Abstract
Herein, I describe pyridylamination for versatile analysis of sugar chains. The reducing ends of the sugar chains are tagged with 2-aminopyridine and the resultant chemically stable fluorescent derivatives are used for structural/functional analysis. Pyridylamination is an effective “operating system” for increasing sensitivity and simplifying the analytical procedures including mass spectrometry and NMR. Excellent separation of isomers is achieved by reversed-phase HPLC. However, separation is further improved by two-dimensional HPLC, which involves a combination of reversed-phase HPLC and size-fractionation HPLC. Moreover, a two-dimensional HPLC map is also useful for structural analysis. I describe a simple procedure for preparing homogeneous pyridylamino sugar chains that is less laborious than existing techniques and can be used for functional analysis (e.g., sugar-protein interaction). This novel approach was applied and some of the results are described: i) a glucosyl-serine type sugar chain found in blood coagulation factors; ii) discovery of endo-β-mannosidase (EC 3.2.1.152) and a new type plant α1,2-l-fucosidase; and iii) novel substrate specificity of a cytosolic α-mannosidase. Moreover, using homogeneous sugar chains of a size similar to in vivo substrates we were able to analyze interactions between sugar chains and proteins such as enzymes and lectins in detail. Interestingly, our studies reveal that some enzymes recognize a wider region of the substrate than anticipated.
Keywords: pyridylamination, sugar chains, two-dimensional HPLC, reversed-phase, fluorescent
1. Introduction
Sugar chains are characterized by their structural complexity and heterogeneity, which make them difficult to analyze. However, the chemical structures of the sugar chains must be determined in order to fully understand their function. Component sugars are infrequently recovered quantitatively in the acid hydrolysates of sugar chains. Consequently, this type of analysis often leads to incorrect molar ratios of the various types of sugars. Color reactions for detection and quantitative measurements of sugars were laborious procedures and not particularly sensitive for sugar chains obtained from living matter. Moreover separation of sugar chains with isomeric structures was difficult.
For the structure determination of sugar chains, homogeneous sugar chains are required. Considerable effort has been devoted to achieving resolution of isomeric structures using partition and adsorption chromatography and electrophoresis. Barker et al. and Frahn and Mills were first to introduce a new idea in which separation principles are classified into two kinds i.e., separation according to (i) molecular size or (ii) isomeric structure.1),2) The former procedure was realized by adding a positive charge to a sugar chain, allowing the derivatives to be separated by paper electrophoresis based on their molecular size. If this procedure is coupled with partition chromatography, effective separation of isomeric structures can be achieved. Moreover, analysis of this separation gives information concerning the structure of the sugar chain.1) Using this idea a new procedure of two-dimensional separation was eventually realized.3) In addition to these separation principles, I attempted to introduce a new concept into the field of sugar chain analysis as described in section 2.
2. Introduction of a new concept for the structural analysis of sugar chains
To solve the problems outlined above and to avoid ambiguity caused by the analytical manipulations, we introduced the new concept of pyridylamination3) in 1978. This procedure facilitates structure determination of sugar chains with less laborious experimental manipulations and high sensitivity.
3. Tagging of sugar chains with 2-aminopyridine
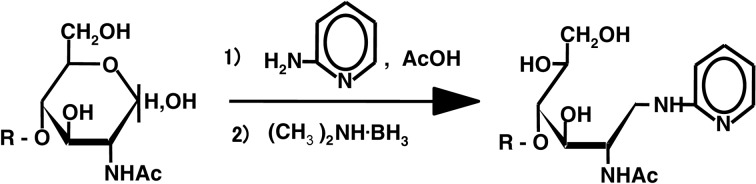
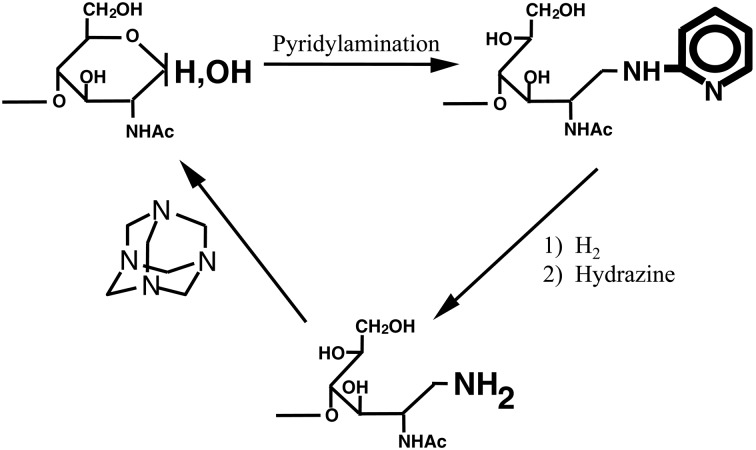
The concept was realized by a procedure which involves tagging a sugar chain with a fluorescent compound.3) This derivatization facilitates easy detection of sugars and provides a positive charge to separate the derivatized sugar chains according to their molecular size by paper electrophoresis. We selected 2-aminopyridine as the fluorescent label and aimed to tag the free aldehyde group of the sugar chain (i.e., reducing end) by reductive amination.3) Using this procedure, the pyridylamino derivative (PA) of the sugar chain is obtained almost quantitatively (Fig. 1).4) Recently, the pyridylamination reaction has been achieved by gas-phase reaction.5) The pyridylamino group of derivatives are fluorescent (excitation wavelength of 320 nm, emission wavelength of 400 nm, a blue spot under a UV-lamp) and sufficiently stable for structural determination. Thus, structure determination can be performed without loss of fluorescence.
Fig. 1.
Tagging of the reducing end of a sugar chain with 2-aminopyridine. The pyridylaminated sugar chain thus obtained is fluorescent. Under a UV-lamp a blue spot is observed. A spot containing >0.2 nmol of a PA-sugar chain can be detected by eye. R, sugar residues.
After our original paper on the fluorescent labeling of sugar chains in 1978 more than 40 derivatives based on the same concept have been reported in the literature.6) Our novel method affords the analysis of complex sugar chains owing to: (i) highly effective separation by reversed-phase HPLC; (ii) sensitive and convenient detection of the fluorescent tag. The fluorescence labeled or pyridylaminated (PA-) sugar chains were then used for structural determination. By following this procedure, only a small amount of sample is required and the lengthy protocol for analytical procedures is considerably simplified. The procedure also acts as a link between analytical methodologies, used for structural determination, and analyses of sugar chains by complementarity (e.g., by employing specific antibodies or lectins).
After introducing the present concept, new procedures were derived such as two dimensional sugar mapping, structural analysis by partial degradation and differential display of glycans, and so on. Some results obtained by the present method from the author’s laboratory are described below.
4. New developments introduced by pyridylamination
Derivatization of the sugar chains facilitated some new developments in the analysis of sugar chains.
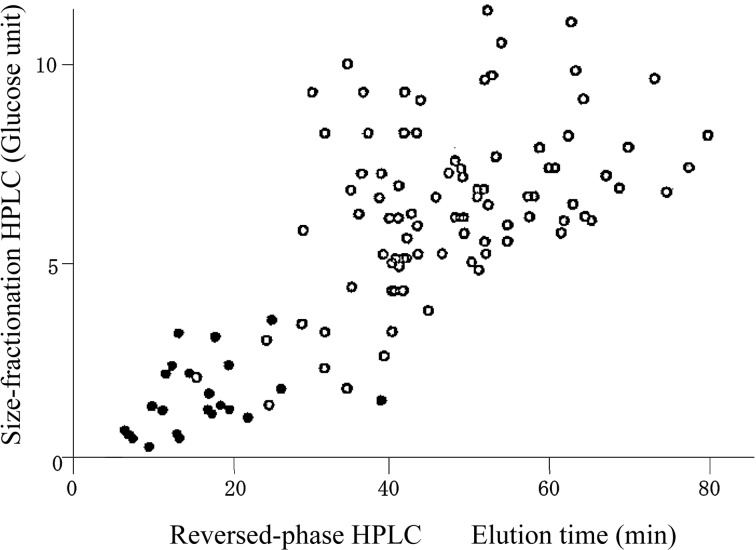
Two-dimensional HPLC: Separation of the sugar chains by means of two different approaches was realized by using the corresponding PA-sugar derivatives. Specifically, separation according to molecular size was done by paper electrophoresis using the positive charge of the PA-group. Separation of isomeric structures is made possible due to the formation of a borate complex (Fig. 2).3) Two-dimensional paper electrophoresis was later replaced by HPLC (Fig. 3).7),8) Size-fractionation HPLC, introduced by Mellis and Baenziger for separation by molecular size,9) was selected. Reversed-phase HPLC for separation of isomers was employed as described below.
