Abstract
Objective
We evaluated the safety of coronary reactivity testing (CRT) in symptomatic women with evidence of myocardial ischemia and no obstructive coronary artery disease (CAD).
Background
Microvascular coronary dysfunction (MCD) in women with no obstructive CAD portends an adverse prognosis of 2.5% annual major adverse cardiovascular event (MACE) rate. The diagnosis of MCD is established by invasive CRT, yet the risk of CRT is unknown.
Methods
We evaluated 293 symptomatic women with ischemia and no obstructive CAD, who underwent CRT at three experienced centers. Microvascular function was assessed using a Doppler wire and injections of adenosine, acetylcholine, and nitroglycerin in the left coronary artery. CRT-related serious adverse events (SAE), adverse events (AE), and follow-up MACE (death, nonfatal myocardial infarction (MI), nonfatal stroke, or hospitalization for heart failure) were recorded.
Results
CRT-SAEs occurred in 2 women (0.7%) during the procedure: one had coronary artery dissection, and one developed MI associated with coronary spasm. CRT-AEs occurred in 2 women (0.7%) and included one transient air microembolism and one deep venous thrombosis. There was no CRT-related mortality. In the mean follow-up period of 5.4 years, the MACE rate was 8.2%, including 5 deaths (1.7%), 8 non-fatal MIs (2.7%), 8 nonfatal strokes (2.7%), and 11 hospitalizations for heart failure (3.8%).
Conclusions
In women undergoing CRT for suspected MCD, contemporary testing carries a relatively low risk compared to the MACE rate in these women. These results support the use of CRT by experienced operators for establishing definitive diagnosis and assessing prognosis in this at-risk population.
Keywords: coronary reactivity, microvascular dysfunction, endothelial dysfunction
Introduction
In patients undergoing angiography for stable angina, the proportion of women and men with no obstructive CAD is increasing over time (1). Compared to men, women have a higher incidence of signs and symptoms of myocardial ischemia, yet 30–50% of women who undergo coronary angiography do not have obstructive coronary artery disease (CAD) (2–4). The absence of obstructive CAD is not benign, as 38% of women with acute myocardial infarction (MI) and no obstructive CAD have been found to have plaque rupture or ulceration using intravascular ultrasound (5). Women with angina in the absence of obstructive CAD are often inappropriately reassured and even dismissed without further investigation or treatment: yet angina among women regardless of coronary angiographic findings is associated with increased mortality (6–7). The National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women’s Ischemia Syndrome Evaluation (WISE) studies have documented that approximately half of these symptomatic women with no obstructive CAD have microvascular coronary dysfunction (MCD), which produces ischemia and is associated with an adverse cardiovascular prognosis compared to asymptomatic women (3–4, 8–11). Both coronary artery spasm and endothelial dysfunction have been shown to be predictors of morbidity and mortality in patients with angina (12–16). Coronary spasm may result in MI, ventricular arrhythmias and sudden cardiac death (15,17–18). Recent data show that women without obstructive CAD who have a low coronary flow reserve (CFR) are at higher risk of major adverse cardiac events (MACE) compared to those with normal CFR (19). Treatment directed at endothelial function can reduce angina, coronary spasm, heart failure, and stroke (20–23); therefore, it is important to establish the diagnosis in order to institute appropriate medical management.
Invasive coronary reactivity testing (CRT) using vasoactive agents to evaluate macroand microvascular responses is considered the reference-standard for a definitive diagnosis of MCD (24). However, it is not routinely performed for a variety of reasons, including lack of standardized protocols and concerns over catheterization laboratory time. Furthermore, limited data exist on the safety of contemporary CRT in women suspected of having MCD. We evaluated the safety of CRT performed at three experienced centers in women with angina, evidence of myocardial ischemia by stress testing, and no obstructive CAD (3, 25).
