Abstract
We have demonstrated that in rats activities of various enzymes related to gluconeogenesis and amino acid metabolism show circadian rhythms. Based on these results, we have explored the molecular mechanisms underlying circadian oscillation and phase response to light of the master clock located in the dorsomedial subdivision of the suprachiasmatic nucleus (SCN) and found various proteins closely related to phase response such as BIT/SHPS-1 and those of circadian oscillation, some of which are involved in protein-tyrosine phosphorylation.
On the other hand, we have presented several lines of evidence that the ventrolateral subdivision of the SCN includes not only the control center of energy supply to the brain, but also that of homeostasis such as blood glucose, blood pressure, water balance, and body temperature. We have also shown that besides these functions, the latter subdivision is involved in the regulations of hormone secretions such as insulin, glucagon, corticosterone and vasopressin. It has been also shown by electrophysiological means that light exposure to rat eye enhances sympathetic nerve activity, whereas it depresses parasympathetic nerve activity. Thus, environmental light is implicated not only in the phase-shift through the retinohypthalamic tract (RHT), but also control of autonomic nerve activities through the RHT, It is also discussed in this review how the two divisions are interconnected and how environmental light is involved in this interconnection.
Keywords: SCN, circadian rhythm, light, BIT, homeostasis, autonomic nervous system
Introduction
About a half century ago, we found in rat liver that serine dehydratase (SDH) activity showed a circadian rhythm,1) while cystathionine-β-synthase (CBT) activity did not.2) This tempted us to speculate if the two enzyme activities were not catalyzed by a single protein, though CBT was then assumed to be the same as SDH.3) In fact, we confirmed later that the two enzyme proteins were different and also showed that both enzymes required pyridoxal-phosphate as a cofactor.4)–9) Homocysteine, the substrate of CBT reaction is now known to be the highest risk factors of cardiovascular disease in western countries.10)
SDH catalyzes the reaction converting serine to pyruvate that is a substrate for gluconeogenesis. This suggests that gluconeogenesis exhibits a circadian rhythm. In accordance with this, activities of phosphoenolpyruvate carboxykinase (PEPCK), a key gluconeogenic enzyme, also showed circadian rhythms in the liver and kidney in rats.11)–13) In relation to this fact, we presented several lines of evidence that circadian changes of PEPCK activities coincided well with those of in vivo gluconeogenesis both in the liver and kidney14),15) and suggested that the circadian rhythm of hepatic gluconeogenesis might be generated to meet the nutritional demand of animals for blood glucose homeostasis, while renal gluconeogenesis might be generated in close association with the regulation of blood pH.15)
It was also shown that urea formation and activity of argininosuccinate synthetase, a key enzyme of the urea cycle, showed circadian rhythm in rat liver and that the generation of this rhythm was closely related with food intake.16),17)
These findings raised problems of how the signals generated for food intake and circadian rhythms of metabolism are interrelated and thus what other body functions are synchronously regulated with these signals. This review will cover these problems from a standpoint that the suprachiasmatic nucleus (SCN) in the hypothalamus is not only involved in the circadian signal generation, but also plays as an integrating control center of homeostasis and that environmental light functions as an essential controller of both systems.
Suprachiasmatic nucleus as the site of circadian clock of gluconeogenesis and food intake
In 1965, Axelrod and his collaborators18) reported that hydroxyindole O-methyltransferase (HIOMT) activity showed a diurnal rhythm with the highest in the midnight in the rat pineal gland and that this rhythm was controlled by environmental light. Based on these findings, they suggested that the pineal gland might serve as a “biological clock.” This report attracted a number of investigators to explore where master clock was located and what was underling molecular mechanism of the circadian temporal signal generation in mammals.
Then we had obtained several lines of evidence that the circadian increase in PEPCK activity in the liver might depend on increase in sympathetic nerve activity and/or decrease in parasympathetic nerve activity, while its circadian decrease might depend on the opposite changes in these nerve activities.12) These findings suggested that the temporal signal to generate the circadian change of the enzyme activity was transmitted to the liver through the autonomic nervous system. Then we examine whether the circadian clock was located in some brain areas related to the autonomic nerve functions.
In 1972, Moore and Eichler19) demonstrated that in rat pineal gland the enzyme whose activity exhibited a circadian rhythm was not HIOMT, but serotonin N-acetyl-transferase and that this rhythm as well as that of plasma corticosterone level disappeared after removal of the SCN. Stephan and Zucker also showed that ablation of the SCN abolished the circadian rhythms of locomotive activity and drinking behavior.20)
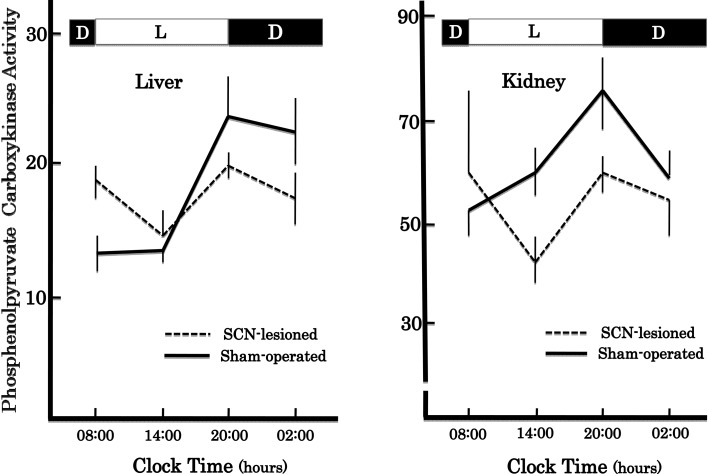
In accordance with these results, we found that the SCN lesions eliminated the circadian rhythm of PEPCK activity both in the liver and kidney17) (Fig. 1). To promote the study of the molecular mechanism underlying circadian oscillation further, we changed our strategy to use other circadian rhythms as markers such as feeding and drinking behaviors, and locomotive activity which could be automatically and continuously monitored, because tedious and time-consuming enzyme assays of too many samples derived from a number of animal groups sacrificed at an interval of 3 to 4 hours had hampered our studies. The new strategy indeed encouraged the process leading to conclusion that master circadian clock was located in the SCN.21)–24) Details will be described later.
Fig. 1.
Effects of bilateral lesions of the SCN on circadian rhythms of phosphoenolpyruvate carboxykinase activities in rat liver and kidney. L and D indicate the light and dark periods, respectively. The enzyme activity is expressed as mμmoles/min/mg protein. A group of 3 to 4 rats was used in one experiment. Vertical bars are the ranges of means ±SEM.
Suprachiasmatic nucleus and feeding behavior
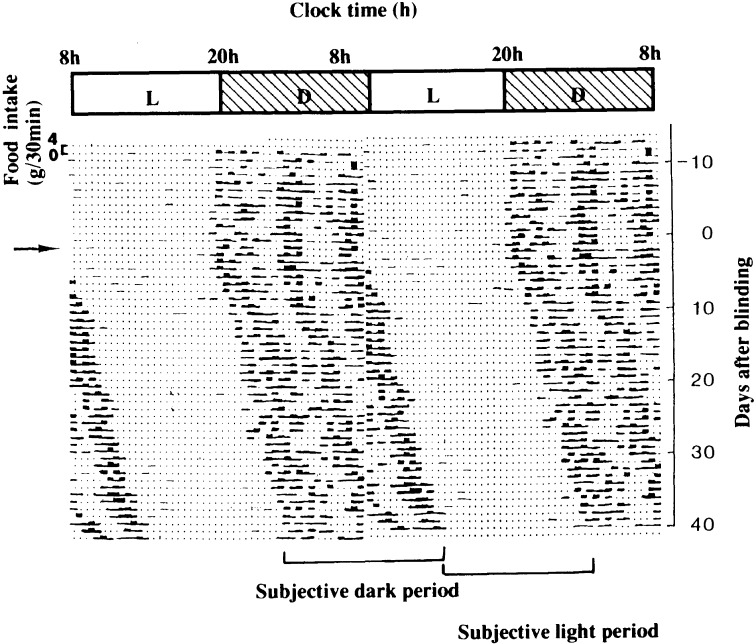
Figure 2 shows circadian feeding pattern of a rat with a 48-hour record in a line depicted by the double-plot method. In this case, rat was maintained in a room at 24 °C under a 12 hour light and 12 hour dark cycle and allowed to eat freely. It should be noteworthy that even rat eats almost 3 times as human does, while nocturnal animals eat mainly in the dark period. Figure 2 also shows that the 3 clusters of food intake run perpendicularly. However, the clusters began to show free-running rhythm with a cycle of longer than 24 hours after the animal was surgically blinded at the day indicated by an arrow.25) This indicates that the animal has own oscillator to drive feeding rhythm with a period of longer than 24 hours, but that the feeding rhythm is entrained to an environmental time cue under a 12 hour light and 12 hour dark cycle.
Fig. 2.
Circadian rhythm of feeding behavior of a rat. This animal was kept in a room illuminated for 12 h (08:00~20:00) and kept at 24 ±1 °C. Food intake was automatically measured by decrease in the total weight of a food container with a plate placed under it to collect the spillover of powdered food. Data are expressed as grams per 30 min. The figure is shown by the double-plot method, replotting the latter 24-h data in the beginning of the next line. At the point of an arrow, the animal is made blind by an operation. The approximate 12-h period when nocturnal animals are active and eat is called a subjective dark period, and the period when they are inactive is called a subjective light period. (From ref. 17.)
On the other hand, a number of report have so far indicated that food intake is regulated by the two hypothalamic areas, “the feeding center” in the lateral hypothalamic area (LHA) and “the satiety center” in the ventromedial hypothalamus (VMH). Some reported that bilateral ablation of the VMH resulted in complete loss of the circadian feeding rhythm. Thus we examined the relationship between the SCN, VMH and LHA in regard to the generations of the circadian temporal signal and showed that bilateral lesions of the SCN by electrolytic and immunological means completely abolished the feeding rhythm.22) but that total food intakes in a day of the operated rats were the same as those in sham-operated ones. We also obtained evidence suggesting that the SCN might be neuronally connected with the LHA and VMH by isolation of the nuclei from neighboring neural connections by knife-cut.23)
From these findings, we suggested that the circadian clock for feeding behavior was located in the SCN and that the temporal signal generated in the SCN was not only transmitted to the “feeding center” to increase their neuronal activities, but also transmitted to the “satiety center” to decrease their activities during the dark period in nocturnal rats.24) In this connection, it was demonstrated electro-physiologically that there were actually the synaptic pathways between the LHA and the SCN, and between the VMH and the SCN.26)
When rats were subjected to place under a restricted feeding schedule in which food was given only for 3 hours during light period in a 12 hour light and 12 hour dark cycle, the clusters of locomotive activity and drinking behavior appeared several hours before food was given, but immediately disappeared after restricted feeding schedule was replaced by free-feeding one. We showed that such “anticipatory response” monitored by Animex activity or drinking behavior was eliminated either by bilateral lesion of the SCN or VMH,26) but that that of wheel-running activity was lost by bilateral VMH lesion, but not by bilateral SCN lesion.25),27) We obtained firm evidence that motivation of wheel-running activity was different from that of activity monitored by Animex.28)
It was demonstrated that in rats VMH lesions not only eliminated food-shifted daily changes of body temperature and blood corticosterone level,29),30) but also “anticipatory response” in wheel-running activity.31) However, our results suggested that the VMH was involved in “anticipatory response” in wheel running rhythm, but that the SCN or/and VMH were related with the anticipatory response represented by Animex activity and drinking behavior.