High sensitivity. PA-sugar chains are detected with high sensitivity. One femtomole of PA-sugar chain can be detected using a commercially available HPLC apparatus coupled with a fluorescence detector. Pyridylamination (fluorescence labeling) is an effective “operating system” that increases the sensitivity of classical procedures and makes the procedures less laborious for structure determination. Moreover, simple detection is achieved by fluorescence, which does not result in loss of samples. Indeed, pyridylamination increased the sensitivity of mass spectrometric analysis for the detection of sugar chains,10) and recently fluorescence labeling more suitable for mass analysis like pyrene derivatives was reported.11)
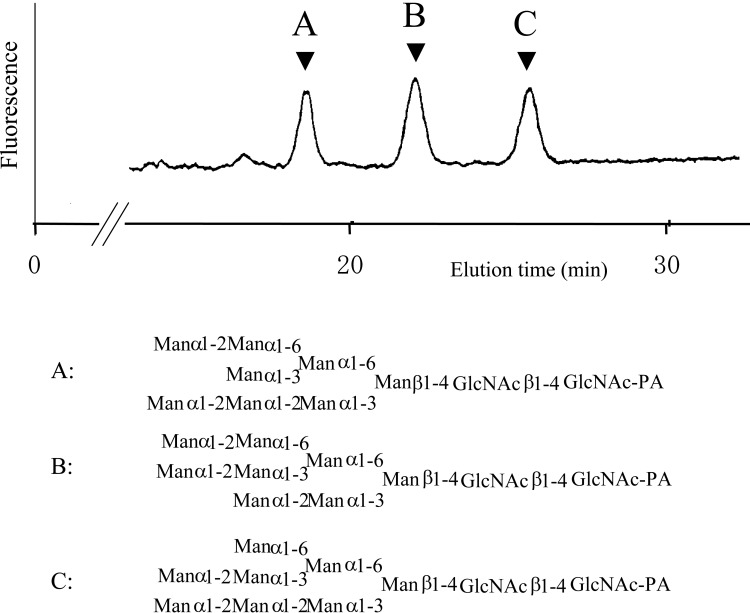
Highly improved separation by reversed-phase HPLC. Introduction of a pyridylamino group increases the hydrophobic character of the molecule. As a result, derivatized isomers can be separated by reversed-phase HPLC with much better resolution than the non-tagged versions (Fig. 4).7),12) Therefore, homogeneous complex PA-sugar chains can be more easily prepared and utilized for structure determination (e.g., chemical procedures, NMR, and mass spectrometry) as well as for functional analyses (e.g., as an enzyme substrate or for binding studies). In addition, high resolution affords differential display of glycans13) and reducing end analysis.14)
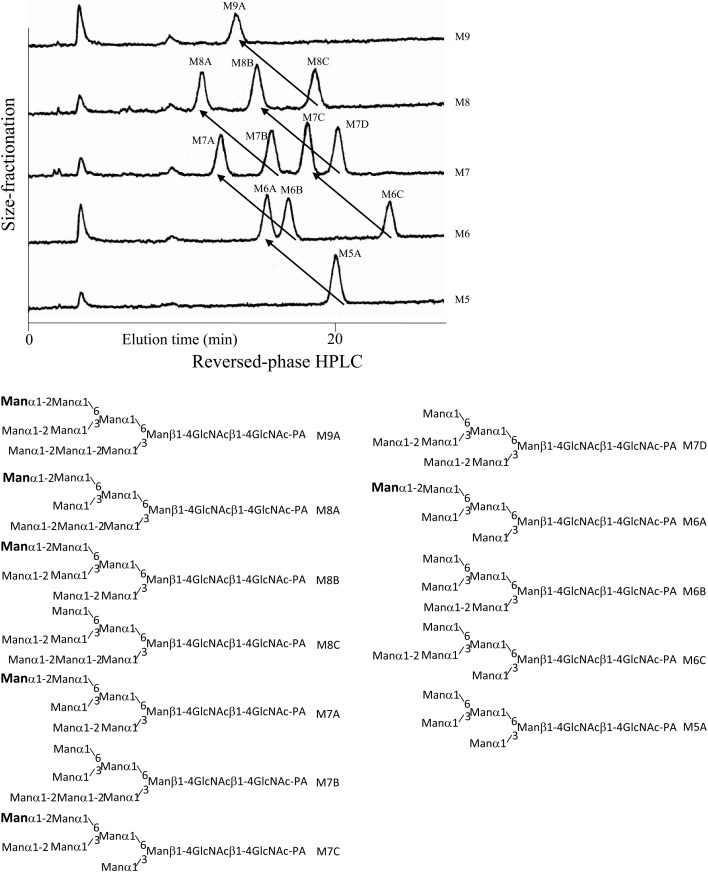
Structural information obtained by reversed-phase HPLC. Structural information is obtained from the elution times obtained by reversed-phase HPLC of derivatized sugar chains.7),15) Therefore this separation technique can act as an alternative to borate electrophoresis, which also yields structural information. If reversed-phase HPLC was performed under a defined set of elution conditions, the elution time of a PA-sugar chain is the sum of partial elution times (contribution of each sugar residue to the elution time) of the all sugar residues of the PA-sugar chain. As PA-sugar chains are separated according to their molecular sizes by size-fractionation HPLC, a sugar residue is expressed as a vector on the two-dimensional HPLC map as shown in Fig. 5.7),15) In this way, a two-dimensional HPLC map is useful for deducing the structures of unknown sugar chains.
Fig. 2.
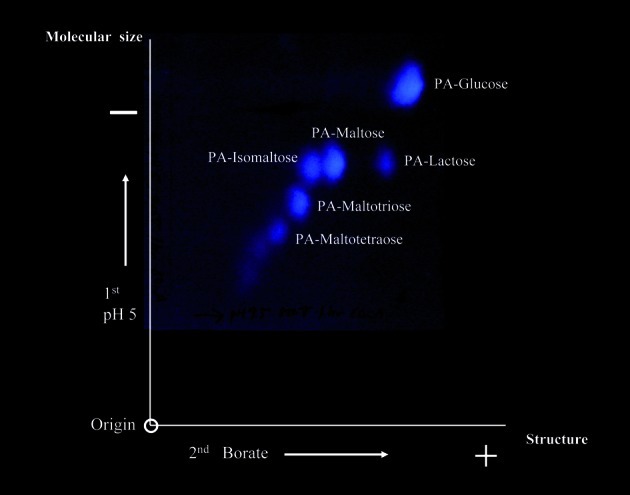
Two-dimensional paper electrophoresis of PA-sugar chains. An artificial mixture of PA-sugar chains was first separated by electrophoresis at pH 5.0 according to their molecular size. For the second dimension, PA-sugar chains were separated by borate electrophoresis according to binding ability of borate ions and sugar chains. After electrophoresis separated PA-sugar chains were visible under a UV-lamp. (Reprinted with slight modification from Biochem. Biophys. Res. Commun. 85, Hase S. et al. 257. Copyright (1978), with permission from Elsevier.)
Fig. 3.
A two-dimensional HPLC map of PA-sugar chains. PA-sugar chains were separated by two different separation principles; size-fractionation HPLC and reversed-phase HPLC. Open circles indicate the elution positions of N-linked sugar chains; closed circles indicate O-linked sugar chains. (Reprinted with slight modification from The Production & Technique. 51, Hase S. 39. Copyright (1999), with permission from the Association for the Advancement of Manufacturing & Technology.)
Fig. 4.
An elution profile of three isomers of Man8GlcNAc2-PA separated by reversed-phase HPLC. (Reprinted with slight modification from Anal. Biochem. 167, Hase S. et al. 321. Copyright (1987), with permission from Elsevier.)
Fig. 5.
A two-dimensional HPLC of twelve high mannose PA-sugar chains. An artificial mixture of twelve high mannose PA-sugar chains was first separated by size-fractionation HPLC and fractions corresponding to Man5GlcNAc2-PA (M5) ~ Man9-GlcNAc2-PA (M9) were collected. Each fraction obtained was next separated by reversed-phase HPLC. Addition of a sugar residue to a PA-sugar chain is represented by a vector on the two-dimensional HPLC map. Arrows in the figure indicate the replacement caused by the addition of the mannose residue indicated by bold faces in the structures. (Reprinted from Anal. Biochem. 167, Hase S. et al. 321. Copyright (1987), with permission from Elsevier.)