Methods
Women with angina and evidence of myocardial ischemia underwent CRT at three experienced clinical centers that participate in WISE: the University of Pittsburgh, the University of Florida, Gainesville, and Cedars-Sinai Medical Center. Inclusion criteria: women with angina, myocardial ischemia by stress testing, absence of obstructive CAD (<50% luminal obstruction in one or more epicardial coronary arteries on angiography). Exclusion criteria: contraindications to angiography and invasive CRT (hypersensitivity of contrast media, active bleeding, bleeding diathesis, renal dysfunction), prior or planned percutaneous coronary intervention or coronary artery bypass grafting, acute MI within 30 days, primary valvular heart disease, cardiogenic shock or intra-aortic balloon pump, inability to withhold nitrates, calcium channel agents, and alpha and beta adrenergic blockers for 24 hours prior to testing, New York Heart Association class III or IV heart failure, ejection fraction <40%, hypertrophic obstructive cardiomyopathy, severe lung, renal, or hepatic disease, life expectancy <6 months, age <21 years, or pregnancy. All study participants gave written informed consent before undergoing evaluation. Demographic data were recorded with standardized questionnaires. CRT data were read on site (at the Cedars-Sinai Cardiovascular Intervention Center) or at the WISE Angiographic Core Laboratory (Brown University). The institutional review boards at each site approved the study.
CRT Protocol
Patients fasted for 12 hours and withheld caffeine, long-acting nitrates, and other vasoactive agents for 24 hours prior to testing. Patients were instructed to discontinue nicotine and avoid sublingual nitroglycerin four hours prior to the procedure. Pre-mixed acetylcholine in three concentrations (0.182 mcg/ml, 1.82 mcg/ml, and 18.2 mcg/ml) were prepared within three hours of scheduled procedure.
Outpatient diagnostic coronary angiography was performed via the percutaneous femoral approach. A pigtail catheter measured aortic and left ventricular pressures. Patients with significant CAD, coronary artery anomalies, or bridging were excluded. For borderline lesions, at the discretion of the interventionalist, intravascular ultrasound and/or fractional flow reserve were used to confirm absence of obstructive stenosis.
Following angiography, women were given body-weight adjusted heparin for anticoagulation and ACT was maintained above 250. CRT was performed by infusing vasoactive substances through a guiding catheter placed in the left main coronary artery. Doppler guide wire (0.014-in diameter, FloWire, JOMED/Cardiometrics/Volcano) was positioned in the proximal left anterior descending coronary artery. The following coronary functions were tested:
Non-endothelial dependent microvascular function determination: After an adequate flow reading was obtained by the ComboMap Pressure and Flow System (JOMED/Cardiometrics/Volcano), baseline average peak velocity was recorded. Intracoronary (IC) bolus injections of incremental doses of adenosine (18 mcg, 18mcg and 36 mcg) were administered to create maximal hyperemia. The catheter was flushed with saline after each adenosine injection, and an average peak velocity reading was obtained five seconds after the saline flush. Adenosine CFR was calculated by ComboMap as a ratio of average peak velocity to average baseline velocity. This process was repeated and recorded for each dose of adenosine after the peak velocity returned to baseline. A CFR ≤2.5 in response to adenosine was considered abnormal (14, 19).
Endothelial-dependent micro- and macro-vascular dysfunction determination: Graded IC acetylcholine concentrations of 0.182 mcg/ml and 18.2 mcg/ml were infused (2 ml over 3 minutes). An intermediate dose of 1.82 mcg/ml was infused at the discretion of the angiographer if it was deemed unsafe to proceed directly to a higher dose of 18.2 mcg/ml, based on the coronary reactivity from the lower dose (i.e., 0.182 mcg/ml) of acetylcholine. Doppler measurement of peak velocity was obtained at the end of each acetylcholine infusion. Normal endothelial-dependent microvascular response was defined as a coronary blood flow increase >50% at the highest dose of acetylcholine. Post-acetylcholine cine image was obtained for each concentration for quantitative coronary angiography (QCA). We ensured that coronary flow returned to baseline prior to each infusion. Normal acetylcholine response, or endothelialdependent macrovascular coronary function, was defined as coronary artery dilation >5%. Coronary blood flow response to acetylcholine was calculated from the Doppler-derived time velocity integral and vessel diameter by the following equation: Coronary blood flow = π (average peak velocity/2)(vessel diameter/2)2. Vessel diameter was calculated 5 mm distal to the Doppler wire.
Non-endothelial-dependent macrovascular function determination: After completion of acetylcholine infusions and the return of coronary flow velocity to baseline, IC nitroglycerin (200 mcg) was injected to evaluate non-endothelial-dependent macrovascular function. Average baseline and peak velocity were recorded. A cine image within 30 seconds of IC nitroglycerin was obtained for QCA. Normal nitroglycerin response was defined as a diameter increase >20%.
