Abstract
Although liver transplantation is a widely accepted treatment for hepatocellular carcinoma (HCC), much controversy remains and there is no generally accepted set of guidelines. An international consensus conference was held on Dec 2–4, 2010, in Zurich, Switzerland, with the aim of reviewing current practice regarding liver transplantation in patients with HCC and to develop internationally accepted statements and guidelines. The format of the conference was based on the Danish model. 19 working groups of experts prepared evidence-based reviews according to the Oxford classification, and drafted recommendations answering 19 specific questions. An independent jury of nine members was appointed to review these submissions and make final recommendations, after debates with the experts and audience at the conference. This report presents the final 37 statements and recommendations, covering assessment of candidates for liver transplantation, criteria for listing in cirrhotic and non-cirrhotic patients, role of tumour downstaging, management of patients on the waiting list, role of living donation, and post-transplant management.
Introduction
Although liver transplantation was first done in human beings by Tom Starzl in 1963, the procedure did not begin to gain wide acceptance until the mid-1980s, when effective immunosuppression with ciclosporin became available. Currently, overall 1-year and 5-year survival after liver transplantation exceeds 85% and 70%, respectively, in most centres.1,2
Hepatocellular carcinoma (HCC) is a major health problem worldwide, which continues to increase because of the association of HCC with hepatitis B and C viruses. HCC was one of the first indications for liver transplantation, because it was postulated that this approach would eliminate the tumour and cure the underlying liver disease. However, it soon became apparent that the success of liver transplantation depends on the tumour load; patients with extensive disease had very poor outcomes, whereas most patients with small tumours could be cured. This led to many controversies around the use of liver transplantation in patients with HCC, such as the selection of patients in the context of worldwide organ shortage, control of the tumour load while patients wait for a graft, use of living donors, and the choice of immunosuppression or adjuvant therapies.
The goal of liver transplantation, regardless of the underlying disease, is providing liver recipients with the maximum benefit possible from the limited resource of deceased and living donor organs, in a fair, ethical, and cost-effective manner. Thus, indications for the procedure and allocation of donor organs are closely scrutinised by all stakeholders in liver transplantation. With the endorsement of most international societies concerned with liver transplantation or HCC, we organised a conference that aimed to reach wide consensus throughout the medical and non-medical population on various aspects of the use of liver transplantation for patients with HCC, based on the best available evidence.
Methods
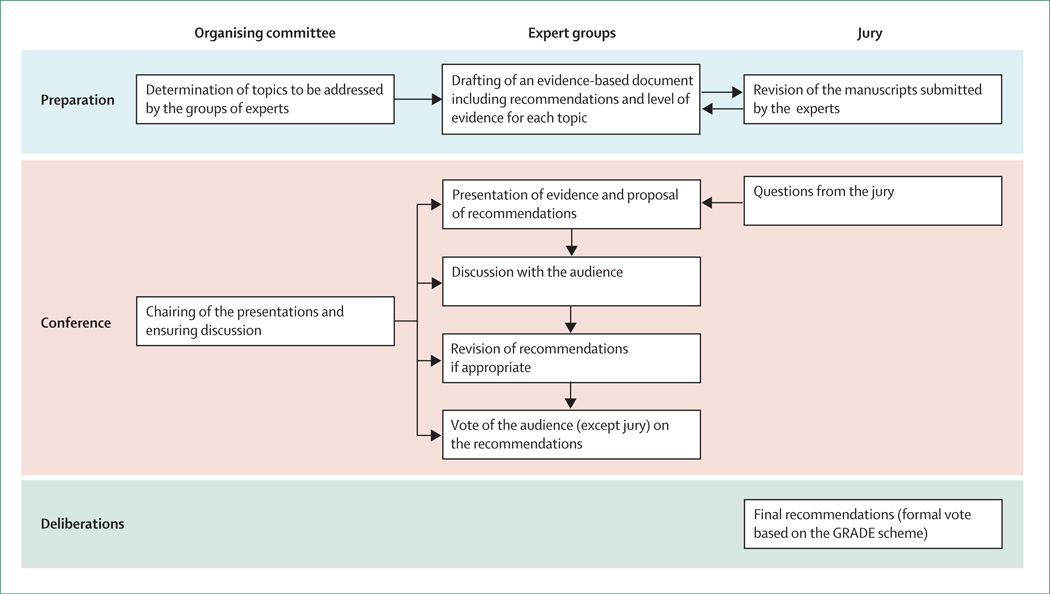
An international consensus conference on liver transplantation for HCC was held on Dec 2–4, 2010, in Zurich, Switzerland, under the auspices of ten international societies focused on liver diseases or transplantation, with the aim of establishing a consensus regarding indications for liver transplantation in patients with HCC and to provide internationally accepted statements and guidelines for the conduct of liver transplantation programmes. For this purpose, we developed a novel format based on the Danish model (figure).3 The organising committee identified key topics and appointed 19 working groups composed of four to six experts from various areas of medicine, selected on the basis of their scientific and clinical records, to prepare reviews of the evidence and draft recommendations. Working groups were asked to follow the Oxford classification of levels of evidence.4 The material prepared by the various panels is available as a supplement of Liver Transplantation.5–24 Each working group did an English language literature search of Pubmed, Embase, and Scopus databases, and the Cochrane central register of controlled trials. Text, keywords, and medical subject heading terms were used for titles and abstracts. Manual cross-referencing was also used to find further relevant articles. Search terms and dates varied according to topic (webappendix). A jury of nine members, who were selected from various clinical and academic areas and who were not actively involved in liver transplantation, was appointed to review and comment on the submitted papers and to finalise and grade the final recommendations.
Figure 1. Format of the HCC consensus conference.
The role of the organising committee, expert groups, and jury at each step of the conference. HCC=hepatocellular carcinoma. GRADE=grading of recommendations assessment, development, and evaluation.
Around 300 participants from five continents attended the meeting in Zurich. Each of the 19 working groups, assisted by members of the jury, prepared statements associated with a level of evidence from their review of the literature. The chair of each working group gave a 15-min presentation covering specific questions, followed by questions from the jury and the audience. The prepared statements could be modified in real time according to the debates during the conference. Then, a poll of the audience was obtained through an anonymous electronic voting system to inform the jury of the strength of consensus.
The jury met independently after the conference to produce the final recommendations, based on the expert reports, debates, and attendee voting. The jury concluded by voting to assign a level of evidence for each recommendation, according to the Oxford classification, with respect to diagnosis, prognosis, or therapy. They also determined the strength of each recommendation according to the grading of recommendations assessment, development, and evaluation system (GRADE).25 From the initial statements and recommendations proposed by the panels of experts, 27 were dropped or merged with other statements or recommendations, ten were left unchanged, and another 27 were reworded. Among the definitive 37 statements and recommendations, the level of evidence proposed by the experts was modified in three cases by the jury after discussion with the statistician (PMMB).
The present review was prepared by a writing committee, which included the president (AP), vice president (BL), and statistician (PMMB) of the jury, along with three members of the organising committee (ML, GJG, P-AC). The manuscript was circulated among the working groups for confirmation of accuracy of the data with no possibility to alter the recommendations. The final version was approved by each member of the jury. The recommendations are presented in table 1.
Table 1.
