Abstract
Problem
Virtually all women who have cervical cancer are infected with the human papillomavirus (HPV). Of the 275 000 women who die from cervical cancer every year, 88% live in developing countries. Two vaccines against the HPV have been approved. However, vaccine implementation in low-income countries tends to lag behind implementation in high-income countries by 15 to 20 years.
Approach
In 2011, Rwanda’s Ministry of Health partnered with Merck to offer the Gardasil HPV vaccine to all girls of appropriate age. The Ministry formed a “public–private community partnership” to ensure effective and equitable delivery.
Local setting
Thanks to a strong national focus on health systems strengthening, more than 90% of all Rwandan infants aged 12–23 months receive all basic immunizations recommended by the World Health Organization.
Relevant changes
In 2011, Rwanda’s HPV vaccination programme achieved 93.23% coverage after the first three-dose course of vaccination among girls in grade six. This was made possible through school-based vaccination and community involvement in identifying girls absent from or not enrolled in school. A nationwide sensitization campaign preceded delivery of the first dose.
Lessons learnt
Through a series of innovative partnerships, Rwanda reduced the historical two-decade gap in vaccine introduction between high- and low-income countries to just five years. High coverage rates were achieved due to a delivery strategy that built on Rwanda’s strong vaccination system and human resources framework. Following the GAVI Alliance’s decision to begin financing HPV vaccination, Rwanda’s example should motivate other countries to explore universal HPV vaccine coverage, although implementation must be tailored to the local context.
Résumé
Problème
Pratiquement toutes les femmes touchées par le cancer du col de l’utérus sont infectées par le virus du papillome humain (VPH). Parmi les 275 000 femmes qui meurent chaque année d'un cancer du col utérin, 88% d’entre elles vivent dans des pays en voie de développement. Deux vaccins contre le VPH ont été approuvés. Toutefois, l'instauration du vaccin dans les pays à revenu faible reste à la traîne depuis 15 à 20 ans par rapport à sa mise en œuvre dans les pays à revenu élevé.
Approche
En 2011, le ministère de la Santé du Rwanda a créé un partenariat avec Merck pour offrir le vaccin Gardasil contre le VPH à toutes les filles d'âge approprié. Le ministère a établi un «partenariat communautaire public-privé» pour en assurer une délivrance efficace et équitable.
Environnement local
Grâce à un effort national majeur en matière de renforcement des systèmes de santé, plus de 90% de tous les nourrissons rwandais âgé de 12 à 23 mois reçoivent l’ensemble des vaccins de base recommandés par l'Organisation mondiale de la Santé.
Changements significatifs
En 2011, le programme rwandais de vaccination contre le VPH a atteint une couverture de 93,23% après la première cycle de trois doses de vaccin parmi les filles de niveau CM2. Cela a été rendu possible grâce à une vaccination en milieu scolaire et à l'implication communautaire dans l'identification des filles absentes ou non inscrites à l'école. Une campagne nationale de sensibilisation a précédé l'administration de la première dose.
Leçons tirées
Grâce à une série de partenariats novateurs, le Rwanda a réduit l'écart historique de deux décennies à seulement cinq ans quant à l'introduction d'un vaccin dans les pays à revenu élevé et faible. Des taux de couverture élevés ont été atteints grâce à une stratégie de délivrance basée sur un système de vaccination solide au Rwanda et à une organisation efficace des ressources humaines. Suite à la décision de l'Alliance GAVI d'initier le financement de la vaccination contre le VPH, l'exemple du Rwanda devrait inciter d'autres pays à envisager une couverture totale du vaccin contre le VPH, bien que la mise en œuvre doive être adaptée au contexte local.
Resumen
Situación
Casi todas las mujeres que tienen cáncer cervicouterino están infectadas con el virus del papiloma humano (VPH). De las 275 000 mujeres que fallecen anualmente debido al cáncer cervicouterino, el 88% vive en países en desarrollo. Se han aprobado dos vacunas contra el VPH, no obstante, la implantación de la vacuna en países de ingresos bajos tiende a quedar entre 15 y 20 años por detrás de su aplicación en países de ingresos altos.