The angles, skew rotation, and table height were kept constant during the procedure. QCA measurements were made in the segment 5mm distal to the tip of the Doppler wire. For each time interval, the diameter was measured in the same segment. Heart rate and blood pressure were recorded before and after administration of adenosine, acetylcholine, and nitroglycerin.
Peri-procedural Adverse Events
Adverse events (AE) and serious adverse events (SAEs) during and immediately post- CRT were recorded. SAEs were defined as those that required termination of the protocol and immediate hospitalization (hemodynamic instability, coronary artery dissection, myocardial infarction, stroke, and death). AEs were defined as events related to the procedure which did not require hospitalization (such as deep venous thrombosis, transient coronary air embolism, nonsustained arrhythmias, and transient hypotension not requiring treatment). SAEs and AEs were adjudicated by a clinical events committee.
Outcomes during Follow-up
As per WISE protocol, women were followed 6 weeks and then annually for death, nonfatal MI, non-fatal stroke, and hospitalization for heart failure. MACE rate was calculated as the percentage of patients with a first event.
Results
The patient demographics are shown in Table 1. Results of CRT are reported in Table 2; not all patients underwent all aspects of reactivity testing, as this was site-dependent. CRT related SAEs occurred in 2 women (0.7 %) and included one coronary artery dissection (0.3%) and one ST-elevation MI due to coronary artery spasm (0.3%) (Table 3). CRT-related AEs occurred in 2 women (0.7 %), and included one with transient air microembolism (0.3%) and one with deep venous thrombosis on the side of the groin access site (0.3%), greater than 30 days after the CRT (Table 4). The combined CRT-related AE/SAE rate was 1.4%. There was no CRT-related mortality.
Table 1.
Patient Demographics
| Baseline Characteristics |
TOTAL (n = 293) |
|---|---|
| White/Caucasian | 247 (84 %) |
| Age (years ±SD) | 54 ±10 |
| History of smoking | 140 (48 %) |
| Dyslipidemia | 94 (32 %) |
| Hypertensive | 102 (35 %) |
| Diabetics | 29 (10 %) |
Table 2.
Results of Coronary Reactivity Testing
| Total (%) | |
|---|---|
|
Abnormal Non-endothelial Microvascular Function (CFR ≤ 2.5 in response to 18 mcg adenosine) |
138/293 (47%) |
|
Abnormal Endothelial Microvascular Function (≤ 50% change in CBF in response to high-dose acetylcholine) |
112/220 (51%) |
|
Abnormal Endothelial Macrovascular Function (<5% increase in diameter in response to high-dose acetylcholine) |
127/220 (58%) |
|
Abnormal Smooth Muscle Function (Non-endothelial Macrovascular Function) (<20% increase in diameter to nitroglycerin) |
136/225 (60%) |
|
Coronary Vasospasm (>50% reduction in diameter to high-dose acetylcholine compared to baseline) |
11/220 (5%) |
|
Coronary Vasospasm (>70% reduction in diameter to high-dose acetylcholine compared to post-nitroglycerin diameter) |
5/220 (2.3%) |
Table 3.
Coronary Reactivity Testing Related Serious Adverse Events
| Patient Age |
Target Vessel |
Complication | Treatment |
|---|---|---|---|
| 53 | LAD | Prior to initiation of protocol, a focal spasm in the LCX was visualized while the Doppler wire was in the LAD, causing an MI and prolonged chest pain. | Patient was given IC nitroglycerin and verapamil, as well as sublingual and intravenous nitroglycerin. She was admitted with a peak CPK 532 and positive MB fraction. |
| 58 | LAD | A nonflow-limiting coronary dissection of the mid LAD resulted from advancement of the Doppler wire. Focal vasospasm and staining were visualized after the acetylcholine injection. IC nitroglycerin appropriately dilated the vessel. | TIMI-3 flow was present, and no intervention was needed. Patient was given clopidogrel and monitored overnight. She did not experience additional chest pain. There were no electrocardiographic changes. |
LAD= left Anterior descending artery, LCX= left circumflex artery, CPK= creatine phosphokinase, TIMI=Thrombolysis in Myocardial Infarction
Table 4.
