Abstract
Sjögren’s syndrome (SjS) patients often have a variety of extraglandular manifestations including neurological and gastrointestinal involvement. In this study we evaluated the diagnostic performance of luciferase immunoprecipitation system (LIPS) that employs mammalian cell-produced recombinant antigens for analyzing SjS autoantibody responses. LIPS testing of mammalian cell-produced La, Ro60 and Ro52 recombinant antigens with defined commercial antibodies demonstrated highly specific immunoprecitation of each antigen without cross-reactivity. Next, sera from 57 SjS and 25 volunteers were evaluated by LIPS against a panel of human autoantigens. LIPS detected robust anti-La antibody levels in 43/57 SjS patients (75% sensitivity) and markedly outperformed an ELISA (46% sensitivity). Profiling of other SjS-associated autoantigens revealed the presence of anti-Ro60, anti-Ro52 in 63% and 61%, of SjS patients, respectively. Interestingly, a C-terminal fragment of Ro52 (Ro52-Δ2), a protein fragment not previously found to be antigenic by ELISA, also showed positive immunoreactivity in 42/57 SjS patients (65% sensitivity). Additional profiling of other autoantigens revealed that certain SjS patients also showed positive immunoreactivity with thyroid peroxidase (14%), AQP-4 (12%) and the H+/K+ gastric ATPase (16%) suggesting potential autoantibody attack of thyroid, neuronal and gastric parietal cells, respectively. These heterogeneous autoantibody responses detected by LIPS in SjS will likely be useful for diagnosis and for evaluating extraglandular manifestations.
Keywords: Autoantibodies, Autoantigen, Diagnosis Sjögren’s syndrome, SSA, SSB
Introduction
Sjögren’s syndrome (SjS) is a common autoimmune disorder associated with epithelial inflammation and exocrine glands dysfunction [1]. The etiology of SjS is unknown and genetic and environmental factors are both thought to play a role in triggering the autoimmune response. The presence of multiple autoantibodies and oligoclonal and polyclonal B cell activation is characteristic of SjS. The spectrum of SjS extends from an organ-specific autoimmune disease with sicca symptoms of dry eyes and mouth, to systemic disease with diverse extraglandular manifestations involving other organs such as the thyroid, lungs, gastrointestinal and the peripheral nervous system.
The current American-European consensus classification for primary SjS is based on six criteria [2]. Signs of ocular and oral dryness, evidence of inflammation from minor salivary gland biopsy, and the presence of autoantibodies towards the extractable nuclear SSA and SSB antigens are included in the criteria. These antibodies are not specific to SjS, however, as they are detectable in SLE (systemic lupus erythematosus), myositis and other autoimmune diseases [3]. SSA is composed of structurally unrelated Ro52 and Ro60, while SSB, also known as La, is a single 48 kDa protein. In the 2002 classification standards for SjS diagnosis, positive SSA and SSB autoantibody titers was the only mandatory criteria for primary SjS if the salivary gland biopsy was negative [2]. Current ELISA-based immunoassays detect a rate of approximately 70% SSA and 40% SSB immunoreactivity in SjS [1]. However, few autoantibody tests exist to evaluate the diverse extraglandular manifestations that are commonly found in SjS.
Most ELISA-based immunoassays for evaluating autoantibodies, including those for SSA and SSB, employ either native proteins or protein complexes, such as those purified from calf thymus [4], or ones produced recombinantly in bacteria or baculovirus. Immunoassays that utilize proteins from these sources may contain contaminating proteins or macromolecular aggregates that limit the accuracy of these tests due to high backgrounds and/or false signals. We recently developed a highly sensitive immunoprecipitation technology called Luciferase Immunoprecipitation System (LIPS) that utilizes mammalian cell-produced, recombinant fusion protein antigens for efficiently evaluating antibody responses [5–11]. In a limited number of side-by-side comparisons, LIPS showed improved diagnostic performance compared to existing immunoassays for detecting antibodies to infectious agents [10,11] and provided new tools to monitor drug treatment [11] and sub-stratify disease states [9]. In addition, LIPS is highly useful for profiling autoimmunity [7,8] and in one study showed several advantages over a highly sensitive radioactive in vitro transcription/translation assay for detecting anti-IA2 autoantibodies associated with type I diabetes [8]. In the present study, LIPS was evaluated for its diagnostic performance in detecting autoantibodies to SSA and SSB and for screening additional autoantibody responses in primary SjS and control volunteers.
Material and methods
Patients and methods
The sera were randomly selected from a cohort of patients with primary SjS participating in a longitudinal natural history study. These sera included 57 well-characterized patients diagnosed with primary SjS and 25 healthy volunteers evaluated under Institutional Review Board-approved protocols at the SjS clinic of the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD. An additional 15 serial samples from the primary SjS patients were tested for further validation of the LIPS tests but were not used for calculations of sensitivity and specificity. As part of a clinical protocol, all SjS patients and volunteers underwent medical evaluation and standardized tests for salivary (unstimulated and stimulated glandular salivary flow rate, whole salivary flow rate) and lacrimal (Schirmer’s test) gland function, and minor salivary gland biopsy. The age, gender, focus score, salivary flow and clinical laboratory findings of the volunteers and primary SjS patients are summarized in Table I. SjS patients were also documented for other covariants including peripheral and central nervous system disease, gammopathy, and lymphoma. For the focus score [12], the histopathological ranking score ranged from 0 to 12 (severe). Focus score, a marker of lymphocytic infiltrate, is defined as the number of lymphocytic foci containing more than 50 lymphocytes per 4mm2 of glandular tissue and range from 0 (no infiltrate) to 12 (confluent).
Table I.
Clinical characteristics of 57 patients diagnosed with primary SjS and 25 healthy volunteers.
| SjS (n = 57) | NV (n = 25) | |
|---|---|---|
| Age, years (mean, ± SD) | 53 (± 13) | 48 (± 14) |
| Percent Female | 83% | 60% |
| Unstimulated Salivary Flow, mL/15 min (mean, ± SD) | 1.30 (± 2.30) | 3.39 (± 3.39) |
| Focus Score* (mean, ± SD) | 4 (± 4) | 0 (± 1) |
| Schirmer’s test, mm/5 min (mean, ± SD) | 5 (± 7) | 14 (± 11) |
| Anti-SSA positive by ELISA† (%) | 72 | 0 |
| Anti-SSB positive by ELISA† (%) | 46 | 0 |
| RF positive‡ (%) | 60 | 8 |
| ESR, mm/h (mean, ± SD) | 27 (± 26) | 16 (± 13) |
| Serum IgG, mg/dL (mean, ± SD) | 1640 (± 857) | 1162 (± 291) |
Minor salivary gland biopsy and focus score data was available for all subjects. A focus score of 1 or greater is needed to show histopathological evidence of Sjögren’s syndrome.;
Anti-SSA and SSB reference (0–19 EU).;
Rheumatoid Factor reference (< 20 IU/mL).
