Abstract
The transport and sorting of lipids are fundamental to membrane biogenesis. In the synthesis of sphingolipids in mammalian cells, ceramide is newly produced at the endoplasmic reticulum (ER), and transported from the ER to the trans Golgi regions, where it is converted to sphingomyelin. Ceramide transfer protein (CERT) mediates the ER-to-Golgi trafficking of ceramide. It has been suggested that CERT extracts ceramide from the ER and carries it to the Golgi apparatus in a non-vesicular manner and that efficient CERT-mediated trafficking of ceramide occurs at membrane contact sites between the ER and the Golgi apparatus.
Keywords: ceramide, sphingomyelin, endoplasmic reticulum, Golgi, lipid transport
Introduction
Biological membranes are composed of lipids and proteins. Eukaryotic cells have various intracellular membrane compartments or “organelles” including the plasma membrane, endoplasmic reticulum (ER), Golgi apparatus, mitochondrion, and lysosome. In eukaryotic cells, the membranes of different organelles differ in lipid composition, and the various bio-membranes show an asymmetric distribution of lipid types across the membrane bilayer. This heterologous distribution of lipids is regulated, being determined by the location of lipid synthases and their topology in the membrane, as well as several types of trafficking including flip-flop, vesicular trafficking, and non-vesicular trafficking.1) The inter-organelle trafficking of proteins is mediated by transport membrane vesicles and tubules, which load the desired set of proteins and deliver them to the appropriate organelles.2),3) Vesicle-mediated mechanisms also operate in some lipid flux routes such as the endocytosis of plasma membrane lipids.4),5) In contrast, various types of lipid synthesized in the ER are sorted to other organelles by non-vesicular mechanisms.1),6)–9) Little is known, however, about the molecular machinery responsible for the inter-organelle movement of lipids.
A molecular factor playing a key role in the trafficking of the lipid ceramide has been identified, and, based on its characteristics, a model of non-vesicular trafficking of ceramide has been proposed as summarized in this short review.
Biosynthesis of sphingolipids
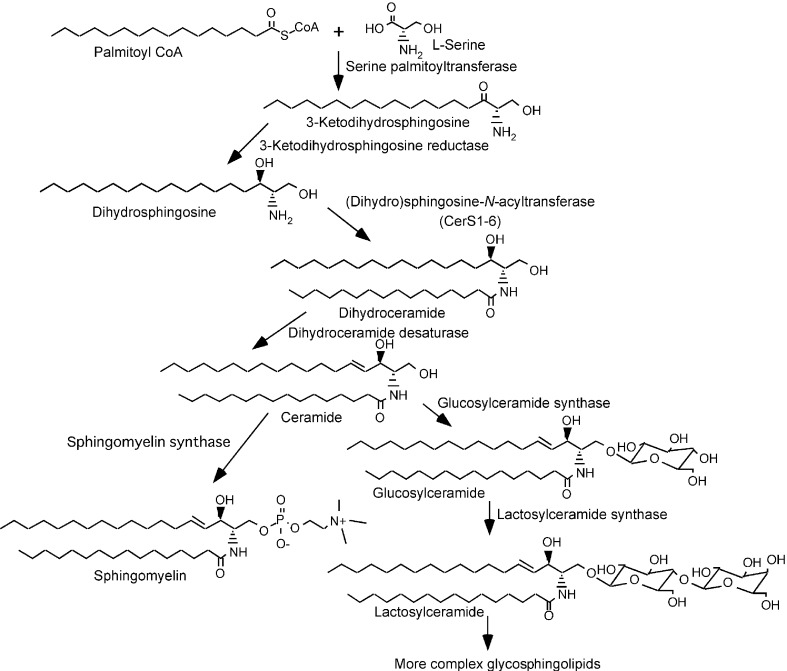
Sphingolipids are ubiquitous constituents of biological membranes of mammalian cells. The phosphosphingolipid sphingomyelin (SM) typically accounts for 2–15% of all the phospholipids in mammalian cells. Glycosphingolipids also occur widely in animal cells but with a remarkable diversity in composition among cell types.10) In mammalian cells, the biosynthesis of sphingolipids occurs as shown in Fig. 1.11),12) Condensation of l-serine and palmitoyl co-enzyme A (CoA) produces 3-ketodihydrosphingosine, which is converted to dihydrosphingosine. Dihydrosphingosine is N-acylated to produce dihydroceramide, which is desaturated to be converted to ceramide. These enzymatic reactions occur at the cytosolic side of the ER membrane. After being transported to the Golgi apparatus, ceramide is converted to SM, or to glucosylceramide (GlcCer) and then to more complex glycosphingolipids.
Fig. 1.
Biosynthetic pathway of sphingolipids in mammalian cells.
Cell mutant defective in intracellular trafficking of ceramide
The cytolytic toxin lysenin, derived from the earthworm Eisenia foetida, binds to SM with high affinity.13) For the isolation of cell mutants defective in SM metabolism, lysenin-resistant mutants were selected from CHO-K1 cells (a permanent cell line derived from a Chinese hamster ovary) after chemical mutagenesis, and a novel type of cell mutant (designated LY-A) was obtained.14) LY-A cells have reduced levels of SM.14) Metabolic labeling experiments with radioactive precursors showed that LY-A cells are severely defective in the synthesis of SM, but not other sphingolipid types.15) However, no defect was observed in the synthesis of ceramide and phosphatidylcholine (precursors of SM synthesis) or in the activity of SM synthase.15) Hence, it was hypothesized that LY-A cells had a defect in the transport of ceramide from the site of its synthesis to the site of the synthesis of SM.
Several lines of evidence supported this hypothesis.15) First, when cells were treated with the fungal metabolite brefeldin A to merge the Golgi apparatus and the ER, the synthesis of SM in LY-A cells was restored to the wild-type level, consistent with the notion that, in brefeldin A-treated cells, ceramide is accessible to the normally Golgi-localized SM synthase without ER-to-Golgi trafficking events. Second, pulse-chase experiments with C5-DMB-ceramide, a fluorescent analog of ceramide, in intact cells demonstrated that transport of C5-DMB-ceramide from the ER to the Golgi apparatus is far slower in LY-A cells than in wild-type cells (Fig. 2). Interestingly, treatment of wild-type cells with energy poisons inhibited ER-to-Golgi transport of C5-DMB-ceramide to the level in LY-A cells (Fig. 2). These results revealed that LY-A cells are defective in energy (probably ATP)-dependent trafficking of ceramide from the ER to the Golgi compartment. ER-to-Golgi trafficking of proteins in LY-A cells appeared to be normal.
Fig. 2.