Coronary Reactivity Testing Related Adverse Events
| Patient Age |
Target Vessel |
Complication | Treatment |
|---|---|---|---|
| 66 | LAD | An air microembolism to RCA was noticed during insertion of an infusion catheter, causing chest pain for 2 minutes. | Supplemental oxygen was delivered by face mask, with spontaneous recovery. |
| 52 | LAD | Patient was diagnosed with a deep venous thrombosis more than 30 days after her coronary reactivity study. | Anticoagulation was initiated as indicated. |
LAD= left anterior descending Artery, RCA = right coronary artery
The prevalence of epicardial coronary vasospasm in our women was 5 %, when vasospasm was defined as acetylcholine response of >50% coronary artery diameter reduction from baseline diameter (26). When defined as >70% coronary artery diameter reduction to acetylcholine from baseline, vasospasm occurred in 2 patients (0.9%). We also compared acetylcholine response to post-nitroglycerin diameter. 5 patients (2.3%) had a >70% reduction in diameter to acetylcholine compared to post-nitroglycerin diameter.
The cohort was then followed for a period of 5.4 years, with 32 MACE observed in 24 women, including 5 deaths (1.7%), 8 non-fatal MIs (2.7%), 8 nonfatal strokes (2.7%), and 11 hospitalizations for heart failure (3.8%). The composite MACE rate to first event was 8.2% (24/293).
Discussion
While invasive CRT is used to diagnose MCD in patients without obstructive CAD, the safety of CRT has not been well established, especially among women. The results of our study demonstrate that invasive CRT is relatively safe to evaluate MCD in symptomatic women with evidence of ischemia but no obstructive CAD. Compared to diagnostic coronary angiography, which carries a less than 2% risk of complications (26–27), addition of CRT does not appear to significantly raise procedural risk. Specifically, the combined CRT-related AE/SAE risk (1.4%) was substantially lower than the 5.4 year follow-up MACE rate (8.2%). Prior studies have documented that MCD is associated with an adverse cardiovascular prognosis compared to asymptomatic women (3–4, 8–10). While clinical trials testing whether medical therapy reduces MACE in patients with MCD are needed, existing intermediate outcome trials suggest that endothelial function improves with treatment (20–23), as do signs and symptoms of ischemia (28). Accordingly, establishment of the diagnosis of MCD in these patients is important for appropriate medical management.
Coronary blood flow is regulated by various endothelial-dependent and non-endothelial-dependent factors. Non-endothelial-dependent factors include myocardial metabolism, myocardial compressive forces, aortic pressure, and neurohumoral substances (29). We measured CFR directly by the Doppler wire in response to adenosine, a non-endothelial dependent vasodilator (30). Acetylcholine was used to test endothelium-dependent function, as it stimulates nitric oxide release from the endothelial cells. Nitroglycerin response was used to test non-endothelial dependent macrovascular function. Procedural success rates were high in our study, and half of patients had an abnormal CRT in our patient population (Table 2).
Safety of IC Doppler flow measurement
IC Doppler measurement currently has a class IIb recommendation for assessment of the severity of coronary flow abnormalities in patients with angina, ischemia by stress testing, but no obstructive CAD (31). The Doppler wire used in our study is a 0.014-inch diameter flexible, steerable guide wire with a piezoelectric ultrasound transducer integrated into the tip (32–33). The Doppler wire may cause coronary spasm, which was previously seen in 1% of patients undergoing IC Doppler examination (34). Wire-induced spasm was relieved by IC nitroglycerin, similar to our contemporary study findings. A smaller study of 44 patients reported that no Doppler wire-related complications occurred in patients with normal or mild CAD (35). In our study, there was one coronary artery dissection (0.3%) that may be due to the Doppler wire. The dissection likely occurred during placement of the Doppler wire before reactivity testing. Focal vasospasm and staining of the mid left anterior descending artery were then noted during acetylcholine injection. No intervention was needed as the dissection was stable with no limitation of flow.
Safety of IC adenosine
The safety and use of IC boluses of adenosine is well established (36–38). In a study of 39 patients by Wilson et al (39), IC boluses (2–16 mcg) produced small, brief, dose-dependent reductions in mean arterial pressure and did not significantly change the PR, QRS, or QT intervals on the electrocardiogram, even when the drug was injected directly into the right coronary artery. However, in a study by Qian et al (34) of 906 patients, 14 patients experienced arrhythmias (7 asystole, 4 2nd degree AV block, 1 3rd degree AV block, 1 severe sinus bradycardia, 1 ventricular fibrillation), all whom received IC bolus of 12 mcg adenosine in the right coronary artery. One patient experienced sinus bradycardia and hypotension after 18 mcg of IC adenosine in the left anterior descending artery after stent implantation (34). In our study of 293 patients, neither the 18mcg nor the 36mcg dosages of adenosine resulted in any arrhythmias, and all cases were performed in the left coronary artery, showing that contemporary testing safety has improved and is safe.
