Abstract
Purpose
Protein phosphatase 4 (PP4) has been reported to be overexpressed in breast and lung cancers. PP4 plays an important role in the regulation of centrosome maturation, DNA repair, NFκB and JNK signaling pathways. However, the expression and functions of PP4 in pancreatic cancer have not been studied.
Experimental design
We examined the expression of PP4 catalytic subunit (PP4C) protein in 133 patients with stage II pancreatic ductal adenocarcinoma (PDAC) and their paired benign pancreatic samples (N=113) by immunohistochemistry (IHC). To confirm the IHC results, we measured PP4C protein and mRNA levels by Western blotting and real time RT-PCR. Using univariate and multivariate analysis, we correlated PP4C expression with survival and other clinicopathologic features.
Results
PP4C was overexpressed in 75 of 133 (56.4%) stage II PDAC samples, which was significantly higher than the paired benign pancreatic tissue (15%, 17/113). PP4C mRNA expression levels were also higher in PDAC samples than the paired benign pancreatic tissue. Overexpression of PP4C in PDAC samples was associated with higher frequencies of distant metastasis (p=0.02) and poor disease-free and overall survivals in patients with stage II PDAC (p = 0.006 and 0.02) independent of tumor size, margin status, and lymph node status (stage).
Conclusions
Our study showed that PP4C is overexpressed in PDAC. Overexpression of PP4C in PDAC samples is associated with poor prognosis in patients with stage II PDAC. Therefore, targeting PP4 signaling pathway may represent a new approach for the treatment of PDAC.
Impact
Our study demonstrated that PP4C is an independent prognostic factor in patients with stage II PDAC.
Keywords: PP4, Pancreatic cancer, Survival, Prognosis
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer death in the United States with less than 5% 5-year survival rate (1). Surgical resection remains the only hope for long time survival in patients with PDAC. However, at the time of diagnosis, only 15–20% of patients with PDAC are surgically resectable. Even for the patients who underwent pancreatectomy, the disease commonly recurs and the prognosis is poor with the long-term survival rate of 10%–20% (2). Therefore identifying new markers and therapeutic targets are urgently needed.
Protein phosphatases (PP) are divided into three major groups based on their substrate specificity: serine/threonine phosphatases, tyrosine phosphatases and dual-specificity phosphatases (3). Protein serine/threonine phosphatases have been further classified into five major groups: PP type 1, 2A, 3, 5, 7, based on their biochemical characteristics, sensitivity to specific inhibitors, ability to dephosphorylate specific substrates and the requirement for divalent cations (4). Protein phosphatase 4 (PP4, also known as PPX) is a PP2A-like phosphatase, and shares 65% amino acid sequence identity with PP2A (5). Like PP2A, PP4 is a holoenzyme comprised of catalytic subunit (PP4C), structural and regulatory subunits PP4R1 and PP4R2. PP2A has been shown to regulate cellular processes as diverse as metabolism, signal transduction, DNA replication, transcription, translation, cell cycle, development, and transformation (6). However, many of these functional studies of PP2A have relied upon the use of the phosphatase inhibitor okadaic acid (OA). PP4 contains a putative binding domain for OA and OA inhibits PP4 with a similar range of concentration (IC50 = 0.1 nM in vitro) to PP2A (7, 8). Therefore, some of the previously identified functions assigned to PP2A may in fact be the functions of PP4, rather than PP2A.
Recent study showed that PP4C is overexpressed in human breast and lung cancer (9). Inhibition of PP4C expression sensitized breast and lung cancer cells to cisplatin treatment (9), suggesting the functions of PP4 overexpression in cancer progression. However, the expression and the prognostic significance of PP4 expression in patients with PDAC have not been studied. In this study, we examined PP4C protein expression by immunohistochemistry in 133 patients with stage II PDAC and their matched benign pancreatic tissue samples (N=113). In addition, we measured PP4 mRNA expression levels by real time RT-PCR and PP4 protein expression by Western blotting. We correlated PP4C expression with survival and other clinicopathologic parameters in patients with stage II PDAC. Our data suggest that PP4 plays an important role in the progression of PDAC. Targeting PP4 signaling pathway may represent a new approach for the treatment of PDAC.
MATERIALS AND METHODS
Patient population
Our study population consisted of 133 patients with stage II PDAC who underwent pancreatectomy and did not receive any form of neoadjuvant chemoradiation therapy at our institution from 1990 to 2010. One patient each with stage IA, IB, or III disease and 2 patients with stage IV disease were excluded from this study because the case number was too small to be representative for these disease stages. One hundred and thirteen patients (85.0%) underwent pancreaticoduodenectomy, 18 patients (13.5%) underwent distal pancreatectomy, and 2 patients (1.5%) received total pancreatectomy. There were 78 male and 55 female patients with age ranging from 24.9 to 84.8 years (median age: 64.6 years). Post-operatively, 25 patients (18.8%) received adjuvant chemotherapy alone, 73 patients (54.9%) received combined chemoradiation therapy and 35 patients (26.3%) did not receive adjuvant therapy. The study was approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center.