Enfoque
En 2011, el Ministerio de Sanidad de Rwanda se asoció con Merck para ofrecer la vacuna Gardasil contra el VPH a todas las niñas en la edad adecuada. El Ministerio formó una «asociación comunitaria público-privada» para garantizar un reparto eficaz y equitativo de la misma.
Marco regional
Más del 90% de los niños de 12 a 23 meses de edad en Rwanda ha recibido todas las vacunas básicas recomendadas por la Organización Mundial de la Salud gracias a los esfuerzos gubernamentales por mejorar los sistemas sanitarios.
Cambios importantes
El programa de vacunación contra el VPH en Rwanda alcanzó una cobertura del 93,23% entre las niñas de sexto curso en el año 2011 al cabo de la primera ronda de vacunación con las tres dosis. Esto fue posible a través de la vacunación escolar y gracias a la participación de la comunidad para identificar a las niñas que faltaban o no estaban matriculadas en el colegio. Una campaña de sensibilización a escala nacional precedió la entrega de la primera dosis.
Lecciones aprendidas
A través de una serie de asociaciones innovadoras, Rwanda redujo a tan solo cinco años la brecha histórica de dos décadas en cuanto a la introducción de la vacuna entre países de ingresos altos y bajos. Una estrategia de reparto construida sobre el sólido sistema de vacunación y el marco de recursos humanos de Rwanda permitió alcanzar una cobertura elevada. Siguiendo la decisión de la GAVI Alliance de comenzar a financiar la vacuna contra el VPH, el ejemplo de Rwanda debería animar a otros países a investigar la cobertura de la vacuna universal contra el VPH, aunque su aplicación deberá adaptarse al contexto local.
ملخص
المشكلة
جميع السيدات المصابات بسرطان عنق الرحم تقريباً مصابات بعدوى فيروس الورم الحليمي البشري (HPV). وتعيش نسبة 88 % من إجمالي 275000 امرأة تتعرض للوفاة من جراء سرطان عنق الرحم سنويًا في البلدان النامية. وتمت الموافقة على لقاحين لمكافحة فيروس الورم الحليمي البشري. ومع ذلك، يميل تنفيذ اللقاح في البلدان منخفضة الدخل إلى التأخر عن التنفيذ في البلدان مرتفعة الدخل بنحو 15 إلى 20 سنة.
الأسلوب
في عام 2011، عقدت وزارة الصحة الرواندية شراكة مع ميرك لتوفير لقاح فيروس الورم الحليمي البشري Gardasilلجميع الفتيات من الأعمار المناسبة. وشكلت الوزارة "شراكة بين المجتمع المحلي العام والخاص" لضمان إيتاء اللقاح بشكل فعال وعادل.
المواقع المحلية
بفضل التركيز الوطني القوي على تعزيز النظم الصحية، يحصل ما يزيد عن 90 % من جميع الرضع الروانديين الذين تتراوح أعمارهم بين و 12 23 شهرا على جميع خدمات التمنيع التي توصي بها منظمة الصحة العالمية.
التغيّرات ذات الصلة
في عام 2011، حقق برنامج التمنيع ضد فيروس الورم الحليمي البشري في رواندا تغطية بنسبة 93.23 % بعد أول دفعة من جرعات اللقاح الثلاث بين الفتيات في الصف السادس. وتحقق هذا من خلال التمنيع المدرسي وإشراك المجتمع المحلي في تحديد الفتيات المتغيبات عن المدرسة أو غير المسجلات بها. وسبق إيتاء الجرعة الأولى حملة توعية على الصعيد الوطني.
الدروس المستفادة
خفضت رواندا من خلال سلسلة من الشراكات الابتكارية ثغرة العقدين التاريخية بالبدء في استعمال اللقاح بين البلدان مرتفعة ومنخفضة الدخل إلى خمس سنوات فقط. وتعزى معدلات التغطية العالية التي تم تحقيقها إلى إستراتيجية الإيتاء التي قامت على أساس نظام التمنيع وإطار الموارد البشرية القويين في رواندا. وفي أعقاب قرار التحالف العالمي من أجل اللقاحات والتمنيع ببدء تمويل التمنيع ضد فيروس الورم الحليمي البشري، ينبغي أن يدفع نموذج رواندا البلدان الأخرى إلى بحث التغطية الشاملة للقاح فيروس الورم الحليمي البشري على الرغم من ضرورة ملائمة التنفيذ للسياق المحلي.
