between middle-aged and mature runners. Note that only the Run-Stop animals were tested in Maximal Exercise Tests 4 (week 30) and 5 (week 36).
With exercise training, both middle-aged and more mature adults became progressively more fit, with the subsequent submaximal exercise tests inducing little or no rise in plasma lactate concentrations by the end of week 12 in middle-aged monkeys, but not until the end of week 24 in the mature monkeys (Fig. 3 A, B). Furthermore, exercise resulted in significant increases in maximal speed attained in the subsequent maximal exercise tests (p<0.05; Fig. 4 A, B).
When exercise training was terminated at the end of week 24 of the study, the animals in the run-stop groups experienced a decrease in fitness over the following 12 week sedentary period, as indicated by a decline in maximal speed attained during the maximal exercise tests performed at 30 and 36 weeks to levels comparable to those measured at the initiation of the study (Fig. 4 A, B). In the post-exercise training sedentary period there were no significant differences between middle-aged and more mature adult run-stop animals detected during the maximal exercise tests. While maximal speed obtained showed a decline during the sedentary period, an accompanying increase in plasma lactate levels with submaximal exercise was not apparent (Fig. 3A, B: RS columns).
There were no significant differences between middle-aged adult and more mature adult animals in other parameters measured over the course of the study. There was, however, a significant decrease in body weight in both runners (7% decrease, p=0.001) and controls (9% decrease, p=0.05) from the beginning to the end of the study. There was also a slight, but not significant increase in calorie consumption in both runners (6% increase, p=0.089) and controls (9% increase, p=0.276) over the course of the study. All animals maintained relatively regular menstrual cycles throughout the study, with no differences between runners (4.6±0.3 menstrual cycles/5 months) and controls (3.9±0.4 menstrual cycles/5 months).
Cognitive Testing
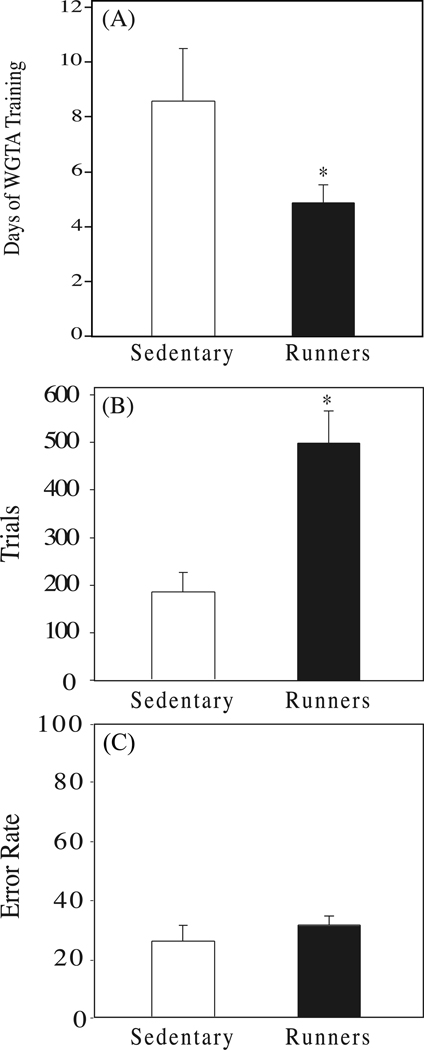
Cognitive testing data from the two age groups were combined for all analyses of the effects of exercise because no differences were found between the groups in any aspect of their performances. Runners learned to move plaques to retrieve rewards in the WGTA twice as fast as the sedentary control animals (Fig. 5A, p=0.05). Runners also showed a higher level of participation in the first cognitive test, the spatial delayed response task, performing significantly more trials (Fig. 5B, p=0.001) than control animals. However, the runners also made more errors than the control animals (p=0.003), and thus had an overall error rate comparable to sedentary control animals (Fig. 5C, p=0.26). There were no significant differences between runners and sedentary controls in the object recognition reversal task (data not shown). During the initial learning of this task runners and sedentary controls performed similarly with regard to the number of trials performed to meet criterion (90% correct; p=0.794), and the number of errors made (p=0.753). Both runners and controls showed a significant increase in the number of trials needed (runners, p=0.002; controls, p=0.003) and number of errors made (runners, p=0.001; controls, p=0.001) in meeting criterion after the first reversal, but there were no significant differences between the runners and sedentary controls. During the second and third reversals the runners and sedentary controls both improved their performance, requiring fewer trials and making fewer errors, but again there were no differences between runners and sedentary controls.