Summary of recommendations and statements
| Level of evidence |
Strength of recommendation |
|
|---|---|---|
| Assessment of candidates with HCC for liver transplantation | ||
| 1. When considering treatment options for patients with HCC, the BCLC staging system is the preferred staging system to assess the prognosis of patients with HCC | 2b (P) | Strong |
| 2. The TNM system (7th edn) including pathological examination of the explanted liver, should be used for determining prognosis after transplantation with the addition of assessment of microvascular invasion | 2b (P) | Strong |
| 3. Either dynamic CT or dynamic MRI with the presence of arterial enhancement followed by washout on portal venous or delayed imaging is the best non-invasive test to make a diagnosis in cirrhotic patients suspected of having HCC and for preoperative staging | 1b (D) | Strong |
| 4. Extrahepatic staging should include CT of the chest, and CT or MRI of the abdomen and pelvis | 3b (D) | Strong |
| 5. Tumour biopsy is not required in cirrhotic patients considered for liver transplantation who have high-quality dynamic CT or MRI findings typical for HCC and a lesion larger than 1 cm according to current AASLD guidelines | 1b (D) | Weak |
| 6. For patients with lesions smaller or equal to 10 mm, non-invasive imaging does not allow an accurate diagnosis and should not be used to make a decision for or against transplantation | 1b (D) | Strong |
| Criteria for listing candidates with HCC in cirrhotic livers for DDLT | ||
| 7. Liver transplantation should be reserved for HCC patients who have a predicted 5-year survival comparable to non-HCC patients | NA | Weak |
| 8. Preoperative assessment of the size of the largest tumour or total diameter of tumours should be the main consideration in selecting patients with HCC for liver transplantation | 2a (P) | Strong` |
| 9. The Milan criteria are currently the benchmark for the selection of HCC patients for liver transplantation, and the basis for comparison with other suggested criteria | 2a (P) | Strong |
| 10. A modest expansion of the number of potential candidates may be considered on the basis of several studies showing comparable survival for patients outside the Milan criteria | 3b (P) | Weak |
| 11. Patients with worse prognosis may be considered for liver transplantation outside the Milan criteria if the dynamics of the waiting list allow it without undue prejudice to other recipients with a better prognosis | NA | Weak |
| 12. α-fetoprotein concentrations add prognostic information in HCC patients and may be used for making decisions regarding transplantation in combination with imaging criteria | 2b (P) | Weak |
| 13. Biomarkers other than α-fetoprotein cannot yet be used for clinical decision making regarding liver transplantation for HCC | 2b (P) | Strong |
| 14. Indication for liver transplantation in HCC should not rely on microvascular invasion because it cannot be reliably detected prior to transplantation | 2b (P) | Strong |
| Criteria for HCC candidates with non-cirrhotic livers | ||
| 15. The Milan criteria and its modifications are not applicable to patients with HCC developing in a non-cirrhotic liver. Such patients with non-resectable HCC and absence of macrovascular invasion and extrahepatic spread may be considered as appropriate candidates for liver transplantation | 4 (P) | Weak |
| 16. Patients with HCC in non-cirrhotic liver who were treated by resection, and have intrahepatic recurrence of HCC and no evidence of lymph node or macrovascular invasion, may be considered for salvage transplantation | 4 (P) | Weak |
| Role of downstaging | ||
| 17. Transplantation may be considered after successful downstaging | 5 (P) | Weak |
| 18. Liver transplantation after successful downstaging should achieve a 5-year survival comparable to that of HCC patients who meet the criteria for liver transplantation without requiring downstaging | 5 (P) | Strong |
| 19. Criteria for successful downstaging should include tumour size and number of viable tumours | 4 (P) | Strong |
| 20. α-fetoprotein concentrations before and after downstaging may add additional information | 4 (P) | Weak |
| 21. Based on existing evidence, no recommendation can be made for preferring a specific locoregional therapy for downstaging over others | NA | None |
| Managing patients on the waiting list | ||
| 22. Periodic waiting-list monitoring should be performed by imaging (dynamic CT, dynamic MRI, or contrast-enhanced ultrasonography) and α-fetoprotein measurements | 5 (P) | Strong |
| 23. Based on current absence of evidence, no recommendation can be made on bridging therapy in patients with UNOS T1 (≤2 cm) HCC | NA | None |
| 24. In patients with UNOS T2 (one nodule 2–5 cm or three or more nodules each ≤3 cm) HCC (Milan criteria) and a likely waiting time longer than 6 months, locoregional therapy may be appropriate | 4 (P) | Weak |
| 25. No recommendation can be made for preferring any type of locoregional therapy to others | NA | None |
| 26. Patients found to have progressed beyond criteria acceptable for listing for liver transplantation should be placed on hold and considered for downstaging | 5 (P) | Strong |
| 27. Patients with progressive disease in whom locoregional intervention is not considered appropriate, or is ineffective, should be removed from the waiting list | 5 (P) | Strong |
| Role of LDLT | ||
| 28. LDLT is acceptable for HCC patients who have an expected 5-year survival similar to comparably staged patients receiving a deceased donor liver. In LDLT, careful attention should be given to psychosocial considerations regarding both donor and recipient | NA | Weak |
| 29. LDLT must be restricted to centres of excellence in liver surgery and liver transplantation to minimise donor risk and maximise recipient outcome | NA | Strong |
| 30. In patients following LDLT for HCC within the accepted regional criteria for DDLT, retransplantation for graft failure is justified | 5 (P) | Weak |
| 31. In patients following LDLT for HCC outside the accepted regional criteria for DDLT, retransplantation for graft failure using a deceased donor organ is not recommended | 5 (P) | Strong |
| Post-transplant management | ||
| 32. Post-transplant monitoring may include 6–12 monthly contrast-enhanced CT or MRI imaging and α-fetoprotein measurements | 5 (P) | Weak |
| 33. There is currently insufficient evidence from clinical trials to base a recommendation for choosing the type or dose of immunosuppression therapy to influence the incidence of HCC recurrence or its prognosis | NA | None |
| 34. Based on current evidence, no recommendation can be made on the use of mTOR inhibitors solely to reduce the risk of HCC recurrence outside clinical trials | NA | None |
| 35. The current evidence does not justify the routine use of adjuvant antitumour therapy after liver transplantation for HCC outside of a controlled clinical trial | NA | Weak |
| 36. HCC recurrence after liver transplantation may be treated by surgery for resectable lesions or by locoregional therapy or systemic therapy (including sorafenib) for unresectable lesions | 4 (P) | Weak |
| 37. Liver retransplantation is not appropriate treatment for recurrent HCC | NA | Strong |
Level of evidence for each recommendation refers to the Oxford classification.4 HCC=hepatocellular carcinoma. BCLC=Barcelona Clinic Liver Cancer. TNM=tumour, node, metastasis. P=prognosis. D=diagnosis. AASLD=American Association for the Study of Liver Diseases. NA=not applicable. OLT=orthotopic liver transplantation. UNOS=United Network for Organ Sharing. LDLT=living donor liver transplantation. DDLT=deceased donor liver transplantation.
Assessment of candidates with HCC for liver transplantation
The purpose of cancer staging is to accurately predict prognosis and to link tumour stage with specific therapeutic interventions. The ideal staging system for HCC should take into account tumour stage, liver function, and functional status of the patient. Several staging systems have been developed over the past three decades, although none has gained worldwide acceptance (table 2).26–34 The Barcelona Clinic Liver Cancer (BCLC) and Cancer of the Liver Italian Program (CLIP) staging systems have been the most popular in Europe and the USA, and the Japan Integrated Staging Score (JIS) in Japan. The BCLC is the only system that links prognosis with treatment recommendations, and thereby was selected in several major trials of HCC therapy. For example, the role of portal hypertension in selecting patients for major liver resection versus liver transplantation has been validated in Japan35 and the USA.36
Table 2.
Staging systems for stratification of patients with hepatocellular carcinoma
| TNM (7th ed) | Okuda | CLIP | BCLC | JIS | GRETCH | CUPI | Tokyo | |
|---|---|---|---|---|---|---|---|---|
| Year introduced | 2010 | 1985 | 1998 | 1999 | 2003 | 1999 | 2002 | 2005 |
| Liver function | No | Bilirubin, albumin, ascites | CTP class | CTP class, portal hypertension | CTP class | Bilirubin, alkaline phosphatase | Bilirubin, alkaline phosphatase, ascites | Bilirubin, albumin |
| Performance status | No | No | No | Yes, ECOG status | No | Yes, Karnofsky | No | |
| Tumour burden | Number of nodules, tumour size, PV invasion, metastasis | Tumour > or <50% of liver | Tumour > or <50% of liver, PV invasion, metastasis | Number of nodules, tumour size, PV invasion, metastasis | TNM stage, define by LCSGJ | PV thrombosis | TNM stage | Number of nodules, tumour size |
| α-fetoprotein concentration | No | No | Yes, cutoff 400 ng/mL | No | No | Yes, cutoff 25 ng/mL | Yes, cutoff 500 ng/mL | No |
| Staging categories | I, II, III, IV | Score 0–4; stage I, II, III | 0–6 | 0, A, B, C, D | 0–5 | Score 0–3 risk groups | Score 0–12; three risk groups | 0–8 |
| Other parameters | +/− symptom disease |
The Up-to-7 criteria system was not selected since it does not include clinical performance, liver function, or tumour markers. Furthermore, since the system is dependent on microvascular invasion, it is not clinically applicable. TNM=tumour, node, metastasis. CLIP=Cancer of the Liver Italian Program. BCLC=Barcelona Clinic Liver Cancer. JIS=Japan Integrated Scoring. GRETCH=Groupe d’Etude et de Traitement du Carcinoma Hepatocellulaire. CUPI=Chinese University Prognostic Index. CTP=Child-Pugh-Turcotte. ECOG=Eastern Cooperative Oncology Group. PV=portal vein. LCSGJ=Liver cancer study group of Japan.