Tissue microarrays and immunohistochemical analysis for PP4C expression
The tissue microarrays used in this study were constructed using formalin-fixed, paraffin-embedded archival tissue blocks from the pancreatectomy specimens from 133 patients with stage II PDAC. The matched hematoxylin & eosin (H & E) stained slides were reviewed to identify the representative areas for tumor and benign pancreas. For each patient, two cores of tumor and one core of paired benign pancreatic tissue were sampled from representative areas using a 1.0-mm punch. The tissue microarrays were constructed using a tissue microarrayer (Beecher Instruments, Sun Prairie, WI) as described previously (10).
Immunohistochemical staining for PP4C was performed on 5-μm unstained sections from the tissue microarray blocks. To retrieve the antigenicity, the tissue sections were treated at 100°C in a steamer containing 10 mmol citrate buffer (pH, 6.0) for 35 min. The sections were then immersed in 3% hydrogen peroxidase for 20 min to block the endogenous peroxidase activity and were incubated in 2.5% blocking serum for 30 min to reduce nonspecific binding. The sections were then incubated with a rabbit polyclonal antibody against PP4C (Novus Biologicals, CO) at a 1:300 dilution at 4°C overnight, washed and then incubated with secondary antibody at room temperature for 60 min. Standard avidin-biotin immunohistochemical analysis of the sections was done according to the manufacturer’s recommendations (Vector Laboratories, Burlingame, CA). The immunohistochemical stained slides for PP4C were reviewed independently by two pathologists (D.C. and H.W.), who graded PP4C expression in both tumor and their paired benign pancreatic samples. Since the immunohistochemical staining for PP4C showed diffuse staining in PDAC samples that were positive for PP4C, PP4C expression was scored based on the staining intensity as negative, weak, moderate, and strong. All cases with a discrepancy in the grading of PP4C expression between the pathologists were re-reviewed together and the consensus results for PP4C expression were used. For the statistical analysis, we categorized our cases into two groups based on the staining intensity for PP4C: PP4C-high (moderate or strong staining for PP4C in tumor cells) and PP4-low (negative or weak staining for PP4C in tumor cells).
Western blotting
All tissue samples used for Western blots were selected by a pathologist from pancreatectomy specimens based on frozen sections prepared from frozen tissue blocks. Antibodies against PP4 and GAPDH, which was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Protein expression wasanalyzed by 10% SDS-PAGE, which was electroblotted onto PVDF membranes (Novex, San Diego, CA), blocked in 5% skim milk in 1 × TBS, and probed with the primary antibodies as indicated in the figures. Proteinswere detected using an enhanced chemiluminescence (ECL) kit (Amersham-Pharmacia Biotech, Piscataway, NJ).
RNA isolation and measurement of PP4C mRNA expression
Frozen OCT-embedded human samples of primary PDAC and benign pancreatic tissue were microdissected and total RNA isolation was performed as described previously (11). One microgram of total RNA was reverse transcribed using the avian myeloblastosis virus reverse transcriptase kit (Promega, Madison, WI) according to the manufacturer’s protocol. Briefly, total RNA was denatured for 5 min at 70°C and cooled for 5 min on ice, reverse transcriptase was added to a total volume of 20 μL, and reverse transcription was conducted at 42°C for 60 min. Quantitative reverse transcription-PCR (QRT-PCR) was performed using a set of primers specific for PP4C (QuantiTect Primer Assays, Qiagen, Inc. Valencia, CA) according to the manufacturer’s instructions. To correct for quantitative differences between samples and possible PCR artifacts, we used primers specific for Ribosomal protein S6 (RPS6) (forward, 5′-AAGGAGAGAAGGATATTCCTGGAC-3′; reverse, 5′-AGAGAGATTGAAAAGTTTGCGGAT-3′) as an internal control for each sample. Amplification was performed using a thermal cycler (Bio-Rad Laboratories, Inc.) for 40 cycles consisting of 20 s at 95°C (denaturation), 1 min at 60°C (annealing), and 1 min at 60°C (extension). Each QRT-PCR was performed in triplicate, and the mean value was used to calculate the ratio of PP4 to RPS6, with a value of one used as the control. All assays were repeated three times.