Figure 5.
(A) Days of training required to learn to use the WGTA in runners (solid bar; n=8) and sedentary controls (open bar; n=8), (B) number of trials and (C) errors/trial made by runners and sedentary controls in the spatial delayed response task. Asterisk indicates a significant difference between groups.
Vascularity in the Motor Cortex
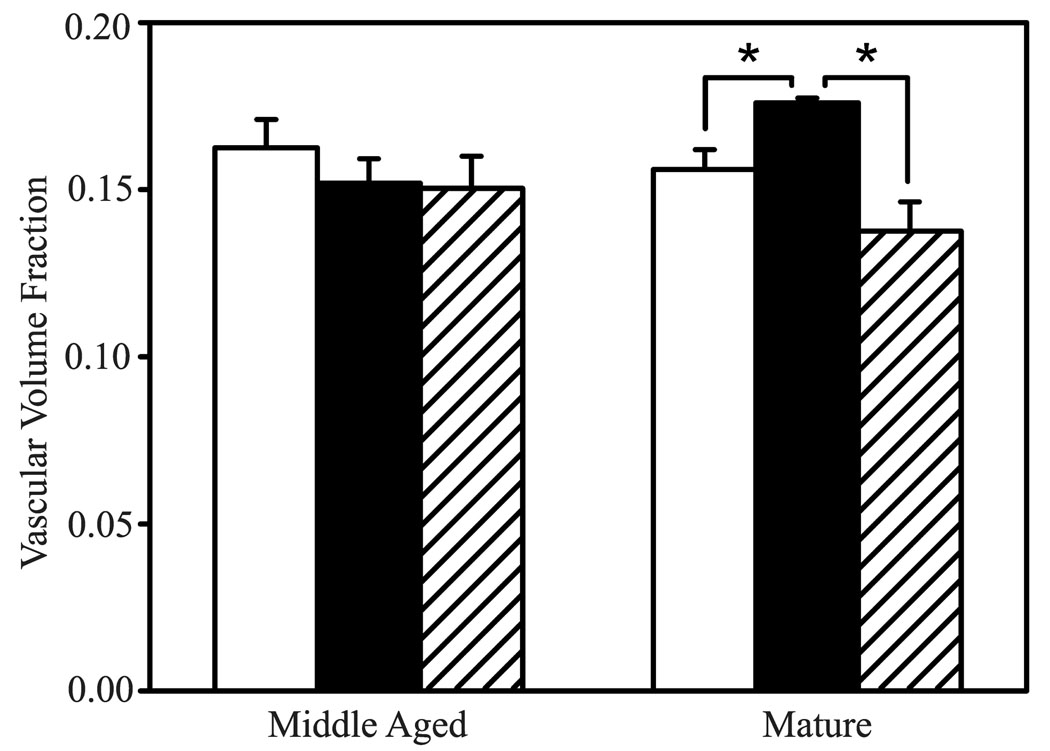
The vascular volume fraction of the mature adult monkey running group was increased significantly compared to the mature adult control monkeys (p=0.029; Fig. 6). These changes in vascular volume fraction were observed throughout the full cortical depth (superficial to deep: Zone A, p=0.043; Zone B, p=0.012; Zone C, p=0.039; Zone D, p=0.077). However, the increased volume fraction induced by running was reversed during the sedentary period that occurred after 20 weeks of running. The vascular volume fractions of the middle-aged adult monkeys did not show any statistically significant differences.
Figure 6.
Effects of exercise on volume fraction of CD31-immunostained vascular tissue in motor cortex of runners (solid bars), run-stops (striped bars) and controls (open bars). Asterisks indicate a significant difference between groups.
Discussion
This is the first study to demonstrate the effects of exercise training on cognitive function in a nonhuman primate. We found that exercising animals learned to use the WGTA significantly faster than sedentary control animals and that the exercising animals performed significantly more trials during a spatial delay task compared to sedentary controls. Our data support findings from human studies that show positive effects of exercise on a broad range of cognitive tests (Martin et al., 1997; Etnier and Berry, 2001; Khatri et al., 2001; Colcombe and Kramer, 2003; Colcombe et al., 2003, 2006; Gordon et al., 2008). This is also the first report of an exercise-induced increase in vascular volume in a primate species. It is consistent with the finding that people participating in a long-term exercise program show an improved hemodynamic response (Colcombe et al., 2004). Together, these findings indicate that a level of exercise that is recommended for improving fitness in middle-aged humans is sufficient to increase both the rate of learning and blood flow to the cerebral cortex, at least during periods of regular exercise training.