Pathological features in the explanted liver that have prognostic value for staging HCC include tumour size and number of nodules, satellite lesions, vascular invasion (macroscopic or microscopic), and lymph-node metastases. However, staging systems to guide therapy before liver transplantation are necessary and are mainly based on imaging information. Preoperative and post-transplant liver staging systems can be used in assessing outcome. This led to recommendations 1 and 2: (1) when considering treatment options for patients with HCC, the BCLC staging system is the preferred staging system to assess the prognosis of patients with HCC; (2) the TNM system (7th edn), including pathological examination of the liver, should be used for determining the prognosis after transplantation with the addition of the assessment of microvascular invasion.
Despite incremental technological advances in cross-sectional imaging techniques (ultrasonography, CT, and MRI), standard imaging methods can underestimate or overestimate the extent of HCC in up to 25% of cases, compared with pathological findings of the explanted liver.18 Recent reports suggest that either dynamic CT or MRI, including unenhanced, arterial, portal venous, and delayed phases, provide improved sensitivity and specificity compared with standard techniques of the past. Currently, there is no data showing the superiority of either MRI or CT. Conclusive imaging features rely on the presence of arterial enhancement followed by washout on portal venous or delayed imaging.37 Such examinations should follow established protocols, which define the amount and rate of contrast given, the precise individualised timing of image acquisition, and image reconstruction with minimum slice thickness. Dynamic ultrasonography has improved the accuracy of ultra sonography, but is less useful than CT or MRI because of the inability to reliably acquire images of the entire liver during a particular contrast phase. Bone scintigraphy has been used for evaluating bone metastases; however, the technique is poor in terms of cost-effectiveness when used routinely. There is insufficient data to propose 18F-fluorodeoxyglucose (FDG)-PET for staging HCC before liver transplantation. These finding are summarised in recommendations 3 and 4: (3) either dynamic CT or dynamic MRI with the presence of arterial enhancement followed by washout on portal venous or delayed imaging is the best non-invasive test to make a diagnosis in cirrhotic patients suspected of having HCC and for preoperative staging; (4) extrahepatic staging should include CT of the chest, and CT or MRI of the abdomen and pelvis.
Because of improvements in the accuracy of non-invasive imaging, the need for liver biopsy in the work-up of cirrhotic patients with HCC being considered for liver transplantation has changed in recent years. The specificity of liver biopsy is close to 100%, but sensitivity varies depending on location of the tumour, needle size (86–90% with an 18 gauge cutting needle, 67% with 21–22 gauge needle), and tumour size (>90% for nodules >1 cm vs 83% for nodules <1 cm). A positive tumour biopsy is clinically relevant to rule in a diagnosis of HCC, but a negative biopsy is less useful. There is also the risk of tumour seeding after liver biopsy, which has been reported to be 2·7% (95% CI 1·8–4·0) with a median time interval between biopsy and seeding of 17 months (IQR 7–48).38
The American Association for the Study of Liver Disease (AASLD) has proposed an algorithm for diagnosis of HCC based on availability of state-of-the-art CT or MRI.39 These data are summarised as recommendations 5 and 6: (5) tumour biopsy is not required in cirrhotic patients considered for liver transplantation who have high-quality dynamic CT or MRI findings typical for HCC and a lesion larger than 1 cm according to current AASLD guidelines; (6) for patients with lesions smaller or equal to 10 mm or atypical findings, non-invasive imaging does not allow an accurate diagnosis, and should not be used to make a decision for or against transplantation.
Criteria for listing candidates with HCC in cirrhotic livers for deceased-donor liver transplantation
In the context of shortage of available grafts, decisions have to take into account the collective benefit of all potential liver recipients, in addition to the benefit for the individual patient. Liver transplantation achieves excellent results in patients with limited tumour load. Patients with solitary HCC of less than 5 cm or with up to three nodules of less than 3 cm (the Milan criteria40) have a 5-year survival of 70% after liver transplantation, with recurrence in less than 10%. This survival matches post-transplant survival of most other indications for liver transplantation.1,2
In the context of organ shortage for liver transplantation, an extension of the boundaries of transplantation for HCC must take into account the benefit for individual HCC patients as well as the consequences for all potential liver recipients. Whether, and to what extent, post-transplant survival could be lowered, because of expansion of the HCC receiver pool, while remaining acceptable, depends on the effect on mortality for non-HCC patients awaiting transplantation. Even though a survival opportunity considerably lower than that achieved in non-HCC patients might be considered worth the risk of surgery for some patients with HCC, the negative effects on others on the donor list must be taken into consideration. This led to recommendation 7: liver transplantation should be reserved for HCC patients who have a predicted 5-year survival comparable to non-HCC patients.
Among criteria used for listing patients with HCC, gross features of tumours are important. Burroughs and colleagues41 did a meta-analysis of 101 studies assessing the effect of HCC staging in terms of size and number of nodules on post-transplant recurrence and survival. 74 of the studies, comprising 22 432 patients, were used for quantitative analysis. There was variability in the reporting of tumour characteristics, with information missing for time on the waiting list, immunosuppression, and bridging therapy. Two-thirds of the studies were based on explant findings, and few compared pretransplant imaging with explant data. If multiple versus single nodules were considered, the definition of multiple was poorly reported. Burroughs and colleagues41 concluded that assessment of the diameter of the largest nodule or total diameter of nodules was the best predictor of outcome, and that total tumour size (sum of diameters) of 10 cm or larger (vs <10 cm) was associated with a four-times increased risk of death or recurrence. Measurement of volume might be a better way to assess tumour burden, but the data to support this are not yet available.42 A study using the Organ Procurement and Transplant Network (OPTN) database suggested that a total tumour volume cutoff at 115 cm3 and α-fetoprotein concentration higher than 400 ng/mL could discriminate between patients with acceptable outcome and those with poor outcome.43 There is insufficient evidence regarding the effect of number of nodules on outcome of liver transplantation.
This led to recommendation 8: preoperative assessment of the size of the largest tumour or total diameter of tumours should be the main consideration in selecting HCC patients for liver transplantation. In 1996, after a period of unrestricted indications for liver transplantation, associated with overall 5-year survival below 50%, the Milan criteria were introduced. These criteria set restrictive limits for size and number of tumours in candidates for liver transplantation.40 Mazzaferro and colleagues44 did a systematic review that included 90 studies, comprising 17 780 patients over 15 years (1612 patients in level 1b studies, 16 043 in level 2 studies, and 125 in level 4 studies). Their review showed that the Milan criteria are an independent prognostic factor for outcome after liver transplantation. Application of the Milan criteria for patients with HCC with chronic hepatitis and cirrhosis achieves similar post-transplant survival to that in non-HCC indications. In the European Liver Transplant Registry (ELTR), Organ Procurement and Transplantation Network (OPTN), and Australia and New Zealand Liver Transplant Registry (ANZLTR), 5-year survival for non-HCC was 65–87%.1,2,45 The jury concluded with recommendation 9: the Milan criteria are currently the benchmark for selection of HCC patients for liver transplantation, and the basis for comparison with other suggested criteria.
As evidence accumulated of good outcomes in some patients outside the Milan criteria, there was a drive to identify expanded criteria and to increase the number of eligible candidates for liver transplantation. Among the many proposals, only the University of California San Francisco (UCSF) criteria (one tumour ≤6·5 cm, three nodules at most with the largest ≤4·5 cm, and total tumour diameter ≤8 cm) have been prospectively validated by the proponent group, with outcome data comparable to those from other retrospective studies.46,47 This is summarised in recommendation 10: a modest expansion of the number of potential candidates may be considered on the basis of several studies showing comparable survival for patients outside the Milan criteria.
It is essential to consider how expansion of criteria beyond the Milan criteria might affect the survival of candidates for liver transplantation who do not have HCC. According to studies based on Markov models48 using data from the USA, patients outside the Milan criteria would need to achieve 5-year survival of 60% or higher to prevent a substantial decrement to the life-years available to the entire population of candidates for liver transplantation. The effect on non-HCC patients could vary widely, depending on the composition of the population on the waiting list and the scarcity of available donor livers in a transplant region and in individual centres. Any decision by a centre to expand criteria should take into account the current mortality on the waiting list, and should only be done if a low mortality will not be substantially increased by additional expanded-criteria cases. For example, a centre with very low or no mortality on the waiting list might expand their criteria for patients with HCC with lower post-transplant survival than that expected for non-HCC candidates, whereas such strategy would not be acceptable in another centre with a high death rate on the waiting list. Thus, the jury proposed recommendation 11: patients with a worse prognosis may be considered for liver transplantation outside the Milan criteria if the dynamics on the waiting list allow it without undue prejudice to other recipients with a better prognosis.