摘要
问题
患有宫颈癌的妇女几乎都受到人类乳头瘤病毒(HPV)感染。每年有 27.5 万名妇女死于宫颈癌,其中88%生活在发展中国家。两种预防HPV的疫苗已通过审批。然而,较之高收入国家,低收入国家的疫苗实施情况往往要落后15 至20 年。
方法
2011 年,卢旺达卫生部与默克公司合作,为所有适龄女孩提供加德西HPV疫苗。卫生部形成“公私社区合作伙伴关系”,以确保公平有效地进行疫苗接种。
当地状况
由于国家对强化卫生系统的大力关注,卢旺达所有12到23个月年龄的婴儿中超过90%接受了世界卫生组织推荐的所有基本免疫接种。
相关变化
2011 年,卢旺达在对六年级的女孩进行了前三个疫苗剂量的接种之后, 其HPV疫苗接种计划取得了93.23%的覆盖率。这是通过基于学校的疫苗接种和社区参与确定女孩是否缺课或是没有入学得以实现的。在进行第一剂量接种之前开展了全国性的皮试活动。
经验教训
通过一系列的创新合作伙伴关系,卢旺达把历史上高收入和低收入国家之间疫苗引进方面二十年的差距缩短为短短五年。由于在卢旺达强有力的疫苗接种系统和人力资源框架中建立了实施战略,所以取得了高覆盖率。在全球疫苗免疫联盟决定开始为HPV免疫筹资之后,尽管实施方案必须因地制宜,卢旺达的例子仍会调动其他国家探索普遍的HPV疫苗接种覆盖率。
Резюме
Проблема
Практически все женщины, у которых диагностирован рак шейки матки, инфицированы вирусом папилломы человека (ВПЧ).Из275 000женщин, которые ежегодно умирают от рака шейки матки,88%живут в развивающихся странах. Были одобрены две вакцины против ВПЧ.Тем не менее, страны с низким уровнем дохода имеет тенденцию отставать в вопросах вакцинации от стран с высоким доходом на 15-20 лет.
Подход
В 2011 году Министерство здравоохранения Руанды в сотрудничестве с компанией Merck начало программу вакцинации для всех девочек соответствующего возраста вакциной Гардасил (Gardasil) против ВПЧ. Министерство создало«государственно-частное партнерство», чтобы обеспечить эффективную и равную вакцинацию.
Местные условия
Благодаря пристальному вниманию государства к укреплению систем здравоохранения более 90% детей в Руанде получают все основные прививки, рекомендованные Всемирной организацией здравоохранения.
Осуществленные перемены
В 2011 г.программа вакцинации против ВПЧ в Руанде охватила первой из трех доз вакцины 93,23% девочек, учащихся в шестых классах. Это стало возможным благодаря школьной вакцинации и участию общественности в нахождении девочек, которые отсутствовали или вообще не ходили в школу. Введению первой дозы предшествовала общенациональная кампания сенсибилизации.
Выводы
С помощью ряда инновационных партнерств Руанде удалось за пять лет сократить исторический разрыв в вопросах вакцинации размером в два десятилетия, существующий между странами с высоким и низким уровнем дохода. Высокие показатели охвата были достигнуты за счет стратегии, построенной на сильной системе вакцинации в Руанде и высоком человеческом потенциале. Вслед за решением Альянса GAVI начать финансирование вакцинации против ВПЧ, пример Руанды должен мотивировать другие страны рассмотреть вопрос универсального охвата вакциной против ВПЧ, однако реализация данных мероприятий должна быть адаптирована к местным условиям.