5. Conversion reactions for analysis of function
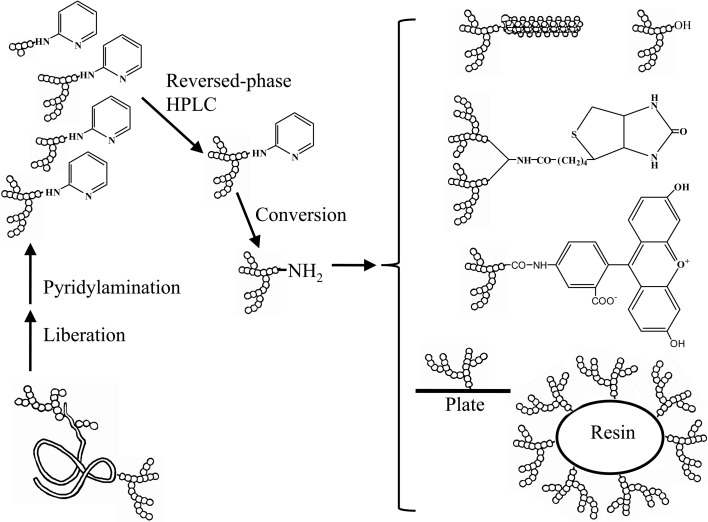
Although the pyridylamino group is chemically stable under conditions used for structure determination, it is unsuitable for further reactions such as coupling to resins, plates or lipids. For this reason conversion reactions were introduced to obtain 1-amino-1-deoxy derivatives16) (Fig. 6). The derivatives can then be coupled with chemically activated resins, plates and lipids that are commercially available. These probes, comprising homogeneous complex sugar chains, are useful for accurate functional analyses of proteins that bind the sugar chains (Fig. 7).17) 1-Amino-1-deoxy derivatives can further be converted to reducing sugar chains (Fig. 6).18) The chemical reaction of pyridylamination and the conversion reaction from PA-sugar chains to 1-amino-1-deoxy derivatives proceed almost quantitatively,4),16) however the yield of the conversion reaction from the 1-amino-1-deoxy derivative of Glc1Man9GlcNAc2 to the corresponding reducing sugar chain was about 30%.18)
Fig. 6.
Conversion reactions for pyridylaminated sugar chains. (Reprinted from J. Biochem. 134, Takahashi, C. et al. 51. Copyright (2003), with permission from The Japanese Biochemical Society.)
Fig. 7.
Preparation of homogeneous sugar chain probes. Functional analysis using homogeneous sugar chains is feasible. (Reprinted from Sugar Chain Analysis by Pyridylamination for Carbo-diversity edited by Hase, S. Osaka University Press. Copyright (2009), with permission from Osaka University Press.)
6. Some discoveries led by pyridylamination
Three examples where the application of pyridylamination has led to interesting discoveries in our laboratory are described below. In each case, I focus on the advantages of the present methodology.
a. A glucosyl-Ser type sugar chain
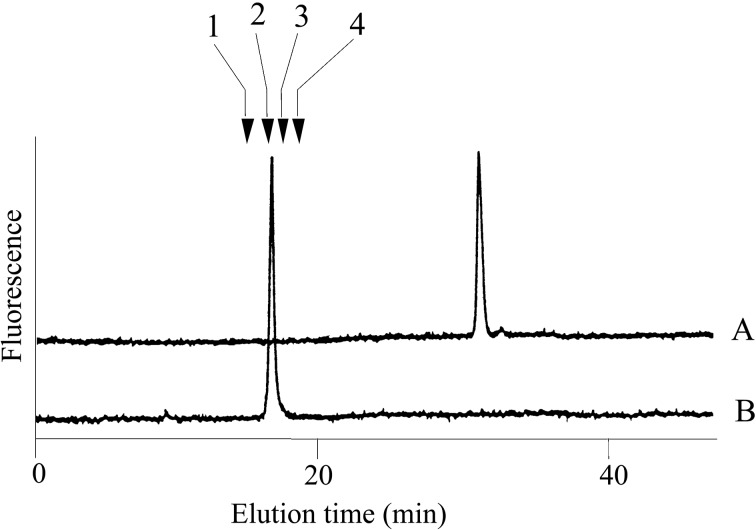
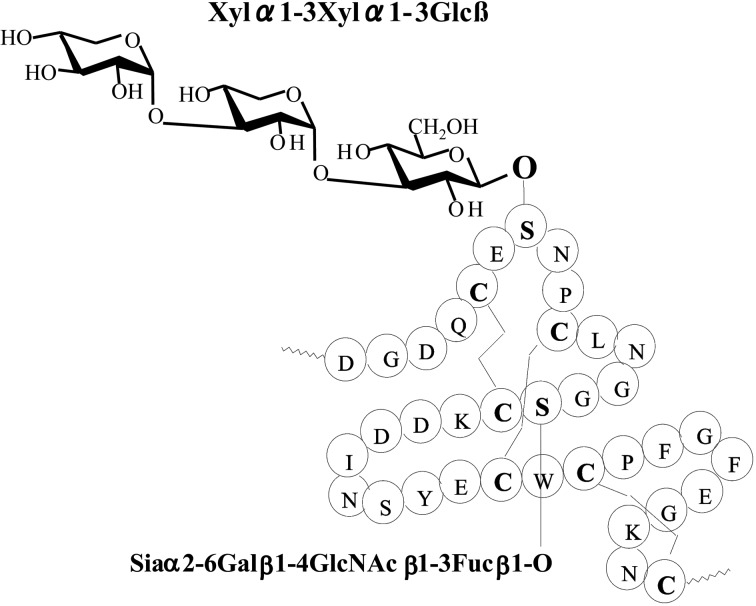
Edman degradation, amino acid analysis and mass spectrometry suggested that an unknown substituent was bound to a serine residue of the peptides isolated from human blood coagulation factor VII and IX.19) The quantity of sample (0.2 nmol) was far too small for component sugar analysis by gas-liquid chromatography. It is possible to perform such an investigation after pyridylamination. However, component sugar analysis using such a small quantity of material is exquisitely sensitive to contamination. If the unknown material is a sugar chain that is O-glycosidically linked to the serine residue, the sugar chain should be liberated by treating with hydrazine by the β-elimination reaction. The reaction mixture was pyridylaminated and the products purified by reversed phase HPLC. A single fluorescent peak appeared and was collected. The reducing end of the sugar chain was then analyzed after acid hydrolysis. A new peak appeared at the elution position of PA-glucose20) under the separation conditions of component sugar analysis21) (Fig. 8). This result indicated that the unknown material is a sugar chain with glucose at the reducing end. Further analysis of the PA-sugar chain showed a novel structure Xylα1-3Xylα1-3Glcβ1-O-Ser in the first EGF-like domain22) (Fig. 9). This type of sugar chains was subsequently found in several proteins.23) Surprisingly, a part of the structure Xylα1-3Glc was reported in human urine before our results were published.24)
Fig. 8.
HPLC of the pyridylaminated product (A) obtained from the oligopeptide and its acid hydrolysates (B). An oligopeptide from human blood coagulation factor IX was hydrazinolyzed and the products were pyridylaminated. Arrows indicate the elution positions; 1, pyridylaminated galactose; 2, pyridylaminated glucose; 3, pyridylaminated mannose; 4, pyridylaminated xylose. (Reprinted from J. Biochem. 104, Hase, S. et al. 867. Copyright (1988), with permission from The Japanese Biochemical Society.)
Fig. 9.
The structures of Xylα1-3Xylα1-3Glc found in human blood coagulation factor IX.
b. Endo-β-mannosidase (EC 3.2.1.152) and plant α1,2-l-fucosidase
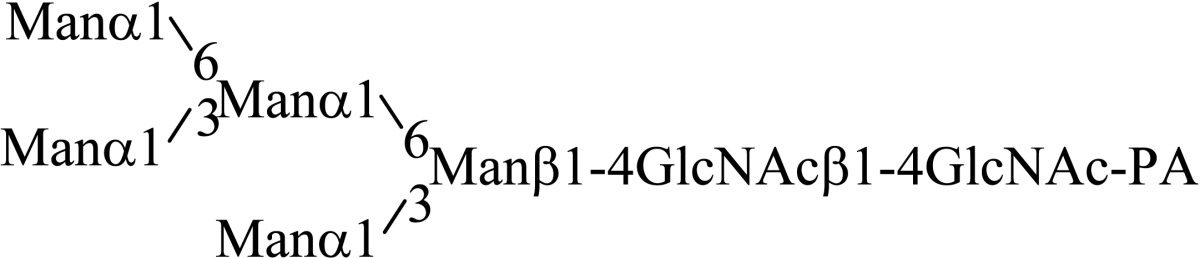
Asparagine linked sugar chains of glycoproteins are usually larger than Man5GlcNAc2. These complex sugar chains larger than Man5GlcNAc2 are generally analyzed because isolation of smaller sugar chains is technically difficult due to potential contamination of the sample from the reagents used. Pyridylamination facilitates the isolation of sugar chains from monosaccharides. Indeed, sugar chain analysis of S-RNase from Pyrus pyrifolia was carried out using this method, which identified a novel N-linked sugar chain, GlcNAcβ1-4GlcNAc, as the major (54 mole %) component. The next sugar chain larger than GlcNAcβ1-4GlcNAc was Manα1-6(Manα1-3)Manβ1-4GlcNAcβ1-4GlcNAc (Table 1).25) However Manα1-6Manβ1-4GlcNAcβ1-4GlcNAc, Manα1-3Manβ1-4GlcNAcβ1-4GlcNAc, Manβ1-4GlcNAcβ1-4GlcNAc having an intermediate molecular size could not be detected. These results suggested GlcNAcβ1-4GlcNAc is not derived from Manα1-6(Manα1-3)Manβ1-4GlcNAcβ1-4GlcNAc by digestion with α-mannosidase(s) present in the cell. Rather, an unknown enzyme that hydrolyzes Manα1-6(Manα1-3)Manβ1-4GlcNAcβ1-4GlcNAc to Manα1-6(Manα1-3)Man and GlcNAcβ1-4GlcNAc was proposed. When the homogenates of Lilium longiflorum flowers were used as an enzyme source, some PA-sugar chains were hydrolyzed and GlcNAcβ1-4GlcNAc-PA was detected. Manα1-6Manβ1-4GlcNAcβ1-4GlcNAc-PA showed the highest activity among the PA-sugar chains tested.26) Using this sugar chain as a substrate, the enzyme was purified from lily flowers to homogeneity. Precise analyses of substrate specificity using homogeneous PA-sugar chains showed that the enzyme is an endo type hydrolase and can hydrolyze Mann-Manα1-6Manβ1-4GlcNAcβ1-4GlcNAc with variation of reducing end modification such as a pyridylamino group, reducing aldehyde group and peptides, but cannot hydrolyze sugar chains containing the Manα1-3Manβ1 structure such as Mann-Manα1-6(Manα1-3)Manβ1-4GlcNAcβ1-4GlcNAc (Table 2).27)
Table 1.