Recently, some investigations reported that the dorsomedial hypothalamus (DMH) might be the site of the putative clock of “food-shifted daily changes of body functions” in mice and rats.32)–34) However, other group presented the negative results against “DMH theory”.35),36) Thus the actual site of such food-entrainable circadian rhythm or “anticipatory response” remains undefined yet.
Molecular oscillation mechanisms in circadian clock in suprachiasamtic nucleus
Explorations of the clock genes were initiated by a report of Konopka and Benzar published in 1971.37) They obtained 3 clock mutants in regard to circadian period of locomotive activity by treating a wild strain of Drosophila melanogaster with ethyl methane sulfonate, of which one was arrhythmic, another had a period of 19 hours and a third had a period of 28 hours and concluded that all these mutation might be derived from the same functional gene on the X chromosome. These 3 putative mutant genes were named pero, pers and per l. In 1984, the per region DNA was isolated independently by two different laboratories.38),39) It was also shown that a portion of per transcript was strongly homologous to mouse DNA.40)
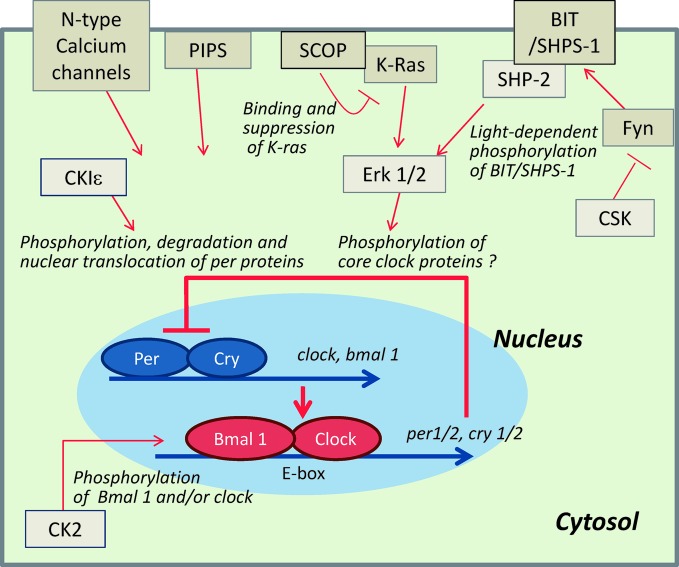
Clock was the first mammalian gene cloned by Takahashi and his collaborators in 1997.41) Subsequently, several genes such as per1-3, crypt1-2, and bmal1 were cloned and their transcripts were shown to be present in the SCN of mammals.42)–48) Figure 3 depicts a presumptive molecular mechanism of the circadian temporal signal generation, consisting of transcriptional and translational positive–negative feedback loop in mammals. This loop is considered to be essentially common to different animal species. The loop is established from the negative and positive elements, and the negative elements suppress transcriptions of the positive elements. The negative elements are period (per1-3) and cryptochrome (cry1-2) and their expressed transcripts and proteins showed the robust circadian oscillation, while the positive elements are clock and bmal 1 (brain and muscle Arnt-like protein 1) and their expressed transcripts and proteins show relative weak circadian or no rhythm. Positive elements, Bmal1 and Clock proteins, form heterodimer and bind to E-box of enhancer of per and cry genes and activate their transcriptions. The resulting enhancement of the expressions of per and cry inhibits their own transcripts. Numerous other proteins including protein kinases and phosphatases were also shown to be implicated in the circadian clock mechanism.
Fig. 3.
Regulation of core circadian oscillator in the SCN. In mammals, the core circadian oscillator is the feedback loop composed of Per/Cry and Clock/Bmal 1 heterodimers. These proteins create spontaneous circadian rhythms of their own expression essentially by transcriptional control. In addition, in order to adapt to internal and external conditions, the core circadian oscillator is regulated by numerous factors including environmental changes, hormones and neutritional signals, which are transmitted to the core circadian clock through signal transduction proteins that regulate the core oscillator by a variety of mechanisms such as phosphorylation.
During the period when attention began to be focus to identify the clock genes, our project turned to the following three points to analyze the molecular oscillation mechanism of the circadian clock in mammals; 1) exploration of possible oscillator candidates based on the effects of the agents inducing significant changes in the circadian rhythm upon their infusions into the brain, 2) isolation of proteins from the brain whose amounts or enzyme activities show circadian rhythms, and 3) isolation of proteins unique to the SCN.
In studies of the first category, we found that when a solution of insulin, ZnCl2, Ca ionophore A23187, NG-methylarginine (nitric oxide synthetase inhibitor) or ω-conotoxin caused significant disturbance of the circadian rhythm when it was infused into rat brain constantly through a catheter implanted into the 3rd ventricle from an Alzet’s osmotic minipump.17),49)–51) From the findings about A23187, it was later shown that N-type Ca2+-channel was involved in the circadian signal generation.51) This will be described later in connection of the circadian rhythm setting.
It is well known that insulin signaling is mediated by protein tyrosine kinases and the Zn+ is a potent inhibitor of protein tyrosine kinase. These findings tempted us to find out protein tyrosine kinase closely related to circadian rhythm generation. Consequently, we isolated a protein kinase named c-src kinase (CSK) from rat brain.52)–56) CSK has been shown to contribute to various signal transduction pathways in cooperation with Src-family kinases.56) In mammals, several Src-family kinases including Fyn and Src are highly expressed in the brain, and have been implicated in neuronal functions such as regulation of N-methyl-D-aspartate (NMDA) receptor signaling at the synaptic region.57) Moreover, Fyn-like immunoreactive substance was detected in the SCN. Then, we examined whether Fyn-deficient mice showed any disturbance in circadian rhythm of locomotive activity and found that under constant dark condition, the animals showed free-running rhythm with significant longer period than control animals. These findings suggest that Fyn is involved in the regulation of circadian oscillation system in the SCN.58) We also explored other molecular targets of tyrosine kinases in the SCN and found that BIT/SHPS-1 was one of those undergoing tyrosine phosphorylation in the SCN, as discussed in the later section.
In the studies on the second category, we identified three types of histone-H1 kinase whose activities showed circadian oscillations in rat SCN and termed periodically fluctuating protein kinases (FPKs). We showed that one of these proteins might be participated in the feed-back loop by inactivating CLOCK through phosphorylation of its Ser-Pro rich region.59) Recently, one of these kinases was identified as casein kinase (CK) 2 alpha and shown that this phosphorylated BMAL 1, suggesting that CK2 might be also an essential regulator of the mammalian circadian system.60)
In regard to the third strategy, Per1-interacting protein of the SCN (PIPS) was identified in mouse and several lines of evidence that it might be involved in the feedback loop of circadian rhythm by interacting with Per1 were presented.61) Shimizu et al.62) isolated a novel gene product, scop (SCN circadian oscillatory protein) from rat brain and found that it was expressed with a circadian manner in the SCN. SCOP is a 200 kDa protein which is composed of a pleckstrin homology (PH) domain, a leucine-rich repeat (LRR), a protein phosphatase 2C (PP2C)-like domain, and a glutamine (Q)-rich domain. The LRR domain of SCOP binds to K-Ras to negatively regulate the K-Ras-dependent ERK 1,2 activation.63) It is degraded in neurons by calpain, a calcium-dependent peptidase, upon stimulation with N-methyl-D-aspartate (NMDA), brain-derived neurotrophic factor (BDNF) or KCl-induced depolarization, resulting in enhancement of ERK 1,2 activation.64) Thus it seems quite likely that SCOP is a modulator of the signaling pathway from neuronal electrical activities to ERK 1,2 activation. These raised a possibility that the rhythmic change in SCOP protein was the cause of circadian changes in ERK1,2 activities in the SCN. SCOP is also distributed in various other brain regions than the SCN, suggesting that it plays broader roles in neural functions including long-term memory.63)
Takano et al.65),66) also demonstrated that casein kinase 1 epsilon (CK1 epsilon) catalyzed phosphorylation of mPer1, resulting in acceleration of nuclear translocation of mPer1 which seemed to be critical for circadian signal generation.67) Tamaru et al.60) demonstrated that casein kinase (CK)-2 alpha phosphorylated BMAL1 and suggested that CK2 might be also an essential regulator of the mammalian circadian system.
The main and auxiliary feedback loops are now being established, but the problem of how circadian temporal signal generated is transmitted to other parts of the brain and peripheral organs remain to be solved. We noticed this problem during an effort of investigations on the role of SCN on regulation of blood pressure. It is known that arginine vasopressin (AVP) neurons are abundant in the dorsal region of the SCN, while VIP neurons (vasoactive intestinal peptide-like immunoreactive substance) are abundant in the ventral region. Then we examined the effects of bilateral lesions of the SCN on the plasma AVP concentration in rats and found that the lesions suppressed the increase in the plasma AVP level by hypertonic saline and that this increase was enhanced by VIP injection into the brain, but suppressed by a VIP antagonist.68) To study the role of VIP in the SCN further, we examined the effect of injection to the SCN of a conjugate of anti-VIP IgG and A chain of ricin fragment, which was shown to eliminate the neurons with VIP-receptors in the presence of VIP. This procedure caused selective lesions of the two types of neurons in the SCN; the VIP neurons and the AVP neurons with VIP receptors. Interestingly, rats with latter lesions lost circadian drinking rhythm completely, while those with former lesions showed no change in the rhythm.69) This suggests that the AVP neurons with VIP receptors plays a role in transduction of the circadian signal rather than in its generation. Recent comment on this problem can be seen in Bittman’s review entitled “vasopressin: more than just an output of the circadian pacemaker?”.70)
Suprachiasmatic nucleus as center integrating autonomic nervous functions and energy metabolism regulation
As previously mentioned, circadian rhythm of PEPCK activity showed the circadian rhythm both in the liver and kidney. It was also described that this rhythm was generated under the regulation of autonomic nervous system and that it was abolished by bilateral SCN lesions (see Fig. 1). These findings raised the problem of how the SCN and the autonomic nervous system were interconnected. The following findings gave us a clue to challenge this problem.
The brain essentially requires glucose as a sole source of energy in mammalian brains including human beings. Moreover, it requires a large quantity of energy. For example, the human adult brain utilizes about 20% of total body energy consumption in a resting state, corresponding to glucose utilization of 120 g/day. Glucose is converted to carbon dioxide and water completely in the brain, where storage of glycogen is only slight (less than 0.2% of brain weight) and gluconeogenesis does not take place. Then, it must be constantly supplied from the peripheral organs.