In our cognitive testing studies, we found that exercising monkeys showed increased participation in the initial cognitive tests, but that the performances of the exercising and sedentary monkeys were similar on tests later in the training period. Specifically, we found that exercising monkeys learned to use the testing apparatus twice as fast as sedentary controls (Fig. 5A), and exercising monkeys completed 2.5 times as many trials as the sedentary monkeys in the spatial delay task (Fig. 5B). However, by the end of the 20 weeks of regular exercise we found no difference in rate of learning or performance between the exercising and sedentary groups even though the task we used at this time, the object reversal task, was the most complex and required the greatest degree of executive control. Although with small group sizes (n=4/group) one is always concerned that low sample sizes may lead to false negative data, our findings parallel the findings reported in rodent species. In both rats and mice, exercise training for 1 week to 3 months has been shown to improve cognitive performance using the Morris water maze and the radial arm maze (Fordyce and Farrar, 1991; Fordyce and Wehner, 1993; Anderson et al., 2000; Cotman and Berchtold, 2002; Vaynman et al., 2004; Leggio et al., 2005). Interestingly however, Anderson et al. (2000) found in rats that the initial rate of learning is influenced by exercise but that later criterion level performance was not enhanced in the exercising group. They suggested that this might reflect an ability of practice effects to compensate for the initial group differences between exercising and sedentary animals. Our findings, like those of Anderson et al. (2000), suggest that mental training may obscure effects of exercise on cognitive function. The idea that staying mentally active is a lifestyle factor that is recognized to maintain cognitive function as people age (Fillet et al., 2002), supports the conclusion that the performance of cognitive testing over the course of this study obscured the evaluation of exercise effects on cognitive function by the end of the study.
We found that there were no significant differences on cognitive test performance between our middle-aged monkeys and more mature animals, despite the fact that the older animals were initially less ‘fit’ than the middle-aged group, as indicated by our maximal exercise test results, and took longer to become fit (Figs. 3 and 4) during the exercise training period. This supports findings by Radak et al. (2001) who found that conditioned pole-jumping avoidance learning was improved by exercise in both young and middle-aged rats. However in studies using more complex cognitive tasks, Barnes et al. (1991) showed no effect of exercise training on spatial memory in old rats and Van Boxtel et al. (1997) showed an interaction between age and cognitive performance in humans on several complex tasks. It is possible that we did not see a difference in the effect of exercise on cognitive performance as a function of age because our more complex cognitive tasks were performed later in the experiment and performance may have been influenced by the mental training acquired by the earlier cognitive testing (as discussed above, Bherer et al., 2008).
This report also shows a structural change in the brain related to exercise. Specifically, we found an increase in brain vascular density with exercise in a primate species. Preliminary data from our laboratories suggest that this increase in vascular density might be generated by newborn vascular cells (Kohler et al, 2007; 2009). The increase in vascular density occurs with reasonable levels of exercise that are commonly recommended for humans by the College of Sports Medicine and the American Heart Association (Haskell et al., 2007). The increase in motor cortex vascular density was only found in the mature group of monkeys (15–17 yrs of age), not in the middle-aged (10–12 yrs of age) monkeys, possibly because of differences between monkeys in these two age groups in activation of angiogenic signaling factors such as vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), or angiopoietins, all of which are stimulated by exercise (Carro et al., 2000; Ding et al., 2004a, 2004b, 2006, Llorens-Martin et al., 2009). The exercise-induced, increased vascular density that we show in the motor cortex in the present study also appears to occur in the monkey striatum (Kohler et al., 2007) and in the motor cortex of rats (Kleim et al., 2002; Swain et al., 2003). The link between exercise and increased vascularity in motor areas of the brain supports the commonly held assumption of a functional relationship between behavior neural activity and brain structure. It is reasonable to assume that such a relationship occurs as well in brain areas related to cognitive function, such as the prefrontal cortex, which might explain improvements in the learning rate with WGTA testing in our exercised animals.
As humans age, morphological and physiological changes suggestive of declining function in the vascular system have been reported. These include decreased capillary density (Abernethy et al. 1993; Sonntag et al. 1997) and increased vascular anomalies including perivascular collagen deposits, basement membrane thickening, and decreased number of mitochondria (Farkas and Luiten 2001). The exercise-induced impact on brain vasculature may be more profound when baseline vascular integrity has already begun to decline. This may contribute to the increased responsiveness of the vascular volume fraction of the more mature monkeys.