Safety of IC acetylcholine
Acetylcholine has been used to evaluate coronary vasomotor function. Sueda et al (40) performed 1000 acetylcholine tests in Japanese men with and without obstructive CAD from 1991 to 2004. Incremental doses of 20/50/80 mcg into the right coronary artery and 20/50/100 mcg into the left coronary artery were injected over 20 seconds. They reported 17/1000 patients (1.7%) who experienced a major adverse reaction during acetylcholine infusion, including 11 nonsustained ventricular tachycardia (1.1%), one sustained ventricular tachycardia (0.1%), one ventricular fibrillation (0.1%), 3 shock due to left main stem spasm (0.3%), and one cardiac tamponade (0.1%). No serious complications, such as death, stroke or acute MI, were observed in this study.
More recently, the CASPAR (Coronary Artery Spasm in Patients with Acute Coronary Syndrome) study investigators injected incremental doses of acetylcholine (2/20/100 mcg over 3 minutes) into the left coronary artery and/or right coronary artery of 86 patients (15). Coronary vasospasm was detected in 42 patients (49%). Ischemic ST-segment changes were seen in 20 patients (17 ST depressions, 3 ST elevation), but there were no clinical adverse events. In a study of nifedipine’s effect on endothelial dysfunction, investigators infused acetylcholine (0.36, 3.6, and 18 mcg/ml at 2 mL/min for 3 min) in either the left anterior descending artery or circumflex artery of 641 patients (41). Transient electrocardiographic changes were reported in 5 patients, while diffuse coronary vasoconstriction with hemodynamic instability occurred in 5 patients (0.78%) (one [0.16 %] which required resuscitation). One patient (0.16%) developed acute coronary syndrome and cardiac arrest in the catheterization laboratory, possibly related to acetylcholine.
In our similarly-sized study, acetylcholine infusions were well tolerated without significant hemodynamic changes, again suggesting that contemporary testing safety has improved. Although some patients did experience chest pain at higher doses of acetylcholine, we were careful in monitoring coronary flow throughout acetylcholine infusion to assess for significant spasm. Five patients (2.3%) developed acetylcholine-induced vasospasm which immediately resolved after nitroglycerin injection, with no further sequelae. The higher doses of acetylcholine used in the CASPAR study (2/20/100 mcg) likely caused more coronary vasospasm than the lower doses of acetylcholine used in our study (0.364/3.64/36.4 mcg). Our case of coronary artery dissection was likely due to the Doppler wire rather than vasospasm from acetylcholine.
Limitations
As per WISE protocol, CRT was performed in the left anterior descending coronary artery in all patients. Safety of CRT in the right coronary artery or left circumflex artery was not evaluated. Acetylcholine is not directly infused into the left anterior descending artery but rather infused through the guiding catheter in the left main coronary artery, and thus the concentration of the acetylcholine may be diluted in the left anterior descending artery. Since vasoactive substances are infused in the left main artery, both the circumflex and the left anterior descending artery are susceptible to vasoconstrictive effects. The protocol stipulated for the Doppler wire to be maintained in the proximal left anterior descending artery. Therefore, the safety of CRT when the Doppler wire is placed more distally is unknown. It is also difficult to accurately perform QCA in distal vessels due to smaller diameter.
Conclusion
In women undergoing CRT for suspected MCD, contemporary testing is relatively safe with a low adverse event rate when using standardized protocols for IC adenosine, acetylcholine, and nitroglycerin delivery in experienced centers. These results support the use of CRT by experienced operators for diagnostic and prognostic purposes in patients with persistent angina, evidence of myocardial ischemia, and no obstructive CAD. Prior studies investigating therapy directed at improvement of MCD have shown reduction of angina, vasospasm, heart failure, and stroke. Additional studies are needed to demonstrate improvement in cardiovascular outcomes.