Since gross features of tumours are unable to define subclasses of patients with better biology and outcome, several biomarkers have been studied—eg, G3, EpCAM, miR26, and poor-survival gene signatures. However, these biomarkers have not been studied in the setting of liver transplantation.49 Several studies highlighted the value of preoperative α-fetoprotein concentrations in predicting outcome after liver transplantation,43,50,51 but there is no agreement on the cutoff values to consider, which ranged from 200 to 1000 ng/mL. A recent study using the Scientific Registry of Transplant Recipients (SRTR) data suggested adding α-fetoprotein concentration to total tumour volume as a predictor of outcome after liver transplantation.43 In several studies, α-fetoprotein concentration lower than 400 ng/mL has been used in selecting patients for liver transplantation after downstaging protocols, in addition to criteria based on tumour size and number.52 Another group found that dynamic changes in α-fetoprotein concentrations higher than 15 ng/mL are the most relevant preoperative predictor of recurrence and overall survival after orthotopic liver transplantation.50 Recommendations 12 and 13 summarise this topic: (12) α-fetoprotein concentrations add prognostic information in HCC patients and may be used for making decisions regarding transplantation in combination with imaging criteria; (13) biomarkers other than α-fetoprotein cannot yet be used for clinical decision making regarding liver transplantation for HCC.
HCC often involves branches of portal or hepatic veins (or both), causing a tumour thrombus. Vascular invasion of HCC affects prognosis after liver transplantation.53–55 Macrovascular invasion involves branches of portal or hepatic veins, or both. It can be detected using high-quality imaging and in a pathological specimen, and is generally considered an absolute contraindication for liver transplantation. Micro vascular invasion, which is identified only by microscopic observation, is associated with poorer outcome or increased recurrence rates after liver transplantation. Although it correlates with larger tumour size (>3 cm), multiple nodules, histological grade (moderately vs poor differentiated), and gross features such as confluent multinodular type,56 microvascular invasion cannot be reliably detected before transplantation and is therefore not useful for clinical decision making.
Conventional imaging modalities are ineffective for preoperative detection of microvascular invasion. Only one study suggested a value for PET in predicting microvascular invasion.57 Several studies from Japan suggested that serum concentrations of des-gamma-carboxyprothrombin (also known as protein induced by vitamin K absence [PIVKA]) correlate well with microvascular invasion.58,59 Positive and negative correlations of α-fetoprotein with microvascular invasion have been reported. The available data led to recommendation 14: indication for liver trans plantation in HCC should not rely on microvascular invasion because it cannot be reliably detected prior to transplantation.
Criteria for HCC candidates with non-cirrhotic livers
Although most HCC occurs in patients with liver cirrhosis, about 10% of cases arise in absence of cirrhosis. In such patients, diagnosis is often made at an advanced stage. Resection is currently the preferred therapeutic option, when feasible, since patients with good liver reserve have high tolerance for extensive liver resection.60 However, as in cirrhotic patients, the risk of local recurrence is high, ranging from 30 to 73%, and affects 5-year overall survival (25–81%) and disease-free survival (24–54%).61,62 Several pathological features are associated with poor prognosis, such as the presence of satellite or multiple nodules, microvascular and macrovascular invasion, and R1 resection. The subtype of fibrolamellar HCC seems to have similar outcomes.63
Data from the ELTR suggest that liver transplantation might be appropriate in some non-cirrhotic patients with recurrent HCC after resection, with long-term survival approaching 60% at 5 years.64 In this study, the absence of cirrhosis or fibrosis, and seronegativity for hepatitis C and hepatitis B virus was confirmed in all patients. Macrovascular and lymph-node invasion, and early recurrence (<1 year) after resection were significant risk factors for poor outcome. Tumour diameter and Milan criteria of the initial HCC were unrelated to outcome.
There are few reports of cases of primary liver transplantation in non-cirrhotic patients with HCC.64,65 The data available suggest that the risk factors for poor outcomes after liver transplantation are the same as those identified for recurrent disease after resection. Patients without these risk factors had a 5-year survival of 67%, even though most had tumours outside the Milan criteria. This information led to recommendations 15 and 16: (15) the Milan criteria and its modifications are not applicable to patients with HCC developing in a non-cirrhotic liver. Such patients with non-resectable HCC and absence of macrovascular invasion and extrahepatic spread may be considered as appropriate candidates for liver transplantation; (16) patients with HCC in a non-cirrhotic liver, who were treated by resection and have intrahepatic recurrence of HCC and no evidence of macrovascular invasion or extrahepatic spread, may be considered for salvage transplantation.
Role of downstaging of HCC
The goal of downstaging using locoregional therapy—eg, alcohol injection, radiofrequency ablation (RFA), transarterial chemoembolisation (TACE), transarterial radioembolisation (TARE), or liver resection—is to decrease the tumour size and number in patients initially presenting with tumours that do not meet locally acceptable criteria for liver transplantation.
Success in downstaging has been reported in many studies, although most of these are uncontrolled observational studies with no method emerging as superior and each carrying some risk. The largest experience is with TACE and RFA.66 Two prospective studies showed that survival after liver transplantation in patients with large tumour burden successfully treated by downstaging was similar to survival in patients who initially met the criteria for transplantation.52,67 These issues are addressed in recommendations 17 and 18: (17) transplantation may be considered after successful downstaging; (18) liver transplantation after successful downstaging should achieve a 5-year survival comparable to that of HCC patients who meet the criteria for liver transplantation without requiring downstaging.
There is debate about how best to assess successful downstaging. European Association for the Study of the Liver (EASL) guidelines suggest that such assessment should be exclusively based on the amount of viable tumour, as differentiated from necrosis by contrast CT or MRI.68 Most available reports have used the Milan criteria to define successful downstaging.52,67,69 There is currently no well defined upper limit for size and number of lesions as eligibility criteria for downstaging, although the presence of vascular invasion and extrahepatic disease are generally considered absolute con train dications. Some groups add serum α-fetoprotein concentrations for assessment of downstaging; however, there is no agreement on a threshold. Recommendations 19 and 20 address downstaging: (19) criteria for successful downstaging should include tumour size and number of viable tumours; (20) α-fetoprotein concentrations before and after downstaging may add additional information.
Once a tumour has been successfully downstaged to within acceptable criteria, a minimum observation period of 3 months is often recommended before considering liver transplantation. It is crucial to define criteria for failure of downstaging, which may include, before listing: failure to achieve listing criteria, tumour progression with development of vascular invasion, extrahepatic spread, or tumour size and number remaining beyond inclusion criteria; and, after listing: tumour progression requiring delisting and recurrence of HCC after liver transplantation. This consideration led to statement 21: based on existing evidence, no recommendation can be made for preferring a specific locoregional therapy for downstaging over others.
Managing patients on the waiting list
With increases in waiting times for liver transplantation in many centres, it has become common practice to monitor patients with HCC to ensure that they remain within the acceptability criteria for liver transplantation. Strategies have also been developed to treat patients whose HCC is at risk or shows signs of progression while waiting for a graft. There is no agreement about specific timing or optimum imaging methods to use for these patients, although a 3-month interval is common.
Recommendations are based on the known capabilities of the imaging methods used for diagnosis and staging of HCC (see recommendations 3 and 12). There are also reports showing that a rise in α-fetoprotein concentration is associated with poorer outcome after liver transplantation, and of reduction in that risk by successful locoregional therapy. This information is reflected in recommendation 22: periodic waiting-list monitoring should be performed by imaging (dynamic CT, dynamic MRI, or contrast-enhanced ultrasonography) and serum α-fetoprotein measurements.
Dropout of HCC patients on the waiting list is common because of cancer progression or other medical reasons. Locoregional therapy as a bridging strategy for patients on the waiting list aims to decrease tumour-related dropout rates and reduce the incidence of recurrent diseases after liver transplantation. Several cohort studies and a preliminary analysis of large registries suggest that bridging strategies with locoregional therapy are likely to be beneficial for patients having to wait 6 months or longer.70 There is, however, no evidence that bridging strategies are of any benefit in patients with United Network for Organ Sharing (UNOS) T1 tumours (<2 cm). Bridging strategies might be appropriate for patients with UNOS T2 lesions (one nodule 2–5 cm or three or fewer nodules each ≤3 cm) who are likely to wait 6 months or longer. The presence of larger tumours and high α-fetoprotein concentrations seem to predict a higher risk for dropout.