In nocturnal animals such as rats and mice, the main source of glucose supplied to the brain is food in the dark period, while glycogen stored in the liver (glycogen in the muscle is not converted to glucose due to lack of glucose 6-phosphatase) and glucose derived from gluconeogenesis are the main sources of energy supplied to the brain in the light period. In diurnal animals including human beings, the situation is vice versa. Then, we examined in rats what was brought by if glucose utilization in the brain was inhibited by 2-deoxy D-glucose (2DG), a potent inhibitor of glucose utilization.
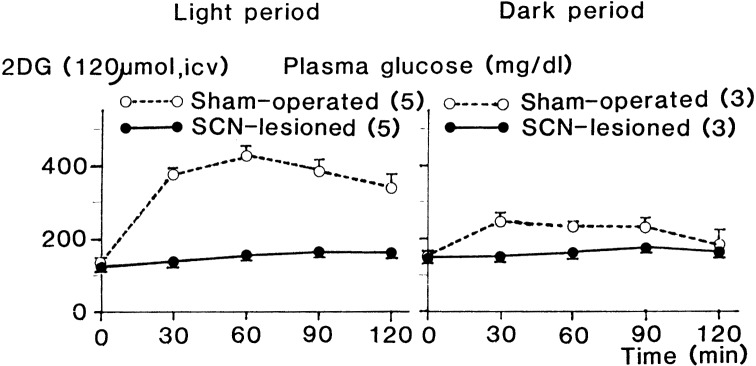
2DG induced time-dependent hyperglycemia that was more prominent in the light period, whereas glucose and saline as controls showed slight hyperglycemia only in the dark period and almost no response, respectively, when each agent was injected into the lateral cerebral ventricle (LCV).71) These responses were completely eliminated by bilateral lesions of the SCN, as shown in Fig. 4,72) suggesting that more potent signal from the SCN is generated to prevent deficiency of energy supply to the brain in the light period when no food is given. It was also revealed that lipolytic response to systemic administration of 2DG was time-dependent, showing increase in plasma FFA (free fatty acid) concentration only in the light period. This suggests that in the light period, FFA, an energy source utilized in the peripheral organs, is mobilized to save glucose consumption in the peripheral organs. This response was also abolished by SCN lesions.73) Electric stimulation of the SCN resulted in hyperglycemia.74) These findings indicate that the SCN is involved in energy supply to the brain. Then we examined what pathway transmitted the signal generated in the SCN to the peripheral organs.
Fig. 4.
Effect of bilateral lesions of the SCN on plasma concentration of glucose after 2DG injection into the lateral cerebral ventricle in the light and dark periods. Rats were maintained in a room illuminated for 12 h (02:00~14:00). 2DG was injected into the lateral cerebral ventricle of SCN-lesioned and control (sham-operated) rats at 10:00 (light period) and 17:00 (dark period) after 2 h starvation. Values are means ±SEM. Numbers of animals used are indicated in parentheses. (From ref. 73.)
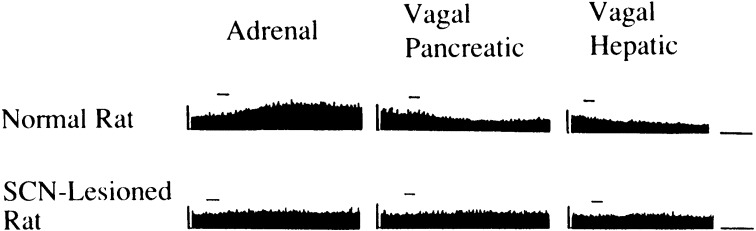
It was previously shown that the hyperglycemia due to 2DG was completely inhibited by adrenalectomy either in the light period or dark period.75) It was also shown that it was inhibited by various blockers of the sympathetic and parasympathetic nervous systems.76),77) These findings suggest that the autonomic nervous system is involved in time-dependent-hyperglycemic response to 2DG. To substantiate this suggestion, we examined direct recordings of the neural activities of sympathetic efferent fibers to the adrenal gland, pancreas and liver and those of the parasympathetic efferent fibers to the pancreas and liver in collaboration with Dr. Akira Niijima, Emeritus Professor of the Niigata University. These revealed that in the light period all neural activities of the sympathetic efferent fibers increased in response to intracranial administration of 2DG, while those of the parasympathetic efferent fibers decreased and that these responses were eliminated by bilateral SCN lesions.25)
In respect to neural pathways from other parts to the SCN, it was confirmed that there existed actually two separate populations of presympathetic and preparasympathetic neurons in the SCN by means of injection of retrograde trans-synaptic tracer pseudorabies virus (PRV) into the adrenal for the former and into the liver with denervated sympathetic branch for the latter.78) These findings support our previous hypothesis that the SCN play a key role in energy supply from the peripheral organs to the brain through autonomic nervous system.
In addition, recent investigations by Nagai and his colleagues using the direct recording system of electrical activities of peripheral fibers revealed that injection of carnosine79) or adiponectin,80) and olfactory stimulation with certain kinds of scents,81) induced changes in sympathetic and parasympathetic nerve activities and that these effects were abolished by bilateral lesion of the SCN.79)–81)
Different localization of circadian clock and regulation center of energy metabolism in the suprachiasamtic nucleus
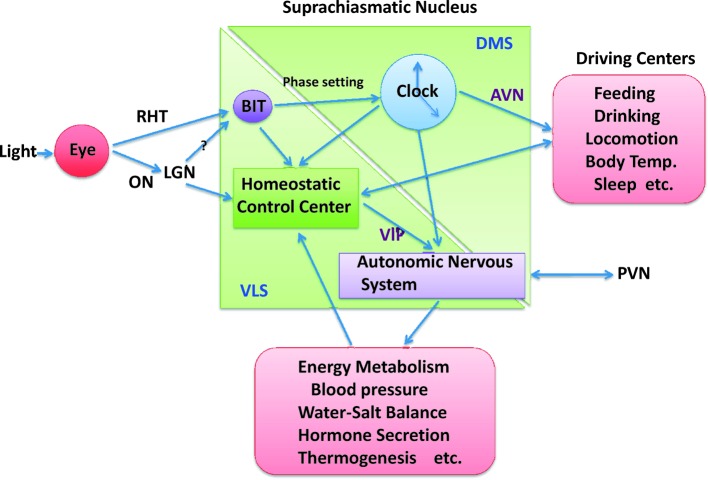
In mammals, circadian rhythms of behaviors, hormone levels, neural functions, metabolism and other physiological phenomena are synchronized with an environmental LD cycle which is created by rotation of the earth. The light signal of this LD cycle is transmitted to the SCN from the retina through the retinohypothalamic tract (RHT).82) The RHT is projected to the ventrolateral part of the contralateral SCN.83) These relationships are illustrated in Fig. 5.
Fig. 5.
Diagram of interrelationship between the two subdivisions of the SCN in regard to regulations of circadian rhythms and homeostasis. AVN: arginine-vasopressinergic neurons. PVN: pareventricular nucleus. VIP: vasoactive intestinal peptide. DMS and VLS: dorsomedial and ventrolateral subdivisions of the SCN, respectively.
Otani and his collaborators created hereditary microphthalmic rats.84) These animals are congenitally blind, because they lack optic nerves and the RHT. They have structurally abnormal SCN, but circadian clock is apparently present in it, because locomotive activity shows free-running circadian rhythm. In collaboration with his group, we examined the effect of intracerebroventricular injection of 2DG. In contrast to the findings in normal rats, 2DG caused no and only slight hyperglycemia in male and female rats with hereditary microphthalmia, respectively.85) In the SCN of the mutant rats, AVP neurons and VIP neurons were present in the ventrolateral and dorsomedial parts, respectively, as in normal rats. However, the number of the VIP neurons were significantly reduced.86) Taken together, these findings raised a possibility that the VIP neurons in the ventrolateral subdividion of the SCN is essential to the hyperglycemic response to 2-DG.
The role of the VIP neurons in metabolic regulation was further supported by the findings that suppression of hyperglycemic response due to 2DG by bilateral SCN lesions were restored by intracerebro-ventricular injection of VIP and that the infusion of VIP antisense oligonucleotide into the third ventricle with Alzet’s osmotic minipump not only inhibited the expression of VIP in the SCN, but also abolished the hyperglycemia due to 2DG injection.87)–90) These findings suggest that VIP neurons in the SCN are involved in regulation of energy metabolism.
Roles of environmental light as synchronizer of circadian clock and regulator of autonomic nervous functions
As previously described, circadian rhythms under the present discussion are entrained to environmental light-dark cycle generated by the earth rotation and light signal is transmitted from the eye to contralateral SCN through the RHT. On the other hand, it is generally accepted that environmental light induces shift of circadian phase of a rhythm, phase advance, phase delay or no response, depending on what phase it is illuminated, and using this system, organisms including human beings adjust their circadian period to 24 hour period. However, it remains yet what mechanism is involved in it.
During the study of the functional roles of thiamine pyrophosphatase in rat brain, we happened to find a glycoprotein unique to the brain among the proteins cross-reacting with crude anti-thiamine pyrophosphatase preparation and named 1D4 antigen.91)–93) This gave us a key to solve the relationship between lighting, phase shift of circadian rhythm and autonomic nervous functions.
1D4 antigen is now known as BIT/SHPS-1 (brain immuno-globulin-like molecule with tyrosine-based activation motifs/substrate of SH2-containing protein tyrosine phosphatase 2). This name is derived from the structure of 1D4 antigen, which is a transmembrane glycoprotein with three immunoglobulin-like domains in the extracellular part and two signaling motif containing tyrosine phosphorylation sites in the intracellular part.94) Later, we obtained several lines of evidence that tyrosine-phosphorylation of BIT/SHPS-1 might be involved in the mechanisms of entrainment of the circadian rhythm to environmental light-dark cycle.
It was found that tyrosine phosphorylation of BIT was higher in the light period than in the dark period in the SCN of rats under 12 hour light and 12 hour dark cycle while the amount of the protein was constant, and that light exposure increased its phosphorylation during the dark period. It was also found that tyrosine phosphorylated form of BIT is bound to the Src homology 2 domain of a protein tyrosine phosphatase, SHP-2. Moreover, it was shown that injection of monoclonal antibody against BIT/SHPS-1 into the SCN to stimulate its tyrosine-phosphorylation caused time-dependent phase shift of circadian locomotive rhythm; phase-delay at CT15 and phase-advance at CT20. In addition, it was revealed that pretreatment of rats with MK801, a non-competitive antagonist of NMDARs (receptors for NMDA) diminished the effect of the phase-delay at CT15 due to the antibody injection, whereas it did not affect those of the phase-advance at CT20.95) These findings suggest that tyrosine-phosphorylation of BIT plays a critical role in the entrainment of circadian rhythms to environmental light-dark cycle through NMDARs. Moreover, infusion of NG-methylarginine (nitric oxide synthetase inhibitor) into the brain caused not only disturbance, but also shift of the drinking rhythm under the constant darkness, as previously mentioned.51)
BIT/SHPS-1 is distributed along the neuronal cell periphery in the entire brain regions as detected by immunohistochemichal analysis. In the SCN, the immunoreactivity is especially high in the ventrolateral region where VIP neurons are located and a portion of optic nerves are innervated (unpublished observation). It was also reported that retinal ganglion cells had axo-somatic and axo-dendritic synaptic contacts with VIP-neurons in the SCN. It is thus possible that, when the VIP neurons receive light signals from the optic nerves, tyrosine phosphorylation of BIT/SHPS-1 is enhanced in its own cells to modulate the expression of clock genes. However, it is yet to be determined in which region of the SCN BIT/SHPS-1 is actually tyrosine-phosphorylated in response to light-exposure.