Sera were kept at −80°C, thawed, aliquoted, then stored at 4°C, and measured as coded samples. Sera were evaluated by LIPS for anti-La, anti-Ro60 anti- Ro52, anti-Jo1, and anti-AQP-4 antibody titers without pre-knowledge of disease status. Assays for anti-TPO, anti-AQP-1, anti-AQP-5, anti-gastric ATPase and antibodies to two recombinant fragments of Ro52 antibodies were determined thereafter. For comparison, SSA (anti-Ro52 and anti-Ro60 antibodies) and SSB (anti-La antibody) tests were measured in the Laboratory of Clinical Medicine, NIH using a commercial ELISA obtained from BioRad (Hercules, CA) that employs native, extractable bovine nuclear antigens [4].
Generation of Ruc-antigen fusion constructs
A mammalian Renilla luciferase (Ruc) expression vector, pREN2, was used to generate all plasmids [6]. Human cDNA clones were amplified by PCR with specific linker-primer adapters as described [6–8]. Unlike the other autoantigens tested, which represented full-length proteins, a fragment of thyroid peroxidase (TPO) was fused to the carboxy terminus of Ruc. In the case of the Ro52, two deletion constructs were also tested, Ro52-Δ1 and Ro52-Δ2, spanning amino acid residues 2–277 and 278 – 475, respectively. DNA sequencing was used to confirm the integrity of all the plasmid constructs. PCR primer sequences that were used to generate each construct are available on request.
Validation of LIPS tests for detecting anti-La, anti-Ro52 and anti-Ro60
As previously described for other autoantigens [6], the specificity of the LIPS tests for La, Ro-52 and Ro60 were validated using known positive control commercial antibodies. The monoclonal anti-La (catalogue # sc-80656) and polyclonal anti-Ro52 (catalogue # sc-20960), anti-Ro60 (SC-20961) antibodies were obtained from Santa Cruz Biotechnology, Ca, USA. For the Ruc-La and Ruc-Ro60 LIPS tests all three antibodies were used at 200 ng, while for the Ruc-Ro52 LIPS test only 20 ng of each of three antibodies were used. Results are shown from the average of two experiments and protein A/G bead background without antibody addition was subtracted and normalized as described [5].
LIPS analysis
The LIPS assay was performed in a 96-well plate format at room temperature as described [7]. First, a “master plate” was constructed by diluting patient sera 1:10 in assay buffer A (20mM Tris, pH 7.5, 150mM NaCl, 5mM MgCl2, 1% Triton X100) in a 96well polypropylene microtiter plate. Additional sera dilutions were required for measuring anti-Ro52 and anti-Ro60 antibody titers. For evaluating antibody titers by LIPS, 40 µl of buffer A, 10 µl of diluted human sera (1 µl equivalent), and 50 µl of 1 × 107 light units (LU) of Ruc-antigen Cos1 cell extract diluted in buffer A, were added to each well of a polypropylene plate and incubated for 1 hour at room temperature. Next, 7 µl of a 30% suspension of Ultralink protein A/G beads (Pierce Biotechnology, Rockford, IL) in PBS were added to the bottom of each well of a 96well filter HTS plate (Millipore, Bedford, MA). To this filter plate, the 100 µl antigen-antibody reaction mixture was transferred and incubated for 1 hour at room temperature on a rotary shaker. The washing steps of the retained protein A/G beads were performed on a BioMek FX [7] work station (Beckman Coulter, Fullerton, CA) using an integrated vacuum manifold. After the final wash, LU were measured in a Berthold LB 960 Centro microplate luminometer (Berthold Technologies, Bad Wilbad, Germany) using coelenterazine substrate mix (Promega, Madison, WI). All LU data were obtained from the average of at least two independent experiments and the LU values used without subtracting the buffer blank as described [8].
Statistical and data analysis
The GraphPad Prism software (San Diego, CA) was used for statistical analysis, including the evaluation of receiver operator characteristics (ROC) and test performance by area under the curve (AUC). Mann-Whitney U-tests were used to compare the antibody titers among the different groups. It should be noted that the duplicate LIPS tests were performed separately for each antigens and the interassay coefficient of variation was below 20%. For each test, the mean + standard deviation (SD) of LU derived from 2 separate determinations was calculated and the 3SD and 5SD cut-off points determined; both of which are indicated in the figures. Due to the robust nature of the autoantibody values found in the SjS patients, the mean plus 3SD was consistently used for most calculation of sensitivity and specificity.
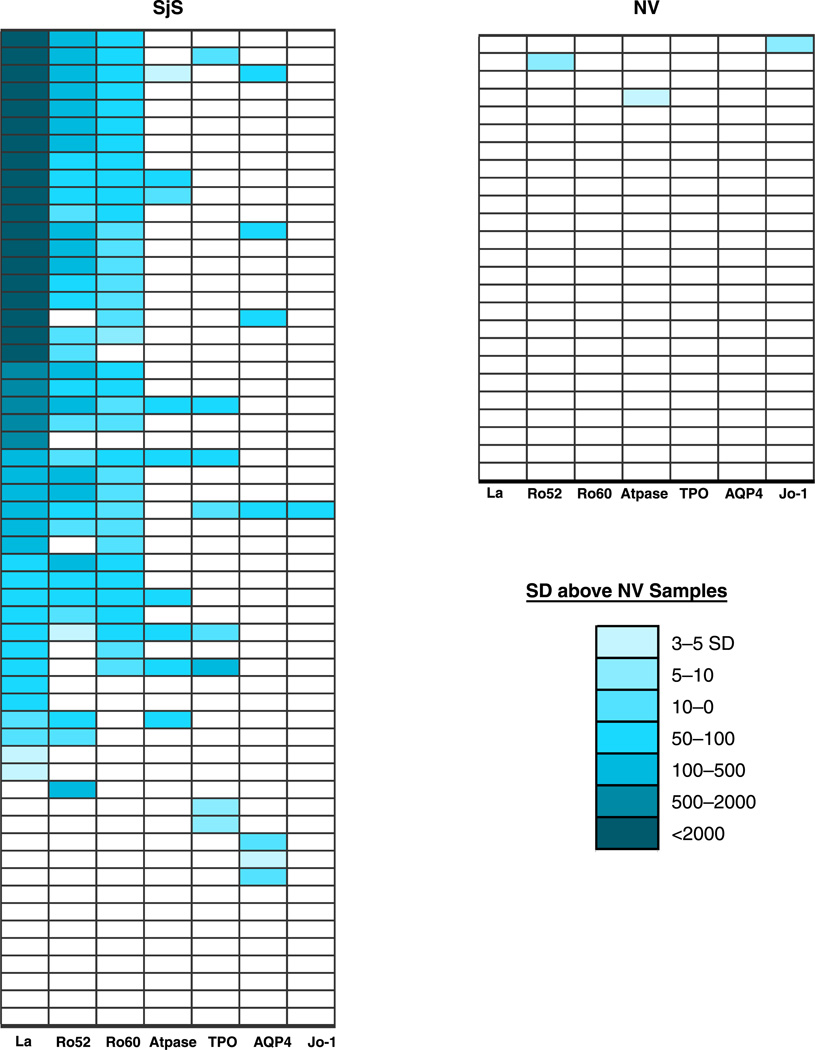
Data transformation and a heatmap were used to visualize the autoantibody profiles of the participants as a single graphic (Figure 5). In order to create this heatmap, the mean and standard deviation of the antibody titers for each antigen in the 25 control samples was first generated as a reference scale. Next antibody titer values for each antigen-antibody measurement greater than the control mean plus 3 SD were color coded to signify the relative number of standard deviations above these cut-off values. Lastly, the samples were rank ordered with respect to anti-La antibodies, the most informative SjS autoantigen.