Background
Human papillomavirus (HPV) is a sexually transmitted virus found in virtually all cases of cervical cancer,1 which kills 275 000 women every year2 and is the biggest contributor to years of life lost from cancer among women in the developing world.3 Two HPV vaccines developed in recent years – Merck’s quadrivalent Gardasil and GlaxoSmithKline’s bivalent Cervarix – have been prequalified by the World Health Organization (WHO) and approved by national governments in many countries, including Rwanda. These vaccines represent a milestone in biomedical progress towards mitigating the burden of cervical cancer.4 Vaccination programmes have changed the landscape of global health drastically by enabling countries to re-evaluate funding priorities and to invest in prevention when cost-effective.5 However, 15 to 20 years usually pass between the introduction of a vaccine in rich and poor countries.6
In recent decades, routine screening programmes have reduced cervical cancer morbidity and mortality in high-income countries, and this trend is expected to accelerate as vaccination coverage is scaled up.7 As a result, 77% of new cases8 and 88% of deaths from the disease now occur in developing countries.2 A five-year delay in introducing the HPV vaccine to these countries would result in 1.5 to 2 million preventable deaths.2
Cervical cancer is the most common cancer among women in Rwanda,9 a country in eastern African with more than 11 million inhabitants. In 2010, 986 cases of cervical cancer were diagnosed in Rwanda and 678 women died from the disease.9 That same year, Rwanda evaluated options for HPV vaccination rollout and decided to pursue a partnership with Merck to offer Rwanda’s young girls the opportunity to receive a life-saving vaccine.
A growing body of scientific literature has emerged in recent years concerning the use of the HPV vaccine in the developing world,2,10 but very little evidence on the success and replicability of nationwide delivery programmes, like Rwanda’s, has been published. In this article, we describe the process by which Rwanda became the world’s first low-income country to provide universal access to the HPV vaccine.
Context
Before 2011, neither cervical cancer screening nor HPV vaccination was available in public health facilities in Rwanda; only a few private clinics and nongovernmental organizations offered screening services. Aware that cervical cancer is highly prevalent in Rwanda, the Ministry of Health considered the overwhelmingly positive evidence of the effectiveness of the HPV vaccine to be a call to action.
In April 2009, Rwanda’s First Lady met with senior Merck officials to initiate advocacy for the HPV vaccine on behalf of women in Rwanda. An internal technical discussion then occurred between Merck and the Ministry of Health, followed by conversations with development partners in Rwanda’s health sector. In April and October 2010, Merck representatives visited Rwanda to work under the leadership of the Ministry of Health in developing a national cervical cancer prevention strategy. Rwanda’s National Strategic Plan for the Prevention, Control, and Management of Cervical Lesions and Cancer11 includes screening and vaccination programmes for women between 35 and 45 years of age, the specifics of which will be detailed in future articles. A memorandum of understanding signed in December 2010 guaranteed Rwanda three years of vaccinations at no cost and concessional prices for future doses.
On 26 and 27 April 2011, 93 888 Rwandan girls in primary grade six received their first shot of Gardasil with no out-of-pocket payment. Data collected by the Ministry of Health during the first round of vaccination showed that 94 141 girls were present at school and that 4 651 girls were either absent or not enrolled, resulting in a total of 98 792 eligible girls across Rwanda. The programme’s first round achieved 95.04% coverage, the second 93.90% and the third, 93.23% (Table 1).
Table 1. Cumulative human papillomavirus vaccination coverage, by vaccination round, Rwanda, 2011.