The structures of smaller N-linked sugar chains obtained from S-RNase
| Structure | Molar ratio (% of total) |
|---|---|
| G1cNAcβ1-4G1cNAc | 54.0 |
| Manβ1-4G1cNAcβ1-4G1cNAc | Not detected |
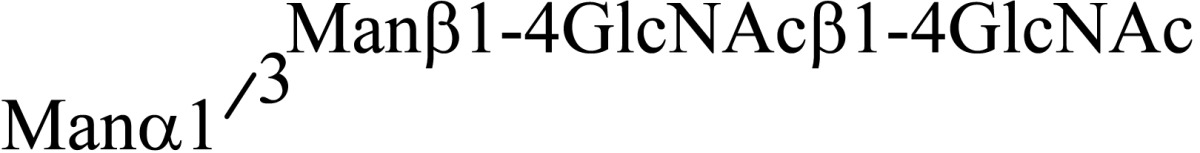
|
Not detected |
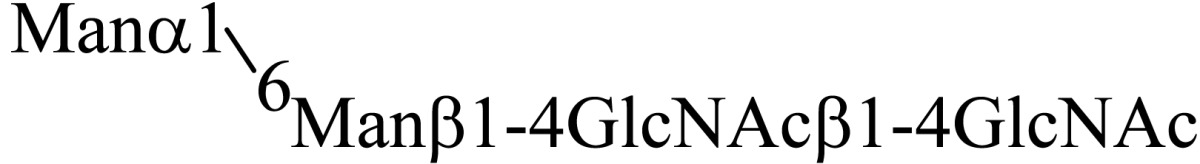
|
Not detected |
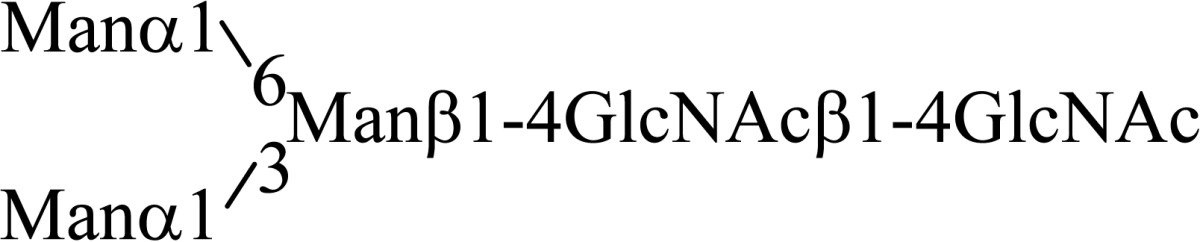
|
0.9 |
Table 2.
Substrate specificities of endo-β-mannosidase
| Substrate | Relative hydrolysis ratio (%) |
|---|---|

|
100 |
|
| |
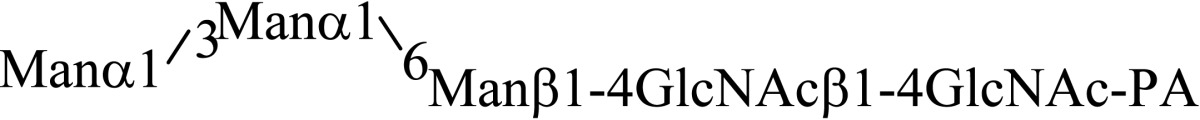
|
48 |
|
| |
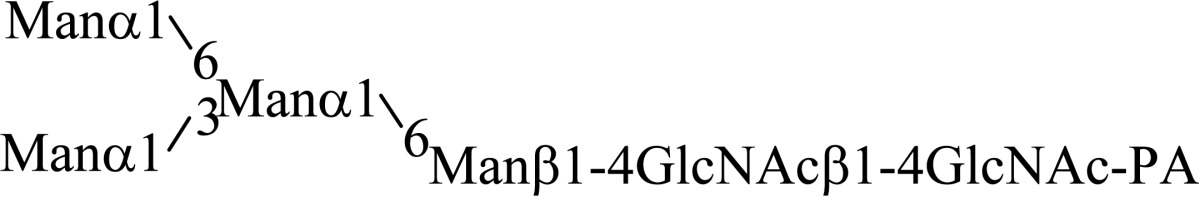
|
42 |
|
| |
| Manβ1-4G1cNAcβ1-4G1cNAc-PA | 4 |
|
| |
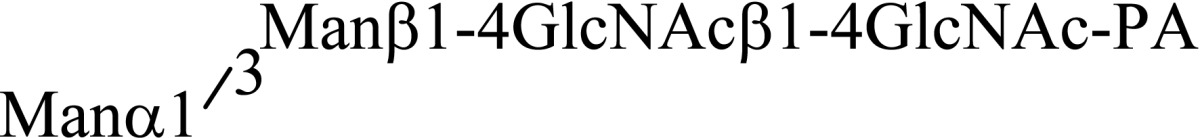
|
<0.01 |
|
| |
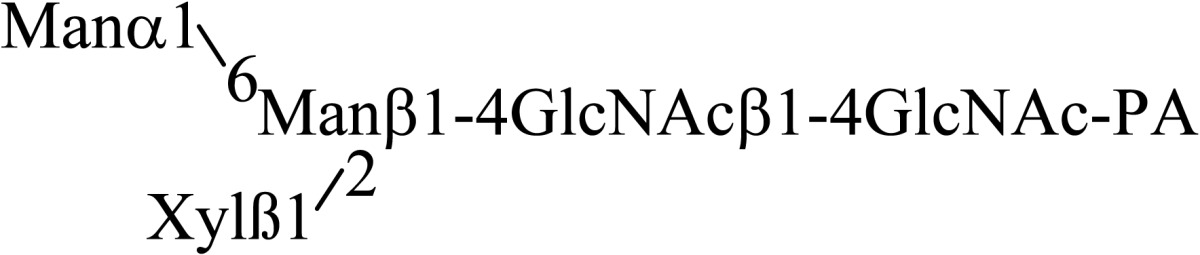
|
<0.01 |
|
| |
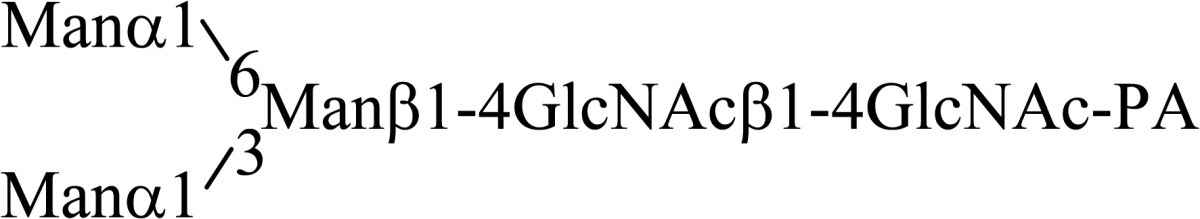
|
<0.01 |
|
| |
|
<0.01 |
|
| |
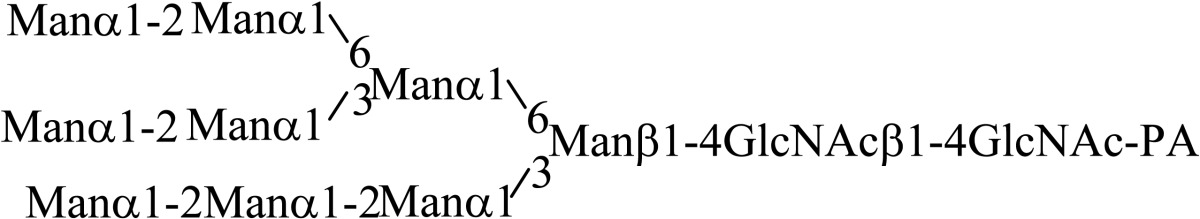
|
<0.01 |
|
| |
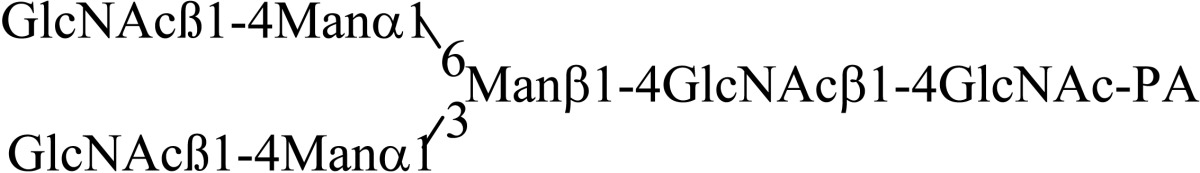
|
<0.01 |
|
| |
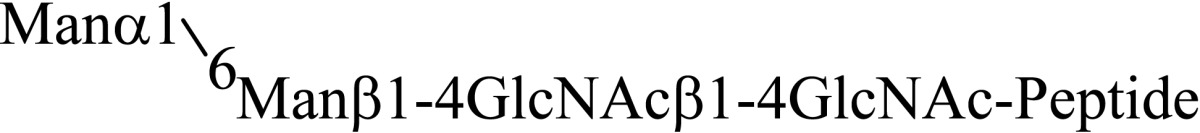
|
300 |
|
| |
| Manβ1-4G1cNAcβ1-4G1cNAc-Peptide | 9.8 |
|
| |
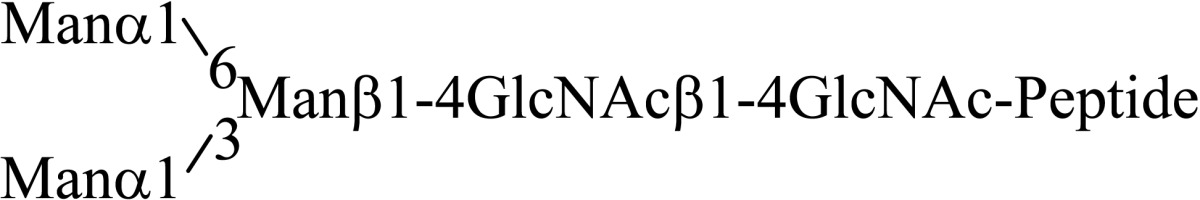
|
<0.01 |
|
| |
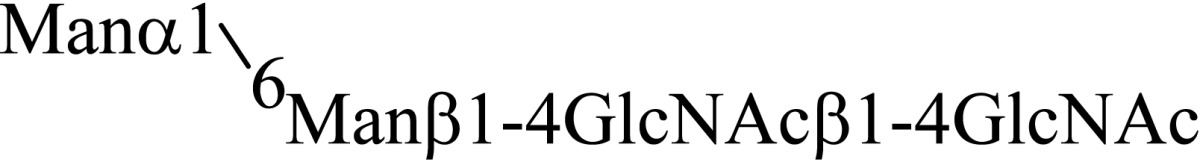
|
86 |
|
| |
| Manβ1-4Manβ1-4Manβ1-4Manβ1-4Manβ1-4Man | <0.01 |
| p-Nitrophenyl αMan | <0.01 |
| p-Nitrophenyl βMan | <0.01 |
Cited from Reference 26) with permission from The Japanese Biochemical Society.
Partial amino acid sequence derived from the purified enzyme allowed the cloning of lily endo-β-mannosidase cDNA. Subsequent sequence analysis of the gene revealed it to encode a protein consisting of 952 amino acid residues. The Arabidopsis gene was also cloned and its recombinant enzyme showed the same endo-β-mannosidase activity. A database search found some homologous genes confined to plant species with unknown function. However, genes displaying slight homology were found to be β-mannosidases in species other than plant.28) From these results, it is concluded that endo-β-mannosidase is specific to plants and does not exist in animal species.
Sugar chains with the Manα1-3Manβ structure that are not hydrolyzed by endo-β-mannosidase can easily be hydrolyzed by plant α-mannosidases such as jack bean α-mannosidase.29) These results suggested that endo-β-mannosidase and plant α-mannosidase may cooperatively hydrolyze N-linked sugar chains like Mann-Manβ1-4GlcNAcβ1-4GlcNAc to GlcNAcβ1-4GlcNAc and oligomannosides.30) Enzyme analysis (pH optimum of 4.5) combined with proteomics studies31) confirmed that endo-β-mannosidase resides in the vacuole. Thus the degradation of sugar chains may take place in this organelle.