Figure 5.
Heatmap representation of SjS patient autoantibody profiles. Autoantibody titers to 7 autoantigens are shown for each of the 25 NVand 57 SjS patients. The titer values greater than the mean of the 25 normal volunteers plus 3 standard deviations were color-coded from clear to dark blue to signify the relative number of standard deviations above these reference values.
Results
Validation of LIPS tests for detecting anti-La, anti-Ro52 and anti-Ro60 antibodies
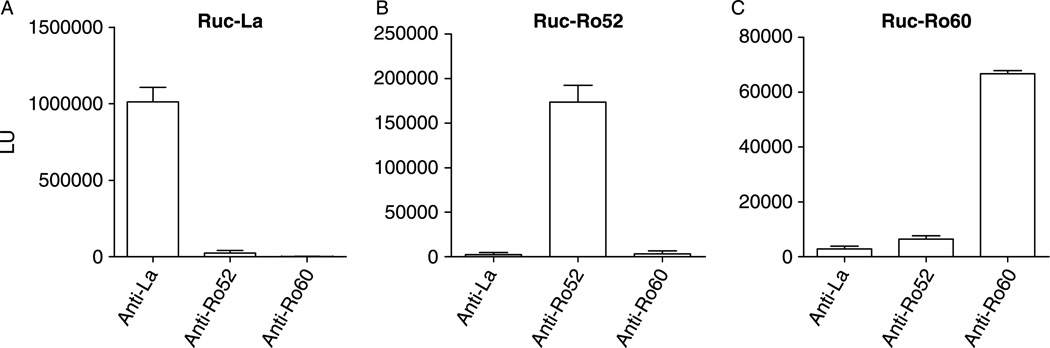
Here we fused cDNAs in-frame for human La, Ro52 and Ro60, encoding full-length proteins, to the C-terminus of Renilla luciferase (Ruc) using the mammalian expression vector, pREN2. Following transfection of these individual constructs into Cos1 cells, cell lysates containing high levels of expression of the Ruc-antigen fusions were obtained. For example, the Ruc-La lysate yielded 1 × 109 LU per 100mm2 plate of Cos1 cells (sufficient for ~ 300 serological tests). These Ruc-antigen lysates were next used in the standard LIPS format to evaluate specificity with commercial anti-La, anti-Ro52 and anti-Ro60 antibodies. In the standard LIPS format, 1 µg of anti-La monoclonal antibody immunoprecitatated approximately 10% of the Ruc-La input, but this antibody did not immunoprecipitate the Ruc-Ro52 or Ruc-Ro60 fusion proteins (Figure 1A). In contrast a polyclonal anti-Ro52 antibody efficiently immunoprecipitated Ruc-Ro52, but did not immunoprecipitate the Ruc-La or Ruc-Ro60 fusion proteins (Figure 1B). Lastly, an anti-Ro60 antibody did not immunoprecipitate the Ruc-La or Ruc-Ro52 fusion proteins, but efficiently immunoprecipitated the Ruc-Ro60 extract (Figure 1C). These results suggest that the Ruc-La, Ruc-Ro52 and Ruc-Ro60 antigen fusions contain the expected linear and/or conformational epitopes and may be useful in LIPS tests for detecting autoantibodies to these proteins in SjS.
Figure 1.
Validation of LIPS tests for detecting anti-La, anti-Ro52 and anti-Ro60 antibodies. As described in the material and methods, commercial antibodies to La, Ro52 and Ro60 were mixed with extract from lysates containing Ruc-La (A), Ruc-Ro52 (B) and Ruc-Ro60 (C) for 1 hour, incubated with protein A/G beads for an additional hour, processed and light units measured. The mean LU plus standard error is shown for each immunoprecipitation determination.
LIPS detection of anti-La autoantibodies in SjS
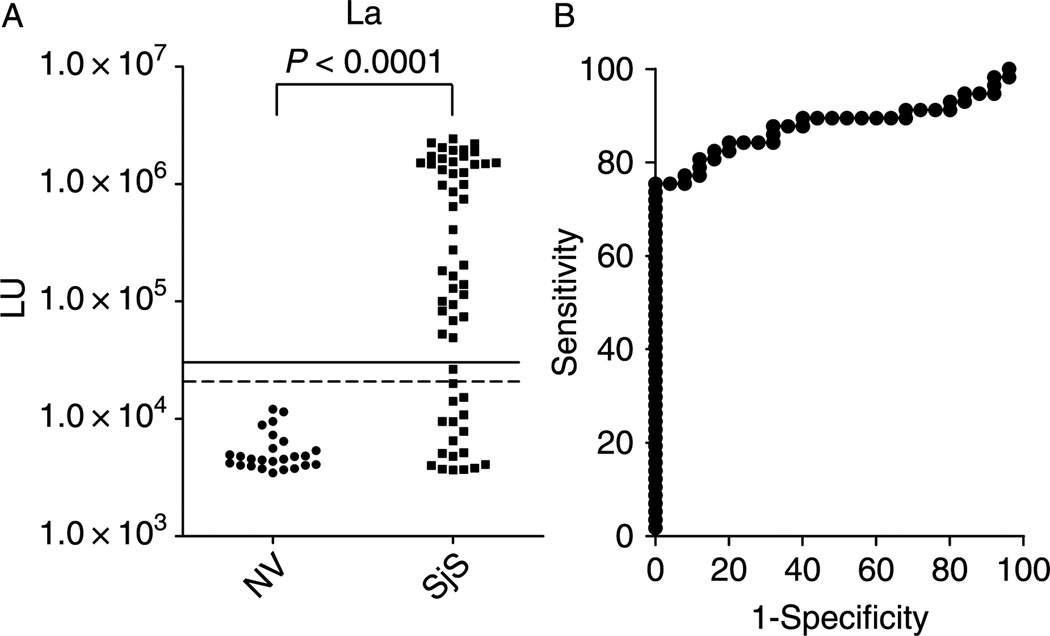
LIPS testing of 82 serum samples with the Ruc-La antigen yielded anti-La antibody titers ranging from 3,468 to 2.4 million LU in the (Figure 2A). The mean + SD of the anti-La antibody titer in the 25 controls was 5561 + 2424 LU and it was significantly different (p < 0.0001) from the mean value for 57 SjS samples of 686,471 + 794,507 LU. The results from these duplicate interassay LIPS tests for anti-La antibodies were highly reproducible and had a coefficient of variation of 16%.