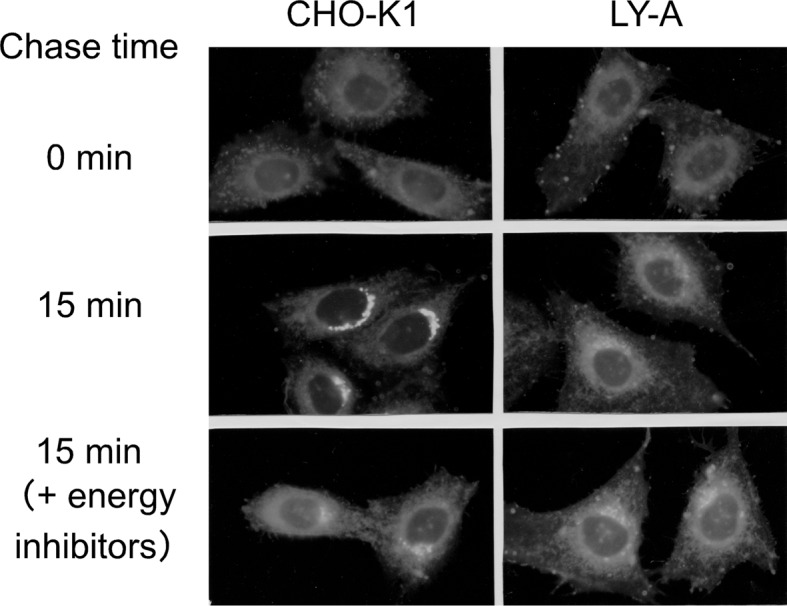
Redistribution of C5-DMB ceramide in intact CHO cells.15) Cells were labeled with 1 μM C5-DMB ceramide at 4 °C for 30 min, washed and chased at 33 °C for 15 min. For energy inhibition, cells were pretreated with energy inhibitors (50 mM deoxy-d-glucose and 5 mM NaN3) at 33 °C for 15min, chilled, labeled, and chased in the presence of the energy inhibitors.
Reconstitution of ceramide trafficking in semi-intact CHO cells
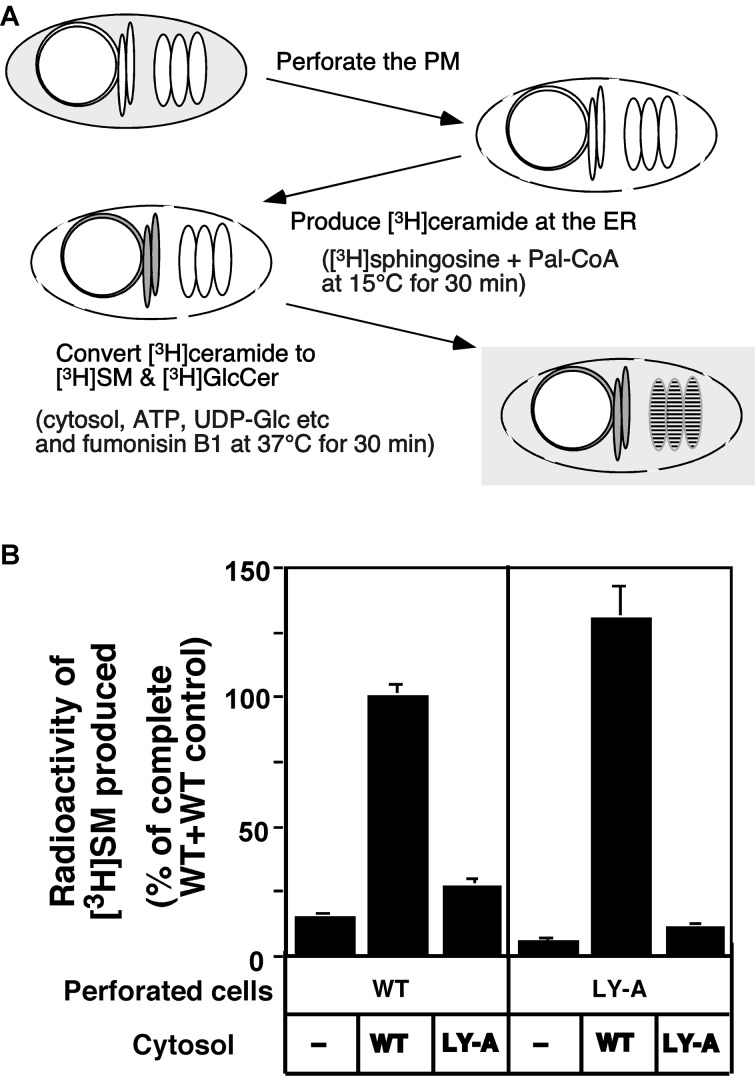
Transport of ceramide from the ER to the Golgi site for SM synthesis was reconstituted within semi-intact cells (Fig. 3A).16) Semi-intact CHO cells, prepared by mild perforation of the plasma membrane, retain the ER and Golgi apparatus but not cytosolic soluble proteins. In the reconstitution system, [3H]ceramide is pulse-labeled with d-erythro-[3H]sphingosine and palmitoyl CoA in perforated cells at 15 °C, and the prelabeled semi-intact cells are chased in the presence of the cytosolic fraction and an ATP-regeneration system at 37 °C to convert [3H]ceramide to [3H]SM.
Fig. 3.
Reconstitution of ER-to-Golgi trafficking of ceramide in perforated cells.16) (A) The procedure for the reconstitution of ceramide trafficking in semi-intact CHO cells is represented schematically. (B) Reconstitution of ceramide transport from the ER to the Golgi site for SM synthesis in the semi-intact cell system. Perforated wild-type (WT) and LY-A cells pulsed with [3H]sphingosine were chased at 37 °C for 30 min in the transport reaction mixture with the indicated combination of perforated cells (40 μg protein) and cytosolic fraction (100 μg protein). For the cytosol-minus experiments (−), no cytosolic fraction was added to the reaction mixture. The data are expressed as a percentage of the mean value of the wild-type control, where wild-type perforated cells (40 μg protein) were chased in the transport reaction mixture, containing wild-type cytosol (100 μg protein).
Several lines of evidence indicate that the assay of [3H]ceramide’s conversion to [3H]SM using the semi-intact cell system is a faithful in vitro assay for the activity to transport ceramide from the ER to the site of SM synthesis.16) First, long-chain [3H]ceramide produced by metabolic conversion of [3H]sphingosine in semi-intact cells is expected to be embedded in the membrane in the same way as natural ceramide synthesized at the ER in intact cells. Second, the rates of conversion of [3H]ceramide to [3H]SM in intact and semi-intact cells are similar. Third, ceramide-to-SM conversion in semi-intact cells is dependent on ATP, consistent with the observation that ATP-depletion in intact CHO-K1 cells by energy inhibitors inhibits ER-to-Golgi trafficking of ceramide. Fourth, the rate-determining step for the production of [3H]SM from [3H]ceramide in vitro as well as in vivo is the transport of ceramide not the reaction involving SM synthase, because ceramide was converted to SM efficiently in brefeldin A-treated intact and semi-intact cells even under low temperature and ATP-depleted conditions unlike in untreated controls. Most importantly, the semi-intact cell system reproduces the phenotype of mutant LY-A cells; the rate of ceramide-to-SM conversion in semi-intact LY-A cells is only ~20% of the wild-type level (Fig. 3B).