From the findings that environmental light might be involved in the regulation of glucose metabolism through the control of autonomic functions, on the other hand, we examined whether and how it affected energy supply to the brain and found that light stimulation with 2,000 lux for 10 minutes to the left eye of rat increased efferent activities of the pancreatic, hepatic, splenic, adrenal and renal branches of the splanchnic (sympathetic) nerve and suppressed those of the pancreatic, hepatic and gastric branches of the vagus nerve, irrespective of environmental lighting condition and that these changes were eliminated by bilateral lesions of the SCN as in case of 2-DG injection (Fig. 6).96)–98) These findings suggest that in diurnal animals including human environmental light stimulates energy supply to the brain by activating the sympathetic nerve functions and that appropriate light exposure can correct imbalanced function of autonomic nervous system occurring in such situations as stress and jet lag.
Fig. 6.
Effects of light exposure on the efferent activity of the adrenal branch of the splanchnic nerve, and the pancreatic branch of vagal nerve in normal rats and rats with bilateral lesions of the SCN. Upon horizontal bars; light stimulation at 2,000 lux for 10 min to the left eye; horizontal bars; 30 min; vertical bars; 100 impulses/5 s. (From ref. 96.)
In short, the environmental light projected to the ventrolateral subdivision of the SCN is involved not only in phase shift of the circadian rhythm, depending on when it is illuminated, through tyrosine phosphorylation of BIT/SHPS-1, but also regulations of glucose supply to the brain through autonomic nervous functions with the VIP neurons as mediator.
The suprachiasmatic nucleus and homeostatic regulation
During these investigations, we also found that beside energy metabolism, the SCN played critical roles in homeostasis of body functions such as fluid balance, blood pressure, endocrine secretion such as insulin, glucagon and vasopressin and body temperature. In this respect, the relationship between circadian rhythm and homeostasis will be discussed.
As depicted in Fig. 2, in rat, a nocturnal animal, three clusters of food-intake were differentiated during the dark period in 12 hour light and 12 hour dark cycle and these clusters showed free-running with a period more than 24 hours in a constant dark condition. This fact suggests that the three clusters of food-intake are induced by the circadian temporal signal from the SCN. From this, it also seems likely that a habit of three meals a day in human being, diurnal animal, might be induced by the mechanism similar to rat in which the SCN is involved.
It is well known that insulin secretion is enhanced to assimilate the nutrients derived from food into body during meal, while glucagon secretion is enhanced to degrade body constituents to supply energy between meals. From these facts, it can be expected that in rat insulin secretion is higher during the dark period when the animal is eating, while glucagon secretion is higher during light period when it is starved. In fact, plasma insulin concentration showed a daily change with the highest value in the middle of the dark period and the lowest in the middle of light period. In contrast, the plasma glucagon concentration changed inversely to that of insulin.99) Moreover, we obtained firm evidence that daily change in the plasma insulin concentration was driven by endogenous oscillator in the SCN. On the other hand, plasma glucagon concentration showed daily change and bilateral lesions of the SCN lowered it to the baseline level either in the light and dark periods. 2DG injection into the brain caused significant increase in plasma glucagon concentration whose levels were the same both during the light and dark periods and the increase was abolished by bilateral lesions of the SCN.99),100) These findings suggest that glucagon secretion is under the control of the SCN as in case of insulin secretion, and that it is not directly driven by circadian temporal signal from endogenous oscillator in the SCN, but rather by food intake or its related events.
In regard to insulin secretion, it was reported that in human glucose tolerance was lower in the late evening.101) On the other hand, it was shown that in rats, glucose tolerance was higher during the dark period than during the light period, irrespective of the way of glucose administration, orally or intravenously and bilateral lesions of the SCN abolished the time-dependent difference in glucose tolerance.102) These findings indicate that insulin sensitivity of tissues is also under the control of circadian temporal signal from the SCN.
It has been reported that in rat plasma corticosterone concentration shows circadian rhythm with the highest level at the end of the light period and the lowest level at the end of the dark period and that the rhythm is abolished by bilateral lesions of the SCN.19),103) In line with these findings, bilateral lesions of the SCN eliminated circadian rhythm of plasma ACTH.104) In human beings, it was shown that plasma level of cortisol, the predominant glucocorticoid, showed circadian rhythm with highest level just before awaking and the lowest one in the midnight105) and that the rhythm showed free-running with a period of 25.5 hours in blind people.106) These findings suggest that circadian rhythm of plasma cortisol level is driven by endogenous oscillator with dark-light cycle as the environmental synchronizer. Other hormones such as plasma aldosterone, testosterone, luteinizing hormone, growth hormone, prolactin, thyroid stimulating hormone and follicle-stimulating hormone are also known to show daily variations.107) Such daily hormonal variations are considered to be generated under the control of the time signal from the SCN in even human beings.
As described previously, vasopressinergic neurons were concentrated in the dorsomedial subdivision of the SCN where circadian clock involved in generation of the circadian time signal is located. On the other hand, it is well known that vasopressin is implicated in regulations of the blood osmosis and pressure. It is known that AVP is produced not only in the SCN, but also in the paraventricular nucleus and the supraoptic nucleus, and AVP in the latter two nuclei are transported to the neurohypophysis through neural axons. On the other hand, it was reported that neural structures in the preoptic-SCN region was related to the regulation of water-salt balance108) and arterial blood pressure109) and also that paraventricular-SCN lesions prevented experimentally induced hypertension.110) These findings raised the problem of how different roles AVP produced in different nuclei played in and which subdivision of the SCN was related to water-salt balance, if AVP produced in the SCN is involved in such functions.
In regard to the former problem, we obtained evidence that AVP-neurons in the paraventricular nucleus inhibited energy supply to the brain by suppressing the sympathetic activities, but that AVP-neurons in the SCN enhanced on the sympathetic activities.
We obtained further evidence that AVP-neuron in the SCN was involved in water-salt balance and blood pressure, using hereditary blind microphthalmic rats. In normal rats, twenty four hour water deprivation induced increase in the plasma arginine-vasopressin (AVP)111) and renin levels.112) However, these increases were greatly suppressed in hereditary blind microphthalmic rats with morphologically abnormal structure in the ventrolateral subdivision of the SCN, but with free-running circadian locomotive activity.113) This clearly indicates that the other region of the SCN than that including the circadian oscillator, ventrolateral subdivision, is related to these functions.
The SCN is also involved in maintenance of body temperature. When rats or mice are exposed to cold, heat production was stimulated mainly by three means; shivering thermogenesis, increased gluconeogenesis and increased heat production in the brown adipose tissue. It is well known that the brown adipose tissue is abundantly innervated by sympathetic nerve.114) On the other hand, we showed that in rats cold-exposure caused remarkable increase in liver PEPCK activity whose level far exceeded the peak activity of circadian rhythm of the enzyme activity115) and also that both of sympathetic nerve and thyroid hormone were involved in this response.116) These findings suggest that increased gluconeogenesis is needed for body temperature maintenance at such a severe environmental temperature as cold-exposure and that it is induced by enhanced sympathetic outflow from the SCN. Recently, it was found that in rats acute cold exposure resulted in enhanced tyrosine phosphorylation of BIT through NMDA receptor not only in the SCN, but also in other nuclei including the paraventricular nucleus, lateral hypothalamic area, ventromedial hypothalamus and arcuate nucleus.117) Conversely, it was found that administration of mAb 1D4 (anti-BIT) into the third cerebral ventricle enhanced the electrical activity of the renal sympathetic nerves, while it suppressed that of the gastric parasympathetic nerves. It was also shown that temperatures of the abdomen and brown adipose tissue as well as blood pressure increased in response to the mAb 1D4 injection.118) These results indicate that BIT/SHPS-1 is involved in the hypothalamic regulations of thermogenesis and blood pressure via the autonomic nervous system.
In summary, in the SCN the dorsomedial subdivision includes master circadian oscillator and the ventrolataral subdivision includes homeostatic control center. The latter detects environmental and endogenous signals and relayed them to autonomic nervous system to maintain homeostasis. When needed, circadian temporal signal system is switched to connect homeostatic control center, but the former is neglected when maintenance of homeostasis is strongly requested in such case of cold-exposure. These relationships are depicted in Fig. 6.
Relationship between clock genes in the SCN and peripheral tissues
We have so far mentioned the close relationship between circadian rhythm of glucose supply to the brain and the circadian oscillator in the SCN. We have also suggested that circadian temporal signal is transmitted through the autonomic nervous system to peripheral tissues. It was also mentioned that circadian locomotive activity in hereditary blind microphthalmic rat with morphological abnormal structure in the ventrolateral subdivision of the SCN showed free-running circadian rhythm. This indicates that the circadian time signal from the SCN controls hierarchically peripheral circadian rhythms through autonomic nervous system. Recently, clock genes were found in the peripheral tissues and their expressions showed free-running circadian rhythms.119) It was also shown that, when food intake was restricted to the light period in rat, the central and peripheral clocks were desynchronized.120) These findings suggest that circadian rhythms of peripheral metabolism may partly be created by transcriptional regulation by peripheral clock genes and thus that disturbance of peripheral metabolism be brought about by free-running of circadian rhythms of some enzyme activities. This suggestion is supported by the findings that mice bearing a mutation of a clock-related gene show metabolic disorders such as obesity121) and our findings on fat deposition in hereditary blind microphthalmic rats.25)
On the other hand, the Clock gene product was found to catalyze acetylation of proteins including histone and BMAL1.122) This activity shows a circadian rhythm and is counteracted by NAD+-dependent histone deacetylase, SIRT1.123) In addition, an NAD+-synthesizing enzyme, nicotinamide phosphoribosyltransferase (NAMPT), is under the transcriptional control of clock.124) These indicate that clock genes and peripheral metabolism are closely linked each other.
Other functions of the SCN
Leibowitz et al.125) reported that in rats macro-nutrient selection exhibited the daily change under an 12 hour light and 12 hour dark cycle; carbohydrate was mainly selected in the early dark period, protein-intake was higher during the period from the middle to late dark period and lipid was mainly selected in the late dark period and suggested that serotonin in the SCN as well as the paraventricular nucleus (PVN) and VMH might be involved in macronutrient selection. As described earlier, plasma glucagon level showed daily change, whose pattern was very similar to protein-intake. This tempted us to examine the effects of bilateral lesions of the SCN and hereditary blind microphthalmic rats on macro-nutrient selection. Consequently, we found that in intact rats intraventricular and subcutaneous infusions of glucagon caused significant increase in protein intake, while they caused compensatory decrease in carbohydrate intake. We also found that the changes were negated either in bilateral SCN-lesioned rats or hereditary blind microphthalmic rats.126) These findings suggest that the SCN might be involved in macronutrient selection through glucagon.