Figure 2.
LIPS detection of autoantibodies against La in 25 normal volunteers and 57 primary SjS patients. (A). Each circle or square symbol represents the anti-La antibody titer of a normal volunteer (NV) or SjS patient sample, respectively. For determining sensitivity and specificity for this anti-La antibody test, the dashed line represents the cut-off level derived from mean plus 3 SD of the antibody titer of the 25 normal volunteers, while the solid line is the cut-off for the mean plus 5 SD. p-values were calculated using the Mann Whitney U-test. (B). Roc plot analysis of anti-La antibodies detected by the LIPS test. Area under the curve (AUC) = 0.88.
ROC analysis of the anti-La antibody test showed a calculated area under the curve (AUC) value of 0.88, which reflected the high sensitivity and specificity of this assay (Figure 2B). A cut-off of the mean plus 3 SD of the control subjects revealed 75% sensitivity (43/57) and 100% specificity in detecting positive anti-La autoantibodies in the SjS samples, while a lower cut-off of the mean plus 2 SD yielded 76% sensitivity with 92% specificity. An even higher cut-off of the mean plus 5 SD of the control subjects yielded a 72% and 100% specificity. In comparison to the SSB ELISA, LIPS using the mean plus 3SD cut-off showed a dramatically greater sensitivity than ELISA (i.e., 76% for LIPS versus 46% for ELISA) for detecting anti-La antibody positive SjS samples (Table II).
Table II.
Performance of ELISA and/or LIPS for the diagnosis of primary SjS.
| Test | Sensitivity | Specificity |
|---|---|---|
| ELISA | ||
| SSA (Ro52 and Ro60)* | 72% (41/57) | |
| SSB (La)† | 46% (26/57) | |
| SSA & SSB‡ | 77% (44/57) | |
| LIPS¶ | ||
| La (mean plus 3 SD) | 75% (43/57) | 100% |
| La (mean plus 5 SD) | 72% (41/57) | 100% |
| Ro52 (mean plus 3 SD) | 61% (35/57) | 96% |
| Ro52 (mean plus 5 SD) | 60% (34/57) | 100% |
| Ro60 (mean plus 3 SD) | 63% (36/57) | 96% |
| Ro60 (mean plus 5 SD) | 61% (35/57) | 100% |
| Ro52 & Ro60 (mean plus 3 SD) | 68% (39/57) | 92% |
| Ro52 & Ro60 (mean plus 5 SD) | 68% (39/57) | 100% |
| La & Ro52 (mean plus 3 SD) | 77% (44/57) | 96% |
| La & Ro52 (mean plus 5 SD) | 74% (42/57) | 100% |
| TPO (mean plus 3 SD) | 14% (8/57) | 100% |
| TPO (mean plus 5 SD) | 11% (6/57) | 100% |
| H + /K + ATPase (mean plus 3 SD) | 16% (9/57) | 96% |
| H + / K + ATPase (mean plus 5 SD) | 14% (8/57) | 100% |
| AQP-4 (mean plus 3 SD) | 12% (7/57) | 100% |
| AQP-4 (mean plus 5 SD) | 11% (6/57) | 100% |
| La, Ro52, TPO & AQP-4 (mean plus 3 SD) | 86% (49/57) | 96% |
| La, Ro52, TPO & AQP-4 (mean plus 5 SD) | 79% (45/57) | 100% |
Includes 1 ELISA borderline positive cases.;
Includes 3 ELISA borderline positive case.;
Includes 2 ELISA borderline positive case.;
For LIPS, the cut-off limit for calculating sensitivity and specificity for each antigen was derived from the value of the mean plus 3 or 5 SDs of the 25 control samples.
Anti-Ro60 and anti-Ro52 antibody titers in SjS
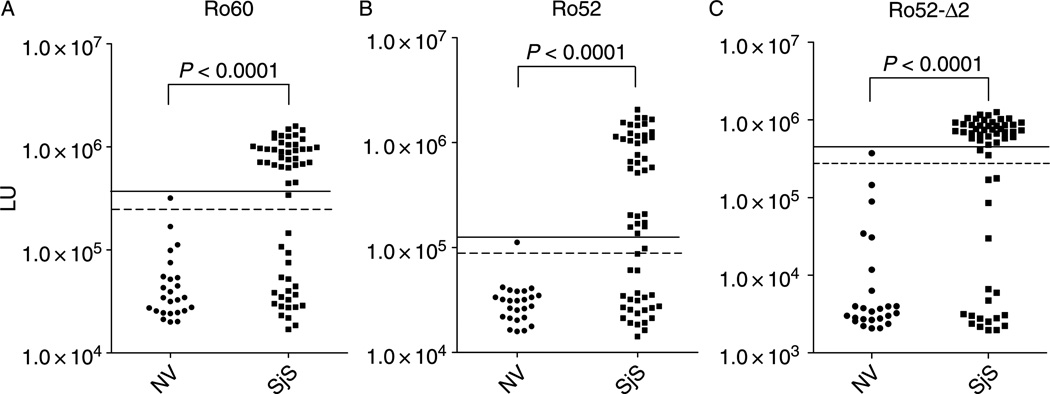
The Ro52 and Ro60 proteins of the SSA autoantigen are generally measured together in ELISAs using extracts of purified, native proteins. Since separate analysis of these two antigens could improve the sensitivity, recombinant Ruc-Ro60 and Ruc-Ro52 fusion proteins were analyzed independently. A pilot set of controls and SjS samples showed a uniformly high immunoprecipitation activity in the sera suggesting that the anti-Ro60 antibodies in nondiluted sera were in excess (data not shown). Dilution of the sera to 1:200 (equivalent to 5 nanoliters of serum/assay) was required to accurately determine anti-Ro60 antibody titers in the linear range of the LIPS assay. The diluted sera yielded anti-Ro60 antibody titers ranging from 16,981 to 1,590,939 LU in the 82 samples (Figure 2A). While the 25 normal control samples showed anti-Ro60 antibody titers with a mean and SD of 57,389 + 64,381 LU, the 57 SjS had a much higher mean and SD of 609,065 + 495,736 LU. A Mann-Whitney U-test showed a marked difference in anti-Ro60 antibody titers between the controls and SjS patients (p < 0.0001). The cut-off value determined from the control mean plus 3 SD identified 36/57 (63% sensitivity and 96% specificity) of SjS subjects with positive anti-Ro60 antibody titers (Table II). A higher cut-off of the control mean plus 5 SD would identify 61% (35/57) of the SjS patients as positives with 100% specificity (Figure 2A).