Analysis with the in vitro reconstitution system showed that the ATP-dependent trafficking of ceramide requires cytosol (Fig. 3B).16) In addition, cytosol-exchange experiments demonstrated that, when perforated LY-A cells were chased with the wild-type cytosol, the [3H]ceramide-to-[3H]SM conversion was completely rescued (Fig. 3B), indicating that the mutant phenotype of LY-A cells results from a deficiency of a cytosolic factor.16)
Identification of CERT as the factor impaired in LY-A cells
Because cholesterol has a stronger affinity for SM than for glycerophospholipids, altering the level of coexisting SM affects the behavior of cholesterol in artificial model membranes and biological membranes.17) Through functional rescue experiments exploiting the hyper-sensitivity of LY-A cells to the cholesterol-adsorbent methyl-β-cyclodextrin,18) CERT was isolated as the factor that restores the defect in the mutant.19)
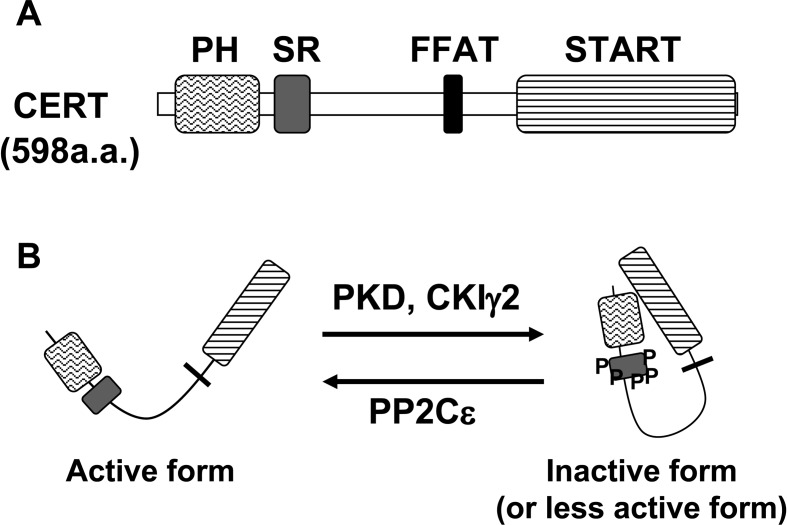
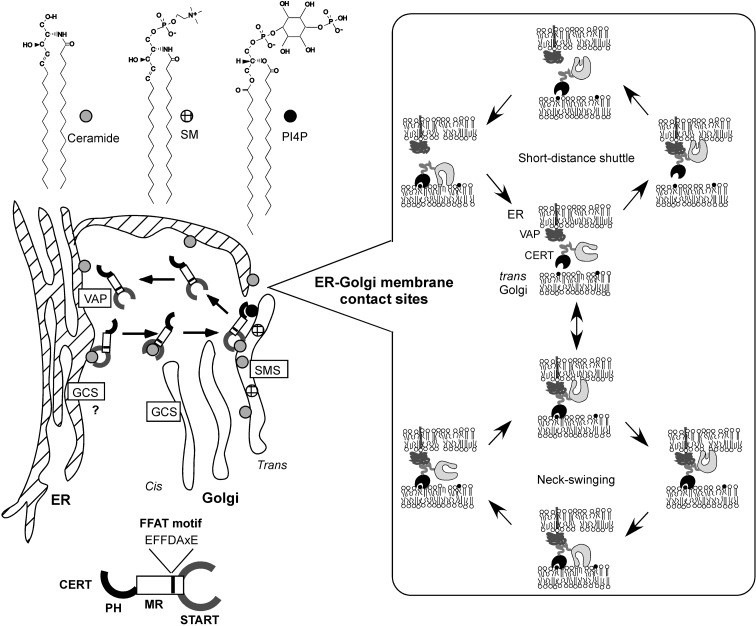
CERT, a hydrophilic 68-kDa protein, consists of three parts (Fig. 4A). The amino terminal ~120 amino acid region forms a pleckstrin homology (PH) domain.20),21) The carboxyl terminal ~230 amino acid region forms a steroidogenic acute regulatory protein (StAR)-related (START) domain.22) The ~250 amino acid region between the two domains is predicted to form no globular domains, but may have various roles as described below. Among model organisms in genome projects, vertebrates and invertebrates have CERT orthologues, whereas plants, unicellular eukaryotes (such as fungi and protozoa), and prokaryotes have no close relatives of CERT.
Fig. 4.
(A) Domains and motifs of CERT. SR, serine-repeat. (B) Regulation of CERT activity. The hyperphosphorylation of SR motif by PKD and CKIγ2 inactivates CERT, and the dephosphorylation by PP2Cɛ activates CERT.
PH domains are protein modules of ~120 amino acid residues that have a common core fold consisting of a seven-stranded β-sandwich structure with a C-terminal α-helix. A well-characterized function of PH domains is the binding of phosphatidylinositol phosphates.23) The PH domain of CERT specifically binds to phosphatidylinositol 4-phosphate. (PI4P).19) PI4P is thought to be mainly distributed to the Golgi apparatus, as several PH domains (including the PH domain of CERT) which selectively recognize PI4P were shown to target the Golgi apparatus or the trans Golgi network (TGN).19),24)–26) The CERT of LY-A cells has one point mutation, G67E, which destroys the PI4P-binding activity of the PH domain.19) Thus, CERT having the G67E mutation can not target the Golgi apparatus.