Although the most popular bridging strategy is TACE, pathological studies show a marginal advantage for RFA in terms of tumour necrosis.71,72 Liver resection before transplantation in patients with well preserved liver function, and newer strategies such as a combination of TACE with RFA and use of 90yttrium radioembolisation or targeted therapies, have shown some benefits in preliminary studies. This topic is summarised by the following three statements from the jury: (23) based on current absence of evidence, no recommendation can be made on bridging therapy in patients with UNOS T1 (≤2 cm) HCC; (24) in patients with UNOS T2 (one nodule 2–5 cm or three or fewer nodules each ≤3 cm) HCC (Milan criteria) and a likely waiting time of longer than 6 months, locoregional therapy may be appropriate; (25) no recommendation can be made for preferring one type of locoregional therapy to others. The topic also generated two additional recommendations: (26) patients found to have progressed beyond criteria acceptable for listing for liver transplantation should be placed on hold and considered for downstaging; (27) patients with progressive disease, in whom locoregional therapy intervention is not considered appropriate or is ineffective, should be removed from the waiting list.
Role of living-donor liver transplantation for HCC
Living-donor liver transplantation (LDLT) using the right or left hemiliver of a healthy donor is the only option for liver transplantation in some countries, particularly in Asia, where there is limited or no availability of deceased-donor organs. LDLT has also been used in other countries with well established programmes for organ donation from brain dead or non-heart-beating donors, because of organ shortage, long waiting times associated with deaths on the waiting list, drop-out due to medical reasons, or progression of tumours beyond acceptable criteria.
The main issue in LDLT is donor safety, because of the risk of complications or death, even if small. The concept of double equipoise was proposed to describe the balance between the recipient’s survival benefit with LDLT and the risk of a complication or death of a healthy donor.73 The risks and benefits need to be openly discussed and understood by all parties involved in such cases, and meet the test of equipoise.
Some studies have suggested a higher risk of tumour recurrence with the use of partial grafts from living relatives versus whole grafts from deceased donors. Six studies compared deceased-donor liver transplantation (DDLT) and LDLT for HCC, including a report from a multicentre US consortium of LDLT centres.74–79 No convincing difference in outcome could be identified according to type of graft, although a higher risk of recurrence was noted in fast-tracked patients, since a short delay between diagnosis and liver transplantation might not allow enough time for the biological behaviour of the tumour to manifest. Therefore, it might be important to consider a period of observation (eg, 3 months) when offering LDLT in recipients with HCC. This topic is covered in recommendations 28 and 29: (28) LDLT is acceptable for HCC patients who have an expected 5-year survival similar to comparably staged patients receiving a deceased-donor liver. In LDLT, careful attention should be given to psychosocial considerations regarding both donor and recipient; (29) LDLT must be restricted to centres of excellence in liver surgery and liver transplantation to minimise donor risk and maximise recipient outcome.
Should LDLT be offered to patients with tumour stages beyond the accepted criteria for listing for deceased donation? This might seem to be ethically acceptable, since unlike deceased-donor donation, other listed patients are not adversely affected by this process. However, the question raises ethical concerns regarding the double equipoise principle, since the risk to the donor might not be acceptable below a certain expected survival threshold for the recipient. There was considerable disagreement among the experts at the conference on what that threshold might be. Those who set a priority on donor protection would not consider offering LDLT to patients with a tumour beyond criteria for DDLT, whereas others supported the procedure even for patients with a very dismal prognosis, to maximise individual patient benefit and patients’ choice.
The jury recognised that there are differences in current practice and suggested that any institution using LDLT should take a clear position, make it known to the public, and, when endorsing criteria beyond those accepted for deceased donation, provide rigorous safeguards to guarantee full disclosure to donor and recipients and to prevent donor coercion and increased risk-taking by the surgical team.
The use of deceased-donor organs is usually justified for graft failure after LDLT.80 The panel of experts supported use of deceased-donor graft for failed LDLT, even if extended criteria were used. Rates of retrans plantation because of graft failure after LDLT are typically low, and when needed, outcomes are excellent. The jury agreed with the use of retransplantation in recipients who received a living graft within the accepted criteria for liver transplantation. However, based on utility, justice, and equity, they did not support retransplantation for patients who were beyond these criteria, because these patients would not have qualified for DDLT in the first place, and their acceptance of LDLT did not benefit patients on the DDLT waiting list. The vote of the audience at the conference was in line with this re com mendation (46% strongly disagreed and 25% disagreed with the use of retransplantation in patients beyond the accepted criteria). This discussion led the jury to formulate recommendations 30 and 31: (30) in patients following LDLT for HCC within the accepted regional criteria for DDLT, retransplantation for graft failure is justified; (31) in patients following LDLT for HCC outside the accepted regional criteria for DDLT, retransplantation for graft failure using a deceased-donor organ is not recommended.
Post-transplant management
The main concern after liver transplantation for HCC is the risk of tumour recurrence, which occurs in 8–20% of recipients.81 HCC recurrence is usually seen within the first 2 years after liver transplantation, and is associated with a median survival of less than 1 year (IQR 7–18 months) from the time of diagnosis.82 The adoption of routine imaging and α-fetoprotein monitoring has led to the detection of early recurrence, with a possibility of cure with ablation therapies in up to a third of cases.83 However, studies addressing the issue of protocols for monitoring are scarce. A limitation for the use of routine imaging examination (CT or MRI) is the high cost or poor cost-effectiveness to detect HCC recurrence, and most centres have limited their application to every 6 months or yearly intervals for the first 3–5 years after liver transplantation or in the presence of abnormal α-fetoprotein concentrations, leading to recommendation 32: post-transplant monitoring may include 6–12-monthly contrast-enhanced CT or MRI imaging and α-fetoprotein measurements.
There is debate about how to adjust the immunosuppression in HCC patients after liver transplantation. Immunosuppressive drugs are associated with oncogenic properties in experimental models, and most programmes are careful to balance the inherent risks of rejection and tumour recurrence.84 However, there are no randomised controlled trials (RCTs) that have shown that lowering immunosuppression reduces the risk of HCC recurrence after liver transplantation.
One class of immunosuppressive drugs, the mTOR inhibitors, might be of particular relevance for patients with HCC who receive a liver transplantation, since experimental studies have shown that this drug has strong immunosuppressive effects with concomitant anti-neoplastic properties.85 Uncontrolled pilot trials and retrospective analyses have suggested that sirolimus, an mTOR inhibitor, was associated with lower tumour recurrence and improved survival after liver transplantation;86–88 however, these results have not been confirmed in an RCT. This topic is covered in statements 33 and 34: (33) there is currently insufficient evidence from clinical trials to base a recommendation for choosing the type or dose of immunosuppression therapy to influence the incidence of HCC recurrence or its prognosis; (34) based on current evidence, no recommendation can be made on the use of mTOR inhibitors to reduce the risk of HCC recurrence outside clinical trials.
To assess whether adjuvant therapies are effective to decrease post-transplantation tumour recurrence and improve overall survival, the experts identified eight uncontrolled studies (226 patients)89–96 and four RCTs (213 patients).96–100 Difficulties in interpreting these studies include variability in the drugs tested (doxorubicin, cisplatin, fluorouracil, gemcitabine, or mithoxanthrone) and in the inclusion criteria and endpoints selected, and small sample sizes. Single-arm, retrospective studies suggest a benefit for using adjuvant therapy, but their level of evidence is too low (level 4) to support recommendations. Controlled studies have also provided inconsistent results.
Two compounds show some early promise: sorafenib and licartin. Sorafenib, a multitargeted tyrosine-kinase inhibitor, has been shown to have an antitumour effect in patients with advanced HCC,101 and is currently being studied as adjuvant therapy after resection or ablation of HCC in a multicentre phase 3 trial (STORM trial). Licartin, a 131I-radiolabelled murine monoclonal antibody that specifically binds HCC cells, was shown to have a positive effect on prevention of tumour recurrence and on survival, in a small, placebo-controlled, randomised, double-blind study.97 This led to recommendation 35: current evidence does not justify the routine use of adjuvant antitumour therapy after liver transplantation for HCC outside of a controlled clinical trial.
There is considerable debate about how to treat HCC recurrence after liver transplantation. Most recurrences are associated with systemic tumour dissemination, thus retransplantation is not indicated. A distinction must be made for de-novo HCC, which typically occurs at a later stage and most often in the setting of recurrent hepatitis C and advancing fibrosis. Such cases should probably be treated as having new tumours and retransplantation might be justified;102 however, the data to support this approach are limited.
Locoregional therapy for HCC recurrence, including liver resection, radiofrequency ablation, or TACE, has been successfully used in selected patients with limited disease, and might be considered when technically feasible.103 Sorafenib has been used with limited side-effects after liver transplantation, sometimes in conjunction with mTOR inhibitors, and might be considered when systemic treatment is warranted.104 This topic is summarised in the last two recommendations: (36) HCC recurrence after liver transplantation may be treated by surgery for resectable lesions or by locoregional therapy or systemic therapy (including sorafenib) for unresectable lesions; (37) liver retransplantation is not appropriate treatment for recurrent HCC.
