Abstract
1α,25(OH)2-Vitamin D3 (1,25D) modulates osteoblast gene expression of bone matrix proteins via a nuclear vitamin D receptor (VDR) and also modifies the electrical state of the plasma membrane through rapid nongenomic mechanisms still not fully understood. The physiological significance of 1,25D membrane-initiated effects remains unclear. To elucidate whether the VDR is required for 1,25D-promoted electrical responses, we studied 1,25D modulation of ion channel activities in calvarial osteoblasts isolated from VDR knockout (KO) and WT mice. At depolarizing potentials, Cl- currents were significantly potentiated (13.5 ± 1.6-fold increase, n = 12) by 5 nM 1,25D in VDR WT but not in KO (0.96 ± 0.3 fold increase, n = 11) osteoblasts. L-type Ca2+ currents significantly shift their peak activation by -9.3 ± 0.7 mV (n = 10) in the presence of 5 nM 1,25D in VDR WT but not in KO cells, thus facilitating Ca2+ influx. Furthermore, we found that 1,25D significantly increased wholecell capacitance in VDR WT (ΔCap = 2.3 ± 0.4 pF, n = 8) but not in KO osteoblasts (ΔCap = 0.3 ± 0.1 pF, n = 8); this corresponds to a rapid (1–2 min) fusion in WT of 71 ± 33 versus in KO only 9 ± 6 individual secretory granules. We conclude that, in calvarial osteoblasts, 1,25D modulates ion channel activities only in cells with a functional VDR and that this effect is coupled to exocytosis. This is a demonstration of the requirement of a functional classic steroid receptor for the rapid hormonal modulation of electric currents linked to secretory activities in a target cell.
Nongenomic responses to the steroid 1α,25(OH)2-vitamin D3 (1,25D) develop at the plasma membrane of various target cells and comprise rapid (seconds to minutes) changes in ion channel activities, activation of second messenger pathways, and elevation of cytosolic calcium concentrations (1–3). At a different level, 1,25D regulates the expression of tissue-specific genes via a nuclear vitamin D receptor (VDR) that functions as a transcription factor (4, 5). Although these two mechanisms of action are known to occur through different signaling pathways, there is no clear consensus yet whether rapid, membrane-related actions of 1,25D are initiated by a unique membrane receptor of unknown molecular identity or whether the classic VDR is involved (6–9).
In bone, 1,25D elicits physiological responses at both the genomic and nongenomic levels. Osteoblasts, which are secretory cells, produce a variety of bone matrix proteins and actively participate in the mineralization process under the influence of hormones involved in mineral metabolism, such as 1,25D. Recently, several strains of functional VDR knockout (KO) mice have been created by means of deletion of selected portions of the VDR gene (10–13). They constitute a valuable tool for the study of a vast range of physiological conditions that arise from an impaired 1,25D physiology. Nuclear VDR KO mice, with abrogated 1,25D genomic actions, develop a genotype typical of rickets type II, characterized by decreased growth, hypocalcemia, hyperparathyroidism, and reduced bone formation (14).
Nongenomic effects have been described in several cell types for different steroid hormones including 1,25D (15, 16). Their physiological significance at the single-cell level is currently a field of intense study. Typically, rapid 1,25D effects such as transcaltachia in the intestinal epithelium (17) and insulin secretion by β-pancreatic cells (18, 19) have been studied at the tissue level. In osteoblasts, the bone-forming cells, 1,25D stimulates the production of bone matrix proteins that are constitutively secreted to the extracellular matrix (20, 21). The 1,25D hormone has multiple modulatory effects on the electrical activity of several ion channel types present in the osteoblast plasma membrane, where it acts through different molecular mechanisms, which may include the participation of cytoplasmic second messengers and a putative membrane receptor molecule (22).
With the purpose to elucidate whether a functional classic VDR is needed for the onset of rapid electrical responses to 1,25D, we studied the modulation by the hormone of Cl- and Ca2+ channel activities in the plasma membrane of calvarial osteoblasts isolated from newborn VDR WT and KO mice. In addition, we investigated the physiological significance of 1,25D-promoted electrical effects and characterized single-cell secretory activities by means of measurement of changes in whole-cell capacitance in VDR WT and KO osteoblasts in the absence and presence of the hormone. This article reports the rapid induction of individual exocytotic events by 1,25D in single primary osteoblasts and the requirement for a functional classic VDR for the development of rapid, membrane-associated electrical responses to 1,25D.