START domains are protein modules of ~230 amino acid residues that have similarity to StAR,22) which mediates the transport of cholesterol to mitochondria and/or from the outer to inner membrane of mitochondria for the production of steroid hormones.27)–29) The human genome has 15 START domains, and invertebrates and plants also have START domains.30)–32) Several START domains have been shown to bind specific lipid ligands: for example, cholesterol for StAR and metastatic lymph node 64 protein,33),34) phosphatidylcholine for phosphatidylcholine-transfer protein (PCTP),35),36) phosphatidylcholine (and phosphatidylethanolamine with less efficiency) for PCTP-like protein/StarD10/CGI-52,37) and carotenoid for the silkworm carotenoid-binding protein.38) The START domain of CERT can extract ceramide from membranes and transfer the bound ceramide to membranes.19) CERT can also catalyze inter-membrane transport of dihydro-ceramide and phytoceramide, natural isoforms of ceramide, but not other types of lipids such as cholesterol, phosphatidylcholine, sphingosine and SM.39)
Vesicle-associated membrane protein-associated protein (VAP) is an ER-resident membrane protein, homologs of which are widely distributed from yeast to human.40) A short peptide motif interacting with VAP proteins has been identified.41) Based on the conserved sequence (EFFDAxE), the motifs are referred to as FFAT motifs (two phenylalanines in an acidic tract).41) Interestingly, an FFAT motif is present in CERT (Fig. 4A).41),42) VAP co-immuno-precipitated with CERT, and mutations in the FFAT motif of CERT disrupted not only VAP-CERT interaction but also the CERT-mediated ER-to-Golgi transport of ceramide.42)
Crystal structure of the START domain of CERT
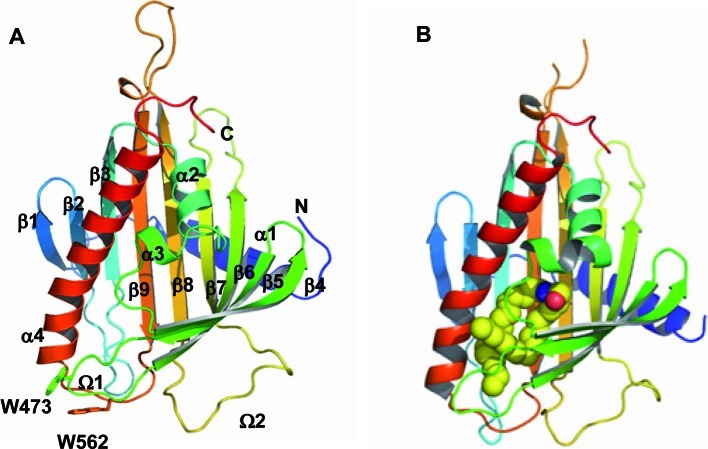
X-ray crystallography has revealed the structure of the START domain of CERT; N- and C-terminal α-helices with a curved antiparallel β-sheet consisting of nine β-strands and two short α-helices (Fig. 5).43) A hydrophobic cavity is formed by the β-strands covered by three helices (the C-terminal and two short helices) in the START domain (Fig. 5A). The co-crystal structure of the START domain of CERT in a complex with ceramide showed that one ceramide molecule is buried in a long amphiphilic cavity and that at the far end of the cavity, the amide- and hydroxyl-groups of ceramide form a hydrogen bond network with specific amino acid residues (Fig. 5B).43) There is no extra space to accommodate an additional bulky group at the C1 position of ceramide, which explains why CERT can not recognize SM.43) The two aliphatic chains of ceramide are surrounded by the hydrophobic wall of the cavity, whose size and shape dictate the acyl chain length limit of cognate ceramides.43)
Fig. 5.
Crystal structure of the START domain of CERT.43) (A) The apo-form of the CERT START domain in a ribbon representation. α-Helices (α1-α4), β-strands (β1-β9), and Ω loops (Ω1 and Ω2) are numbered from the N to C terminus. (B) The CERT START domain in a complex with C16-ceramide. The ceramide molecule is represented as space-filling spheres in which yellow, blue, and red spheres represent C, N, and O atoms, respectively.
Non-vesicular model of CERT-mediated trafficking of ceramide
Based on the molecular features of CERT described above, a model of ceramide trafficking from the ER to the Golgi apparatus has been proposed (Fig. 6).19),42),44) In this model, CERT extracts newly synthesized ceramide from the ER, depending on its START domain, carries the ceramide molecule through the cytosol in a non-vesicular manner, and targets the Golgi apparatus, dependent on its PH domain recognizing PI4P. Interaction of CERT with the ER is probably facilitated by the binding of the FFAT motif of CERT with the ER membrane protein VAP, as described above. After the release of ceramide at the Golgi apparatus, CERT might in turn bind diacylglycerol generated during SM synthesis, because CERT can poorly but significantly catalyze the inter-membrane transfer of diacylgly-cerol in vitro.39)
Fig. 6.
A model of CERT-mediated trafficking of ceramide from the ER to the trans Golgi region. SMS, SM synthase; GCS, GlcCer synthase. Inset, efficient trafficking of ceramide through short-distance shuttling by CERT or through the ‘neck-swinging’ movement of the START domain might occur at the sites of contact between the ER and trans Golgi cisternae.
Subdomains of the ER are suggested to be spatially very close to trans Golgi stacks.45),46) CERT might quickly shuttle between the two organelle membranes at the sites of contact (Fig. 6). If the PH domain and the FFAT motif of CERT can simultaneously associate with the Golgi apparatus and the ER, respectively, transport of ceramide from the ER to Golgi apparatus might be attained by the ‘neck-swinging’ movement of the START domain (Fig. 6).
Involvement of CERT in the synthesis of GlcCer
It is controversial whether CERT plays a key role in the conversion of ceramide to GlcCer. When analyzed with radioactive serine, LY-A cells showed a mild deficiency in metabolic labeling of GlcCer.19) When CERT was knocked down in MEB4 cells (a subline of mouse B16 melanoma), a mild deficiency in metabolic labeling of GlcCer with radioactive serine was also observed.47) Interestingly, when metabolic labeling with radioactive sphingosine, which allows the de novo synthesis of sphingoid bases to be bypassed in the labeling of ceramide, was carried out, LY-A cells showed an ~50% reduction in the labeling of SM and ~100% increase in the labeling of GlcCer, compared with wild-type cells.19) The mild reduction in GlcCer in CERT-deficient cells might be due to a repression of the formation of sphingoid bases as a compensatory response to the reduced consumption of ceramide, although the mechanism of repression remains unknown. Alternatively, CERT could also be important for the transport of de novo synthesized ceramide from the ER to the site of GlcCer synthesis. Ceramide produced from exogenous sphingosine might exist in different pools: sphingosine-derived ceramide may be transported from one pool to the site of SM production in a CERT-dependent manner and from another pool to the site of GlcCer production in a CERT-independent manner.