These studies were performed with female monkeys only. However, previous studies in rodents, examining the effects of exercise on cognitive function (Cotman and Berchtold 2002), as well as vascularity (Swain et al. 2003) have shown comparable effects of exercise in males and females. This has also been true for human studies, thus we have no reason to believe that gender differences would be expected in the response to exercise in nonhuman primates.
In summary, we show in this report that regular exercise training at a level that is recommended for improving fitness in middle-aged humans is sufficient to increase both the rate of learning and vascular density in the brain in a primate species, two changes that have been well documented in rodent studies. As it has been difficult to judge the equivalence of exercise regimens used in rodents to those recommended for humans (Dishman et al., 2006; Cotman et al., 2007), the finding that a level of exercise comparable to that recommended for humans has clear effects on the brain supports the concept that other effects of exercise that have been documented in the rodent brain, including exercise-induced increases hippocampal dentate gyrus neurogenesis (van Praag et al., 1999; Rhodes et al., 2003; van Praag et al., 2005), and neurotrophic factor expression (Neeper et al., 1995; Gomez-Pinella et al., 1997; Klintsova et al., 2005; Griesback et al., 2009), may also be happening in human brain when people participate in regular exercise programs. Future studies using this nonhuman primate model of exercise training would be very useful in testing these possibilities.
Acknowledgements
We would like to thank Dr. Jocelyne Bachevalier and the members of her laboratory who provided very helpful guidance in setting up WGTA testing in our laboratory for these studies. The animal care services provided by the University of Pittsburgh Division of Animal Resources, were also appreciated. The statistical assistance of Dr. Lee Jy of Korea University is gratefully acknowledged. The assistance of Georgina Aldridge and Julie Markham is also acknowledged. This work was supported by a grant from the Retirement Research Foundation, and by grants from the National Institute of Aging (AG10154) and National Institute on Diabetes, Digestive and Kidney Disorders (DK55819).
Abbreviations
- WGTA
Wisconsin General Testing Apparatus
- TBS
Tris buffered saline
- NGS
normal goat serum
- PBS
phosphate-buffered saline
- DAB
diaminobenzidine
- 2X SSC
50% formamide saline-sodium citrate buffer
- ANOVA
analysis of variance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abernethy WB, Bell MA, Morris M, Moody Microvascular density of the human paraventricular nucleus decreases with aging but not hypertension. Exp Neurol. 1993;121:270–274. doi: 10.1006/exnr.1993.1095. [DOI] [PubMed] [Google Scholar]
- Albelda SM, Oliver PD, Romer LH, Buck CA. EndoCAM: a novel endothelial cell-cell adhesion molecule. J Cell Biol. 1990;110:1227–1237. doi: 10.1083/jcb.110.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BJ, Rapp DN, Baek DH, McCloskey DP, Coburn-Litvak PS, Robinson JK. Exercise influences spatial learning in the radial arm maze. Physiol Behav. 2000;70:425–429. doi: 10.1016/s0031-9384(00)00282-1. [DOI] [PubMed] [Google Scholar]
- Balady GJ, Weiner DA. Exercise testing for sports and the exercise prescription. Cardiol Clin. 1987;5:183–196. [PubMed] [Google Scholar]
- Barnes CA, Forster MJ, Fleshner M, Ahanotu EN, Laudenslager ML, Mazzeo RS, Maier SF, Lal H. Exercise does not modify spatial memory, brain autoimmunity, or antibody response in aged F344 rats. Neurobiol Aging. 1991;12:47–53. doi: 10.1016/0197-4580(91)90038-l. [DOI] [PubMed] [Google Scholar]
- Bherer L, Kramer AF, Peterson MS, Colcombe S, Erickson K, Becic E. Transfer effects in task-set cost and dual-task cost after dual-task training in older and younger adults: further evidence for cognitive plasticity in attentional control in late adulthood. Exp Aging Res. 2008;34:188–219. doi: 10.1080/03610730802070068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebeller cortex of adult rats. Proc Natl Acad Sci USA. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA. Lactate production under fully aerobic conditions: the lactate shuttle during rest and exercise. Fed Proc. 1986;45:2924–2929. [PubMed] [Google Scholar]
- Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, Brooks WH, Swerdlow RH. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bytheway JA, Lange H, Lamb J, McCormick K, Williams NI, Greenough WT, Cameron JL. Exercise training leads to increased participation in cognitive testing in female cynomolgus monkeys. Society for Neuroscience Abstract. 2003;920:3. [Google Scholar]
- Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–669. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- Clifton DK, Bremner WJ, Steiner RA. An automated technique for the radiographic determination of bone age. J Med Primatol. 1982;11:147–154. [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson SI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. TRENDS Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Ding Y, Li J, Luan X, Ding YH, Lai Q, Rafols JA, Phillis JW, Clark JC, Diaz FG. Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regional angiogenesis and cellular overexpression of neurotrophin. Neuroscience. 2004a;124:583–591. doi: 10.1016/j.neuroscience.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Ding YH, Li J, Zhou Y, Rafols JA, Clark JC, Ding Y. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res. 2006;3:15–23. doi: 10.2174/156720206775541787. [DOI] [PubMed] [Google Scholar]
- Ding YH, Luan XD, Li J, Rafols JA, Guthinkonda M, Diaz FG, Ding Y. Exercise-induced overexpression of angiogenic factors and reduction of ischemia/reperfusion injury in stroke. Curr Neurovasc Res. 2004b;1:411–420. doi: 10.2174/1567202043361875. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of exercise. Obesity (Silver Spring) 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Etnier JL, Berry M. Fluid intelligence in an older COPD sample after short-and long-term exercise. Med. Sci. Sports Exerc. 2001;33:1620–1628. doi: 10.1097/00005768-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Fillit HM, Butler RN, O’Connell AW, Albert MS, Birren JE, Cotman CW, Greenough WT, Gold PE, Kramer AF, Kuller LH, Perls TT, Shagan BG, Tully T. Achieving and maintaining cognitive vitality with aging. Mayo Clin Proc. 2002;77:681–696. doi: 10.4065/77.7.681. [DOI] [PubMed] [Google Scholar]
- Fong GH. Regulation of angiogenesis by oxygen sensing mechanisms. J Mol Med. 2009;87:549–560. doi: 10.1007/s00109-009-0458-z. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Farrar RP. Physical activity effects on hippocampal and parietal cortical cholinergic function and spatial learning in F344 rats. Behav Brain Res. 1991;43:115–123. doi: 10.1016/s0166-4328(05)80061-0. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Wehner JM. Physical activity enhances spatial learning performance with an associated alteration in hippocampal protein kinase C activity in C57BL/6 and DBA/2 mice. Brain Res. 1993;619:111–119. doi: 10.1016/0006-8993(93)91602-o. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Dao L, So V. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain Res. 1997;764:1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- Gordon BA, Rykhlevskaia EI, Brumback CR, Lee Y, Elavsky S, Konopack JF, McAuley E, Kramer AF, Colcombe S, Gratton G, Fabiani M. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45:825–838. doi: 10.1111/j.1469-8986.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesback GS, Hoyda DA, Comez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNS activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a ystematic review of prospective evidence. Psychol Med. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Lee I-M, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- Khatri P, Blumenthal JA, Babyak MA, Craighead WE, Herman S, Baldewicz T, Madden DJ, Doraiswamy M, Waugh R, Krishnan KR. Effects of exercise training on cognitive functioning among depressed older men and women. J Aging Phys Activ. 2001;9:43–57. [Google Scholar]
- Kleim JA, Cooper NR, VandenBerg PM. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 2002;934:1–6. doi: 10.1016/s0006-8993(02)02239-4. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004;1028:92–104. doi: 10.1016/j.brainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Kohler SJ, Jennings V, Todd SV, Rhyu I, Williams NI, Cameron J, Greenough WT. Exercise increases capillary volume in the neostriatum of macaque monkeys. San Diego, CA: Society for Neuroscience; 2007. 589.11 2007. Online. [Google Scholar]
- Kohler SJ, Boklewski JL, Stanton GB, Kleczek AS, Jennings VL, Cameron JL, Greenough WT. Characterization of adult born cells in the precentral gyrus of nonhuman primates. Chicago, IL: Society for Neuroscience; 2009. 308.20 2009. Online. [Google Scholar]