Conclusion
The 37 recommendations and statements presented here cover the most controversial topics surrounding liver transplantation for HCC, and may guide transplantation programmes around the world to improve management of their HCC patients. The phrasing of many recommendations permits adjustment according to variations in programme and regional circumstances, among which might be team experience and the availability of donor organs or living donation. Although the conference was not designed to address future areas of research, the existence of weak recommendations or absence of recommendation in a controversial area might serve as the basis to identify key questions that urgently require convincing answers.
Acknowledgments
This consensus conference was endorsed and sponsored by the American Association for the Study of Liver Disease (AASLD), American Society of Transplant Surgeons (ASTS), European Association for the Study of the Liver (EASL), European-African Hepato-Pancreato-Biliary Association (E-AHPBA), European Liver and Intestine Transplant Association (ELITA), International Hepato-Pancreato-Biliary Association (IHPBA), International Liver Cancer Association (ILCA), International Liver Transplantation Society (ILTS), and The Transplantation Society (TTS). We acknowledge the generous financial support from the industry including Bayer HealthCare, Novartis, Astellas, Johnson & Johnson, Roche, MSD, AstraZeneca, Nycomed, B Braun, Baxter, MeVis, CAScination, and Microsulis Medical. These companies were neither involved in the selection of topics, nor in the choice of experts or members of the jury. The conference was also supported by a grant from the University of Zurich and the Liver and Gastro-Intestinal Foundation. Finally, we thank Carol De Simio and Stefan Schwyter (University Hospital Zurich) for their help in preparing the figure.
OLT for HCC consensus group
Organising committee—J Belghiti (France); J Bruix (Spain); P-A Clavien (Switzerland); G Gores, D W Hanto, G Klintmalm, M Charlton (USA); C-M Lo (China); R J Porte (Netherlands).
Jury—A Perrier (president; Switzerland); B Langer (vice-president; Canada); G Barritt, (Australia); P Bossuyt (Netherlands); R Finn, B Hippen (USA); A Hisham (Malaysia); P Marcellin (France); A Sexton Dobby (Switzerland).
Chairs of the expert panels—A K Burroughs (UK); C Duvoux, D Samuel (France); A S H Gouw (Netherlands); D Grant, P Greig, N Kneteman (Canada); J M Lee (Korea); J Lerut (Belgium); J Llovet (Spain); V Mazzaferro (Italy); P Majno, B Müllhaupt (Switzerland); R Freeman, K Olthoff, E Pomfret, M Schwartz, J Trotter, F Yao (USA).
Details of members of the expert panels, local organising committee, and writing committee can be found in the webappendix p1.
Footnotes
Contributors
P-AC and ML contributed to conference organisation and collection and analysis of data. PMMB contributed to the methods of the consensus conference and writing of recommendations. GJG contributed to conference organisation. AP and BL wrote recommendations. All authors wrote the manuscript.
Conflicts of interest
The authors declared no conflicts of interest.
Contributor Information
Pierre-Alain Clavien, Department of Surgery, Swiss HPB and Transplant Centers, University Hospital Zurich, Zurich, Switzerland.
Mickael Lesurtel, Department of Surgery, Swiss HPB and Transplant Centers, University Hospital Zurich, Zurich, Switzerland.
Patrick M M Bossuyt, Department of Clinical Epidemiology and Biostatistics, Academic Medical Center, Amsterdam, Netherlands.
Gregory J Gores, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA.
Bernard Langer, Department of Surgery, University of Toronto, Toronto, ON, Canada.
Arnaud Perrier, Department of Internal Medicine, University Hospital of Geneva, Geneva, Switzerland.
References
- 1.European Liver Transplant Registry. Results. [accessed Jan 1, 2011];2011 www.eltr.org/spip.php?rubrique37.
- 2.Organ Procurement and Transplantation Network. OPTN/SRTR Annual Report. [accessed Jan 1, 2011]; www.ustransplant.org/annual_reports.
- 3.Grundahl J. The Danish consensus conference model. In: Joss S, Durant J, editors. Public participation in science: the role of consensus conferences in Europe. London: Science Museum; 1995. [Google Scholar]
- 4.Oxford Centre for Evidence-based Medicine. Levels of evidence. [accessed Nov 1, 2010];2009 Mar; http://www.cebm.net/index.aspx?o=1025. [Google Scholar]
- 5.Lesurtel M, Clavien PA. 2010 international consensus conference on liver transplantation for hepatocellular carcinoma: texts of experts. Liver Transpl. 2011;17(suppl 2):S1–S5. doi: 10.1002/lt.22350. [DOI] [PubMed] [Google Scholar]
- 6.Samuel D, Colombo M, El-Serag H, Sobesky R, Heaton N. Toward optimizing the indications for orthotopic liver transplantation in hepatocellular carcinoma. Liver Transpl. 2011;17(suppl 2):S6–S13. doi: 10.1002/lt.22423. [DOI] [PubMed] [Google Scholar]
- 7.Müllhaupt B, Durand F, Roskams T, Dutkowski P, Heim M. Is tumor biopsy necessary? Liver Transpl. 2011;17(suppl 2):S14–S25. doi: 10.1002/lt.22374. [DOI] [PubMed] [Google Scholar]
- 8.Olthoff KM, Forner A, Hübscher S, Fung J. What is the best staging system for hepatocellular carcinoma in the setting of liver transplantation? Liver Transpl. 2011;17(suppl 2):S26–S33. doi: 10.1002/lt.22352. [DOI] [PubMed] [Google Scholar]
- 9.Lee JM, Trevisani F, Vilgrain V, Wald C. Imaging diagnosis and staging of hepatocellular carcinoma. Liver Transpl. 2011;17(suppl 2):S34–S43. doi: 10.1002/lt.22369. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Bhoori S, Sposito C, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17(suppl 2):S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 11.Germani G, Gurusamy K, Garcovich M, et al. Which matters most: Number of tumors, size of the largest tumor, or total tumor volume? Liver Transpl. 2011;17(suppl 2):S58–S66. doi: 10.1002/lt.22336. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Paradis V, Kudo M, Zucman-Rossi J. Tissue biomarkers as predictors of outcome and selection of transplant candidates with hepatocellular carcinoma. Liver Transpl. 2011;17(suppl 2):S67–S71. doi: 10.1002/lt.22340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gouw AS, Balabaud C, Kusano H, Todo S, Ichida T, Kojiro M. Markers for microvascular invasion in hepatocellular carcinoma: where do we stand? Liver Transpl. 2011;17(suppl 2):S72–S80. doi: 10.1002/lt.22368. [DOI] [PubMed] [Google Scholar]
- 14.Prasad KR, Young RS, Burra P, et al. Summary of candidate selection and expanded criteria for liver transplantation for hepatocellular carcinoma: a review and consensus statement. Liver Transpl. 2011;17(suppl 2):S81–S89. doi: 10.1002/lt.22380. [DOI] [PubMed] [Google Scholar]
- 15.Lerut J, Mergental H, Kahn D, et al. Place of liver transplantation in the treatment of hepatocellular carcinoma in the normal liver. Liver Transpl. 2011;17(suppl 2):S90–S97. doi: 10.1002/lt.22393. [DOI] [PubMed] [Google Scholar]
- 16.Majno P, Lencioni R, Mornex F, Girard N, Poon RT, Cherqui D. Is the treatment of hepatocellular carcinoma on the waiting list necessary? Liver Transpl. 2011;17(suppl 2):S98–S108. doi: 10.1002/lt.22391. [DOI] [PubMed] [Google Scholar]
- 17.Yao FY, Breitenstein S, Broelsch CE, Dufour JF, Sherman M. Does a patient qualify for liver transplantation after the down-staging of hepatocellular carcinoma? Liver Transpl. 2011;17(suppl 2):S109–S116. doi: 10.1002/lt.22335. [DOI] [PubMed] [Google Scholar]
- 18.Kneteman N, Livraghi T, Madoff D, de Santibañez E, Kew M. Tools for monitoring patients with hepatocellular carcinoma on the waiting list and after liver transplantation. Liver Transpl. 2011;17(suppl 2):S117–S127. doi: 10.1002/lt.22334. [DOI] [PubMed] [Google Scholar]
- 19.Pomfret EA, Lodge JP, Villamil FG, Siegler M. Should we use living donor grafts for patients with hepatocellular carcinoma? Ethical considerations. Liver Transpl. 2011;17(suppl 2):S128–S132. doi: 10.1002/lt.22356. [DOI] [PubMed] [Google Scholar]
- 20.Grant D, Fisher RA, Abecassis M, McCaughan G, Wright L, Fan ST. Should the liver transplant criteria for hepatocellular carcinoma be different for deceased donation and living donation? Liver Transpl. 2011;17(suppl 2):S133–S138. doi: 10.1002/lt.22348. [DOI] [PubMed] [Google Scholar]