On January 17th 1995, the Kobe earthquake which was counted as one of the worst in Japan’s history occurred. Then, mouse circadian diagrams recorded drastic increases in locomotive activities during sleep and active periods before the earthquake began.127) Japanese anecdote tells that some animals such as catfish and mice become rampageous before earthquake probably because they have an ability to catch any telltale signs of an immediate earthquake. This allures us to speculate that the SCN is implicated in such animals’ perception.
Concluding remarks
We have so far reviewed that regulation centers of the two apparently contradictory phenomena, constancy of internal environment (homeostasis) and circadian changes of various bodily functions, both of which are essential for our survival, are located in the SCN. This tempts us to speculate what benefits are expected from this connection. We will try to explain this on the basis of energy supply to the brain as an example.
Blood glucose level is a typical example of maintenance of homeostasis. Energy supply to the brain is a rationale behind this. As previously mentioned, the brain consumes glucose as a sole source of energy in a fed state, and its consumption is so great that it accounts approximately 50 and 20% of total energy consumptions in infantile and adult human, respectively. Moreover, brain energy consumption during sleeping period is almost the same as during active period.128) For the purpose of continuous supply of such a large quantity of glucose to the brain, animals must eat during active period, irrespective of whether they are diurnal or nocturnal. Liver glycogen stored after food-intake might be degraded to glucose between meals, and enhanced gluconeogenesis is required during resting or sleeping period owing to limitation of glycogen storage in the liver. In order to switch from eating to gluconeogenesis and vice versa, the body must receive circadian time signal and environmental changes as quickly as possible. In this sense, the structural characteristics that the neurons involved in the regulation of energy metabolism are located close to both the neurons involved in time signal generation and those involved in receiving neural signals of environmental LD information from the retinal ganglion cells might facilitate this and are thus physiological essential.
Various hormones are involved in maintenance of homeostasis. Among these, some show circadian rhythms in their plasma levels under the direct control of the temporal signal from the SCN, while others show daily change probably due to something related to food-intake but without participation of circadian oscillator in the SCN. Glucocorticoid and insulin are the former cases and glucagon is the later case. In both cases, the substrates controlling hormonal secretions are located in the ventrolateral subdivision of the SCN. This raises a question of what determines the receptivity of circadian temporal signal from the SCN. This must await further investigations.
We human have a habit of eating three times a day. Judging from three clusters of food-intake in rats during the dark period (Fig. 2), it is possible that even in human the time signals issued for eating are sent from the circadian oscillator not only to “feeding center” and “satiety center,” but also to the regulation center of homeostasis three times during the subjective day period, from the signal of the latter is further relayed to peripheral organs through the autonomic nervous system. This is partly supported by many of recent observational studies that three meals a day induces more appropriate insulin sensitivity and lipid profiles in comparison with irregular meal frequency in human129) and that skipping breakfast is possibly leads to weight and deleterious alterations in risk factors for diabetes and cardiovascular disease.130) In line with this possibility, observations in human indicated that large meals in small number of food-intake apparently promoted the deposition of fat and overweight, decreased glucose tolerance and hypercholesterolemia.131) In addition, it was reported that significant higher fasting total cholesterol and LDL cholesterol concentrations were observed when breakfast was skipped. Thus it is suggested that skipping breakfast causes impairments of fasting lipids and postprandial insulin sensitivity.132)
It remains unsolved yet whether the feeding time of a person is determined genetically or his or her life-style. However, it seems very likely that once his or her feeding time is determined, his or her body prepares to utilize efficiently food constituents around the feeding time so that their digestion, and transport in the intestine, hormonal secretions and other related functions are well harmonized. In this regard, it should be noteworthy that in rats even intestinal dissacharidase activities show daily changes synchronized with feeding rhythm.133)–137)
Conversely, understanding such harmonizing effects of the two subdivisions of the SCN must be needed to solve the relevant problem of the effects of breakfast skipping on human body.
Light is essential for evoking visual sensation. Light is also involved in biosynthesis of Vitamin D3 in the body. As described previously, it is also important for synchronization of the phase of the endogenous clock as an environmental LD cycle. Its fourth function is related to the regulation of the autonomic nervous system through the SCN; it stimulates the sympathetic nervous activity, while it suppresses the parasympathetic nervous activity. It was reported that bright light with more than 2,500 lux was needed for facilitating phase-shift of circadian rhythm in human,138) though 1,000~2000 lux were used in rats in our cases.
In the latter sense, exposure to bright light is important for balancing the autonomic nervous system. It is generally accepted that human sympathetic nervous activity is enhanced, while parasympathetic nervous activity is suppressed during light or active period and vice versa during dark or sleeping period. Moreover, bright light is needed not only for facilitating energy supply to the brain, but also preventing obesity due to enhanced lipolysis by stimulating sympathetic nervous activity. In this connection, we found that fat composition in the hereditary blind microphthalmic rats was remarkably higher than that in the control littermate, whereas no significant difference was found between body weights and naso-anal lengths of the blind and control rats.25) It was also reported that an increase in insulin sensitivity and change in glucose metabolism were sometimes observed in congenitally blind humans and that the altered glucose metabolism in patients with cataracts was reverted to normal after their sights was recovered after surgically.139) These findings suggest the possibility that functional inability of the SCN is causative factor of some of type 2 diabetes mellitus.
Nowadays air pollution is debated worldwide as a threat not only to human health, but also to the Earth’s ecosystems. Of various causative effects, much attention should be placed on the facts that they interfere light emitted from the sun. Furthermore, there are many people live in the contemporary buildings with closed windows and artificial illumination. These situations provide us to decreased opportunity for exposure to sunlight. Thus we should encourage vigorous efforts to decrease pollutants and find chances in exposing to sunlight for our own mental health and survival.
Acknowledgements
We wish to thank late Professor Masami Suda, the founder of the Division of Protein Metabolism, Institute of Protein Research, Osaka University who inspired us to develop this project and our collaborators who had participated in these studies. Special thanks also to Dr. Akira Niijima, Emeritus Professor of the Niigata University, for his help of our works with his excellent electro-physiological technique.
Biographies
Profile
Hachiro Nakagawa was born in Osaka, Japan, in 1931. He graduated from School of Medicine, Osaka University and received M.D. in 1956. After one year internship at Osaka University Hospital, he started his research career in the field of physiological chemistry as a graduate student at the graduate school of Osaka University and received Ph.D. in 1961. He continued his research as a research assistant with Prof. Masami Suda at the Department of Physiological Chemistry, School of Medicine, Osaka University. He was a postdoctoral fellow from 1962 to 1965 at the Department of Physiological Chemistry, the University of Wisconsin where he was involved in molecular analyses of development of urea cycle during metamorphosis of tadpole induced by thyroxine under the supervision of Prof. Phillip P. Cohen. He moved to the Division of Protein Metabolism, Institute for Protein Research, Osaka University in 1967. During a period from returning to his old research place to moving to new one, he isolated cystathionine synthetase from serine dehydratase in rat liver both of whose enzyme reactions were then assumed to be catalyzed by the same protein. He was appointed to be associate professor in 1971, professor in the Division of Protein metabolism in 1973 and then director of the Institute, Osaka University during 1993 and 1995. During his research carrier at the Institute for Protein Research, Osaka University, he isolated newly a number of proteins from mammalian tissues, especially the brain and developed such unique research on their roles in vivo as the way called “whole body biochemistry.” He is now director of the International Institute of Alternative Medicine in Kishiwada, Osaka.

Profile
Nobuaki Okumura was born in Kyoto, Japan, in 1963. He graduated from Faculty of Science, Osaka University in 1985, and became a graduate student of Graduated School of Science, Osaka University. During he was a graduate student, he studied about the signal transduction mechanisms of neurotransmitters and neurotrophic factors in Professor Hachiro Nakagawa’s laboratory, the Division of Protein Metabolism, Institute for Protein Research, Osaka University. In 1989, he moved to Professor Takashi Shimazu’s lab at School of Medicine, Ehime University as an assistant professor, and studied about the regulation of glucose transporters. In 1991, he moved again to Professor H. Nakagawa’s lab. at the Institute for Protein Research, Osaka University, and received PhD in 1994. After 1995, he continued studying with Professor Katsuya Nagai at the same institute and became the associate professor in 2002. During the period in this institute, he studied about molecular mechanisms of circadian rhythms and homeostatic regulation by the hypothalamus, especially focusing on Src-family substrates, BIT/SHPS-1 and β-adducin, and naturaly occuring dipeptide, carnosine.