Statistical analysis
Patient follow-up information through June of 2011 was extracted from the prospectively maintained institutional pancreatic cancer database in the Department of Surgical Oncology at our institution. The clinical and follow up information were verified in all cases by reviewing the medical record and if necessary, by review of the U.S. Social Security Index. For the patients who were alive or died from other causes during follow up, the follow up time ranged from 7.1 to 213.8 months with a median follow up of 38.2 months. For the patients who died of PDAC during follow up, the follow up time ranged from 4.2 to 82.9 months with a median follow up of 18.1 months. Disease-free survival (DFS) was calculated as the time from the date of surgery to the date of first recurrence in patients with recurrence or to the date of last follow-up in patients without recurrence. The overall survival (OS) was calculated as the time from the date of diagnosis to the date of death or the date of last follow-up if death did not occur. The Fisher’s exact tests were used to compare categorical data. DFS and OS curves were constructed using the Kaplan-Meier method, and the log-rank test was used to evaluate the statistical significance of differences. Univariate Cox regression analysis was used to examine the prognostic significance of PP4C and clinicopathologic parameters. Coxproportional hazards models were fitted for multivariate analysis. After interactions between variables were examined, a backward stepwise procedure was used to derive the best-fitting model. Statistical analysis was performed using Statistical Package for Social Sciences software (for Windows 12.0, SPSS Inc., Chicago, IL). We used a two-sided significance level of 0.05 for all statistical analyses.
RESULTS
PP4C is overexpressed in pancreatic ductal adenocarcinoma compared to the paired benign pancreatic tissue
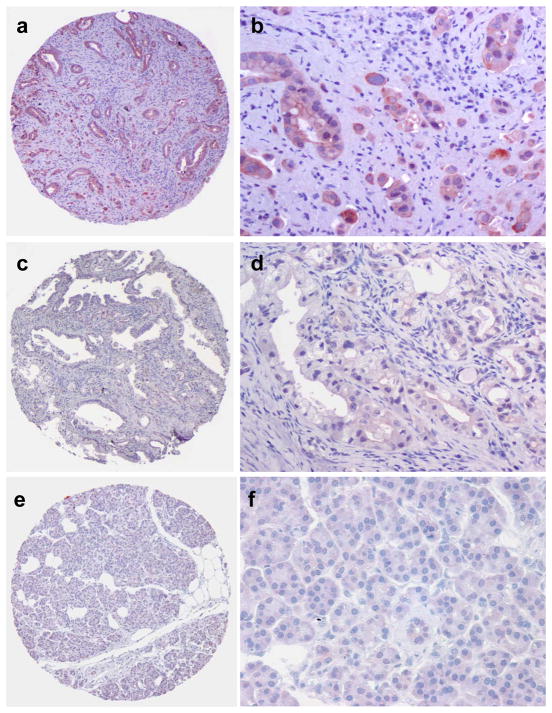
For the PDAC samples that were positive for PP4C, diffuse cytoplasmic and focal nuclear staining for PP4C was observed in the tumor cells (Figs 1A & 1B). No expression of PP4C was detected in tumor stromal cells (Fig 1A–1D). Among the 133 PDAC cases, negative, weak, moderate, and strong staining for PP4C was detected in 15 (11.3%), 43 (32.3%), 44 (33.1%) and 31 (23.3%) PDAC samples respectively.
Figure 1.
Representative micrographs show PP4C expression in pancreatic ductal adenocarcinoma (PDAC) samples and benign pancreatic tissue. A and B, strong cytoplasmic and nuclear staining for PP4C in a moderately to poorly differentiated PDAC; C and D, a moderately differentiated PDAC with weak staining for PP4C; E and F, Representative benign pancreatic tissue that is negative for PP4C (Original magnification, 40× for A, C, and E; 200× for B, D, and F).
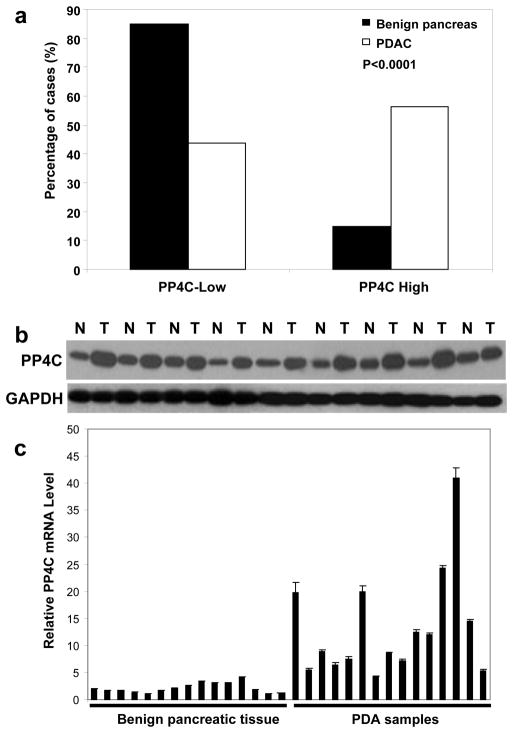
The paired benign pancreatic tissue was available in 113 cases. Fifteen percent (17/113) of the paired benign pancreatic tissue showed moderate to strong staining for PP4C. The frequency of moderate to strong staining for PP4C was significantly lower in benign pancreatic tissue than 56.4% (75/133) in PDAC samples (Fig 2A, p<0.001). When we compared the PP4C expression level in a PDAC sample to its paired benign pancreatic tissue from the same patient, we found that 73.5% (83/113) of the PDAC samples showed higher expression level of PP4C than their paired benign pancreatic tissue by immunohistohemistry. To further confirm our immunohistochemical staining results, we measured PP4C protein expression levels in 9 frozen PDAC samples and their paired benign pancreatic tissue samples by Western blotting. Compared to the paired benign pancreatic tissue samples, increased PP4C protein expression was detected in 9/9 (100%) PDAC samples (Fig 2B). In addition, we also examined PP4C mRNA expression in 15 PDAC and 15 benign pancreatic tissue samples. We found that PP4C mRNA expression level was significantly higher in PDAC samples than benign pancreatic tissue samples by QRT-PCR (Fig 2C). These data show that PP4C was overexpressed in PDAC and suggest that PP4C overexpression in PDAC is due to the up-regulation of PP4C mRNA expression.