Regulation of CERT-mediated trafficking of ceramide
The CERT-dependent movement of C5-DMB-ceramide from the ER to the Golgi apparatus in cells is inhibited by pre-treatment of cells with energy poisons (Fig. 2).15) Also, the transport of ceramide from the ER to the site of SM synthesis in semi-intact cells requires ATP.16) In contrast, the CERT-mediated transfer of ceramide between artificial phospholipid vesicles does not require biological energy.19),39) The dependence on ATP might be attributable to the Golgi-targeting process including the synthesis of PI4P in the Golgi membrane, because the PI4P-dependent Golgi-targeting of the PH domain of four-phosphate-adaptor protein was shown to require ATP in permeabilized COS7 cells (a permanent cell line derived from simian kidney).26) There are four types of phosphatidylinositol 4 kinase (IIα, IIIα, IIβ, and IIIβ) in mammals.48) Among them, type-IIIβ is likely to play a dominant role in the Golgi-targeting of CERT and the ER-to-Golgi transport of ceramide.49) It is also conceivable that ATP is required for the intramembrane transport of ceramide from the cytosolic side to the luminal side in the Golgi lipid bilayers although transbilayer movement of C16-ceramide has been suggested to occur with a half-time of ~1 min in phospholipid vesicles without any biological energy.50)
When expressed in mammalian cells, CERT is phosphorylated multiple times at a serine-repeat motif, a possible site for casein kinase I (CKI), the typical recognition sequence of which is pSer/pThr (or Glu/Asp cluster)–X1–3-Ser/Thr (the underlined Ser or Thr is to be phosphorylated).51) When Ser132, the most N-terminal serine in the serine-repeat motif, was replaced with alanine (S132A), the ptotein was no longer hyperphosphorylated.51) For ER-to-Golgi trafficking of ceramide, the S132A mutant was more active than the wild-type CERT in semi-intact cells.51) In contrast, CERT 10E, a mutant in which all ten serine and threonine residues in the serine-repeat motif are replaced with glutamic acid residues, was almost inactive.51) In cell-free assay systems, both the PI4P-binding and ceramide-transport activities of CERT S132A were higher than those of the wild-type CERT, while these activities of CERT 10E were far lower.51) Interestingly, PH domain-deleted CERT 10E had similar ceramide-transport activity to CERT S132, while START domain-deleted CERT 10E showed similar PI4P-binding activity to CERT S132A.51) Collectively, these results indicate that the phosphorylation of the serine-repeat motif induces an autoinhibitory interaction between the PH and START domains and consequently inactivates both the PI4P-binding and ceramide-transport activities of CERT (Fig. 4B).51) When expressed in human cervical HeLa-S3 cells, wild-type CERT is distributed throughout the cytoplasm with significant concentration at the Golgi complex, while the CERT S132A mutant is localized to the Golgi complex at the fluorescence microscopic level,51) supporting the hypothesis that CERT acts at the ER-Golgi membrane contact sites.
Protein kinase D (PKD) is likely to be a major kinase which phosphorylates S132 of CERT, because knockdown of both PKD1 and 2 reduced the phosphorylation of CERT in human embryonic kidney-derived HEK293T cells.52) After phosphorylation of S132, CERT is further phosphorylated by the CKI family; the γ2 isoform of CKI plays a major role in the hyperphosphorylation of CERT in HeLa-S3 cells (Fig. 4B).53) The hyperphosphorylation of CERT at the serine repeat motif is likely to be a crucial step in the regulation of the activity.53)
Protein phosphatase 2Cɛ (PP2Cɛ), an integral membrane protein located at the ER, is relevant to the dephosphorylation of CERT in cells (Fig. 4B).54) By yeast two-hybrid screening, VAP was found to bind PP2Cɛ.54) Overexpression of PP2Cɛ dephosphorylated co-expressed CERT when VAP was also overexpressed.54) In addition, CERT dephosphorylated by PP2Cɛ selectively bound to VAP.54) Over-expression of PP2Cɛ also redistributed CERT to the Golgi,54) which is consistent with the finding that dephosphorylation of CERT enhanced its binding to PI4P.51) These observations suggest that only dephosphorylated CERT can interact with both the ER and Golgi, and act at the ER-Golgi membrane contact sites.
CERT and Goodpasture antigen-binding protein (GPBP)
CERT is identical to GPBPΔ26, a splicing variant of GPBP. Goodpasture disease is an autoimmune disorder. In Goodpasture patients, autoantibodies against the non-collagenous carboxyl terminus domain of collagen IV α3 cause a progressive glomerulonephritis. Raya et al. screened for human recombinant proteins able to bind the amino terminal peptide of the non-collagenous carboxyl terminus domain using the phage display method, and found a 70-kD protein, which they named GPBP.20) GPBPΔ26, which has 26 fewer amino acid residues than GPBP, is a more common splicing variant and is expressed widely in various tissues.21) COL4A3BP (standing for collagen, type IV, alpha 3-binding protein) is the nomenclature for the genes encoding GPBPs/CERTs in humans. The CERT/GPBPΔ26 transcript does not contain the 11th exon, which encodes a serine-rich region consisting of 26 amino acids.
It has recently been shown that various types of human cells express two GPBP isoforms resulting from canonical (77-kDa) and noncanonical (91-kDa) mRNA translation initiation, and that the 77-kDa GPBP behaves as a soluble secretable protein and the 91-kD GPBP as a membrane-bound protein.55) For the secretion of the 77-kDa GPBP, the FFAT motif and the 26-residue serine-rich region are required.55) Thus, GPBP may primarily act in the extracellular compartment, while CERT/GPBPΔ26 mediates ceramide trafficking in the cytosol. It is worth noting that the larger splicing variant CERTL, which is identical to GPBP, also catalyzes inter-membrane transfer of ceramide in vitro and rescues LY-A cells.19)
CERT mutant animals
As described above, CERT plays an important role in the transport of ceramide from the ER to the Golgi apparatus for the synthesis of SM in cultured cells. It has been demonstrated that Drosophila melanogaster mutant flies lacking a functional CERT protein display a >70% decrease in ceramide phosphoethanolamine (the SM analog in Drosophila) with increased fluidity of the plasma membrane.56) The mutant flies show susceptibility to oxidative stress and have a short lifespan, compared with control flies.56)
Gene disruption of CERT (and also GPBP) in mice resulted in death around embryonic day 11.5.57) An analysis of embryos at embryonic day 10.5 showed the total SM content to be reduced by ~60% in the mutant, compared with the wild-type control.57) Although total ceramide content did not differ between the wild-type and mutant embryos, the ceramide content of the ER was 2-fold higher in the mutant.57) Cells of the mutant embryos showed abnormal dilation of the ER and degenerating mitochondria, which probably affect organogenesis.57) Hence, experiments in vivo and in vitro indicated that the primary physiological function of CERT/GPBPΔ26 is most likely to mediate intracellular trafficking of ceramide.
In zebrafish development, GPBP is expressed at an earlier stage than CERT/GPBPΔ26.58) Knockdown of both GPBP and CERT/GPBPΔ26 results in loss of myelinated tracks in the central nervous system and extensive apoptosis and tissue loss in the brain and somites.58) The defects are rescued by expression of GPBP, but not CERT/GPBPΔ26, indicating that GPBP and CERT/GPBPΔ26 have different functions during zebrafish development.58) If zebrafish GPBP is also secreted from cells, extracellular GPBP might play a crucial role in the development of zebrafish.