- Larsson E, Mandel RJ, Klein RL, Muzyczka N, Lindvall O, Kokaia Z. Suppression of insult-induced neurogenesis in adult rat brain by brain-derived neurotrophic factor. Exp Neurol. 2002;177:1–8. doi: 10.1006/exnr.2002.7992. [DOI] [PubMed] [Google Scholar]
- Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, Petrosini L. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav Brain Res. 2005;163:78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Llorens-Martin M, Torres-Aleman I, Trejo JL. Mechanism mediating brain plasticity: IGF1 and adult hippocampal neurogenesis. Neuroscientist. 2009;15:134–148. doi: 10.1177/1073858408331371. [DOI] [PubMed] [Google Scholar]
- Martin RF, Bowden DM. Primate Brain Maps: Structure of the Macaque Brain. Amsterdam: The Netherlands: Elsevier; 2000. [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychol. 1996;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- Miller RG., Jr . Nonparametric techniques in simultaneous statistical inference. 2nd edition. New York: Springer-Verlag; 1981. pp. 129–188. [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Plate KH, Warnke PC. Vascular endothelial growth factor. J Neurooncol. 1997;35:365–372. doi: 10.1023/a:1005845307160. [DOI] [PubMed] [Google Scholar]
- Podewils LH, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- Radak Z, Kaneko T, Tahara S, Nakamoto H, Pucsok J, Sasvari M, Nyakas C, Goto S. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem Int. 2001;38:17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Van Praag H, Jeffrey S, Garland I, Mitchell GS, Mitchell T, Jr, Gage F. Exercise Increases Hippocampal Neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behavioral Neuroscience. 2003;117(5):1006–1016. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, Riviere D, Vellas B. Exercise program for nursing home residents with Alzheimer's disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55:158–165. doi: 10.1111/j.1532-5415.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Willemer C, Kruger K, Duning T, Warnecke T, Sommer J, Volker K, Ho HV, Mooren F, Knecht S, Floel A. Physical activity and memory functions: An interventional study. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Sasco AJ, Paffenbarger RS, Jr, Gendre I, Wing AL. The role of physical exercise in the occurrence of Parkinson's disease. Arch Neurol. 1992;49:360–365. doi: 10.1001/archneur.1992.00530280040020. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y. Physical activity, diet, and risk of Alzheimer disease. Jama. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Eckberg K, Lauterbur PC, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- Swindler DR. Nonhuman primate dental development and its relationship to human dental development. In: Watts ES, editor. Nonhuman Primate Models for Human Growth and Development. New York: Alan R. Liss, Inc.; 1985. pp. 67–94. [Google Scholar]
- Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, Kukull WA, LaCroix AZ, McCormick W, Larson EB. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trail. JAMA. 2003;290:2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- Uranishi R, Awadallah NA, Ogunshola OO, Awad IA. Further study of CD 31 protein and messenger ribonucleic acid expression in human cerebral vascular malformations. Neurosurgery. 2002;50:110–116. doi: 10.1097/00006123-200201000-00019. [DOI] [PubMed] [Google Scholar]
- Uranishi R, Baev NI, Ng PY, Kim JH, Awad IA. Expression of endothelial cell angiogenesis receptors in human cerebrovascular malformations. Neurosurgery. 2001;48:359–367. doi: 10.1097/00006123-200102000-00024. [DOI] [PubMed] [Google Scholar]
- Van Boxtel MPJ, Paas FGWC, Houx PJ, Adam JJ, Teeken JC, Jolles J. Aerobic capacity and cognitive performance in a cross-sectional aging study. Med Sci Sports Exerc. 1997;29:1357–1365. doi: 10.1097/00005768-199710000-00013. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293:270–276. doi: 10.1152/ajpendo.00108.2007. [DOI] [PubMed] [Google Scholar]
- Williams NI, Caston-Balderrama AL, Helmreich DL, Parfitt DB, Nosbisch C, Cameron JL. Induction of menstrual cycle disturbances in cynomolgus monkeys during strenuous exercise training: Longitudinal changes in reproductive hormones and menstrual cyclicity. Endocrinology. 2001a;142:2381–2389. doi: 10.1210/endo.142.6.8113. [DOI] [PubMed] [Google Scholar]
- Williams NI, Caston-Balderrama AL, Helmreich DL, Parfitt DB, Cameron JL. Induction of menstrual cycle disturbances in cynomolgus monkeys during strenuous exercise training: Evidence for a causal role of low energy availability. J Clin Endocrinol Metab. 2001b;86:5184–5193. doi: 10.1210/jcem.86.11.8024. [DOI] [PubMed] [Google Scholar]