Figure 2.
PP4C is overexpressed in pancreatic ductal adenocarcinoma (PDAC). A, Immunohistochemical staining results for PP4C expression in PDAC (N=133) and their paired benign pancreatic tissue samples (N=113). The expression of PP4C is significantly higher in PDAC samples than their paired benign pancreatic tissue (P<0.0001). B. 150 μg of cell lysates from each of 9 PDAC samples and their matched benign pancreatic tissue samples were resolved by 10% SDS-PAGE and immunoblotted with anti-PP4C or anti-GAPDH as loading control. C. Bar-graph showing the relative PP4C mRNA expression levels in benign pancreatic tissue samples and PDAC samples measured by real time QRT-PCR. Relative PP4C mRNA expression levels that were normalized by RPS6 mRNA levels (internal control) were plotted. PP4C mRNA was overexpressed in PDAC samples compared to the benign pancreatic tissue samples. The experiments were repeated three times.
Overexpression of PP4C is associated with poor prognosis in patients with stage II PDAC
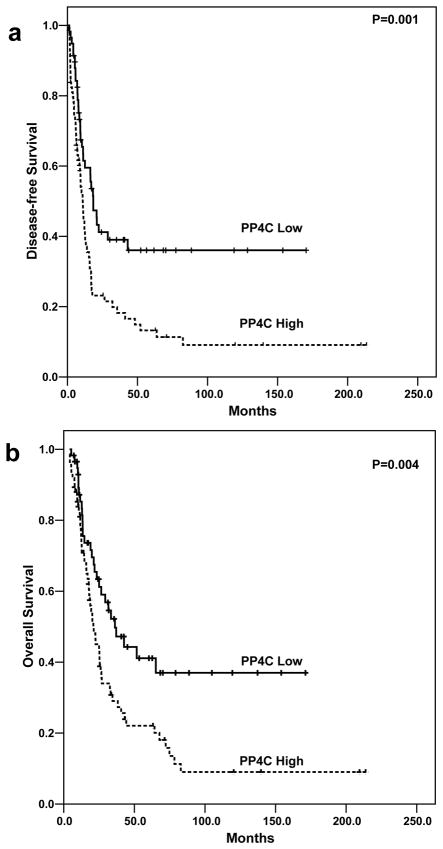
Clinicopathologic correlations of PP4C expression in patients with stage II PDAC are summarized in Table 1. In patients whose tumor was PP4C-high, 62.6% (47/75) developed distant metastasis during follow up, which was significantly higher than those patients whose tumor was PP4C-low (41.4%, 24/58) (p=0.02). However, we did not observe any significant association between PP4C overexpression and lymph node metastasis, locoregional recurrence and other clinicopathologic features (Table 1). The patients whose tumors were PP4C-high had shorter DFS and OS than those whose tumors were PP4C-low. The median DFS was 10.9 months [95% confidence interval (CI): 8.7–13.2 months] for patients whose tumor were PP4C-high compared to 18.4 months (95% CI: 13.4–23.4 months) for patients whose tumors were PP4C-low (p= 0.001, log-rank method, Fig. 3A). The median OS was 20.9 months (95% CI: 14.9–26.9 months) for patients whose tumors were PP4C-high, which was significantly shorter than 36.2 months (95% CI: 21.2–51.2 months) in patients whose tumors were PP4C-low (p= 0.004, log-rank method, Fig. 3B). In univariate analysis, both DFS and OS correlated significantly with tumor size, lymph node metastasis (stage), and PP4C overexpression. In addition, OS also showed significant correlation with resection margin status (p=0.04, Table 2). No significant correlations of either DFS or OS with the other clinicopathologic parameters were found (P>0.05). In multivariate analysis, high level of PP4C expression (PP4C-high) was associated with shorter DFS (p = 0.006) and OS (p=0.02) independent of tumor size, resection margin status, and lymph node metastasis (stage) (Table 3).