References
- 1).Kato A., Matsuzawa T., Suda M., Nakagawa H., Ishizuka J. (1964) Control mechanism in the rat liver enzyme system converting L-methionine to L-cystine. Enzymatic basis for the methionine-sparing action of L-cystine. J. Biochem. 55, 401– 409 [DOI] [PubMed] [Google Scholar]
- 2).Nakagawa H. (1971) Enzymatic investigations of nutrition: The roles of serine dehydratase and cystathionine β-synthetase. SEIKAGAKU (J. Jap. Biochem. Soc.) 43, 245–266 (in Japanese). [PubMed] [Google Scholar]
- 3).Nagabhushanam A., Greenberg D. M. (1965) Isolation and properties of a homogeneous preparation of cystathionine synthetase-L-serine and L-threonine dehydratase. J. Biol. Chem. 240, 3002–3008 [PubMed] [Google Scholar]
- 4).Nakagawa H., Kimura H., Suda M. (1967) Crystallization and characteristics of serine dehydratase from rat liver. Biochem. Biophys. Res. Commun. 28, 359–364 [DOI] [PubMed] [Google Scholar]
- 5).Nakagawa H., Kimura H. (1969) The properties of crystalline serine dehydratase of rat liver. J. Biochem. 66, 669–683 [PubMed] [Google Scholar]
- 6).Kimura H., Nakagawa H. (1968) Purification and properties of cystathionine synthetase from rat liver. Biochem. Biophys. Res. Commun. 32, 599–601 [DOI] [PubMed] [Google Scholar]
- 7).Nakagawa H., Kimura H, Suda M. (1968) Separation of serine dehydratase and cystathionine synthetase. InSymposium on Pyridoxal Enzyme, Maruzen Co. Ltd., Tokyo, pp. 101–103 [Google Scholar]
- 8).Kimura H., Nakagawa H. (1971) Studies on cystathionine synthetase characteristics of purified rat liver enzyme. J. Biochem. 69, 711–723 [DOI] [PubMed] [Google Scholar]
- 9).Nakagawa H., Nagai K. (1971) Cold adaptation I. Effect of cold exposure on gluconeogenesis. J. Biochem. 69, 923–934 [DOI] [PubMed] [Google Scholar]
- 10).Carmel R, Jacobsen D. W. (eds.) (2001) Homocysteine in Health and Disease. Cambridge University Press, Cambridge, UK [Google Scholar]
- 11).Suda M., Nagai K., Nakagawa H. (1973) Studies on the circadian rhythm of phosphoenolpyruvate carboxykinase activity in rats. I. Mechanism of circadian increase in liver enzyme with special reference to hormonal and dietary effects. J. Biochem. 73, 727–738 [DOI] [PubMed] [Google Scholar]
- 12).Nagai K., Suda M., Nakagawa H. (1973) Studies of circadian rhythm of phosphenolpyruvate carboxykinase activity in rats. II. Effect of the autonomic nervous system on the rhythm in liver. J. Biochem. 74, 863–871 [PubMed] [Google Scholar]
- 13).Nagai K., Suda M., Yamagishi O., Toyama Y., Nakagawa H. (1975) Studies on the circadian rhythm of phosphophenolpyruvate carboxykinase III. Circadian rhythm in the kidney. J. Biochem. 77, 1249–1254 [PubMed] [Google Scholar]
- 14).Kida K., Nishio T., Yokozawa T., Nagai K., Matsuda H., Nakagawa H. (1980) The circadian change of gluconeogenesis in the liver in vivo in fed rats. J. Biochem. 88, 1009–1013 [DOI] [PubMed] [Google Scholar]
- 15).Kida K., Nakajo S., Kamiya F., Toyama Y., Nishio T., Nakagawa H. (1978) Renal net glucose release in vivo and its contribution to blood glucose in rats. J. Clin. Invest. 62, 721–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Kato H., Mizutani-Funahashi M., Shiosaka S., Nakagawa H. (1978) Circadian rhythms of urea formation and arginosuccinate synthetase activity in rat liver. J. Nutr. 108, 1071–1077 [DOI] [PubMed] [Google Scholar]
- 17).Nakagawa H., Nagai K., Kida K, Nishio T. (1979) Control mechanism of circadian rhythms of feeding behavior and metabolism influenced by food intake. InBiological Rhythms and Their Central Mechanism (eds. Suda M., Hayaishi O., Nakagawa H.). Elsevier North-Holland Biomedical Press, Amsterdam, pp. 283–297 [Google Scholar]
- 18).Axelrod J., Wurtman R. J., Solomon H. S. (1965) Control of hydroxyindole O-methyltransferase activity in the rat pineal gland by environmental lighting. J. Biol. Chem. 240, 949–954 [PubMed] [Google Scholar]
- 19).Moore R. Y., Eichler V. B. (1972) Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 42, 201–216 [DOI] [PubMed] [Google Scholar]
- 20).Stephan F. K., Zucker I. (1972) Circadian rhythms in drinking behavior and locomotive activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. USA 69, 1583–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Nishio T., Shiosaka S., Nakagawa H., Sakumoto T., Satoh K. (1979) Circadian feeding rhythm after hypothalamic knife-cut isolating suprachiasmatic nucleus. Physiol. Behav. 23, 763–769 [DOI] [PubMed] [Google Scholar]
- 22).Nagai K., Mori T., Nakagawa H. (1982) Application of an immunological technique to behavioral studies: Anti-suprachiasmatic nucleus γ-globulin induced loss of the circadian rhythm. Biomed. Res. 3, 294–302 [Google Scholar]
- 23).Nagai K., Nishio T., Nakagawa H., Nakamura S., Fukuda Y. (1978) Effect of bilateral lesion of the suprachiasamatic nucleus on the circadian rhythm of food-intake. Brain Res. 142, 384–389 [DOI] [PubMed] [Google Scholar]
- 24).Nagai K., Yamamoto H, Nakagawa H. (1985) Circadian rhythm of feeding and metabolism: Suprachiasmatic nucleus as a biological clock and a site of metabolic regulation. InEndogeneous Sleep Substances and Sleep Regulation (eds. Inoue S., Borbély A. A.), Japan Sci. Soc. Press, Tokyo, pp. 113–125 [Google Scholar]
- 25).Nagai K, Nakagawa H. (1992) Central Regulation of Energy Metabolism with Special Reference to Circadian Rhythm. CRC Press, Boca Raton [Google Scholar]
- 26).Oomura Y., Ono T., Nishio H., Kita H., Shimizu N., Ishizuka S, et al. (1979) Hypothalamic control of feeding behavior: Modulation by the suprachiasmatic nucleus. InBiological Rhythms and Their Central Mechanism (eds. Suda M., Hayaishi O., Nakagawa H.). Elsevier North-Holland Biomedical Press, Amsterdam, pp. 295–308 [Google Scholar]
- 27).Mori T., Nagai K., Nakagawa H. (1983) Dependence of memory of meal time upon circadian biological clock in rats. Physiol. Behav. 30, 259–265 [DOI] [PubMed] [Google Scholar]
- 28).Nagai K., Mori T., Nakagawa H. (1982) Different responses of running wheel and animex activity to restrictd feeding and drug-induced anorexia in rats. Biomed. Res. 3, 333–336 [Google Scholar]
- 29).Krieger D. T., Hauser H., Krey L. C. (1977) Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science 197, 398–399 [DOI] [PubMed] [Google Scholar]
- 30).Krieger D. T. (1980) Ventromedial hypothalamic lesions abolish food-shifted circadian adrenal and temperature rhythmicity. Endocrinology 106, 649– 654 [DOI] [PubMed] [Google Scholar]
- 31).Inouye S. T. (1982) Ventromedial hypothalamic lesions eliminate anticipatory activities of restricted daily feeding schedules in the rat. Brain Res. 250, 183–187 [DOI] [PubMed] [Google Scholar]
- 32).Mieda M., Williams S. C., Richardson J. A., Tanaka K., Yanagisawa M. (2006) The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc. Natl. Acad. Sci. USA 103, 12150–12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Gooley J. J., Schomer A., Saper C. B. (2006) The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat. Neurosci. 9, 398–407 [DOI] [PubMed] [Google Scholar]
- 34).Fuller P. M., Lu J., Saper C. B. (2008) Differential rescue of light- and food-entrainable circadian rhythms. Science 320, 1074–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Landry G., Simon M. M., Webb I. C., Mistlberger R. E. (2006) Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1527–R1534 [DOI] [PubMed] [Google Scholar]
- 36).Mistlberger R. E., Buijs R. M., Challet E., Escobar C., Landry G. J., Kalsbeek A., et al. (2009) Standards of evidence in chronobiology: Critical review of a report that restoration of Bmal1 expression in the dorsomedial hypothalamus is sufficient to restore circadian food anticipatory rhythms in Bmal1−/− mice. J. Circadian Rhythms 7, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Konopka R. J., Benzer S. (1971) Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 68, 2112–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Bargiello T. A., Young M. W. (1984) Molecular genetics of a biological clock in Drosophila. Proc. Natl. Acad. Sci. USA 81, 2142–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Reddy P., Zehring W. A., Wheeler D. A., Pirrotta V., Hadfield C., Hall J. C., et al. (1984) Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell 38, 701–710 [DOI] [PubMed] [Google Scholar]
- 40).Shin H. S., Bargiello T. A., Clark B. T., Jackson R. J., Young M. W. (1985) An unusual coding sequence from a Drosophila clock gene is conserved in vertebrates. Nature 317, 445–448 [DOI] [PubMed] [Google Scholar]
- 41).King D. P., Zhao Y., Sagoram A. M., Wilsbacher L. D., Tanaka M., Antoch M. P., et al. (1997) Positional cloning of the mouse circadian clock gene. Cell 89, 641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Tei H., Okamura H., Shigeyoshi Y., Fukuhara C., Ozawa R., Hirose M., et al. (1997) Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature 389, 512–516 [DOI] [PubMed] [Google Scholar]
- 43).Sun Z. S., Albrecht U., Zhuchenko O., Balley J., Eichele G., Lee C. C. (1997) RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell 90, 1003–1011 [DOI] [PubMed] [Google Scholar]
- 44).Shearman L. P., Zylka M. J., Weaver D. R., Kolalowski L. F., Jr., Reppert S. M. (1997) Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron 19, 1261–1269 [DOI] [PubMed] [Google Scholar]
- 45).Ikeda M., Nomura M. (1997) cDNA cloning and tissue-specific expression of a novel basic helix-loop-helix/PAS protein (BMAL1) and identification of alternatively spliced variants with alternative translation initiation site usage. Biochem. Biophys. Res. Commun. 233, 258–264 [DOI] [PubMed] [Google Scholar]
- 46).Homa S., Ikeda M., Abe H., Tanahashi Y., Namihira M., Honma K., et al. (1998) Circadian oscillation of BMAL1, a partner of a mammalian clock gene Clock, in rat suprachiasmatic nucleus. Biochem. Biophys. Res. Commun. 250, 83–87 [DOI] [PubMed] [Google Scholar]
- 47).Ishida N., Kaneko M., Allada R. (1999) Biological clocks. Proc. Natl. Acad. Sci. USA 96, 8819–8820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Kume K., Zylka M. J., Sriram S., Shearman L. P., Weaver D. R., Jin X., et al. (1999) mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98, 193–205 [DOI] [PubMed] [Google Scholar]