| Coverage | Round 1 | Round 2 | Round 3 |
|---|---|---|---|
| Girls vaccinated in school, no. | 91 752 | 89 704 | 88 927 |
| Girls vaccinated outside school, no. | 2 136 | 3 066 | 3 180 |
| Total no. of girls vaccinated | 93 888 | 92 770 | 92 107 |
| Cumulative coverage (%) | 95.04 | 93.90 | 93.23 |
Rwanda’s health sector
Driven by the pursuit of equity, value and quality, the health sector in Rwanda has made immense progress in recent years. Through a decentralization strategy harnessing Rwanda’s 45 000 community health workers, the Ministry of Health and its partners have succeeded in decreasing the burden of major infectious diseases and have scaled up disease prevention programmes efficiently and effectively. Between 2005 and 2010, Rwanda reduced malaria incidence by 70% and under-five mortality by 50%. The country increased the proportion of infants aged 12–23 months receiving all basic WHO immunizations from 75.2% to 90.1% (with all but oral polio vaccine coverage rates exceeding 95% in 2010) and assured universal access to antiretroviral therapy.12,13 Rwanda has also strategically positioned itself to tackle non-communicable diseases by building on its successful integration of infectious disease interventions and primary health care.14,15
Preparation and implementation
Three early decisions were crucial to the success of Rwanda’s HPV vaccine rollout. First, the Ministry of Health decided in September 2010 to widen its technical working group on vaccinations to include the Ministry of Education, the Ministry of Gender and Family Promotion, the Center for Treatment and Research on AIDS, Tuberculosis, Malaria, and other Epidemics, and health workers engaged in cancer care. Multidisciplinary subcommittees were tasked with identifying cold chain requirements; numbers of girls in and out of school; nurse and community health worker training capacity; procurement and distribution logistics; a budget for implementation; education and sensitization requirements; and tools for data collection and social mobilization. Committees met as often as needed – often every other day – for about four months. After the memorandum of understanding between Merck and the government of Rwanda was signed, the following two months were spent devising a strategic plan for rollout preparation, implementation and evaluation.
Concurrently, a nationwide population sensitization campaign was planned and undertaken months in advance of vaccination. Speeches by health-care professionals, local government officials, clergy and the First Lady were made to inform parents and children of the new vaccine. Announcements via newspapers, magazines, radio and television were included in the communications strategy. Teachers were trained and encouraged to discuss cervical cancer and the HPV vaccine with students.
With technical assistance from the US Centers for Disease Control and Prevention (CDC) and the International Center for AIDS Care and Treatment Programs (ICAP) at Columbia University, Rwanda's Ministry of Health conducted a situational analysis in March 2011 using in-depth interviews and focus group discussions to assess the knowledge, attitudes and beliefs of key stakeholders pertaining to HPV vaccination. The systematic inclusion of local leaders, community health workers and teachers in the vaccine delivery strategy combined with Merck’s in-kind support to constitute a “public-private-community partnership” for effective programme implementation specific to the Rwandan context.
The second major decision was to partner with the Ministry of Education to design a school-based strategy to deliver the standard three doses of the HPV vaccine. Because 98% of Rwandan girls attend primary school,16 implementers felt that the highest coverage rates could be attained through schools. Some girls in rural settings are unaware of their exact ages, and a demonstration project in Uganda described difficulties identifying girls through an age-based programme,10 so Rwanda elected to pursue a grade-based strategy.
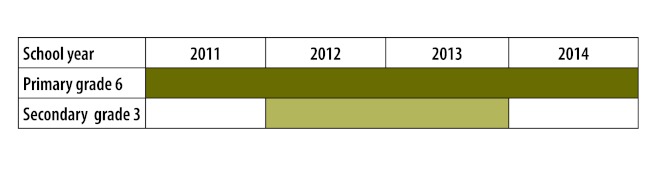
Third, the technical working group decided on a multi-phased vaccination strategy spanning three years (Fig. 1). Every year beginning in 2011, girls enrolled in primary grade six will receive the full three-dose course of HPV vaccine. During the programme’s second and third years, a “catch-up” phase targeting girls in the third year of secondary school will ensure complete coverage of all pre-adolescent and adolescent girls. In 2014 and beyond, only primary grade six vaccinations will be necessary.
Fig. 1.
Human papillomavirus vaccination rollout phases, Rwanda, 2011–2014
Each course of vaccination takes place over six days: school-based vaccinations across the country during the first two days, out-of-school girls are tracked and vaccinated on the third day, and surveillance of adverse events is conducted over the remaining three days. The vaccination is a voluntary opt-out intervention offered to all girls in the targeted age groups, regardless of their school enrolment status. Parents and guardians had been instructed through the massive communications and media campaigns to accompany their daughters to school on the day when the first dose was going to be administered. If a parent or guardian did not come to the school, the girl did not receive the vaccine. On the days when vaccines are administered, which the schools now designate as educational “health days”, local health-care providers and teachers discuss topics pertaining to hygiene, nutrition, infectious diseases and reproductive health.