Similar to the anti-Ro60 LIPS antibody test, the anti-Ro52 antibodies in undiluted sera were in excess. Titration and dilution of sera to 1:400 was needed to obtain values in the linear range (data not shown). Using this 1:400 dilution, the 82 SjS and normal volunteer serum samples showed a wide range of anti-Ro52 antibody titers from 15,862 to 2,068,366 LU (Figure 2B). Analysis of mean and SD of the anti-Ro52 antibody titers between the controls (31,786 + 18,587 LU) and SjS patients (554,249 + 607,002 LU) revealed that they were markedly different (p < 0.0001). The cut-off of the mean plus 3 SD from the 25 control subjects identified a positive anti-Ro52 titer in 61% (35/57) of the SjS samples with 96% specificity (Table II). A higher cut-off of the control mean plus 5 SD would identify 60% (34/57) of the SjS patients as positives with 100% specificity (Figure 2B).
The AUC values for the anti-Ro52 and anti-Ro60 antibody tests were 0.80 and 0.82, respectively. Despite the fact that the AUC values were quite similar, regression analysis of the anti-Ro52, anti- Ro60 and anti-La antibody detected by LIPS revealed weak but statistically significant correlations between the different autoantibody titers in the SjS samples. The strongest association was between the anti-Ro52 antibodies and anti-Ro60 antibodies (R = 0.60; p = 1.0 × 10−6), followed by anti-La antibody and anti-Ro52 antibodies (R = 0.59; P = 1.0 × 10−6), and lastly anti-La antibodies and anti-Ro60 antibodies (R = 0.52; p = 3.0 × 10−5).
The anti-Ro52 and anti-Ro60 antibody titers were also compared to the anti-SSA ELISA values determined in a clinical laboratory, which measures anti-Ro52 and anti-Ro60 as a complex. While the SSA ELISA had a sensitivity of 72%, 1 of the SjS samples was borderline positive. The LIPS test for anti-Ro52 and anti-Ro60 showed a combined sensitivity of 69% sensitivity and 92% specificity using a cut-off of the mean plus 3 SD, while a cut-off of the mean plus 5 SD still showed 69% sensitivity and 100% specificity. These results suggest that LIPS, using defined recombinant antigens, show a similar sensitivity and specificity as the ELISA for detecting anti-SSA antibodies.
Detection of autoantibody responses to the C-terminus of Ro52
Recent biochemical and structural studies suggest that Ro52 functions in regulating the quality control of immunoglobulins via proteosomal destruction of misfolded immunoglobulins [13,14]. Although a C-terminal domain, called the PRYSPRY domain, of Ro52 functions to bind the Fc region of IgG antibodies [15,16], previous ELISA studies have failed to detect autoantibodies to this region [17,18]. Here we used LIPS to test the immunoreactivity of the N- and C-terminal fragments of Ro52, designated Ro52-Δ1 and Ro52-Δ2, containing amino acids 2–277 and 278–475, respectively. In the case of the Ro52-Δ1, this N-terminal protein fragment was found to be highly immunogenic with SjS samples and still required the sera to be diluted (1:40) to obtain values in the linear range (data not shown). Interestingly, the Ro52-Δ2 C-terminal fragment, which no longer required sera to be diluted for detecting values in the linear range, still showed high sensitivity and specificity for detecting SjS samples (Figure 3C). Using a cut-off of the mean plus 3 SD of the normal volunteers, positive anti-Ro52-Δ2 antibody titers were detected in 65% of the SjS samples with 96% specificity (Figure 3C). These results demonstrate that the C-terminal PRYSPRY domain of Ro52 contains useful epitopes for the diagnosis of SjS, but that these antibodies are of lower titer than the N-terminus and are missed by ELISAs which use recombinant proteins.
Figure 3.
LIPS detection of autoantibodies against Ro60, Ro52 and a C-terminal fragment of Ro52 in 25 normal volunteers and 57 primary SjS patients. Each circle or square symbol represents an individual normal volunteer (NV) or SjS patient sample, respectively. For determining sensitivity and specificity, the dashed line represents the cut-off level derived from mean plus 3 SD of the antibody titer of the 25 normal volunteers, while the solid line is the cut-off for the mean plus 5 SD. (A) The anti-Ro60 antibody test. (B) The anti-Ro52 antibody test. (C) The anti-Ro52-Δ2 antibody test. p-values were calculated using the Mann Whitney U-test.
SjS patient autoantibodies against other autoantigens
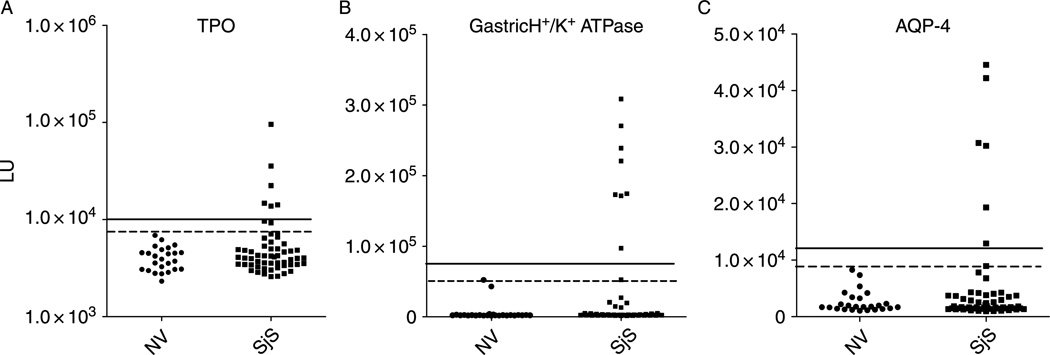
In light of the many extraglandular manifestation found in SjS, 4 additional known autoantigens that are associated with other autoimmune diseases were evaluated by LIPS. These autoantigens included Jo-1, thyroid peroxidase (TPO), gastric H+/K+ ATPase and aquaporin-4 (AQP-4) that are often detected in myositis [19], autoimmune thyroid disease [20], autoimmune gastritis [21] and neuromyelitis optica [22,23], respectively. LIPS analysis examining anti-Jo-1 antibodies revealed only 1 of the 57 SjS subjects had significant antibody titers above the control cut-off level of mean plus 3 SD (data not shown). A much higher percentage of the SjS patients, 14% (8/57), had anti-TPO antibody titers above the cut-off value of the mean plus 3 SDof the 25 control samples (Figure 4A). Testing for anti-gastric H+/K+ ATPase (beta subunit) revealed that 16% (9/57) of the SjS samples had antibody titers above the cut-off value of the mean plus 3 SD of the 25 control samples (Figure 4B). Many of these positive anti-gastric H+/K+ ATPase SjS samples showed robust values that were 15–50-fold higher than the mean of the controls (Figure 4B).