During the course of purifying endo-β-mannosidase a small peak, endo-β-mannosidase II, having endo-β-mannosidase activity was found. The enzyme was purified and its cDNA was cloned from its partial amino acid sequence. The deduced amino acid sequence indicated that the enzyme consists of poly-peptides encoded by two genes; endo-β-mannosidase and another gene of unknown function. However, the protein displayed a weak homology to the fucosidase domain of Bifidobacterium bifidum AfcA.32) Therefore fucosidase activity of endo-β-mannosidase II was analyzed and an α1,2-l-fuosidase activity was detected using as substrate fucose containing PA-deca- and nonaoligosaccharides prepared from xyloglucan (Table 3).33) The amino acid sequence of this enzyme has no significant similarity to known plant α-l-fuosidases indicating the protein represents the first member of a new type of plant α1,2-l-fuosidase. Thus endo-β-mannosidase II has both endo-β-mannosidase activity and α1,2-l-fuosidase activity.
Table 3.
Substrate specificity of fucosidase activity of endo-β-mannosidase II using PA-sugar chains obtained from xyloglycan
| Substrate | Relative hydrolysis rate (%) | |
|---|---|---|
| Endo-β-mannosidase II | Endo-β-mannosidase | |
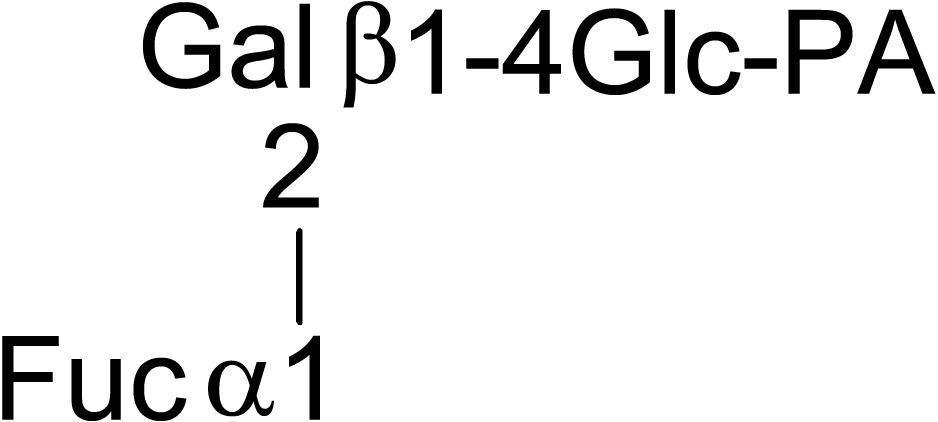
|
100 | < 0.01 |
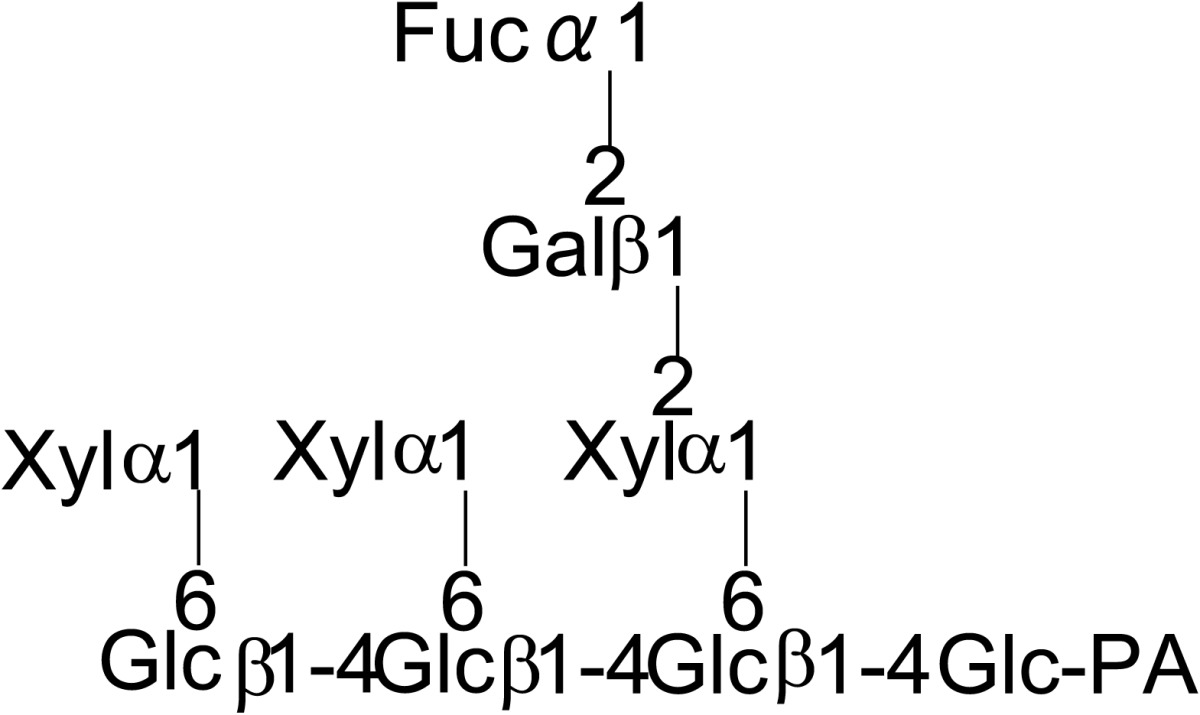
|
66 | < 0.01 |
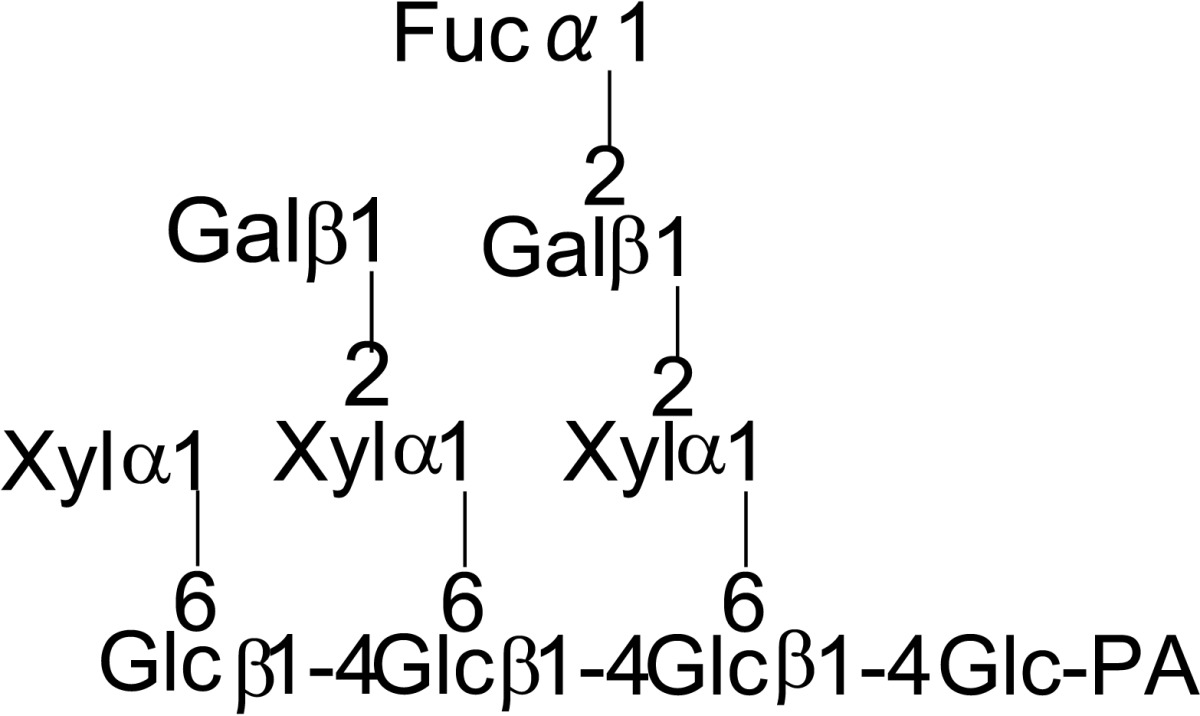
|
40 | < 0.01 |
Cited from Reference 33) with permission from The Japanese Biochemical Society.
Endo-β-mannosidase also catalyzes the reverse reaction,34) facilitating the synthesis of Manβ1-4 linkages, which is rather difficult to achieve by chemical means.
c. Cytosolic α-mannosidase
Manα1-6(Manα1-3)Manβ1-4GlcNAcβ1-4GlcNAc (Man3GlcNAc2) was found to be a major N-linked sugar chain of ovomucoid from Japanese quail egg.35) The structure is far smaller than the reported N-linked sugar chains, which are usually larger than Man5GlcNAc2. This finding suggested the presence of an unknown processing α-mannosidase(s) that hydrolyzes the sugar chains to Man3GlcNAc2. In order to detect the enzyme, a PA-sugar chain with a structure similar to the in vivo substrate, Manα1-6(Manα1-3)Manα1-6(Manα1-3)Manβ1-4GlcNAcβ1-4GlcNAc-PA (Man5GlcNAc2-PA), was chosen instead of p-nitrophenyl α-mannoside, which is commonly used to detect mannosidase activity.