CERT inhibitor
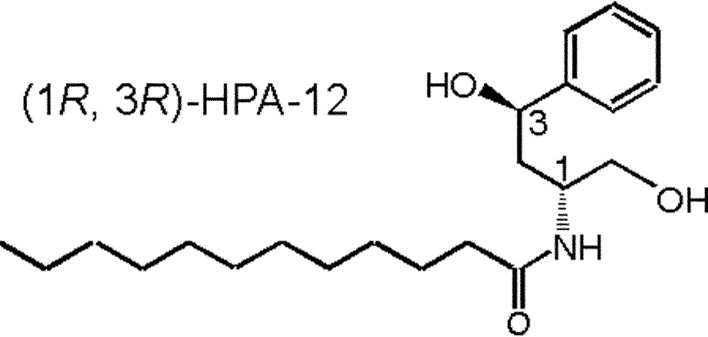
The chemically synthesized compound N-(3-hydroxy-1-hydroxymethyl-3-phenylpropyl)dodecamide (HPA-12) was found to be a selective inhibitor of CERT-mediated ceramide trafficking.39),59) Only the (1R, 3R) isomer among four stereochemical isomers of HPA-12 is active as an inhibitor (Fig. 7),59) and the two hydroxyl groups and also the middle chain lengths (C11–15) are essential for the bioactivity of HPA compounds in cells.60) (1R, 3R)-HPA-12 inhibited de novo SM synthesis in wild-type CHO cells to the level in untreated LY-A cells, while the synthesis in LY-A cells was not affected by the drug.59) (1R, 3R)-HPA-12, but not inactive isomers, inhibited the CERT-mediated inter-membrane transfer of ceramide in a cell-free assay system, indicating this drug to be a direct antagonist of CERT.39)
Fig. 7.
Structure of a CERT inhibitor.59)
CERT and drug-sensitization of cancer cells
Knockdown of COL4A3BP sensitizes various types of cancer cells to multiple cytotoxic agents including paclitaxel.61) Such sensitization also occurs when cancer cells are treated with the CERT inhibitor HPA-12.61) A reduction in CERT/GPBPΔ26 may result in an increase in ceramide in the ER, thereby producing ER stress, which might sensitize cells to paclitaxel.61) Alternatively, when its level in the ER increases, ceramide might flow into mitochondria and permeabilize the mitochondrial outer membrane to proapoptotic proteins.62)
Perspectives
As described above, the activity of CERT is regulated by phosphorylation and dephosphorylation. Treatment of cells with methyl-β-cyclodextrin or sphingomyelinase induces dephosphorylation of CERT.51) Inhibition of the de novo synthesis of sphingolipids also induces such dephosphorylation.51) Although the perturbation of cholesterol/SM rafts at the plasma membrane or the Golgi apparatus is likely to affect the activity of CERT, further study is needed to elucidate the mechanism involved.
The finding that GPBP/CERTL is secreted even without any signal sequence-like motif raises new questions: how is GPBP/CERTL secreted? Are the functions of secreted GPBP/CERTL relevant to PI4P and ceramide (GPBP/CERTL is expected to have an intact PH domain and START domain)? Whereas transgenic overexpression of GPBP/CERTL in mice triggers glomerular abnormalities,63) its physiological role at normal levels remains unknown.
The mechanisms underlying the formation of membrane contact sites between different organelles should also be elucidated, because such sites are anticipated to be crucial for interorganelle transport of small molecules including lipids.64) Recently, mitofusin 2 (a mitochondrial dynamin-related protein) in mammalian cells and the Mmm1/Mdm10/Mdm12/Mdm34 complex in yeast cells were found to play essential roles in the formation of ER-mitochondrial membrane contact sites.65),66) In contrast, it remains unknown what factors are necessary for the formation of ER-Golgi membrane contact sites.
Acknowledgements
I thank Dr. Masahiro Nishijima and all other co-workers, present and past, for invaluable contributions to the study of CERT. This work was supported by a Grant-in-aid from the Japan Society for the Promotion of Science.
Abbreviations
- CERT
ceramide transfer protein
- CKI
casein kinase I
- CoA
co-enzyme A
- ER
endoplasmic reticulum
- FFAT
two phenylalanines in an acidic tract
- GlcCer
glucosylceramide
- GPBP
Goodpasture antigen-binding protein
- HPA-12
N-(3-hydroxy-1-hydroxymethyl-3-phenylpropyl)dodecamide
- PH
pleckstrin homology
- PCTP
phosphatidylcholine-transfer protein
- PI4P
phosphatidylinositol 4-phosphate
- PKD
protein kinase D
- PP2Cɛ
protein phosphatase 2Cɛ
- SM
sphingomyelin
- StAR
steroidogenic acute regulatory protein
- START
StAR-related
- TGN
trans Golgi network
- VAP
vesicle-associated membrane protein-associated protein.
References
- 1).van Meer G., Voelker D. R., Feigenson G. W. (2008) Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Lee M. C., Miller E. A., Goldberg J., Orci L., Schekman R. (2004) Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 20, 87–123 [DOI] [PubMed] [Google Scholar]
- 3).Watson P, Stephens D. J. (2005) ER-to-Golgi transport: form and formation of vesicular and tubular carriers. Biochim. Biophys. Acta 1744, 304–315 [DOI] [PubMed] [Google Scholar]
- 4).Pagano R. E., Puri V., Dominguez M., Marks D. L. (2000) Membrane traffic in sphingolipid storage diseases. Traffic 1, 807–815 [DOI] [PubMed] [Google Scholar]
- 5).Mayor S., Pagano R. E. (2007) Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 8, 603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Ikonen E. (2008) Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 9, 125–138 [DOI] [PubMed] [Google Scholar]
- 7).Holthuis J. C., Levine T. P. (2005) Lipid traffic: floppy drives and a superhighway. Nat. Rev. Mol. Cell Biol. 6, 209–220 [DOI] [PubMed] [Google Scholar]
- 8).Vance J. E. (1991) Newly made phosphatidylserine and phosphatidylethanolamine are preferentially translocated between rat liver mitochondria and endoplasmic reticulum. J. Biol. Chem. 266, 89–97 [PubMed] [Google Scholar]
- 9).Voelker D. R. (2003) New perspectives on the regulation of intermembrane glycerophospholipid traffic. J. Lipid Res. 44, 441–449 [DOI] [PubMed] [Google Scholar]
- 10).Hakomori S. I. (2008) Structure and function of glycosphingolipids and sphingolipids: recollections and future trends. Biochim. Biophys. Acta 1780, 325–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Merrill A. H., Jr (2002) De novo sphingolipid biosynthesis: A necessary, but dangerous, pathway. J. Biol. Chem. 277, 25843–25846 [DOI] [PubMed] [Google Scholar]
- 12).Hanada K. (2003) Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta 1632, 16–30 [DOI] [PubMed] [Google Scholar]
- 13).Yamaji A., Sekizawa Y., Emoto K., Sakuraba H., Inoue K., Kobayashi H., et al. (1998) Lysenin, a novel sphingomyelin-specific binding protein. J. Biol. Chem. 273, 5300–5306 [DOI] [PubMed] [Google Scholar]