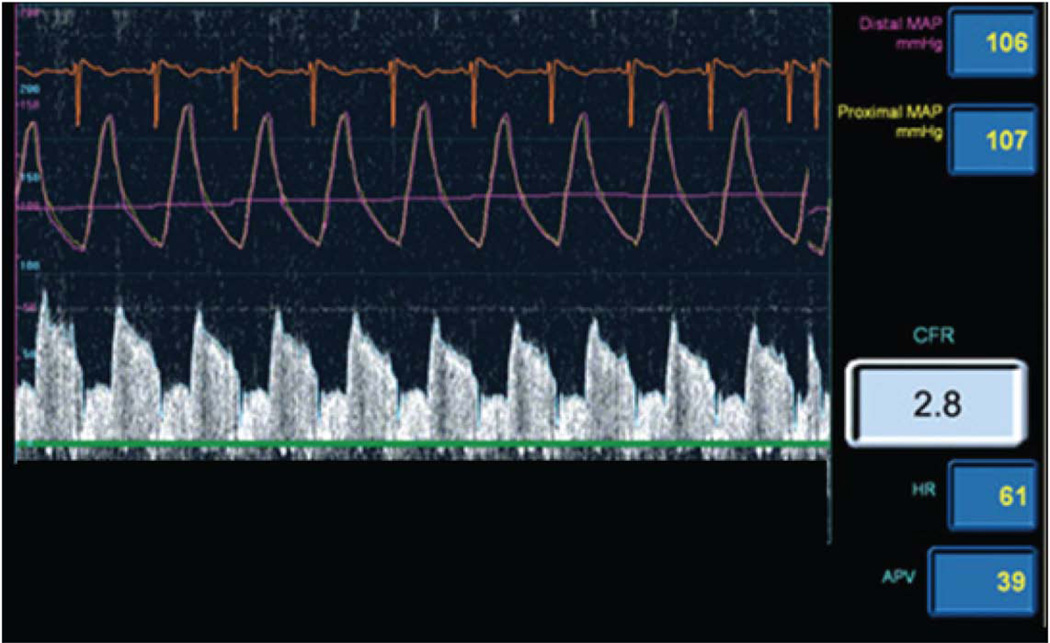
Figure 1. Example Doppler Wire Tracing.
Figure depicts coronary flow velocity map showing an average peak velocity of 39 and a coronary flow reserve of 2.8 in response to adenosine, as determined by a Doppler flow wire in the coronary artery. Adenosine tests non-endothelial dependent microvascular vasodilatory capacity. Coronary blood flow is calculated based on the change in diameter of the vessel and change in velocity in response to acetylcholine. Acetylcholine tests endothelial-dependent vasomotor function.
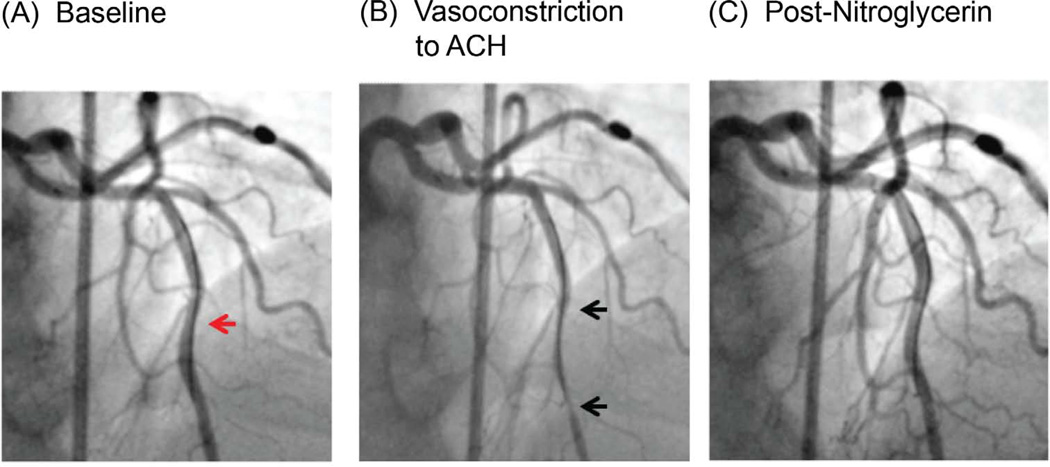
Figure 2. Coronary angiogram and Coronary Reactivity Testing.