The vaccination programme technical working group chose to target girls in primary grade six because most of them are not yet sexually active but have reached an appropriate age for initiating sexual education. In Rwanda, the median age of sexual debut is 20.7 years and only 2.7% of girls have had sex by age 15.13
Delivery challenges
During the first phase of the vaccination rollout, one of the greatest challenges faced by implementers was making the population across the country aware of eligibility guidelines for the HPV vaccination. Some parents wanted to have all of their children immunized, and several female teachers in schools where the vaccine was delivered also asked to be vaccinated. Through radio and on-site communication, representatives of the Ministry of Health acknowledged the potential benefit of vaccinating all women at risk for cervical cancer but explained that this national programme was intended only for girls before sexual debut. They also explained that there would be a “catch-up” phase of HPV vaccination targeting third-year secondary school students.
Targeted action was taken for the girls who were sick or otherwise absent from school on vaccination days, in addition to the small number of girls who should be in primary grade six but were not enrolled in school at the time of the vaccination rollout. To make sure that no eligible girls missed the opportunity to benefit from the vaccine, Rwanda’s 45 000 community health workers were mobilized for active tracing of girls who were enrolled in primary grade six but absent on a vaccination day, as well as the small number of girls who were 12 years old but not enrolled in school. After being identified by community health workers, girls from both groups were vaccinated at the local health centre (Table 2).
Table 2. Human papillomavirus vaccination coverage of girls not enrolled in or absent from school, by vaccination round, Rwanda, 2011.
| Coverage | Round 1 | Round 2 | Round 3 |
|---|---|---|---|
| Girls vaccinated outside school, no. | 2136 | 3066 | 3180 |
| Total girls outside school, no. | 4651 | 3679 | 3734 |
| Coverage of girls outside school (%) | 45.93 | 83.34 | 85.16 |
Resistance
The HPV vaccine rollout in Rwanda, like other progressive public health policies in Africa, has met with some resistance from the international community.17 Concerns over the initially high fixed costs of vaccination have led some to suggest that a focus on cervical cancer prevention might detract scarce resources from other more “cost-effective” child health interventions. Indeed, state-of-the-art health technologies are generally expensive and health ministries face difficult choices when making budget allocations. However, Rwanda acted on its decision to tackle non-communicable diseases in a systematic way by accepting Merck’s offer to provide free vaccines for the first three years of the HPV vaccination programme. High-level leaders are committed to ensuring the long-term integration of a rights-based cervical cancer prevention, care and treatment programme into the basic package of health services. As more low- and middle-income countries follow Rwanda’s example and confront the untallied cost of inaction with respect to cervical cancer, vaccine market dynamics and tiered pricing agreements will most likely drive down vaccine prices in these settings, as has occurred with the pneumococcal conjugate vaccine (which Rwanda was also the first low-income country to roll out).18
Implications
A successful nationwide HPV vaccination programme requires a well-established vaccine delivery system with adequate cold chain, transportation, human resources and monitoring capacity. Collaboration between public and private institutions within the framework of strong national ownership appears to be a prerequisite for long-term sustainability. To assure high vaccine uptake, the health system should have a strong outreach communication capacity to clearly address questions on the part of girls, parents and health workers about vaccine safety and efficacy and the exclusive focus on young adolescent girls.
Favourable outcomes from HPV vaccine rollouts and similar initiatives do not occur by chance; they result from strong coordination, national ownership, strategic planning, comprehensive monitoring (especially the reporting of side-effects and adverse reactions through teacher training) and good managerial capacity. Senior Ministry of Health officials in Rwanda acknowledged that an emphasis on health systems strengthening by the government, The GAVI Alliance, the President’s Emergency Plan for AIDS Relief and The Global Fund to Fight AIDS, Tuberculosis and Malaria was key to the success of HPV vaccine rollout in the country.
We are encouraged by the promising initial results of the HPV vaccination programme in Rwanda, which has attained 93.23% coverage after the first three-dose course of vaccination. Rwanda’s example should motivate other countries to expand their vaccination programmes to include the HPV vaccine, with due customization according to their epidemiological, economic, political, and health system contexts, especially now that the GAVI Alliance has included the vaccine in its immunization package and that Merck has lowered the price to 5 United States dollars per dose for GAVI Alliance eligible countries.19
Competing interests:
None declared.