- 21.Greig PD, Geier A, D'Alessandro AM, Campbell M, Wright L. Should we perform deceased donor liver transplantation after living donor liver transplantation has failed? Liver Transpl. 2011;17(suppl 2):S139–S146. doi: 10.1002/lt.22328. [DOI] [PubMed] [Google Scholar]
- 22.Duvoux C, Kiuchi T, Pestalozzi B, Busuttil R, Miksad R. What is the role of adjuvant therapy after liver transplantation for hepatocellular carcinoma? Liver Transpl. 2011;17(suppl 2):S147–S158. doi: 10.1002/lt.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlitt HJ, Mornex F, Shaked A, Trotter JF. Immunosuppression and hepatocellular carcinoma. Liver Transpl. 2011;17(suppl 2):S159–S161. doi: 10.1002/lt.22318. [DOI] [PubMed] [Google Scholar]
- 24.Davis E, Wiesner R, Valdecasas J, Kita Y, Rossi M, Schwartz M. Treatment of recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2011;17(suppl 2):S162–S166. doi: 10.1002/lt.22361. [DOI] [PubMed] [Google Scholar]
- 25.Schunemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–1110. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chevret S, Trinchet JC, Mathieu D, et al. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire. J Hepatol. 1999;31:133–141. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 27.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 28.Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–1769. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 29.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 30.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 31.Omagari K, Honda S, Kadokawa Y, et al. Preliminary analysis of a newly proposed prognostic scoring system (SLiDe score) for hepatocellular carcinoma. J Gastroenterol Hepatol. 2004;19:805–811. doi: 10.1111/j.1440-1746.2004.03350.x. [DOI] [PubMed] [Google Scholar]
- 32.Tateishi R, Yoshida H, Shiina S, et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419–425. doi: 10.1136/gut.2003.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 34.Wildi S, Pestalozzi BC, McCormack L, et al. Critical evaluation of the different staging systems for hepatocellular carcinoma. Br J Surg. 2004;91:400–408. doi: 10.1002/bjs.4554. [DOI] [PubMed] [Google Scholar]
- 35.Makuuchi M, Sano K. The surgical approach to HCC: our progress and results in Japan. Liver Transpl. 2004;10(suppl 1):46–52. doi: 10.1002/lt.20044. [DOI] [PubMed] [Google Scholar]
- 36.Bilimoria MM, Lauwers GY, Doherty DA, et al. Underlying liver disease, not tumor factors, predicts long-term survival after resection of hepatocellular carcinoma. Arch Surg. 2001;136:528–535. doi: 10.1001/archsurg.136.5.528. [DOI] [PubMed] [Google Scholar]
- 37.Freeman RB, Mithoefer A, Ruthazer R, et al. Optimizing staging for hepatocellular carcinoma before liver transplantation: a retrospective analysis of the UNOS/OPTN database. Liver Transpl. 2006;12:1504–1511. doi: 10.1002/lt.20847. [DOI] [PubMed] [Google Scholar]
- 38.Silva MA, Hegab B, Hyde C, et al. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592–1596. doi: 10.1136/gut.2008.149062. [DOI] [PubMed] [Google Scholar]
- 39.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 40.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 41.Germani G, Gurusamy K, Garcovich M, et al. What matters: number of tumours, size of largest tumour, or total volume of tumour? Liver Transpl. 2011 doi: 10.1002/lt.22336. published online May 16. [DOI] [PubMed] [Google Scholar]
- 42.Toso C, Trotter J, Wei A, et al. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008;14:1107–1115. doi: 10.1002/lt.21484. [DOI] [PubMed] [Google Scholar]
- 43.Toso C, Asthana S, Bigam DL, et al. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009;49:832–838. doi: 10.1002/hep.22693. [DOI] [PubMed] [Google Scholar]
- 44.Mazzaferro V, Bhoori S, Sposito C, et al. Milan Criteria in liver transplantation for HCC: an evidence-based analysis on 15 years of experience. Liver Transpl. 2011 doi: 10.1002/lt.22365. published online June 21. [DOI] [PubMed] [Google Scholar]
- 45.Australia and New Zeland Liver Transplant Registry. 21st report. [accessed Nov 1, 2010]; http://www.anzltr.org/thisYear.
- 46.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 47.Yao FY, Xiao L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 48.Volk ML, Vijan S, Marrero JA. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am J Transplant. 2008;8:839–846. doi: 10.1111/j.1600-6143.2007.02138.x. [DOI] [PubMed] [Google Scholar]
- 49.Villanueva A, Hoshida Y, Toffanin S, et al. New strategies in hepatocellular carcinoma: genomic prognostic markers. Clin Cancer Res. 2010;16:4688–4694. doi: 10.1158/1078-0432.CCR-09-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vibert E, Azoulay D, Hoti E, et al. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant. 2010;10:129–137. doi: 10.1111/j.1600-6143.2009.02750.x. [DOI] [PubMed] [Google Scholar]
- 51.Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726–1732. doi: 10.1097/TP.0b013e31816b67e4. [DOI] [PubMed] [Google Scholar]
- 52.Ravaioli M, Grazi GL, Piscaglia F, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547–2557. doi: 10.1111/j.1600-6143.2008.02409.x. [DOI] [PubMed] [Google Scholar]
- 53.Lohe F, Angele MK, Rentsch M, et al. Multifocal manifestation does not affect vascular invasion of hepatocellular carcinoma: implications for patient selection in liver transplantation. Clin Transplant. 2007;21:696–701. doi: 10.1111/j.1399-0012.2007.00707.x. [DOI] [PubMed] [Google Scholar]
- 54.Parfitt JR, Marotta P, Alghamdi M, et al. Recurrent hepatocellular carcinoma after transplantation: use of a pathological score on explanted livers to predict recurrence. Liver Transpl. 2007;13:543–551. doi: 10.1002/lt.21078. [DOI] [PubMed] [Google Scholar]
- 55.Shah SA, Tan JC, McGilvray ID, et al. Does microvascular invasion affect outcomes after liver transplantation for HCC? A histopathological analysis of 155 consecutive explants. J Gastrointest Surg. 2007;11:464–471. doi: 10.1007/s11605-006-0033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liver Cancer Study Group of Japan. General rules for the clinical and pathological study of primary liver cancer. Tokyo: Kanehara Co; 1997. [Google Scholar]
- 57.Kornberg A, Freesmeyer M, Barthel E, et al. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant. 2009;9:592–600. doi: 10.1111/j.1600-6143.2008.02516.x. [DOI] [PubMed] [Google Scholar]
- 58.Fujiki M, Takada Y, Ogura Y, et al. Significance of des-gamma-carboxy prothrombin in selection criteria for living donor liver transplantation for hepatocellular carcinoma. Am J Transplant. 2009;9:2362–2371. doi: 10.1111/j.1600-6143.2009.02783.x. [DOI] [PubMed] [Google Scholar]
- 59.Shirabe K, Itoh S, Yoshizumi T, et al. The predictors of microvascular invasion in candidates for liver transplantation with hepatocellular carcinoma—with special reference to the serum levels of des-gamma-carboxy prothrombin. J Surg Oncol. 2007;95:235–240. doi: 10.1002/jso.20655. [DOI] [PubMed] [Google Scholar]
- 60.Clavien PA, Petrowsky H, DeOliveira ML, et al. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 61.Chang CH, Chau GY, Lui WY, et al. Long-term results of hepatic resection for hepatocellular carcinoma originating from the noncirrhotic liver. Arch Surg. 2004;139:320–325. doi: 10.1001/archsurg.139.3.320. [DOI] [PubMed] [Google Scholar]
- 62.Lang H, Sotiropoulos GC, Domland M, et al. Liver resection for hepatocellular carcinoma in non-cirrhotic liver without underlying viral hepatitis. Br J Surg. 2005;92:198–202. doi: 10.1002/bjs.4763. [DOI] [PubMed] [Google Scholar]
- 63.Liu S, Chan KW, Wang B, et al. Fibrolamellar hepatocellular carcinoma. Am J Gastroenterol. 2009;104:2617–2624. doi: 10.1038/ajg.2009.440. [DOI] [PubMed] [Google Scholar]
- 64.Mergental H, Porte RJ. Liver transplantation for unresectable hepatocellular carcinoma in patients without liver cirrhosis. Transpl Int. 2010;23:662–667. doi: 10.1111/j.1432-2277.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- 65.Houben KW, McCall JL. Liver transplantation for hepatocellular carcinoma in patients without underlying liver disease: a systematic review. Liver Transpl Surg. 1999;5:91–95. doi: 10.1002/lt.500050201. [DOI] [PubMed] [Google Scholar]