- 49).Nagai K., Mori T., Nishio T., Nakagawa H. (1982) Effect of intracranial insulin infusion on the circadian rhythm of rats. Biomed. Res. 3, 175–180 [Google Scholar]
- 50).Masutani H., Nagai K., Nakagawa H. (1994) Possible involvement of nitric oxide in generation of circadian rhythm. Biol. Rhythm Res. 25, 433–441 [Google Scholar]
- 51).Masutani H., Matsuda Y., Nagai K., Nakagawa H. (1995) Effect of ω-conotoxin, a calcium channel blocker, on the circadian rhythm in rats. Biol. Rhythm. Res. 26, 578–581 [Google Scholar]
- 52).Okada M., Nakagawa H. (1988) Protein tyrosine kinase in rat brain: neonatal rat brain expresses two types of pp60c-src and a novel protein tyrosien kinase. J. Biochem. 104, 297–305 [DOI] [PubMed] [Google Scholar]
- 53).Okada M., Nakagawa H. (1988) Identification of a novel protein tyrosine kinase that phosphorylates pp60c-src and regulates its activity in neonatal rat brain. Biochem. Biophys. Res. Commun. 154, 796–802 [DOI] [PubMed] [Google Scholar]
- 54).Okada M., Nakagawa H. (1989) A protein tyrosine kinase involved in regulation of pp60c-src function. J. Biol. Chem. 264, 20886–20893 [PubMed] [Google Scholar]
- 55).Nada S., Yagi T., Takeda H., Tokunaga T., Nakagawa H., Aizawa S. (1993) Constitutive activation of Src family kinases in mouse embryos that lack Csk. Cell 73, 1125–1135 [DOI] [PubMed] [Google Scholar]
- 56).Nada S., Okada M, Nakagawa H. (1995) C-terminal Src kinase (Vertebrate). InThe Protein Kinase Facts Book, II Protein Tyrosine Kinase (eds. Hardie G., Hanks S.). Academic Press, London, pp. 89–91 [Google Scholar]
- 57).Salter M. W., Kalia L. V. (2004) Src kinases: a hub for NMDA receptor regulation. Nat. Rev. Neurosci. 5, 317–328 [DOI] [PubMed] [Google Scholar]
- 58).Shima T., Yagi T., Isojima Y., Okumura N., Okada M., Nagai K. (2000) Changes in circadian period and morphology of the hypothalamic suprachiasmatic nucleus in fyn kinase-deficient mice. Brain Res. 870, 36–43 [DOI] [PubMed] [Google Scholar]
- 59).Tamaru T., Okada M., Nagai K., Nakagawa H., Takamatsu K. (1999) Periodically fluctuating protein kinases phosphorylate CLOCK, the putative target in the suprachiasmatic nucleus. J. Neurochem. 72, 2191–2197 [DOI] [PubMed] [Google Scholar]
- 60).Tamaru T., Hirayama J., Isojima Y., Nagai K., Norioka S., Takamatsu K., et al. (2009) CK2α phosphorylates BMAL1 to regulate the mammalian clock. Nat. Struct. Mol. Biol. 16, 446–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Matsuki T., Kiyama A., Kawabuchi M., Okada M., Nagai K. (2001) A novel protein interacts with a clock-related protein, rPer1. Brain Res. 916, 1–10 [DOI] [PubMed] [Google Scholar]
- 62).Shimizu K., Okada M., Takano A., Nagai K. (1999) SCOP, a novel gene product expressed in a circadian manner in rat suprachiasmatic nucleus. FEBS Lett. 458, 363–369 [DOI] [PubMed] [Google Scholar]
- 63).Shimizu K., Okada M., Nagai K., Fukada Y. (2003) Suprachiasmatic nucleus circadian oscillatory protein, a novel binding partner of K-Ras in the membrane rafts, negatively regulates MAPK pathway. J. Biol. Chem. 278, 14920–14925 [DOI] [PubMed] [Google Scholar]
- 64).Shimizu K., Phan T., Mansuy I. M., Storm D. R. (2007) Proteolytic degradation of SCOP in the hippocampus contributes to activation of MAP kinase and memory. Cell 128, 1219–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Takano A., Isojima Y., Nagai K. (2004) Identification of mPer1 phosphorylation sites responsible for the nuclear entry. J. Biol. Chem. 279, 32578–32585 [DOI] [PubMed] [Google Scholar]
- 66).Takano H., Hoe H. S., Isojima Y., Nagai K. (2004) Analysis of the expression, localization and activity of rat casein kinase 1ɛ-3. Neuroreport 15, 1461–1464 [DOI] [PubMed] [Google Scholar]
- 67).Kiyama A., Isojima Y., Nagai K. (2006) Role of Per1-interacting protein of the suprachiasmatic nucleus in NGF mediated neuronal survival. Biochem. Biophys. Res. Commun. 339, 514–518 [DOI] [PubMed] [Google Scholar]
- 68).Nagai N., Nagai K., Chun S.-J., Shimizu K., Takezawa K., Tsuji M., et al. (1996) Roles of the suprachiasmatic nucleus and vasoactive intestinal peptide in the response of plasma arginine vasopressin to osmotic challenge. Endocrinology 137, 504–507 [DOI] [PubMed] [Google Scholar]
- 69).Shimizu K., Nagai K., Nakagawa H. (1996) An immunotoxin, anti-VIP antibody-ricin A chain conjugate eliminates neurons in the hypothalamic suprachiasmatic nucleus selectively and abolishes the circadian rhythm of water intake. Brain Res. Bull. 41, 369–378 [DOI] [PubMed] [Google Scholar]
- 70).Bittman E. I. (2009) Vasopressin: more than just an output of the circadian pacemaker? Focus on “Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei”. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R821–R823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Nagai K., Yamamoto H., Nakagawa H. (1982) Time-dependent hyperglycemic actions of centrally administered 2-deoxy-D-glucose, D-manintol, and D-glucose. Biomed. Res. 3, 288–293 [Google Scholar]
- 72).Yamamoto H., Nagai K., Nakagawa H. (1984) Role of the suprachiasmatic nucleus in glucose homeostasis. Biomed. Res. 5, 55–60 [DOI] [PubMed] [Google Scholar]
- 73).Yamamoto H., Nagai K., Nakagawa H. (1984) Bilateral lesions of the SCN abolish lipolytic and hyperphagic responses to 2DG. Physiol. Behav. 32, 1017–1020 [DOI] [PubMed] [Google Scholar]
- 74).Nagai K., Fujii T., Inoue S., Takamura Y., Nakagawa H. (1988) Electric stimulation of the suprachiasmatic nucleus of the hypothalamus causes hyperglycemia. Horm. Metab. Res. 20, 37–39 [DOI] [PubMed] [Google Scholar]
- 75).Yamamoto H., Nagai K., Nakagawa H. (1983) Role of catecholamine in time-dependent hyperglycemia due to 2-deoxyglucose, mannitol, and glucose. Biomed. Res. 4, 505–514 [Google Scholar]
- 76).Yamamoto H., Nagai K., Nakagawa H. (1988) Time-dependent involvement of autonomic nervous system in hyperglycemic due to 2-deoxy-D-glucose. Am. J. Physiol. 255, E928–E933 [DOI] [PubMed] [Google Scholar]
- 77).Fujii T., Inoue S., Nagai K., Nakagawa H. (1989) Involvement of adrenergic mechanism in hyperglycemia due to SCN stimulation. Horm. Metab. Res. 21, 643–700 [DOI] [PubMed] [Google Scholar]
- 78).Buijs R. M., la Fleur S. E., Wortel J., Van Heyningen C., Zuiddam L., Mettenleiter T. C., Kalsbeek A., et al. (2003) The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J. Comp. Neurol. 464, 36–48 [DOI] [PubMed] [Google Scholar]
- 79).Tanida M., Niijima A., Fukuda Y., Sawai H., Tsuruoka N., Shen J., et al. (2005) Dose-dependent effects of L-carnosine on the renal sympathetic nerve and blood pressure in urethane-anesthetized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R447–R455 [DOI] [PubMed] [Google Scholar]
- 80).Tanida M., Shen J., Horii Y., Matsuda M., Kihara S., Funahashi T., et al. (2007) Effects of adiponectin on the renal sympathetic nerve activity and blood pressure in rats. Exp. Biol. Med. 232, 390–397 [PubMed] [Google Scholar]
- 81).Tanida M., Niijima A., Shen J., Nakamura T., Nagai K. (2006) Olfactory stimulation with scent of lavender oil affects autonomic neurotransmission and blood pressure in rats. Neurosci. Lett. 398, 155–160 [DOI] [PubMed] [Google Scholar]
- 82).Moore R. Y. (1979) The retinohypothalamic tract, suprachiasmatic hypothalamic nucleus and central neural mechanisms of circadian rhythm regulation. InBiological Rhythms and Their Central Mechanism (eds. Suda M., Hayaishi O., Nakagawa H.). Elsevier North-Holland, Biomedical Press, Amsterdam: pp. 343–354 [Google Scholar]
- 83).Shibata S., Oomura Y., Kita H., Liou S. Y., Ueki S. (1984) Field potentials in the suprachiasmatic nucleus of rat hypothalamic slice produced by optic nerve stimulation. Brain Res. Bull. 12, 377–379 [DOI] [PubMed] [Google Scholar]
- 84).Kobayashi K., Otani K. (1981) Morphogenesis of the hereditary microphthalmia in a new strain of rat. J. Morphol. 167, 265–276 [DOI] [PubMed] [Google Scholar]
- 85).Nagai K., Sekitani M., Otani K., Nakagawa H. (1988) Little or no induction of hyperglycemia by 2-deoxy-D-glucose in hereditary blind microphthalmic rats. Life Sci. 43, 1575–1582 [DOI] [PubMed] [Google Scholar]
- 86).Tokunaga A., Sugita S., Nagai K., Tsutsui K., Ohsawa K. (1997) Immunohistochemical characterization of the suprachiasmatic nucleus and the intergeniculate leaflet in the hereditary bilaterally microphthalmic rat. Neurosci. Res. 27, 57–63 [DOI] [PubMed] [Google Scholar]
- 87).Shimizu K., Hibino H., Komenami N., Nagai N., Nagai K., Nakagawa H. (1994) Permissive effect of VIP on the hyperglycemic response induced by 2-deoxy-D-glucose. Neurosci. Lett. 175, 157–160 [DOI] [PubMed] [Google Scholar]
- 88).Nagai K., Niijima A., Nagai N., Hibino H., Chun S.-J., Shimizu K., et al. (1996) Bilateral lesions of the hypothalamic suprachiasmatic nucleus eliminated sympathetic response to intracranial injection of 2-deoxy-D-glucose and VIP rescued this response. Brain Res. Bull. 39, 293–297 [DOI] [PubMed] [Google Scholar]
- 89).Chun S.J., Niijima A., Nagai N., Nagai K. (1998) Effect of bilateral lesions of the suprachiasmatic nucleus on hyperglycemia caused by 2-deoxy-D-glucose and vasoactive intestinal peptide in rats. Brain Res. 809, 165–174 [DOI] [PubMed] [Google Scholar]
- 90).Chun S., Niijima A., Shima T., Okada M., Nagai K. (1998) Effect of infusion of vasoactive intestinal peptide (VIP)-antisense oligodeoxy-nucleotide into the third cerebral ventricle above the hypothalamic suprachiasmatic nucleus on the hyperglycemia caused by intracranial injection of 2-deoxy-D-glucose in rats. Neurosci. Lett. 257, 135–138 [DOI] [PubMed] [Google Scholar]
- 91).Sano S., Matsuda Y., Miyamoto S., Nakagawa H. (1984) Thiamine pyrophosphatase and nucleoside diphosphatase in rat brain. Biochem. Biophys. Res. Commun. 118, 292–298 [DOI] [PubMed] [Google Scholar]
- 92).Sano S., Matsuda Y., Nakagawa H. (1989) A novel brain-specific antigen: A glycoprotein electrophoretically similar to but immunochemically different from type B nucleoside diphosphatase. J. Biochem. 105, 457–460 [DOI] [PubMed] [Google Scholar]