Figure 4.
LIPS detection of anti-TPO, gastric H/K ATPase and anti-AQP4 antibodies. Each circle or square symbol represents an individual normal volunteer (NV) or SjS patient sample, respectively. For determining sensitivity and specificity, the dashed line represents the cut-off level derived from mean plus 3 SD of the antibody titer of the 25 normal volunteers, while the solid line is the cut-off for the mean plus 5 SD. (A) Anti-TPO autoantibodies, (B), Anti-H + /K + gastric ATPase autoantibodies (C). Anti-AQP-4 autoantibodies.
Testing for anti-AQP-4 antibodies revealed that 12% (7/57) of the SjS patients showed positive titers using a cut-off value of the mean plus 3 SD of the 25 control samples (Figure 4C). Using this cut-off, none of the controls were positive for anti-AQP-4 antibodies (100% specificity). In contrast, LIPS assays for antibodies to two other aquaporin, AQP-1 and AQP-5, showed little immunoreactivity with no significant antibody titer differences between the SjS and control samples (data not shown). LIPS testing of an additional deletion mutant of AQP-4, missing the N-terminal 53 amino acids, failed to show immunoreactivity suggesting that antibodies to AQP-4 may be conformation dependent (data not shown). Lastly, positive AQP-4 autoantibody titers observed in the SjS samples were not simply a reflection of indiscriminate polyclonal B cell activation, since 3 of 7 anti-AQP-4 antibody positive samples were found in subjects negative for autoantibodies to La, Ro52, and TPO.
Autoantibody profiles in SjS and clinical phenotypes
A heatmap was used to more easily visualize the antibody profiles to the seven most informative autoantigens in all the different participants. For this heatmap, antibody titer values for each antigen-antibody measurement greater than the cut-off of the control mean plus 3 SDwere color coded to signify the relative number of standard deviations above these cut-off values. Analysis of the heatmap revealed several interesting findings. As expected, only a few single weak signals were detected in the normal volunteer controls (Figure 5). In contrast, the SjS patients showed a wide range of titers and heterogeneous immunoreactivity to this seven-antigen panel. The most informative SjS autoantigen was the anti-La antibody test (Figure 5). Although adding the results from the anti-Ro52 antibody test added only 1 additional SjS patient, positive anti-Ro60 antibodies were only detected in SjS samples that were already positive for anti-La antibodies (Figure 5). Anti-gastric ATPase antibodies were always found in SjS patients positive for anti-La and anti-Ro52 antibodies and 4 of the 9 anti-gastric ATPase positive samples were also positive for anti-TPO antibodies. Finally, several anti-AQP-4 and anti-TPO antibody responses were found in anti-La and anti-Ro52 antibody negative patients (Figure 5). Taken together these results, along with the titer differences, highlight the heterogeneity of autoantibody responses in SjS patients.
The antibody titer data were also analyzed to determine whether there were any antibody correlates for focus score, salivary flow, lacrimal gland function and other clinical laboratory findings. While there were no apparent correlations of any antibody titers measured with LIPS for gammopathy, anti-Ro52 antibody titers detected by LIPS weakly correlated (R = 0.56; p < 0.0001) with the amount of inflammation in the salivary gland as determined by the focus score (data not shown). Although the sample size was small, all 3 lymphoma patients showed markedly elevated anti-Ro52 antibody titers (data not shown).
Discussion
LIPS technology provided quantitative measures of autoantibody titers to 8 different human proteins. This simple modular assay system, expressing human autoantigens as a series of Ruc fusion proteins in mammalian cells followed by standardized chemiluminescent detection, efficiently evaluated patient humoral response to these different antigens. In contrast, ELISA and protein microarrays utilize a variety of antigens produced from multiple sources, varying in purity and amount, and/or under different conditions [24]. Given the substantial difference in antibody titers determined by LIPS between the SjS patients and controls, cut-off values derived from the mean of the 25 controls plus 3 or 5 SD yielded relatively similar results (see Table II).
The anti-La antibody test was the most informative achieving 75% sensitivity and 100% specificity. Incorporating anti-Ro52, anti-TPO and anti-AQP-4 antibody tests improved the sensitivity to 86%, still maintaining 96% specificity. Using LIPS, autoantibodies associated with four other autoimmune diseases were evaluated in SjS. Similar to published studies [25], only a minority of patients within our SjS cohort tested positive for anti-Jo-1 antibodies (1/57; 1.7%). The 14% rate of positive TPO autoantibodies found in SjS patients was also similar to the results found in other studies [26,27] and is consistent with autoimmune epithelitis associated with SjS [28]. The findings that some SjS patients without clinical thyroid disease have positive anti-TPO antibodies and others have only anti-AQP-4 antibodies, but undetectable anti-La, anti-Ro52/Ro60 antibodies, also supports the notion that these SjS patients do not have “classical SjS” and that SjS is a heterogeneous disease. Finally, one limitation of our study is the relatively small number of healthy control sera and lack of other rheumatological disease control sera that were tested. Future studies expanding the autoantigen panel and profiling a larger and more diverse set of samples (e.g., rheumatoid arthritis, SLE) are still needed to evaluate the diagnostic utility of these tests.