Figure shows Doppler flow wire in the left anterior descending artery (red arrow) (A). In response to acetylcholine infusion, there is abnormal coronary artery vasoconstriction (black arrows), indicating endothelial dysfunction (B). This is resolved by IC nitroglycerin (C).
Acknowledgments
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, T32HL69751, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad Women’s Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, California, and the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles.
Abbreviations
- AE
Adverse events
- CAD
Coronary artery disease
- CFR
Coronary flow reserve
- CRT
Coronary Reactivity Testing
- IC
Intracoronary
- MACE
Major Adverse Cardiovascular Events
- MCD
Microvascular Coronary Dysfunction
- MI
Myocardial infarction
- SAE
Serious adverse events
- QCA
Quantitative coronary angiography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: There are no relevant conflicts of interest of any of the authors to disclose.
Clinical Trial: WISE; http://clinicaltrials.gov/ct2/show/NCT00832702; NCT00832702
References
- 1.Jespersen L, Hvelplund A, Abildstrom SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RD, Pepine CJ. Gender differences in the treatment for acute myocardial infarction: bias or biology? Circulation. 2007;115:823–826. doi: 10.1161/CIRCULATIONAHA.106.685859. [DOI] [PubMed] [Google Scholar]
- 3.Reis SE, Holubkov R, Conrad Smith AJ, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–741. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 4.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds HR, Srichai MB, Iqbal SN, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–1425. doi: 10.1161/CIRCULATIONAHA.111.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpiuc KT, Wingard DL, Kritz-Silverstein D, Barrett-Connor E. The association of angina pectoris with heart disease mortality among men and women by diabetes status: the Rancho Bernardo Study. J Womens Health (Larchmt) 2010;19:1433–1439. doi: 10.1089/jwh.2009.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemingway H, Langenberg C, Damant J, Frost C, Pyorala K, Barrett-Connor E. Prevalence of angina in women versus men: a systematic review and meta-analysis of international variations across 31 countries. Circulation. 2008;117:1526–1536. doi: 10.1161/CIRCULATIONAHA.107.720953. [DOI] [PubMed] [Google Scholar]
- 8.Johnson BD, Shaw LJ, Pepine CJ, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women's Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27:1408–1415. doi: 10.1093/eurheartj/ehl040. [DOI] [PubMed] [Google Scholar]
- 9.von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 10.Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–850. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanza GA, Buffon A, Sestito A, et al. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol. 2008;51:466–472. doi: 10.1016/j.jacc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 12.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 13.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 14.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 15.Ong P, Athanasiadis A, Hill S, Vogelsberg H, Voehringer M, Sechtem U. Coronary artery spasm as a frequent cause of acute coronary syndrome: The CASPAR (Coronary Artery Spasm in Patients With Acute Coronary Syndrome) Study. J Am Coll Cardiol. 2008;52:523–527. doi: 10.1016/j.jacc.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 16.Wakabayashi K, Suzuki H, Honda Y, et al. Provoked coronary spasm predicts adverse outcome in patients with acute myocardial infarction: a novel predictor of prognosis after acute myocardial infarction. J Am Coll Cardiol. 2008;52:518–522. doi: 10.1016/j.jacc.2008.01.076. [DOI] [PubMed] [Google Scholar]
- 17.Sovari AA, Cesario D, Kocheril AG, Brugada R. Multiple episodes of ventricular tachycardia induced by silent coronary vasospasm. J Interv Card Electrophysiol. 2008;21:223–226. doi: 10.1007/s10840-008-9207-4. [DOI] [PubMed] [Google Scholar]
- 18.MacAlpin RN. Cardiac arrest and sudden unexpected death in variant angina: complications of coronary spasm that can occur in the absence of severe organic coronary stenosis. Am Heart J. 1993;125:1011–1017. doi: 10.1016/0002-8703(93)90108-l. [DOI] [PubMed] [Google Scholar]