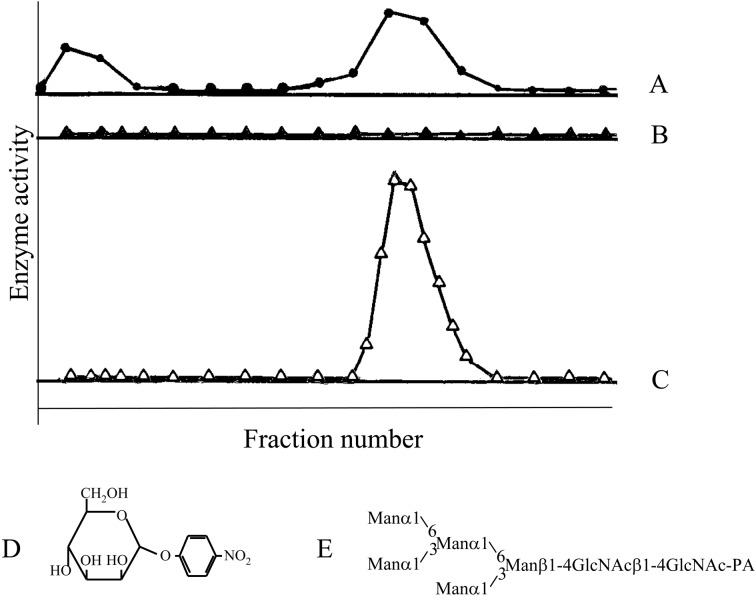
When a homogenate of quail oviduct was fractionated by DEAE-cellulose chromatography, at least two peaks were detected using p-nitrophenyl α-mannoside. By contrast, no activity was detected when Man5GlcNAc2-PA was used as substrate (Fig. 10).36) Man5GlcNAc2-PA hydrolyzing activity was eventually found when Co2+ was added to the reaction mixture. Using this activity as a guide, the enzyme was purified to homogeneity. The substrate specificity of the enzyme was then analyzed using PA-sugar chains available in the author’s laboratory. The results are summarized below.
Fig. 10.
Enzyme activity in the fractions separated by DEAE-cellulose chromatography of a crude enzyme fraction prepared from Japanese quail oviduct. A, p-nitrophenyl αd-mannoside (D) was used as a substrate; B, Manα1-6(Manα1-3)Manα1-6(Manα1-3)Manβ1-4GlcNAcβ1-4GlcNAc-PA (E, Man5GlcNAc2-PA); C, same as in B but in the presence of 1 mM Co2+. (Reprinted with slight modification from J. Biochem. 110, Hase, S. et al. 29. Copyright (1991), with permission from The Japanese Biochemical Society.)
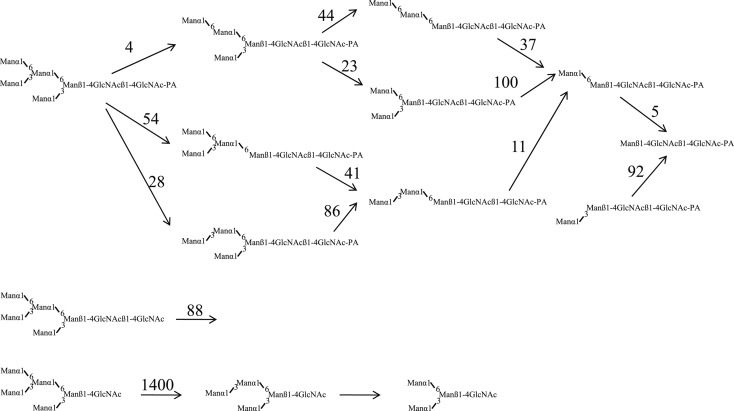
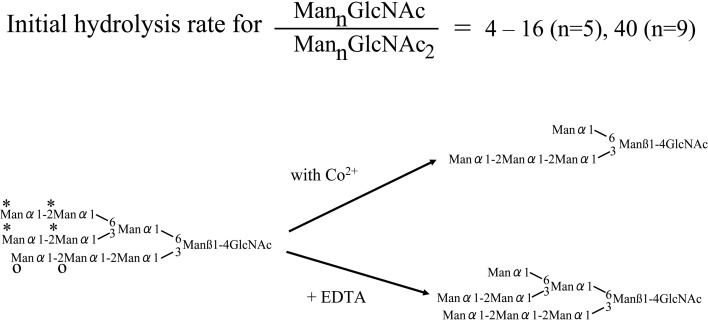
Manα1-6(Manα1-3)Manα1-6(Manα1-3)Manβ1-4GlcNAcβ1-4GlcNAc-PA (Man5GlcNAc2-PA) was hydrolyzed to Manβ1-4GlcNAcβ1-4GlcNAc-PA as a result of hydrolysis of all α-linked mannose residues in the presence of Co2+. However when Manα1-6(Manα1-3)Manα1-6(Manα1-3)Manβ1-4GlcNAc (Man5GlcNAc1) with a single GlcNAc residue at the reducing end was incubated with the enzyme, not all Manα residues were hydrolyzed, and the end product was Manα1-6(Manα1-3)Manβ1-4GlcNAc (Man3GlcNAc). Furthermore Manα1-2Manα1-6(Manα1-2Manα1-3)Manα1-6(Manα1-2Manα1-2Manα1-3)Manβ1-4GlcNAc (Man9GlcNAc1) was hydrolyzed to the end product Manα1-6(Manα1-2Manα1-2Manα1-3)Manβ1-4GlcNAc (Man5GlcNAc1) (Figs. 11, 12).37)–39) The enzyme hydrolyzes only four Manα1-2, Manα1-3, Manα1-6 residues (marked with asterisks in Fig. 12) from the non-reducing ends of Man9GlcNAc, but does not hydrolyze the non-reducing end Manα1-2 residues (marked with O in Fig. 12) of the lower branch.