Table 1.
Clinicopathological correlation of PP4 expression in patients with stage II pancreatic ductal adenocarcinoma
| Characteristics | PP4-low (%)(n=58) | PP4-high (%)(n=75) | p value |
|---|---|---|---|
| Age | 0.07 | ||
| <60 yrs | 20 (34.4) | 19 (25.4) | |
| 60–70 yrs | 27 (46.6) | 28 (37.3) | |
| >70 yrs | 11 (19.0) | 28 (37.3) | |
| Gender | 0.21 | ||
| Female | 20 (34.5) | 35 (46.7) | |
| Male | 38 (65.5) | 40 (53.3) | |
| Tumor differentiation | 0.69 | ||
| Well-Moderate | 44 (75.9) | 54 (72.0) | |
| Poor | 14 (24.1) | 21 (28.0) | |
| Tumor size | 1.00 | ||
| ≤2cm | 9 (15.5) | 12 (16.0) | |
| >2cm | 49 (84.5) | 63 (84.0) | |
| Lymph node(s)/AJCC stage | 1.00 | ||
| Negative (IIA) | 14 (24.1) | 18 (24.0) | |
| Positive (IIB) | 44 (75.9) | 57 (76.0) | |
| Resection margin | 0.26 | ||
| Negative | 50 (86.2) | 58 (77.3) | |
| Positive | 8 (13.8) | 17 (22.7) | |
| Post operative treatment | 0.43 | ||
| No | 13 (22.4) | 22 (29.3) | |
| Yes | 45 (77.6) | 53 (70.7) | |
| Recurrence | 0.02 | ||
| No | 23 (39.6) | 14 (18.7) | |
| Locoregional | 11 (19.0) | 14 (18.7) | |
| Distant metastasis | 24 (41.4) | 47 (62.6) |
Figure 3.
Kaplan-Meier curves for disease-free survival (A) and overall survival (B) by PP4C expression levels in patients with stage II PDAC.
Table 2.
Univariate Cox Regression Analysis of Disease-free and Overall Survival in Relation to Clinicopathologic Features and PP4 Expression
| Characteristics | Number of patients | Disease -free survival
|
Overall survival
|
||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender | |||||
| Females (ref) | 55 | 1.00 | 0.10 | 1.00 | 0.12 |
| Males | 78 | 0.71 (0.47–1.07) | 0.61 | 0.71 (0.47–1.09) | 0.46 |
| Age (yrs) | |||||
| <60 (ref) | 39 | 1.00 | 1.00 | ||
| 60–70 | 55 | 1.00 (0.62–1.62) | 1.00 | 1.09 (0.66–1.81) | 0.73 |
| >70 | 39 | 1.26 (0.74–2.17) | 0.40 | 1.41 (0.80–2.47) | 0.23 |
| Tumor size | |||||
| ≤2cm (ref) | 21 | 1.00 | 1.00 | ||
| >2cm | 112 | 2.78 (1.39–5.55) | 0.004 | 3.11 (1.49–6.47) | 0.002 |
| Margin | |||||
| Negative (ref) | 108 | 1.00 | 1.00 | ||
| Positive | 25 | 1.60 (0.98–2.61) | 0.06 | 1.73 (1.03–2.92) | 0.04 |
| Tumor differentiation | |||||
| Well-moderate (ref) | 98 | 1.00 | 1.00 | ||
| Poor | 35 | 1.02 (0.63–1.63) | 0.95 | 1.10 (0.68–1.78) | 0.71 |
| Lymph nodes metastasis/AJCC stage | |||||
| No (stage IIA, ref) | 32 | 1.00 | 1.00 | ||
| Yes (Stage IIB) | 101 | 2.27 (1.33–3.86) | 0.002 | 2.14 (1.22–3.77) | 0.008 |
| Post operative treatment | |||||
| No (ref) | 35 | 1.00 | 1.00 | ||
| Yes | 98 | 0.68 (0.42–1.11) | 0.12 | 0.67 (0.40–1.11) | 0.12 |
| PP4 expression | |||||
| Low (ref) | 58 | 1.00 | 1.00 | ||
| High | 75 | 1.98 (1.29–3.03) | 0.002 | 1.89 (1.21–2.96) | 0.005 |
Abbreviations: HR, hazard ratio; CI, confidence interval; ref, reference
Table 3.