The detection rate of 75% of the primary SjS patients based upon anti-La antibodies is in sharp contrast to the 46% obtained with the ELISA. LIPS and the ELISA had similar sensitivities and specificities for SSA (Ro52 and Ro60), but it should be noted that this commercial ELISA is performed with natural, cell-derived SSA, which may contain contaminating antigens that cross-react with other autoantibodies in SjS sera. Numerous studies show that recombinant E. coli proteins employed in ELISA have notably lower sensitivity than native proteins. For example, using recombinant E. coli proteins only 55%, 30%, and 30% sensitivities were reported for detecting SjS positive anti-Ro52, anti-Ro60 and anti-La antibodies, respectively [29]. Furthermore, the detection of diagnostically useful (i.e., 65% sensitivity) antibodies to the C-terminus of Ro52 by LIPS, which were completely missed by conventional ELISAs that use recombinant bacterial proteins[17], supports the notion of the improved sensitivity and specificity of the LIPS approach over ELISA. In other commercial ELISAs currently used in most clinical laboratories, native non-human bovine SSA and SSB proteins [4] are used. It is likely that the non-human SSA and SSB antigens used in ELISA fail to present many human specific epitopes. Unlike solid phase ELISA, the solution phase LIPS assay detects many more conformational epitopes. These advantages provided by LIPS result in high sensitivity, high specificity and robust signals providing a substantial and clear distinction between positive and negative sera.
We also report for the first time that autoantibodies are frequently found in SjS patients directed against the plasma membrane-associated gastric H+/K+ ATPase. The 16% detection rate of anti-gastric antibodies found here is consistent with many clinical studies demonstrating multiple gastrointestinal manifestations in greater than 30% of SjS [30]. It should also be noted that this simple high-throughput assay for detecting anti-gastric H+/K+ ATPase autoantibodies will likely be useful for detecting these gastric autoantibodies in other autoimmune diseases including type I diabetes [31] and autoimmune gastritis [21]. Nevertheless, additional larger SjS studies are also needed to clarify whether these antibodies are associated with other clinical phenotypes such as anemia and gastric dysfunction.
Anti-AQP-4 autoantibodies are found in patients with neuromyelitis optica [22,23,32], which is characterized by autoimmune attack on the optic nerve and spinal cord. Here we report that 12% of the SjS patient had positive anti-AQP-4 antibodies, which is consistent with a recent study reporting anti-AQP-4 antibodies in a subset of SjS children showing central nervous system inflammation [33]. Although we have not tested whether these anti-AQP-4 antibody positive sera show immunoreactivity by a standard indirect immunofluorescence assay with mouse cerebellum tissue, a similar liquid phase immunoassay employing green fluorescent protein, in place of Renilla luciferase, shows high sensitivity and specificity for detecting these antibodies in patients with neuromyelitis optica [33,34]. Furthermore, these autoantibodies detected by LIPS in the SjS patients appeared to be specific for AQP-4, since no significant antibodies were detected to either AQP-1 or AQP-5 channels. Interestingly, examination of the clinical information from the seven anti-AQP-4 antibody positive SjS patients revealed that two had peripheral neuropathy; one had peripheral and autonomic neuropathy; one had trigeminal neuralgia and the two others had white matter CNS lesions. Since it has been reported that approximately 20% of SjS patients show neuropathy, optic neuropathy and autonomic nervous system dysfunction [35], future studies using a larger SjS patient cohort will address whether there is a relationship between positive anti-AQP-4 antibodies and neurologic disease in SjS patients.
Acknowledgements
We are grateful to Jason Keller for his technical assistance. We also acknowledge Dr. Kathryn Ching, Dr. Kendall Mitchell, and Brian Bates for critical reading of the manuscript. This research was supported by the Intramural Research Program of the NIDCR and, in part, by a Bench to Bedside award from the NIH Clinical Research Center.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Fox RI. Sjogren’s syndrome. Lancet. 2005;366:321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 2.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franceschini F, Cavazzana I. Anti-Ro/SSA and La/SSB antibodies. Autoimmunity. 2005;38:55–63. doi: 10.1080/08916930400022954. [DOI] [PubMed] [Google Scholar]
- 4.Kapogiannis B, Gussin HA, Teodorescu MR, Teodorescu M. Differences in clinical sensitivity of ELISA tests for autoantibodies with human and bovine extractable nuclear antigens. Lupus. 2000;9:343–352. doi: 10.1191/096120300678828398. [DOI] [PubMed] [Google Scholar]
- 5.Burbelo PD, Ching KH, Mattson TL, Light JS, Bishop LR, Kovacs JA. Rapid antibody quantification and generation of whole proteome antibody response profiles using LIPS (luciferase immunoprecipitation systems) Biochem Biophys Res Commun. 2007;352:889–895. doi: 10.1016/j.bbrc.2006.11.140. [DOI] [PubMed] [Google Scholar]
- 6.Burbelo PD, Goldman R, Mattson TL. A simplified immunoprecipitation method for quantitatively measuring antibody responses in clinical sera samples by using mammalian-produced Renilla luciferase-antigen fusion proteins. BMC Biotechnol. 2005;5:22. doi: 10.1186/1472-6750-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burbelo PD, Groot S, Dalakas MC, Iadarola MJ. High definition profiling of autoantibodies to glutamic acid decarboxylases GAD65/GAD67 in stiff-person syndrome. Biochem Biophys Res Commun. 2008;366:1–7. doi: 10.1016/j.bbrc.2007.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burbelo PD, Hirai H, Leahy H, Lernmark A, Ivarsson SA, Iadarola MJ, Notkins AL. A new luminescence assay for autoantibodies to mammalian cell-prepared insulinoma-associated protein 2. Diabetes Care. 2008;31:1824–1826. doi: 10.2337/dc08-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burbelo PD, Meoli E, Leahy HP, Graham J, Yao K, Oh U, Janik JE, Mahieux R, Kashanchi F, Iadarola MJ, Jacobson S. Anti-HTLV antibody profiling reveals an antibody signature for HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) Retrovirology. 2008;5:96. doi: 10.1186/1742-4690-5-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burbelo PD, Ramanathan R, Klion AD, Iadarola MJ, Nutman TB. Rapid, novel, specific, high-throughput assay for diagnosis of Loa loa infection. J Clin Microbiol. 2008;46:2298–2304. doi: 10.1128/JCM.00490-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramanathan R, Burbelo PD, Groot S, Iadarola MJ, Neva FA, Nutman TB. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. J Infect Dis. 2008;198:444–451. doi: 10.1086/589718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenspan JS, Daniels TE, Talal N, Sylvester RA. The histopathology of Sjogren’s syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol. 1974;37:217–229. doi: 10.1016/0030-4220(74)90417-4. [DOI] [PubMed] [Google Scholar]