- 93).Sano S., Matsuda Y., Nakagawa H. (1990) Oligosaccharide-related epitope specific for a brain-specific glycoprotein, 1D4 antigen. J. Neurochem. 55, 1252–1257 [DOI] [PubMed] [Google Scholar]
- 94).Sano S., Ohnishi H., Omori A., Hasegawa J., Kubota M. (1997) BIT, an immune antigen receptor-like molecule in the brain. FEBS Lett. 411, 327–334 [DOI] [PubMed] [Google Scholar]
- 95).Nakahata Y., Okumura N., Otani H., Hamada J., Numakawa T., Sano S., et al. (2003) Stimulation of BIT induces a circadian phase shift of locomotor activity in rats. Brain Res. 976, 194–201 [DOI] [PubMed] [Google Scholar]
- 96).Niijima A., Nagai K., Nagai N., Nakagawa H. (1992) Light enhances sympathetic and suppresses vagal outflows and lesions including the suprachiasmatic nucleus elimimnate these changes in rats. J. Autonom. Nerv. Syst. 40, 155–160 [DOI] [PubMed] [Google Scholar]
- 97).Niijima A., Nagai K., Nagai N., Nakagawa H. (1993) Effects of light stimulation on the activity of the autonomic nerves in anaestetized rats. Physiol. Behav. 54, 555–561 [DOI] [PubMed] [Google Scholar]
- 98).Nagai K., Nagai N., Shimizu K., Chun S.-J., Nakagawa H, Niijima A. (1996) SCN output drives the autonomic nervous system: With special reference to the autonomic function related to the regulation of glucose metabolism. Prog. Brain Res., III InHypothalamic Integration of Circadain Rhythms (eds. Buijis R. M., Kalsbeek H. A., Romijin C.M.A., Pennartz, Mirmiran M.), Elsevier, Amsterdam, pp. 253–273 [DOI] [PubMed] [Google Scholar]
- 99).Yamamoto H., Nagai K., Nakagawa H. (1987) Role of SCN in daily rhythm of plasma glucose, FFA, insulin and glucagon. Chronobiol. Internat. 4, 483–491 [DOI] [PubMed] [Google Scholar]
- 100).Yamamoto H., Nagai K., Nakagawa H. (1985) Lesions involving the suprachaismatic nucleus eliminate the glucagon response to intracranial injection of 2-deoxy-D-glucose. Endocrinology 117, 468–473 [DOI] [PubMed] [Google Scholar]
- 101).Jarrett R. J., Keen H. (1970) Further observations on the diurnal variation in oral glucose tolerance. Br. Med. J. 4, 334–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102).Yamamoto H., Nagai K., Nakagawa H. (1984) Bilateral lesions of the suprachiasmatic nucleus enhance glucose tolerance in rats. Biomed. Res. 5, 47–54 [Google Scholar]
- 103).Abe K., Kroning J., Greer M. A., Critchlow V. (1979) Effects of destruction of the suprachiasmatic nuclei on the circadian rhythms in plasma corticosterone, body temperature, feeding and plasma thyrotropin. Neuroendocrinology 29, 119–131 [DOI] [PubMed] [Google Scholar]
- 104).Cascio C. S., Shinsako J., Dallman M. F. (1987) The suprachiasmatic nuclei stimulate evening ACTH secretion in the rat. Brain Res. 423, 173–178 [DOI] [PubMed] [Google Scholar]
- 105).Weitzman E. D., Fukushima D., Nogeire C., Roffwarg H., Gallgher T. F., Hellman L. (1971) Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J. Clin. Endocr. Metab. 33, 14–22 [DOI] [PubMed] [Google Scholar]
- 106).Orth D. N., Besser G. M., King P. H., Nicholson I. W. E. (1979) Free-running circadian plasma cortisol rhythm in a blind human subject. Clin. Endocrinol. 10, 603–617 [DOI] [PubMed] [Google Scholar]
- 107).Aschoff J. (1979) Circadian rhythms: general features and endocrinological aspects. InEndocrine Rhythms: Comprehensive Endocrinology (ed. Krieger D. T.), Raven Press, New York, pp. 1–61 [Google Scholar]
- 108).Ernsberger P., Azar S., Tarbell M. (1981) Effects of preoptic – suprachiasmatic lesions on renal excretion of electrolytes. Life. Sci. 28, 1387–1390 [DOI] [PubMed] [Google Scholar]
- 109).Ernsberger P., Azar S., Azar P. (1985) The role of the anteromedial hypothalamus in Dahl hypertension. Brain Res. Bull. 15, 651–656 [DOI] [PubMed] [Google Scholar]
- 110).Azar S., Ernsberger P., Livingston W., Azar P., Iwai J. (1981) Paraventricular–suprachiasmatic lesions prevent salt-induced hypertension in Dahl rats. Clin. Sci. 61, 49s–51s [DOI] [PubMed] [Google Scholar]
- 111).Nagai K., Stoynev A.G., Nagai N., Itoh I., Nakagawa H. (1993) Lack of vasopressin response to water deprivation in blind rats with abnormal suprachiasmatic nuclei. J. Clin. Biochem. Nutr. 14, 163–169 [Google Scholar]
- 112).Nagai K., Stoynev A. G., Nagai N., Nakagawa H. (1993) Reduced increase in plasma renin activity on water-deprivation in blind hereditary microphthalmic rats. Neurosci. Lett. 149, 217–220 [DOI] [PubMed] [Google Scholar]
- 113).Nagai K., Nagai N., Sugahara K., Niijima A., Nakagawa H. (1994) Circadian rhythms and energy metabolism with special reference to the suprachiasmatic nucleus. Neurosci. Biobehav. Rev. 18, 579–584 [DOI] [PubMed] [Google Scholar]
- 114).Joel C. D. (1965) The physiological role of brown adipose tissue. InHandbook of Physiology, Sec. 5, Adipose Tissue (Eds. Renold A. E., Cahill G. F., Jr.). American Physiology Society, Washington, D. C., pp. 59–85 [Google Scholar]
- 115).Nakagawa H., Nagai K. (1971) Cold adaptation I. Effect of cold exposure on gluconeogenesis. J. Biochem. 69, 923–934 [DOI] [PubMed] [Google Scholar]
- 116).Nagai K., Nakagawa H. (1972) Cold adaptation II. Effect of thyroxine on phosphoenolpyruvate carboxykinase in rat liver in normal and cold environments. J. Biochem. 71, 125–131 [DOI] [PubMed] [Google Scholar]
- 117).Taniguchi H., Okumura N., Hamada J., Inagaki M., Nakahata Y., Sano S., et al. (2004) Cold exposure induces tyrosine phosphorylation of BIT through NMDA receptors in the rat hypothalamus. Biochem. Biophys. Res. Commun. 319, 178–184 [DOI] [PubMed] [Google Scholar]
- 118).Taniguchi H., Tanida M., Okumura N., Hamada J., Sano S., Nagai K. (2006) Regulation of sympathetic and parasympathetic nerve activities by BIT/SHPS-1. Neurosci. Lett. 398, 102–106 [DOI] [PubMed] [Google Scholar]
- 119).Kowalska E., Brown S. A. (2007) Peripheral clocks: Keeping up with the master clock. Cold Spring Harbor Symposium on Quantitative Biology 72, 301–305 [DOI] [PubMed] [Google Scholar]
- 120).Damiola F., Le Minh N., Preitner N., Kornmann B., Fleury-Olela F., Schibler U. (2000) Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950– 2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121).Turek F. W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E., et al. (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122).Doi M., Hirayama J., Sassone-Corsi P. (2006) Circadian regulator CLOCK is a histone acetyl-transferase. Cell 125, 497–508 [DOI] [PubMed] [Google Scholar]
- 123).Nakahata Y., Kaluzova M., Grimaldi B., Sahar S., Hirayama J., Chen D., et al. (2008) The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124).Nakahata Y., Sahar S., Astarita G., Kaluzova M., Sassone-Corsi P. (2009) Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324, 654–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125).Leibowitz S.F., Weiss G.F., Walsh U. A., Viswanath D. (1989) Medial hypothalamic serotonin: role in circadian patterns of feeding and macronutrient selection. Brain Res. 503, 132–140 [DOI] [PubMed] [Google Scholar]
- 126).Nagai K., Thibault L., Nishikawa K., Hashida-Okumura A., Otani K., Nakagawa H. (1991) Effect of glucagon in macronutrient self-selection: glucagon-enhanced protein intake. Brain Res. Bull. 27, 409–415 [DOI] [PubMed] [Google Scholar]
- 127).Yokoi S., Ikeya M., Yagi T, Nagai K. (2003) Mouse circadian rhythm before the Kobe earthquake in 1995. Bioelectromagnetics 24, 289–291 [DOI] [PubMed] [Google Scholar]
- 128).Mangold R., Sokoloff L., Conner E., Kleinerman J., Therman P. G., Kety S. S. (1955) The effects of sleep and lack of sleep on the cerebral circulation and metabolism of normal young men. J. Clin. Invest. 34, 1092–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129).Farshchi H. R., Taylor M. A., MacDonald I. A. (2004) Regular meal frequency creates more appropriate insulin sensitivity and lipid profiles compared with irregular meal frequency in healthy lean women. Eur. J. Clin. Nutr. 58, 1071–1077 [DOI] [PubMed] [Google Scholar]
- 130).Fábry P., Tepperman J. (1970) Meal frequency – a possible factor in human pathology. Am. J. Clin. Nutr. 23, 1059–1068 [DOI] [PubMed] [Google Scholar]
- 131).Timlin M. T., Pereira M. A. (2007) Breakfast frequency and quality in the etiology of adult obesity and chronic diseases. Nutr. Rev. 65, 268–281 [DOI] [PubMed] [Google Scholar]
- 132).Farshchi H. R., Taylor M. A., MacDonald I. A. (2005) Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am. J. Clin. Nutr. 81, 388– 396 [DOI] [PubMed] [Google Scholar]
- 133).Saito M., Murakami E., Nishida T., Fujisawa Y., Suda M. (1975) Circadian rhythms in digestive enzymes in the small intestine of rats. I. Patterns of the rhythms in various regions of the small intestine. J. Biochem. 78, 475–480 [DOI] [PubMed] [Google Scholar]
- 134).Saito M., Murakami E., Nishida T., Fujisawa Y., Suda M. (1976) Circadian rhythms of digestive enzymes in the small intestine of the rat. II. Effects of fasting and refeeding. J. Biochem. 80, 563–568 [DOI] [PubMed] [Google Scholar]
- 135).Nishida T., Saito M., Suda M. (1978) Parallel between circadian rhythms of intestinal disacchari-dases and foot intake of rats under constant lighting conditions. Gastroenterology 74, 224–227 [PubMed] [Google Scholar]
- 136).Saito M., Kato H., Suda M. (1980) Parallel between circadian rhythms of intestinal disacchari-dases and foot intake of rats under constant lighting conditions. Am. J. Physiol. Gastrointest. Liver Physiol. 238, G97–G101 [Google Scholar]
- 137).Kaufman M. A., Korsmo H. A., Olsen W. A. (1980) Circadian rhythm of intestinal sucrase activity in rats. Mechanism of enzyme change. J. Clin. Invest. 65, 1174–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138).Czeisler C. A., Kronauer R. E., Allan J. S., Duffy J. F., Jewett M. E., Brown E. N., et al. (1989) Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science 244, 1328–1333 [DOI] [PubMed] [Google Scholar]
- 139).Hollwich F., Hartmann C. (1969) Influence of light through the eyes on metabolism and hormones. Ophthalmologie 4, 385–389 [PubMed] [Google Scholar]