Multivariate Cox Regression Analysis of Disease-free and Overall Survival in Relation to Clinicopathologic Features and PP4 Expression
| Characteristics | Number of patients | Disease -free survival
|
Overall survival
|
||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Tumor size | |||||
| ≤2cm (ref) | 21 | 1.00 | 1.00 | ||
| >2cm | 112 | 1.86 (0.90–3.84) | 0.09 | 2.16 (1.004–4.66) | 0.049 |
| Margin | |||||
| Negative (ref) | 108 | 1.00 | 1.00 | ||
| Positive | 25 | 1.69 (1.005–2.82) | 0.048 | 1.80 (1.05–3.10) | 0.03 |
| Lymph nodes metastasis/AJCC stage | |||||
| No (stage IIA, ref) | 32 | 1.00 | 1.00 | ||
| Yes (Stage IIB) | 101 | 2.12 (1.20–3.74) | 0.01 | 1.80 (0.99–3.27) | 0.06 |
| PP4 expression | |||||
| Low (ref) | 58 | 1.00 | 1.00 | ||
| High | 75 | 1.83 (1.20–2.81) | 0.006 | 1.71 (1.09–2.68) | 0.02 |
Abbreviations: HR, hazard ratio; CI, confidence interval; ref, reference
DISCUSSION
In this study, we examined the expression of PP4C in 133 patients with stage II PDAC and their matched benign pancreatic tissue samples. We found that PP4C was overexpressed in PDAC samples compared to benign pancreatic tissue. PP4C overexpression was associated with shorter DFS and OS and was an independent prognostic factor for patients with stage II PDAC. Our data demonstrate that PP4C may be used as a prognostic factor in patients with stage II PDAC who underwent pancreatectomy.
It has been shown that PP4 is involved in the regulation of microtubule growth or organization at the centrosomes (12) and the centrosome maturation in mitosis and meiosis (13). Nakada et al. reported that depletion of PP4C results in a prolonged checkpoint arrest in human cells, suggesting that PP4c plays a critical role in dephosphorylating γ-H2AX after DNA damage (14). Lee et al. showed that PP4 dephosphorylates replication protein A subunit 2 (RPA2) and plays an essential role in DNA damage response and repair via homologous recombination (15). Previous studies have also showed that PP4 interacts with the members of the Rel/NFκB family of transcription factors c-Rel, RelA and p50, and activates NFκB-mediated transcription (16). PP4 dephosphorylates Thr435 of RelA, which is required for NFκB activation induced by cisplatin and MEK/ERK (17). In addition, PP4 has been found to be a positive regulator of the c-Jun N-terminal kinase (JNK) pathway in TNF-α signaling (18) and regulates hematopoietic progenitor kinase 1 (HPK1) activity in a T-cell receptor (TCR)-dependent manner (19). PP4 interacts with insulin receptor substrate 4 (IRS4) and HDAC3, down-regulates IRS4 and inhibits HDAC3 activity (20, 21). Recently, PP4 has been shown to be overexpressed in human breast and lung cancer and regulates the sensitivity of breast and lung cancer cells to cisplatin treatment (9). PP4 activates JNK-1 in prostate carcinoma cell lines, PC-3 and LNCaP, and increases the activities of c-Jun/AP-1 and EGR-1 (22). In addition, PP4 has also been shown to regulate the survival of both leukemic T-cells and untransformed human peripheral blood T-cells and has been shown to play an important role in the development and progression of leukemia (23). These data suggest that PP4 may play an important role in human malignancies. However, the expression and the function of PP4 in the tumorigenesis and progression of human cancers remain unclear. In this study, we showed that that PP4C expression levels were significantly higher in PDAC samples than their paired benign pancreatic tissue samples. Consistent with our immunohistochemical staining results, we demonstrated that both PP4C protein and mRNA expression levels were significantly higher in PDAC samples than those in benign pancreatic tissue by either Western blotting analysis or real time QRT-PCR. Therefore our data showed that PP4C was overexpressed in human PDAC samples. Increased PP4 mRNA expression in PDAC samples may suggest that PP4C overexpression in PDAC may be due to up-regulation of PP4C gene transcription. Our findings were consistent with the previous report that PP4C is overexpressed in human breast and lung cancers compared to benign breast or lung tissue respectively. PP4C protein has been shown to be expressed in 44/46 (95.7%) breast cancer samples compared to 37.8% (14/37) in benign breast tissue (p<0.01). In the same study, Wang et al showed that PP4C protein is expressed in 52/57 (91.2%) lung cancer samples, which is significantly higher than 67.7% (21/31) in benign lung tissue (p<0.01) (9). Since the expression of PP4C is significantly higher in PDAC samples than the paired benign pancreas tissue in our study, immunohistochemical staining for PP4C may be used as a marker for the diagnosis of invasive PDAC.