- 14).Hanada K., Hara T., Fukasawa M., Yamaji A., Umeda M., Nishijima M. (1998) Mammalian cell mutants resistant to a sphingomyelin-directed cytolysin. Genetic and biochemical evidence for complex formation of the LCB1 protein with the LCB2 protein for serine palmitoyltransferase. J. Biol. Chem. 273, 33787–33794 [DOI] [PubMed] [Google Scholar]
- 15).Fukasawa M., Nishijima M., Hanada K. (1999) Genetic evidence for ATP-dependent endoplasmic reticulum-to-Golgi apparatus trafficking of ceramide for sphingomyelin synthesis in Chinese hamster ovary cells. J. Cell Biol. 144, 673–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Funakoshi T., Yasuda S., Fukasawa M., Nishijima M., Hanada K. (2000) Reconstitution of ATP-and cytosol-dependent transport of de novo synthesized ceramide to the site of sphingomyelin synthesis in semi-intact cells. J. Biol. Chem. 275, 29938–29945 [DOI] [PubMed] [Google Scholar]
- 17).Ohvo-Rekila H., Ramstedt B., Leppimaki P., Slotte J. P. (2002) Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 41, 66–97 [DOI] [PubMed] [Google Scholar]
- 18).Fukasawa M., Nishijima M., Itabe H., Takano T., Hanada K. (2000) Reduction of sphingomyelin level without accumulation of ceramide in Chinese hamster ovary cells affects detergent-resistant membrane domains and enhances cellular cholesterol efflux to methyl-β-cyclodextrin. J. Biol. Chem. 275, 34028–34034 [DOI] [PubMed] [Google Scholar]
- 19).Hanada K., Kumagai K., Yasuda S., Miura Y., Kawano M., Fukasawa M., et al. (2003) Molecular machinery for non-vesicular trafficking of ceramide. Nature 426, 803–809 [DOI] [PubMed] [Google Scholar]
- 20).Raya A., Revert F., Navarro S., Saus J. (1999) Characterization of a novel type of serine/threonine kinase that specifically phosphorylates the human goodpasture antigen. J. Biol. Chem. 274, 12642–12649 [DOI] [PubMed] [Google Scholar]
- 21).Raya A., Revert-Ros F., Martinez-Martinez P., Navarro S., Rosello E., Vieites B., et al. (2000) Goodpasture antigen-binding protein, the kinase that phosphorylates the goodpasture antigen, is an alternatively spliced variant implicated in auto-immune pathogenesis. J. Biol. Chem. 275, 40392–40399 [DOI] [PubMed] [Google Scholar]
- 22).Ponting C. P., Aravind L. (1999) START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends Biochem. Sci. 24, 130–132 [DOI] [PubMed] [Google Scholar]
- 23).Lemmon M. A. (2008) Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol. 9, 99–111 [DOI] [PubMed] [Google Scholar]
- 24).Levine T. P., Munro S. (2002) Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr. Biol. 12, 695–704 [DOI] [PubMed] [Google Scholar]
- 25).Wang Y. J., Wang J., Sun H. Q., Martinez M., Sun Y. X., Macia E., et al. (2003) Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114, 299–310 [DOI] [PubMed] [Google Scholar]
- 26).Godi A., Di Campli A., Konstantakopoulos A., Di Tullio G., Alessi D. R., Kular G. S., et al. (2004) FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat. Cell Biol. 6, 393–404 [DOI] [PubMed] [Google Scholar]
- 27).Christenson L. K., Strauss J. F., 3rd (2000) Steroidogenic acute regulatory protein (StAR) and the intramitochondrial translocation of cholesterol. Biochim. Biophys. Acta 1529, 175–187 [DOI] [PubMed] [Google Scholar]
- 28).Miller W. L. (2007) Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim. Biophys. Acta 1771, 663–676 [DOI] [PubMed] [Google Scholar]
- 29).Rone M. B., Fan J, Papadopoulos V. (2009) Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim. Biophys. Acta 1791, 646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Soccio R. E., Breslow J. L. (2003) StAR-related lipid transfer (START) proteins: mediators of intracellular lipid metabolism. J. Biol. Chem. 278, 22183–22186 [DOI] [PubMed] [Google Scholar]
- 31).Alpy F., Tomasetto C. (2005) Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J. Cell Sci. 118, 2791–2801 [DOI] [PubMed] [Google Scholar]
- 32).Schrick K., Nguyen D., Karlowski W. M., Mayer K. F. (2004) START lipid/sterol-binding domains are amplified in plants and are predominantly associated with homeodomain transcription factors. Genome Biol. 5, R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Kallen C. B., Billheimer J. T., Summers S. A., Stayrook S. E., Lewis M., Strauss J. F., 3rd (1998) Steroidogenic acute regulatory protein (StAR) is a sterol transfer protein. J. Biol. Chem. 273, 26285–26288 [DOI] [PubMed] [Google Scholar]
- 34).Tsujishita Y., Hurley J. H. (2000) Structure and lipid transport mechanism of a StAR-related domain. Nat. Struct. Biol. 7, 408–414 [DOI] [PubMed] [Google Scholar]
- 35).Wirtz K. W. A. (1991) Phospholipid transfer proteins. Ann. Rev. Biochem. 60, 73–99 [DOI] [PubMed] [Google Scholar]
- 36).Kanno K., Wu M. K., Scapa E. F., Roderick S. L., Cohen D. E. (2007) Structure and function of phosphatidylcholine transfer protein (PC-TP)/StarD2. Biochim. Biophys. Acta 1771, 654–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Olayioye M. A., Vehring S., Muller P., Herrmann A., Schiller J., Thiele C., et al. (2005) StarD10, a START domain protein overexpressed in breast cancer, functions as a phospholipid transfer protein. J. Biol. Chem. 280, 27436–27442 [DOI] [PubMed] [Google Scholar]
- 38).Tabunoki H., Sugiyama H., Tanaka Y., Fujii H., Banno Y., Jouni Z. E., et al. (2002) Isolation, characterization, and cDNA sequence of a carotenoid binding protein from the silk gland of Bombyx mori larvae. J. Biol. Chem. 277, 32133–32140 [DOI] [PubMed] [Google Scholar]
- 39).Kumagai K., Yasuda S., Okemoto K., Nishijima M., Kobayashi S., Hanada K. (2005) CERT mediates intermembrane transfer of various molecular species of ceramides. J. Biol. Chem. 280, 6488–6495 [DOI] [PubMed] [Google Scholar]
- 40).Lev S., Ben Halevy D., Peretti D., Dahan N. (2008) The VAP protein family: from cellular functions to motor neuron disease. Trends Cell Biol. 18, 282–290 [DOI] [PubMed] [Google Scholar]
- 41).Loewen C. J., Roy A., Levine T. P. (2003) A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. EMBO J. 22, 2025–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Kawano M., Kumagai K., Nishijima M., Hanada K. (2006) Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. J. Biol. Chem. 281, 30279–30288 [DOI] [PubMed] [Google Scholar]