References
- 1.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papilloma virus is a necessary cause of invasive cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin D. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Agosti JM, Goldie SJ. Introducing HPV vaccine in developing countries – key challenges and issues. N Engl J Med. 2007;356:1908–10. doi: 10.1056/NEJMp078053. [DOI] [PubMed] [Google Scholar]
- 4.Schiffman M, Wacholder S. Success of HPV vaccination is now a matter of coverage. Lancet Oncol. 2012;13:10–2. doi: 10.1016/S1470-2045(11)70324-2. [DOI] [PubMed] [Google Scholar]
- 5.Ehreth J. The global value of vaccination. Vaccine. 2003;21:596–600. doi: 10.1016/S0264-410X(02)00623-0. [DOI] [PubMed] [Google Scholar]
- 6.Raising the profile of pneumococcal disease: collaborations in communications efforts 2003–2008 Baltimore: PneumoADIP & GAVI; 2009. [Google Scholar]
- 7.Human papillomavirus (HPV) vaccine background paper Geneva: World Health Organization; 2008. Available from: http://www.who.int/immunization/documents/HPVBGpaper_final_03_04_2009.pdf [accessed 4 April 2012].
- 8.Forouzanfar M, Foreman K, Delossantos A, Lozano R, Lopez A, Murray C, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet 2011. Epub 14 Sept [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization [Internet]. Human papillomavirus and related cancers in Rwanda: summary report 2010. Geneva: WHO/ICO Information Centre on HPV and Cervical Cancer. Available from: www.who.int/hpvcentre [accessed 4 April 2012].
- 10.LaMontagne DS, Barge S, Le NT, Mugisha E, Penny ME, Gandhi S, et al. Human papillomavirus vaccine delivery strategies that achieved high coverage in low- and middle-income countries. Bull World Health Organ. 2011;89:821–30. doi: 10.2471/BLT.11.08986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Strategic Plan for Prevention, Control, and Management of Cervical Lesions and Cancer Kigali: Ministry of Health; 2010. [Google Scholar]
- 12.Rwanda Demographic and Health Survey 2005 Calverton: National Institute of Statistics of Rwanda & Macro International, Inc.; 2006. [Google Scholar]
- 13.Rwanda Demographic and Health Survey 2010 Calverton: National Institute of Statistics of Rwanda & Macro International, Inc.; 2012. [Google Scholar]
- 14.Price JE, Leslie JA, Welsh M, Binagwaho A. Integrating HIV clinical services into primary health care in Rwanda: a measure of quantitative effects. AIDS Care. 2009;21:608–14. doi: 10.1080/09540120802310957. [DOI] [PubMed] [Google Scholar]
- 15.The AIDS response and the Millennium Development Goals: Rwanda case study Geneva: Joint United Nations Programme for HIV/AIDS; 2010. [Google Scholar]
- 16.Ministry of Education of Rwanda [Internet]. Major achievements (2003–2010). Kigali: ME; 2011. Available from: http://www.mineduc.gov.rw/spip.php?article27
- 17.Binagwaho A, Wagner CM, Nutt CT. HPV Vaccine in Rwanda: Different Disease, Same Double Standard. Lancet. 2011;378:1916. doi: 10.1016/S0140-6736(11)61837-0. [DOI] [PubMed] [Google Scholar]
- 18.Hargreaves J, Greenwood B, Clift C, Goel A, Roemer-Mahler A, Smith R, et al. Making new vaccines affordable: a comparison of financing processes used to develop and deploy new meningococcal and pneumococcal conjugate vaccines. Lancet 2011. Epub 9 June [DOI] [PubMed] [Google Scholar]
- 19.GAVI Alliance [Internet]. GAVI welcomes lower prices for life-saving vaccines Geneva: GAVI; 2011. Available from: http://www.gavialliance.org/library/news/press-releases/2011/gavi-welcomes-lower-prices-for-life-saving-vaccines/ [accessed 4 April 2012].