We showed that overexpression of PP4C correlated with distant metastasis in patients with stage II PDAC. We also showed for the first time that overexpression of PP4C was associated with both shorter DFS and shorter OS and was a prognostic factor for both DFS and OS independent of tumor size, resection margin status, and lymph node status (stage) in patients with stage II PDAC. Consistent with our findings is the recent study which showed that PP2A protein levels in the mononuclear cells at the time of the diagnosis of chronic myeloid leukemia (CML) can consistently predict patients who will progress to blast crisis. Significantly higher levels of PP2A protein expression was detected in patients who will later progress to blast crisis than those patients who do not progress (P < 0.0001) (24). PP2A promotes the growth and survival of pancreatic cancer cells. Li et al demonstrated that inhibition of PP2A induces growth inhibition in pancreatic cancer cells through the activation of c-Jun N-terminal kinase pathway (25, 26). Inhibition of PP2A also induces apoptosis in pancreatic cancer cells through the activation of NF-κB by persistent IKKα phosphorylation (27). In the light of these reports and the fact that PP4 is involved in the activation of both JNK and NF-κB pathways (16–18), overexpression of PP4C in PDAC may play an important role in the progression of PDAC and in predicting the prognosis in patients with stage II PDAC. Inhibition of PP4 either alone or in combination with other treatment modalities may be more effective for pancreatic cancer treatment.
One limitation of this study is that only patients with stage II PDAC were included in this study. This was mainly due to the number of patients of other disease stages was too small to be representative in our patient population. Only one patient each with stage IA, IB, or stage III PDAC and 2 patients with stage IV disease were identified in our patient population. However, our findings are important in that PP4C overexpression may be used as a prognostic marker for patients with stage II PDAC because vast majority of the patients with surgically resectable PDAC have stage II disease and little is known about the prognostic markers in this group of patients.
In conclusion, our study demonstrated that PP4C is overexpressed in PDAC. High level of PP4C expression in PDAC samples is associated with poor prognosis and is an independent prognostic marker for patients with stage II PDAC. Therefore, targeting PP4C signaling pathways may represent a novel target for the treatment of pancreatic cancer.
Acknowledgments
Supported by the National Institutes of Health grant (1R21CA149544-01A1) and G. S. Hogan Gastrointestinal Cancer Research Fund at The University of Texas M.D. Anderson Cancer Center
Footnotes
There are no financial disclosures from all authors.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez JM, Morton CA, Al-Saadi S, Villadolid D, Cooper J, Bowers C, et al. The natural history of resected pancreatic cancer without adjuvant chemotherapy. Am Surg. 2010;76:480–5. [PubMed] [Google Scholar]
- 3.Wera S, Hemmings BA. Serine/threonine protein phosphatases. Biochem J. 1995;311 (Pt 1):17–29. doi: 10.1042/bj3110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen PT. Novel protein serine/threonine phosphatases: variety is the spice of life. Trends Biochem Sci. 1997;22:245–51. doi: 10.1016/s0968-0004(97)01060-8. [DOI] [PubMed] [Google Scholar]
- 5.Brewis ND, Street AJ, Prescott AR, Cohen PT. PPX, a novel protein serine/threonine phosphatase localized to centrosomes. Embo J. 1993;12:987–96. doi: 10.1002/j.1460-2075.1993.tb05739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24:186–91. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- 7.Dounay AB, Forsyth CJ. Okadaic acid: the archetypal serine/threonine protein phosphatase inhibitor. Curr Med Chem. 2002;9:1939–80. doi: 10.2174/0929867023368791. [DOI] [PubMed] [Google Scholar]
- 8.Hastie CJ, Cohen PT. Purification of protein phosphatase 4 catalytic subunit: inhibition by the antitumour drug fostriecin and other tumour suppressors and promoters. FEBS Lett. 1998;431:357–61. doi: 10.1016/s0014-5793(98)00775-3. [DOI] [PubMed] [Google Scholar]