- 66.Lesurtel M, Mullhaupt B, Pestalozzi BC, et al. Transarterial chemoembolization as a bridge to liver transplantation for hepatocellular carcinoma: an evidence-based analysis. Am J Transplant. 2006;6:2644–2650. doi: 10.1111/j.1600-6143.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 67.Yao FY, Kerlan RK, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819–827. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 69.Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 70.Majno PE, Lencioni R, Mornex F, et al. Is treatment of HCC on the waiting list necessary? Liver Transpl. 2011;17(suppl 2):S98–S108. doi: 10.1002/lt.22391. [DOI] [PubMed] [Google Scholar]
- 71.Martin AP, Goldstein RM, Dempster J, et al. Radiofrequency thermal ablation of hepatocellular carcinoma before liver transplantation—a clinical and histological examination. Clin Transplant. 2006;20:695–705. doi: 10.1111/j.1399-0012.2006.00538.x. [DOI] [PubMed] [Google Scholar]
- 72.Pompili M, Mirante VG, Rondinara G, et al. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl. 2005;11:1117–1126. doi: 10.1002/lt.20469. [DOI] [PubMed] [Google Scholar]
- 73.Cronin DC, Millis JM. Living donor liver transplantation: the ethics and the practice. Hepatology. 2008;47:11–13. doi: 10.1002/hep.22150. [DOI] [PubMed] [Google Scholar]
- 74.Di Sandro S, Slim AO, Giacomoni A, et al. Living donor liver transplantation for hepatocellular carcinoma: long-term results compared with deceased donor liver transplantation. Transplant Proc. 2009;41:1283–1285. doi: 10.1016/j.transproceed.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 75.Fisher RA, Kulik LM, Freise CE, et al. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant. 2007;7:1601–1608. doi: 10.1111/j.1600-6143.2007.01802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hwang S, Lee SG, Joh JW, et al. Liver transplantation for adult patients with hepatocellular carcinoma in Korea: comparison between cadaveric donor and living donor liver transplantations. Liver Transpl. 2005;11:1265–1272. doi: 10.1002/lt.20549. [DOI] [PubMed] [Google Scholar]
- 77.Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127(suppl 1):277–282. doi: 10.1053/j.gastro.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 78.Lo CM, Fan ST, Liu CL, et al. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg. 2007;94:78–86. doi: 10.1002/bjs.5528. [DOI] [PubMed] [Google Scholar]
- 79.Vakili K, Pomposelli JJ, Cheah YL, et al. Living donor liver transplantation for hepatocellular carcinoma: increased recurrence but improved survival. Liver Transpl. 2009;15:1861–1866. doi: 10.1002/lt.21940. [DOI] [PubMed] [Google Scholar]
- 80.Olthoff KM, Merion RM, Ghobrial RM, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg. 2005;242:314–323. doi: 10.1097/01.sla.0000179646.37145.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zimmerman MA, Ghobrial RM, Tong MJ, et al. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143:182–188. doi: 10.1001/archsurg.2007.39. [DOI] [PubMed] [Google Scholar]
- 82.Hollebecque A, Decaens T, Boleslawski E, et al. Natural history and therapeutic management of recurrent hepatocellular carcinoma after liver transplantation. Gastroenterol Clin Biol. 2009;33:361–369. doi: 10.1016/j.gcb.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 83.Roberts JP. Tumor surveillance-what can and should be done? Screening for recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2005;11(suppl 2):45–46. doi: 10.1002/lt.20605. [DOI] [PubMed] [Google Scholar]
- 84.Hojo M, Morimoto T, Maluccio M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 85.Soll C, Clavien PA. Inhibition of mammalian target of rapamycin: two goals with one shot? J Hepatol. 2011;54:182–183. doi: 10.1016/j.jhep.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 86.Chinnakotla S, Davis GL, Vasani S, et al. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2009;15:1834–1842. doi: 10.1002/lt.21953. [DOI] [PubMed] [Google Scholar]
- 87.Toso C, Meeberg GA, Bigam DL, et al. De novo sirolimus-based immunosuppression after liver transplantation for hepatocellular carcinoma: long-term outcomes and side effects. Transplantation. 2007;83:1162–1168. doi: 10.1097/01.tp.0000262607.95372.e0. [DOI] [PubMed] [Google Scholar]
- 88.Toso C, Merani S, Bigam DL, et al. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;51:1237–1243. doi: 10.1002/hep.23437. [DOI] [PubMed] [Google Scholar]
- 89.Stone MJ, Klintmalm GB, Polter D, et al. Neoadjuvant chemotherapy and liver transplantation for hepatocellular carcinoma: a pilot study in 20 patients. Gastroenterology. 1993;104:196–202. doi: 10.1016/0016-5085(93)90852-4. [DOI] [PubMed] [Google Scholar]
- 90.Cherqui D, Piedbois P, Pierga JY, et al. Multimodal adjuvant treatment and liver transplantation for advanced hepatocellular carcinoma. A pilot study. Cancer. 1994;73:2721–2726. doi: 10.1002/1097-0142(19940601)73:11<2721::aid-cncr2820731112>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 91.Roayaie S, Frischer JS, Emre SH, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533–539. doi: 10.1097/00000658-200204000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hsieh CB, Chou SJ, Shih ML, et al. Preliminary experience with gemcitabine and cisplatin adjuvant chemotherapy after liver transplantation for hepatocellular carcinoma. Eur J Surg Oncol. 2008;34:906–910. doi: 10.1016/j.ejso.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 93.Olthoff KM, Rosove MH, Shackleton CR, et al. Adjuvant chemotherapy improves survival after liver transplantation for hepatocellular carcinoma. Ann Surg. 1995;221:734–741. doi: 10.1097/00000658-199506000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang ZH, Ma LW, Song SB, et al. Adjuvant chemotherapy after orthotopic liver transplantation for advanced hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 2005;27:45–47. (in Chinese). [PubMed] [Google Scholar]
- 95.Chen GH, Lu MQ, Cai CJ, et al. Clinical study of adjuvant individualized chemotherapy for hepatocellular carcinoma after liver transplantation. Zhonghua Wai Ke Za Zhi. 2004;42:1040–1043. (in Chinese). [PubMed] [Google Scholar]
- 96.Sun J, Hou BH, Jian ZX, et al. Value of perioperative adjuvant therapy in liver transplantation for advanced hepatocellular carcinoma. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:471–473. (in Chinese). [PubMed] [Google Scholar]
- 97.Xu J, Shen ZY, Chen XG, et al. A randomized controlled trial of Licartin for preventing hepatoma recurrence after liver transplantation. Hepatology. 2007;45:269–276. doi: 10.1002/hep.21465. [DOI] [PubMed] [Google Scholar]
- 98.Soderdahl G, Backman L, Isoniemi H, et al. A prospective, randomized, multi-centre trial of systemic adjuvant chemotherapy versus no additional treatment in liver transplantation for hepatocellular carcinoma. Transpl Int. 2006;19:288–294. doi: 10.1111/j.1432-2277.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- 99.Pokorny H, Gnant M, Rasoul-Rockenschaub S, et al. Does additional doxorubicin chemotherapy improve outcome in patients with hepatocellular carcinoma treated by liver transplantation? Am J Transplant. 2005;5:788–794. doi: 10.1111/j.1600-6143.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- 100.Li N, Zhou J, Weng D, et al. Adjuvant adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of liver transplantation in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2007;13:5847–5854. doi: 10.1158/1078-0432.CCR-07-0499. [DOI] [PubMed] [Google Scholar]
- 101.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 102.Kita Y, Klintmalm G, Kobayashi S, et al. Retransplantation for de novo hepatocellular carcinoma in a liver allograft with recurrent hepatitis B cirrhosis 14 years after primary liver transplantation. Dig Dis Sci. 2007;52:392–393. doi: 10.1007/s10620-006-9574-6. [DOI] [PubMed] [Google Scholar]
- 103.Taketomi A, Fukuhara T, Morita K, et al. Improved results of a surgical resection for the recurrence of hepatocellular carcinoma after living donor liver transplantation. Ann Surg Oncol. 2010;17:2283–2289. doi: 10.1245/s10434-010-0999-y. [DOI] [PubMed] [Google Scholar]
- 104.Yoon DH, Ryoo BY, Ryu MH, et al. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Jpn J Clin Oncol. 2010;40:768–773. doi: 10.1093/jjco/hyq055. [DOI] [PubMed] [Google Scholar]