The cytosolic α-mannosidase can hydrolyze Man9GlcNAc and Man5GlcNAc-PA with a single GlcNAc or GlcNAc-PA at the reducing end much faster than those with GlcNAcβ1-4GlcNAc or GlcNAcβ1-4GlcNAc-PA (Figs. 11, 12).
Fig. 11.
Effects of the reducing end structures on the substrate specificities of cytosolic α-mannosidase from Japanese quail oviduct. Figures on the arrows indicate the relative initial hydrolysis rate. (Reprinted with slight modification from J. Biochem. 110, Oku, H. and Hase, S. 982. Copyright (1991), with permission from The Japanese Biochemical Society.)
Fig. 12.
Summary of the substrate specificities of cytosolic α-mannosidase purified from Japanese quail oviduct, bovine liver and hen oviduct.
Judging from the neutral optimal pH and the distribution of the specific activity using Man5-GlcNAc-PA in the presence or absence of Co2+, the enzyme seems to reside in cytosol. Therefore this enzyme appears to be the one reported as a cytosolic α-mannosidase.40) This α-mannosidase is thought to hydrolyze sugar chains with single GlcNAc residues at the reducing ends liberated by digesting with an endo-β-N-acetylglucosaminidase of glycoproteins and/or sugar chains translocated from the endoplasmic reticulum.41) The structures of cytosolic free oligosaccharides, which were thought to be the enzyme products, were analyzed. The structures of most of these oligosaccharides were in agreement with the suggested substrate specificity of the α-mannosidase.42) From these results, the cytosolic α-mannosidase is thought to hydrolyze sugar chains with one GlcNAc residue at the reducing end to the end product, Manα1-6(Manα1-2Manα1-2Manα1-3)Manβ1-4GlcNAc, which is the most abundant high mannose type derived sugar chain in the cytosol.42)–44)
7. Discussion
A novel procedure for analyzing sugar chains was realized that involved pyridylamination. Derivatized isomers were efficiently resolved by reversed-phase HPLC and readily detected.17) Because pyridylamination facilitates the preparation of homogeneous samples, mass analysis10),11) and NMR45) structural determination are greatly simplified. Therefore pyridylamination is considered to be an “operating system” rather than an analytical procedure. Pyridylamination is also suited to the analysis of carbo-diversity or microheterogeneity due to high resolution by comparing the elution profiles obtained by reversed-phase HPLC or two-dimensional HPLC.46),47) The method is considered to be a bridge between the precise structural determinations and analyses by lectins or antibodies. Using this novel approach, several important findings were obtained as described in section 6.
One key observation from analyzing a homogeneous complex sugar chain was that some enzymes recognize a wider region of the substrate than expected.48) Substrate recognition can be analyzed with a high level of precision using homogeneous PA-complex oligosaccharides similar to those found in nature. I believe pyridylamination will assist in deciphering the nature of carbo-diversity in complex carbohydrates.
8. Acknowledgements
I would like to express my gratitude to collaborators and students who have studied with the author in the Organic-Biochemistry Laboratory, Department of Chemistry, Graduate School of Science, Osaka University.
Abbreviations
- PA
pyridylaminated
- HPLC
high-performance liquid chromatography
Profile
Sumihiro Hase was born in 1943. He graduated from Department of Chemistry, Faculty of Science, Osaka University in 1966, and received his Ph.D. degree from Osaka University. He was appointed as research associate in 1971, assistant professor, associate professor, and then full professor of Osaka University, Graduate School of Science, Department of Chemistry in 1989. He has been engaged in the basic research on analyses of sugar chains: i) nitrous oxidation of mannosamine to glucose and its application to structure determination of a polysaccharide; ii) structure analysis of lipid A; and iii) introduction of fluorescence labeling method (pyridylamination) to analyses of sugar chains, and several discoveries of a new type sugar chain and of enzymes by this method as described in this paper.

References
- 1).Barker S.A., Bourne E.J., Grant P.M., Stacey M. (1956) Inophoresis of oligosaccharides as N-benzylglycosylammonium ions. Nature 177, 1125 [Google Scholar]
- 2).Frahn J.L., Mills J.A. (1956) Determination of the molecular weight of aldose sugars by paper ionophoresis. Chem. Ind. 1137–1138 [Google Scholar]
- 3).Hase S., Ikenaka T., Matsushima Y. (1978) Structure analyses of oligosaccharides by tagging of the reducing end sugars with a fluorescent compound. Biochem. Biophys. Res. Commun. 85, 257–263 [DOI] [PubMed] [Google Scholar]
- 4).Kondo A., Suzuki J., Kuraya N., Hase S., Kato I., Ikenaka T. (1990) Improved method for fluorescene labeling of sugar chains with sialic acid residues. Agric. Biol. Chem. 54, 2169–2170 [PubMed] [Google Scholar]
- 5).Nakakita S., Sumiyoshi W., Miyanishi N., Natsuka S., Hase S., Hirabayashi J. (2007) Gas-phase pyridylamination of saccharides: Development and applications. Anal. Chem. 79, 2674–2679 [DOI] [PubMed] [Google Scholar]
- 6).Hase S. (2002) InCarbohydrate analysis by modern chromatography and electrophoresis: Pre-and post-column detection-oriented derivatization techniques in HPLC of carbohydrates (ed. Rassi Z.L.). J. Chromatogr. Library Vol. 66 Elsevier Science B.V., Amsterdam, pp. 1043–1069 [Google Scholar]
- 7).Hase S., Natsuka S., Oku H., Ikenaka T. (1987) Identification method for twelve oligomannose-type sugar chains thought to be processing intermediates of glycoproteins. Anal. Biochem. 167, 321–326 [DOI] [PubMed] [Google Scholar]
- 8).Hase S. (1999) Analysis of structure and function of sugar chains by fluorescence labeling. Production & Technique 51, 39–42 [Google Scholar]
- 9).Mellis S.J., Baenziger J.U. (1983) Size fractionation of anionic oligosaccharides and glycopeptides by high-performance liquid chromatography. Anal. Biochem. 134, 442–449 [DOI] [PubMed] [Google Scholar]
- 10).Okamoto M., Takahashi K., Doi T., Takimoto Y. (1997) High-sensitivity detection and postsource decay of 2-aminopyridine-derivatized oligosaccharides with matrix-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 69, 2919–2926 [DOI] [PubMed] [Google Scholar]
- 11).Amano J., Sugahara D., Osumi K., Tanaka K. (2009) Negative-ion MALDI-QIT-TOFMSn for structural determination of fucosylated and sialylated oligosaccharides labeled with a pyrene derivative. Glycobiology 19, 592–600 [DOI] [PubMed] [Google Scholar]
- 12).Hase S., Ikenaka T., Matsushima Y. (1981) A highly sensitive method for analyses of sugar moieties of glycoproteins by fluorescence labeling. J. Biochem. 90, 407–414 [DOI] [PubMed] [Google Scholar]
- 13).Nakakita S., Menon K.K., Natsuka S., Ikenaka K., Hase S. (1999) β1-4Galactosyltransferase activity of mouse brain as revealed by analysis of brain-specific complex-type N-linked sugar chains. J. Biochem. 126, 1161–1169 [DOI] [PubMed] [Google Scholar]
- 14).Makino Y., Kuraya N., Omichi K., Hase S. (1996) Classification of sugar chains of glycoproteins by analyzing reducing end oligosaccharides obtained by partial acid hydrolysis. Anal. Biochem. 238, 54–59 [DOI] [PubMed] [Google Scholar]
- 15).Yanagida K., Ogawa H., Omichi K., Hase S. (1998) Introduction of a new scale into reversed-phase high-performance liquid chromatography of pyridylamino sugar chains for structural assignment. J. Chromatogr. A 800, 187–198 [DOI] [PubMed] [Google Scholar]