- 9.Wang B, Zhao A, Sun L, Zhong X, Zhong J, Wang H, et al. Protein phosphatase PP4 is overexpressed in human breast and lung tumors. Cell Res. 2008;18:974–7. doi: 10.1038/cr.2008.274. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Wang H, Zhang W, Fuller GN. Tissue microarrays: applications in neuropathology research, diagnosis, and education. Brain Pathol. 2002;12:95–107. doi: 10.1111/j.1750-3639.2002.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Song X, Logsdon C, Zhou G, Evans DB, Abbruzzese JL, et al. Proteasome-mediated degradation and functions of hematopoietic progenitor kinase 1 in pancreatic cancer. Cancer Res. 2009;69:1063–70. doi: 10.1158/0008-5472.CAN-08-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helps NR, Brewis ND, Lineruth K, Davis T, Kaiser K, Cohen PT. Protein phosphatase 4 is an essential enzyme required for organisation of microtubules at centrosomes in Drosophila embryos. J Cell Sci. 1998;111 (Pt 10):1331–40. doi: 10.1242/jcs.111.10.1331. [DOI] [PubMed] [Google Scholar]
- 13.Sumiyoshi E, Sugimoto A, Yamamoto M. Protein phosphatase 4 is required for centrosome maturation in mitosis and sperm meiosis in C. elegans. J Cell Sci. 2002;115:1403–10. doi: 10.1242/jcs.115.7.1403. [DOI] [PubMed] [Google Scholar]
- 14.Nakada S, Chen GI, Gingras AC, Durocher D. PP4 is a gamma H2AX phosphatase required for recovery from the DNA damage checkpoint. EMBO Rep. 2008;9:1019–26. doi: 10.1038/embor.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DH, Pan Y, Kanner S, Sung P, Borowiec JA, Chowdhury D. A PP4 phosphatase complex dephosphorylates RPA2 to facilitate DNA repair via homologous recombination. Nat Struct Mol Biol. 2010;17:365–72. doi: 10.1038/nsmb.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu MC, Tang-Oxley Q, Qiu WR, Wang YP, Mihindukulasuriya KA, Afshar R, et al. Protein phosphatase X interacts with c-Rel and stimulates c-Rel/nuclear factor kappaB activity. J Biol Chem. 1998;273:33561–5. doi: 10.1074/jbc.273.50.33561. [DOI] [PubMed] [Google Scholar]
- 17.Yeh PY, Yeh KH, Chuang SE, Song YC, Cheng AL. Suppression of MEK/ERK signaling pathway enhances cisplatin-induced NF-kappaB activation by protein phosphatase 4-mediated NF-kappaB p65 Thr dephosphorylation. J Biol Chem. 2004;279:26143–8. doi: 10.1074/jbc.M402362200. [DOI] [PubMed] [Google Scholar]
- 18.Zhou G, Mihindukulasuriya KA, MacCorkle-Chosnek RA, Van Hooser A, Hu MC, Brinkley BR, et al. Protein phosphatase 4 is involved in tumor necrosis factor-alpha-induced activation of c-Jun N-terminal kinase. J Biol Chem. 2002;277:6391–8. doi: 10.1074/jbc.M107014200. [DOI] [PubMed] [Google Scholar]
- 19.Zhou G, Boomer JS, Tan TH. Protein phosphatase 4 is a positive regulator of hematopoietic progenitor kinase 1. J Biol Chem. 2004;279:49551–61. doi: 10.1074/jbc.M410317200. [DOI] [PubMed] [Google Scholar]
- 20.Mihindukulasuriya KA, Zhou G, Qin J, Tan TH. Protein phosphatase 4 interacts with and down-regulates insulin receptor substrate 4 following tumor necrosis factor-alpha stimulation. J Biol Chem. 2004;279:46588–94. doi: 10.1074/jbc.M408067200. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Ozawa Y, Lee H, Wen YD, Tan TH, Wadzinski BE, et al. Histone deacetylase 3 (HDAC3) activity is regulated by interaction with protein serine/threonine phosphatase 4. Genes Dev. 2005;19:827–39. doi: 10.1101/gad.1286005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inostroza J, Saenz L, Calaf G, Cabello G, Parra E. Role of the phosphatase PP4 in the activation of JNK-1 in prostate carcinoma cell lines PC-3 and LNCaP resulting in increased AP-1 and EGR-1 activity. Biol Res. 2005;38:163–78. doi: 10.4067/s0716-97602005000200006. [DOI] [PubMed] [Google Scholar]
- 23.Mourtada-Maarabouni M, Williams GT. Protein phosphatase 4 regulates apoptosis in leukemic and primary human T-cells. Leuk Res. 2009;33:1539–51. doi: 10.1016/j.leukres.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas CM, Harris RJ, Giannoudis A, Copland M, Slupsky JR, Clark RE. Cancerous inhibitor of PP2A (CIP2A) at diagnosis of chronic myeloid leukemia is a critical determinant of disease progression. Blood. 2011;117:6660–8. doi: 10.1182/blood-2010-08-304477. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Chen Z, Gong FR, Zong Y, Chen K, Li DM, et al. Growth of the pancreatic cancer cell line PANC-1 is inhibited by protein phosphatase 2A inhibitors through overactivation of the c-Jun N-terminal kinase pathway. Eur J Cancer. 2011;47:2654–64. doi: 10.1016/j.ejca.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Xie L, Chen Z, Zhu Y, Sun Y, Miao Y, et al. Cantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress-independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosis. Cancer Sci. 2010;101:1226–33. doi: 10.1111/j.1349-7006.2010.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Chen Z, Zong Y, Gong F, Zhu Y, Zhu Y, et al. PP2A inhibitors induce apoptosis in pancreatic cancer cell line PANC-1 through persistent phosphorylation of IKKalpha and sustained activation of the NF-kappaB pathway. Cancer Lett. 2011;304:117–27. doi: 10.1016/j.canlet.2011.02.009. [DOI] [PubMed] [Google Scholar]