- 43).Kudo N., Kumagai K., Tomishige N., Yamaji T., Wakatsuki S., Nishijima M., et al. (2008) Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide. Proc. Natl. Acad. Sci. USA 105, 488–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Munro S. (2003) Cell biology: earthworms and lipid couriers. Nature 426, 775–776 [DOI] [PubMed] [Google Scholar]
- 45).Ladinsky M. S., Mastronarde D. N., McIntosh J. R., Howell K. E., Staehelin L. A. (1999) Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J. Cell Biol. 144, 1135–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Marsh B. J., Mastronarde D. N., Buttle K. F., Howell K. E., McIntosh J. R. (2001) Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proc. Natl. Acad. Sci. USA 98, 2399–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Halter D., Neumann S., van Dijk S. M., Wolthoorn J., de Maziere A. M., Vieira O. V., et al. (2007) Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J. Cell Biol. 179, 101–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).D’Angelo G., Vicinanza M., Di Campli A., De Matteis M. A. (2008) The multiple roles of PtdIns(4)P – not just the precursor of PtdIns(4,5)P2. J. Cell Sci. 121, 1955–1963 [DOI] [PubMed] [Google Scholar]
- 49).Toth B., Balla A., Ma H., Knight Z. A., Shokat K. M., Balla T. (2006) Phosphatidylinositol 4-kinase IIIβ regulates the transport of ceramide between the endoplasmic reticulum and Golgi. J. Biol. Chem. 281, 36369–36377 [DOI] [PubMed] [Google Scholar]
- 50).Lopez-Montero I., Rodriguez N., Cribier S., Pohl A., Velez M., Devaux P. F. (2005) Rapid transbilayer movement of ceramides in phospholipid vesicles and in human erythrocytes. J. Biol. Chem. 280, 25811–25819 [DOI] [PubMed] [Google Scholar]
- 51).Kumagai K., Kawano M., Shinkai-Ouchi F., Nishijima M., Hanada K. (2007) Interorganelle trafficking of ceramide is regulated by phosphorylation-dependent cooperativity between the PH and START domains of CERT. J. Biol. Chem. 282, 17758–17766 [DOI] [PubMed] [Google Scholar]
- 52).Fugmann T., Hausser A., Schoffler P., Schmid S., Pfizenmaier K., Olayioye M. A. (2007) Regulation of secretory transport by protein kinase D-mediated phosphorylation of the ceramide transfer protein. J. Cell Biol. 178, 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Tomishige N., Kumagai K., Kusuda J., Nishijima M., Hanada K. (2009) Casein kinase Iγ2 down-regulates trafficking of ceramide in the synthesis of sphingomyelin. Mol. Biol. Cell 20, 348– 357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Saito S., Matsui H., Kawano M., Kumagai K., Tomishige N., Hanada K., et al. (2007) Protein phosphatase 2Cɛ is an endoplasmic reticulum integral membrane protein that dephosphorylates the ceramide transport protein CERT to enhance its association with organelle membranes. J. Biol. Chem. 283, 6584–6593 [DOI] [PubMed] [Google Scholar]
- 55).Revert F., Ventura I., Martinez-Martinez P., Granero-Molto F., Revert-Ros F., Macias J., et al. (2008) Goodpasture antigen-binding protein is a soluble exportable protein which interacts with type IV collagen: Identification of novel membrane-bound isoforms. J. Biol. Chem. 283, 30246– 30255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Rao R. P., Yuan C., Allegood J. C., Rawat S. S., Edwards M. B., Wang X., et al. (2007) Ceramide transfer protein function is essential for normal oxidative stress response and lifespan. Proc. Natl. Acad. Sci. USA 104, 11364–11369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Wang X., Rao R. P., Kosakowska-Cholody T., Masood M. A., Southon E., Zhang H., et al. (2009) Mitochondrial degeneration and not apoptosis is the primary cause of embryonic lethality in ceramide transfer protein mutant mice. J. Cell Biol. 184, 143–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Granero-Molto F., Sarmah S., O’Rear L., Spagnoli A., Abrahamson D., Saus J., et al. (2008) Good-pasture antigen-binding protein and its spliced variant, ceramide transfer protein, have different functions in the modulation of apoptosis during zebrafish development. J. Biol. Chem. 283, 20495– 20504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Yasuda S., Kitagawa H., Ueno M., Ishitani H., Fukasawa M., Nishijima M., et al. (2001) A novel inhibitor of ceramide trafficking from the endoplasmic reticulum to the site of sphingomyelin synthesis. J. Biol. Chem. 276, 43994–44002 [DOI] [PubMed] [Google Scholar]
- 60).Nakamura Y., Matsubara R., Kitagawa H., Kobayashi S., Kumagai K., Yasuda S., et al. (2003) Stereoselective synthesis and structure-activity relationship of novel ceramide trafficking inhibitors, (1R,3R)-N-(3-hydroxy-1-hydroxymethyl-3-phenyl-propyl)dodecanamide (HPA-12) and its analogues. J. Med. Chem. 46, 3688–3695 [DOI] [PubMed] [Google Scholar]
- 61).Swanton C., Marani M., Pardo O., Warne P. H., Kelly G., Sahai E., et al. (2007) Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell 11, 498–512 [DOI] [PubMed] [Google Scholar]
- 62).Stiban J., Caputo L., Colombini M. (2008) Ceramide synthesis in the endoplasmic reticulum can permeabilize mitochondria to proapoptotic proteins. J. Lipid Res. 49, 625–634 [DOI] [PubMed] [Google Scholar]
- 63).Revert F., Merino R., Monteagudo C., Macias J., Peydro A., Alcacer J., et al. (2007) Increased Goodpasture antigen-binding protein expression induces type IV collagen disorganization and deposit of immunoglobulin A in glomerular basement membrane. Am. J. Pathol. 171, 1419–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Levine T. (2004) Short-range intracellular trafficking of small molecules across endoplasmic reticulum junctions. Trends Cell Biol. 14, 483–490 [DOI] [PubMed] [Google Scholar]
- 65).de Brito O. M., Scorrano L. (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–610 [DOI] [PubMed] [Google Scholar]
- 66).Kornmann B., Currie E., Collins S. R., Schuldiner M., Nunnari J., Weissman J. S., et al. (2009) An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325, 477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]