- 13.Keeble AH, Khan Z, Forster A, James LC. TRIM21 is an IgG receptor that is structurally, thermodynamically, and kinetically conserved. Proc Natl Acad Sci USA. 2008;105:6045–6050. doi: 10.1073/pnas.0800159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahata M, Bohgaki M, Tsukiyama T, Kondo T, Asaka M, Hatakeyama S. Ro52 functionally interacts with IgG1 and regulates its quality control via the ERAD system. Mol Immunol. 2008;45:2045–2054. doi: 10.1016/j.molimm.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 15.James LC, Keeble AH, Khan Z, Rhodes DA, Trowsdale J. Structural basis for PRYSPRY-mediated tripartite motif (TRIM) protein function. Proc Natl Acad Sci USA. 2007;104:6200–6205. doi: 10.1073/pnas.0609174104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Eversole T, Lee DJ, Sontheimer RD, Capra JD. Protein-protein interactions between native Ro52 and immunoglobulin G heavy chain. Scand J Immunol. 1999;49:620–628. doi: 10.1046/j.1365-3083.1999.00547.x. [DOI] [PubMed] [Google Scholar]
- 17.Ottosson L, Hennig J, Espinosa A, Brauner S, Wahren-Herlenius M, Sunnerhagen M. Structural, functional and immunologic characterization of folded subdomains in the Ro52 protein targeted in Sjogren’s syndrome. Mol Immunol. 2006;43:588–598. doi: 10.1016/j.molimm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Wahren-Herlenius M, Muller S, Isenberg D. Analysis of B-cell epitopes of the Ro/SS-A autoantigen. Immunol Today. 1999;20:234–240. doi: 10.1016/s0167-5699(99)01458-9. [DOI] [PubMed] [Google Scholar]
- 19.Ascherman DP. The role of Jo-1 in the immunopathogenesis of polymyositis: current hypotheses. Curr Rheumatol Repts. 2003;5:425–430. doi: 10.1007/s11926-003-0052-2. [DOI] [PubMed] [Google Scholar]
- 20.McLachlan SM, Rapoport B. Thyroid peroxidase as an autoantigen. Thyroid. 2007;17:939–948. doi: 10.1089/thy.2007.0169. [DOI] [PubMed] [Google Scholar]
- 21.Toh BH, Gleeson PA, Simpson RJ, Moritz RL, Callaghan JM, Goldkorn I, Jones CM, Martinelli TM, Mu FT, Humphris DC. The 60- to 90-kDa parietal cell autoantigen associated with autoimmune gastritis is a beta subunit of the gastric H + /K(+)-ATPase (proton pump) Proc Natl Acad Sci USA. 1990;87:6418–6422. doi: 10.1073/pnas.87.16.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul F, Jarius S, Aktas O, Bluthner M, Bauer O, Appelhans H, Franciotta D, Bergamaschi R, Littleton E, Palace J, Seelig HP, Hohlfeld R, Vincent A, Zipp F. Antibody to aquaporin 4 in the diagnosis of neuromyelitis optica. PLoS Med. 2007;4:e133. doi: 10.1371/journal.pmed.0040133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharp V, Utz PJ. Technology insight: can autoantibody profiling improve clinical practice? Nat Clin Pract Rheumatol. 2007;3:96–103. doi: 10.1038/ncprheum0404. [DOI] [PubMed] [Google Scholar]
- 25.Feng Y, Ke X, Ma R, Chen Y, Hu G, Liu F. Parallel detection of autoantibodies with microarrays in rheumatoid diseases. Clin Chem. 2004;50:416–422. doi: 10.1373/clinchem.2003.023994. [DOI] [PubMed] [Google Scholar]
- 26.Ramos-Casals M, Garcia-Carrasco M, Cervera R, Gaya J, Halperin I, Ubieto I, Aymami A, Morla RM, Font J, Ingelmo M. Thyroid disease in primary Sjogren syndrome. Study in a series of 160 patients. Medicine. 2000;79:103–108. doi: 10.1097/00005792-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Ruggeri RM, Galletti M, Mandolfino MG, Aragona P, Bartolone S, Giorgianni G, Alesci D, Trimarchi F, Benvenga S. Thyroid hormone autoantibodies in primary Sjogren syndrome and rheumatoid arthritis are more prevalent than in autoimmune thyroid disease, becoming progressively more frequent in these diseases. J Endocrinol Invest. 2002;25:447–454. doi: 10.1007/BF03344036. [DOI] [PubMed] [Google Scholar]
- 28.Moutsopoulos HM, Manoussakis MN. Lumping or splitting autoimmune rheumatic disorders? Lessons from Sjogren’s syndrome. Br J Rheumatol. 1998;37:1263–1264. doi: 10.1093/rheumatology/37.12.1263. [DOI] [PubMed] [Google Scholar]
- 29.Garberg H, Jonsson R, Brokstad KA. The serological pattern of autoantibodies to the Ro52, Ro60, and La48 autoantigens in primary Sjogren’s syndrome patients and healthy controls. Scand J Rheumatol. 2005;34:49–55. doi: 10.1080/03009740510017940. [DOI] [PubMed] [Google Scholar]
- 30.Sheikh SH, Shaw-Stiffel TA. The gastrointestinal manifestations of Sjogren’s syndrome. Amer J Gastroenterol. 1995;90:9–14. [PubMed] [Google Scholar]
- 31.Riley WJ, Toskes PP, Maclaren NK, Silverstein JH. Predictive value of gastric parietal cell autoantibodies as a marker for gastric and hematologic abnormalities associated with insulin-dependent diabetes. Diabetes. 1982;31:1051–1055. doi: 10.2337/diacare.31.12.1051. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi T, Fujihara K, Nakashima I, Misu T, Miyazawa I, Nakamura M, Watanabe S, Shiga Y, Kanaoka C, Fujimori J, Sato S, Itoyama Y. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. 2007;130:1235–1243. doi: 10.1093/brain/awm062. [DOI] [PubMed] [Google Scholar]
- 33.McKeon A, Lennon VA, Lotze T, Tenenbaum S, Ness JM, Rensel M, Kuntz NL, Fryer JP, Homburger H, Hunter J, Weinshenker BG, Krecke K, Lucchinetti CF, Pittock SJ. CNS aquaporin-4 autoimmunity in children. Neurology. 2008;71:93–100. doi: 10.1212/01.wnl.0000314832.24682.c6. [DOI] [PubMed] [Google Scholar]
- 34.Waters P, Vincent A. Detection of anti-aquaporin-4 antibodies in neuromyelitis optica: current status of the assays. InterMSJ/MS Forum. 2008;15:99–105. [PubMed] [Google Scholar]
- 35.Barendregt PJ, van den Bent MJ, van Raaij-van den Aarssen VJ, van den Meiracker AH, Vecht CJ, vanderHeijde GL, Markusse HM. Involvement of the peripheral nervous system in primary Sjogren’s syndrome. Ann Rheum Dis. 2001;60:876–881. [PMC free article] [PubMed] [Google Scholar]