- 19.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonetti PO, Barsness GW, Keelan PC, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–1768. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 21.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 22.Yasue H, Mizuno Y, Harada E, et al. Effects of a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, fluvastatin, on coronary spasm after withdrawal of calcium-channel blockers. J Am Coll Cardiol. 2008;51:1742–1748. doi: 10.1016/j.jacc.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 23.Pauly DF, Johnson BD, Anderson RD, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women's Ischemia Syndrome Evaluation (WISE) Am Heart J. 2011;162:678–684. doi: 10.1016/j.ahj.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 25.Bairey Merz CN, Shaw LJ, Reis SE, et al. Insights from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–S29. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 26.Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1999;33:1756–1824. doi: 10.1016/s0735-1097(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy JW. Complications associated with cardiac catheterization and angiography. Cathet Cardiovasc Diagn. 1982;8:5–11. doi: 10.1002/ccd.1810080103. [DOI] [PubMed] [Google Scholar]
- 28.Mehta PK, Goykhman P, Thomson LE, et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging. 2011;4:514–522. doi: 10.1016/j.jcmg.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bugiardini R, Bairey Merz CN. Angina with "normal" coronary arteries: a changing philosophy. JAMA. 2005;293:477–484. doi: 10.1001/jama.293.4.477. [DOI] [PubMed] [Google Scholar]
- 30.Abebe W, Makujina SR, Mustafa SJ. Adenosine receptor-mediated relaxation of porcine coronary artery in presence and absence of endothelium. Am J Physiol. 1994;266:H2018–H2025. doi: 10.1152/ajpheart.1994.266.5.H2018. [DOI] [PubMed] [Google Scholar]
- 31.Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. Circulation. 1999;99:2345–257. doi: 10.1161/01.cir.99.17.2345. [DOI] [PubMed] [Google Scholar]
- 32.Doucette JW, Corl PD, Payne HM, et al. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85:1899–1911. doi: 10.1161/01.cir.85.5.1899. [DOI] [PubMed] [Google Scholar]
- 33.Bach RG, Kern MJ. Practical coronary physiology. Clinical application of the Doppler flow velocity guide wire. Cardiol Clin. 1997;15:77–99. doi: 10.1016/s0733-8651(05)70320-9. [DOI] [PubMed] [Google Scholar]
- 34.Qian J, Ge J, Baumgart D, et al. Safety of intracoronary Doppler flow measurement. Am Heart J. 2000;140:502–510. doi: 10.1067/mhj.2000.109221. [DOI] [PubMed] [Google Scholar]
- 35.Mechem C, Kern MJ, Aguirre F, Cauley M, Stonner T. Safety and outcome of angioplasty guidewire Doppler instrumentation in patients with normal or mildly diseased coronary arteries. Circulation. 1992;86:I–323. [Google Scholar]
- 36.Rieber J, Jung P, Schiele TM, et al. Safety of FFR-based treatment strategies: the Munich experience. Z Kardiol. 2002;91 Suppl 3:115–119. doi: 10.1007/s00392-002-1321-1. [DOI] [PubMed] [Google Scholar]
- 37.Jeremias A, Whitbourn RJ, Filardo SD, et al. Adequacy of intracoronary versus intravenous adenosine-induced maximal coronary hyperemia for fractional flow reserve measurements. Am Heart J. 2000;140:651–657. doi: 10.1067/mhj.2000.109920. [DOI] [PubMed] [Google Scholar]
- 38.De Bruyne B, Pijls NH, Barbato E, et al. Intracoronary and intravenous adenosine 5'-triphosphate, adenosine, papaverine, and contrast medium to assess fractional flow reserve in humans. Circulation. 2003;107:1877–1883. doi: 10.1161/01.CIR.0000061950.24940.88. [DOI] [PubMed] [Google Scholar]
- 39.Wilson RF, Wyche K, Christensen BV, Zimmer S, Laxson DD. Effects of adenosine on human coronary arterial circulation. Circulation. 1990;82:1595–1606. doi: 10.1161/01.cir.82.5.1595. [DOI] [PubMed] [Google Scholar]
- 40.Sueda S, Oshita A, Nomoto T, et al. Recommendations for performing acetylcholine tests safely: STOP dangerous complications induced by acetylcholine tests (STOP DCIAT) J Cardiol. 2008;51:131–134. doi: 10.1016/j.jjcc.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Luscher TF, Pieper M, Tendera M, et al. A randomized placebo-controlled study on the effect of nifedipine on coronary endothelial function and plaque formation in patients with coronary artery disease: the ENCORE II study. Eur Heart J. 2009;30:1590–1597. doi: 10.1093/eurheartj/ehp151. [DOI] [PMC free article] [PubMed] [Google Scholar]