- 16).Hase S. (1992) Conversion of pyridylamino sugar chains to 1-amino-1-deoxy derivatives, intermediates for tagging with fluorescein and biotin. J. Biochem. 112, 266–268 [DOI] [PubMed] [Google Scholar]
- 17).Hase S. (2009) Sugar Chain Analysis by Pyridylamination for Carbo-diversity (ed. Hase S.). Osaka University Press, Osaka [Google Scholar]
- 18).Takahashi C., Nakakita S., Hase S. (2003) Conversion of pyridylamino sugar chains to corresponding reducing sugar chains. J. Biochem. 134, 51–55 [DOI] [PubMed] [Google Scholar]
- 19).Takeya H., Kawabata S., Nakagawa K., Yamamichi Y., Miyata T., Iwanaga S., et al. (1988) Bovine factor VII. Its purification and complete amino acid sequence. J. Biol. Chem. 263, 14868–14877 [PubMed] [Google Scholar]
- 20).Hase S., Kawabata S., Nishimura H., Takeya H., Sueyoshi T., Miyata T., et al. (1988) A new trisaccharide sugar chain linked to a serine residue in bovine blood coagulation factors VII and IX. J. Biochem. 104, 867–868 [DOI] [PubMed] [Google Scholar]
- 21).Takemoto H., Hase S., Ikenaka T. (1985) Microquantitative analysis of neutral and amino sugars as fluorescent pyridylamino derivatives by high-performance liquid chromatography. Anal. Biochem. 145, 245–250 [DOI] [PubMed] [Google Scholar]
- 22).Hase S., Nishimura H., Kawabata S., Iwanaga S., Ikenaka T. (1990) The structure of (Xylose)2- Glucose-O-Serine 53 found in the first epidermal growth factor-like domain of bovine blood clotting factor IX. J. Biol. Chem. 265, 1858–1861 [PubMed] [Google Scholar]
- 23).Nishimura H., Takao T., Hase S., Shimonishi Y., Iwanaga S. (1992) Human factor IX has a tetrasaccharide O-glycosidically linked to serine 61 through the fucose residue. J. Biol. Chem. 267, 17520–17525 [PubMed] [Google Scholar]
- 24).Lundblad A., Svensson S. (1973) Isolation and characterization of 3-O-α-d-xylopyranosyl-d-glucose and 2-O-α-l-fucopyranosyl-d-glucose from normal human urine. Biochemistry 12, 306–309 [DOI] [PubMed] [Google Scholar]
- 25).Ishimizu T., Mitsukami Y., Shinkawa T., Natsuka S., Hase S., Miyagi M., et al. (1999) Presence of asparagine-linked N-acetylglucosamine and chitobiose in Pyrus pyrifolia S-RNases associated with gametophytic self-incompatibility. Eur. J. Biochem. 263, 624–634 [DOI] [PubMed] [Google Scholar]
- 26).Sasaki A., Yamaguchi M., Mega T., Norioka S., Natsuka S., Hase S. (1999) Partial purification and characterization of a novel endo-β-mannosidase acting on N-linked sugar chains from Lilium longiflrum Thumb. J. Biochem. 125, 363–367 [DOI] [PubMed] [Google Scholar]
- 27).Sasaki A., Ishimizu T., Hase S. (2005) Substrate specificity and molecular cloning of the lily endo-β-mannosidase acting on N-glycan. J. Biochem. 137, 87–93 [DOI] [PubMed] [Google Scholar]
- 28).Ishimizu T., Sasaki A., Okutani S., Maeda M., Yamagishi M., Hase S. (2004) Endo-β-mannosidase, a plant enzyme acting on N-glycan: Purification, molecular cloning, and characterization. J. Biol. Chem. 279, 38555–38562 [DOI] [PubMed] [Google Scholar]
- 29).Oku H., Hase S., Ikenaka T. (1990) Separation of oligomannose-type sugar chains having one to five mannose residues by high-performance liquid chromatography as their pyridylamino derivatives. Anal. Biochem. 185, 331–334 [DOI] [PubMed] [Google Scholar]
- 30).Ishimizu T., Hase S. (2006) Endo-β-mannosidase, a plant enzyme acting on N-glycans. Trends Glycosci. Glycotech. 18, 39–47 [Google Scholar]
- 31).Carter C., Pan S., Zouhar J., Avila E.L., Girke T., Raikhel N.V. (2004) The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 16, 3285–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Katayama T., Sakuma A., Kimura T., Makimura Y., Hiratake J., Sakata K., et al. (2004) Molecular cloning and characterization of Bifidobacterium bifidum 1,2-α-l-fucosidase (AfcA), a novel inverting glycosidase (glycoside hydrolase family 95). J. Bacteriol. 186, 4885–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Ishimizu T., Hashimoto C., Takeda R., Fujii K., Hase S. (2007) A novel α1,2-l-fucosidase acting on xyloglucan oligosaccharides is associated with endo-β-mannosidase. J. Biochem. 142, 721–729 [DOI] [PubMed] [Google Scholar]
- 34).Sasaki A., Ishimizu T., Geyer R., Hase S. (2005) Synthesis of β-mannosides using the trans-glycosylation activity of endo-β-mannosidase from Lilium longiflorum. FEBS J. 272, 1660–1668 [DOI] [PubMed] [Google Scholar]
- 35).Hase S., Okawa K., Ikenaka T. (1982) Identification of the trimannosyl-chitobiose structure in sugar moieties of Japanese quail ovomucoid. J. Biochem. 91, 735–737 [DOI] [PubMed] [Google Scholar]
- 36).Oku H., Hase S., Ikenaka T. (1991) Purification and characterization of neutral α-mannosidase that is activated by Co2+ from Japanese quail oviduct. J. Biochem. 110, 29–34 [DOI] [PubMed] [Google Scholar]
- 37).Oku H., Hase S. (1991) Studies on the substrate specificity of neutral α-mannosidase purified from Japanese quail oviduct by using sugar chains from glycoproteins. J. Biochem. 110, 982–989 [DOI] [PubMed] [Google Scholar]
- 38).Kumano M., Omichi K., Hase S. (1996) Substrate specificity of bovine liver cytosolic neutral α-mannosidase activated by Co2+. J. Biochem. 119, 991–997 [DOI] [PubMed] [Google Scholar]
- 39).Yamashiro K., Itoh H., Yamagishi M., Natsuka S., Mega T., Hase S. (1997) Purification and characterization of neutral α-mannosidase from hen oviduct: Studies on the activation mechanism of Co2+. J. Biochem. 122, 1174–1181 [DOI] [PubMed] [Google Scholar]
- 40).Shoup V.A., Touster O. (1976) Purification and characterization of the alpha-d-mannosidase of rat liver cytosol. J. Biol. Chem. 251, 3845–3852 [PubMed] [Google Scholar]
- 41).Cacan R., Verbert A. (1999) Free and N-linked oligomannosides as markers of the quality control of newly synthesized glycoproteins. Biochem. Bio-phys. Res. Commun. 258, 1–5 [DOI] [PubMed] [Google Scholar]
- 42).Yanagida K., Natsuka S., Hase S. (2006) Structural diversity of cytosolic free oligosaccharides in the human hepatoma cell line, HepG2. Glycobiology 16, 294–304 [DOI] [PubMed] [Google Scholar]
- 43).Iwai K., Mega T., Hase S. (1999) Detection of Man5GlcNAc and related free oligomannosides in the cytosol fraction of hen oviduct. J. Biochem. 125, 70–74 [DOI] [PubMed] [Google Scholar]
- 44).Ohashi S., Iwai K., Mega T., Hase S. (1999) Quantitation and isomeric structure analysis of free oligosaccharides present in the cytosol fraction of mouse liver: Detection of a free disialobiantennary oligosaccharide and glucosylated oligomannosides. J. Biochem. 126, 852–858 [DOI] [PubMed] [Google Scholar]
- 45).Koyama S., Daiyasu H., Hase S., Kobayashi Y., Kyogoku Y., Ikenaka T. (1986) 1H-NMR analysis of the sugar structures of glycoproteins as their pyridylamino derivatives. FEBS Lett. 209, 265–268 [DOI] [PubMed] [Google Scholar]
- 46).Moriguchi K., Takemoto T., Aoki T., Nakakita S., Natsuka S., Hase S. (2007) Free oligosaccharides with Lewis x structure expressed in the segmentation period of zebrafish embryo. J. Biochem. 142, 213–227 [DOI] [PubMed] [Google Scholar]
- 47).Shimizu H., Ochiai K., Ikenaka K., Mikoshiba K., Hase S. (1993) Structures of N-linked sugar chains expressed mainly in mouse brain. J. Biochem. 114, 334–338 [DOI] [PubMed] [Google Scholar]
- 48).Ishimizu T., Hase S. (2005) Substrate recognition by sugar chain-related enzymes: Recognition of a large area of substrate and its strictness and tolerance. Trends Glycosci. Glycotech. 17, 215–227 [Google Scholar]