Materials and Methods
Chemicals. 1,25D, 25(OH)-Vitamin D3 (25D), and the stereoisomer 1β,25(OH)2-vitamin D3 (1β,25D), kindly provided by M. Uskokovic (Hoffmann–La Roche) and W. Okamura (Department of Chemistry, University of California, Riverside), respectively, were stored as stock solutions in ethanol at -20°C in the dark. Nifedipine and (±)-Bay K8644 (Sigma) were used as the specific L-type Ca2+ channel antagonist and agonist, respectively, and added to the extracellular medium from ethanol stock solutions. 4,4′-Diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS, Sigma) was used as a specific Cl- channel blocker and was added to the bath from a stock solution made in the recording medium. Cholesterol and 17β-estradiol (Sigma) were added to the bath from ethanol stock solutions.
Animals. Mice carrying a WT and a functionally KO VDR gene were a kind gift from S. Kato (University of Tokyo, Tokyo) (10). Mice were raised with a standard laboratory rodent diet containing 0.95% calcium, 0.67% phosphorus, 1.67% lactose, and 4.5 units/g vitamin D3. Newborn mice used in this study were generated by breeding heterozygotes to produce offspring of all three genotypes. From all newborns used, a tail sample was obtained during the osteoblast isolation procedures for VDR genotyping. All animal study protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, Riverside.
Osteoblast Isolation and Culture. Osteoblast primary cultures were established from VDR WT and KO mice basically as described by others (23). Osteoblast isolation was carried out on the same day from genotyped VDR WT and KO littermates. Parietal bones were dissected aseptically from 3- to 5-day-old mice. The periosteal layers were carefully stripped off, and the sutures were discarded to reduce the number of contaminant cells. Stripped parietal bones were fragmented (≈1 mm2), washed several times in sterile Hepes buffer (in mM: 150 NaCl/5.6 KCl/2 CaCl2/1 MgCl2/5 glucose/2.4 NaHCO3/25 Hepes, pH 7.3, adjusted with NaOH), and transferred to 35-mm Falcon culture dishes. Cells were grown in Ham's F-12 medium (Sigma) containing 5% FBS (Sigma) and 5% Serum Plus (JRH Biosciences, Woodland, CA), according to previous protocols (2). Osteoblasts migrated from the bone fragments and settled on the bottom of the culture dishes after 1–2 days. The culture medium was changed every 4–5 days. Mature osteoblasts showing a typical polyhedral shape were chosen on the basis of morphology and studied within the first 2–4 weeks in culture.
PCR Genotyping of Mice. The presence of a WT and a deleted nonfunctional KO VDR gene in so-called VDR WT and KO mice, respectively, was assessed with RT-PCR. DNA was isolated from tail samples by using the DNeasy kit (Qiagen, Santa Clarita, CA); the PCR primers were the same as described in ref. 24.
Electrophysiology. Whole-cell patch-clamp experiments were performed essentially as described (2). Primary osteoblasts were washed at least three times with the external recording solution to remove the medium completely. Chloride channel activity was recorded in the presence of (in mM) 150 TEA-Cl, 3.1 KCl, 20 BaCl2, 1 MgCl2, 4 NaHCO3, 10 Hepes, and 20 sucrose (pH 7.4, adjusted with TEA-OH) in the bath and (in mM) 150 Csmethanesulfonate, 15 NaCl, 4 MgCl2, 5 EGTA, and 10 Hepes (pH 7.4, adjusted with NaOH) in the pipette. Calcium channel activity was recorded in the presence of a high barium bath solution containing (in mM) 108 BaCl2 and 10 Hepes [pH 7.6, adjusted with Ba(OH)2] and an internal (pipette) solution consisting of (in mM) 150 CsCl, 5 EGTA, 10 d-glucose, and 10 Hepes (pH 7.3, adjusted with CsOH).
Patch-clamp recordings (25) were performed with a Heka EPC-9 amplifier (ALA Scientific Instruments, Westbury, NY). Patch pipettes of ≈2 MΩ were fabricated with a DMZ Universal (Zeitz Instruments, Munich) micropipette puller from Drummond capillaries (Drummond Scientific, Broomall, PA), coated with Sylgard elastomer (Dow Corning, Midland, MI) to reduce capacitative transients, and fire-polished. Giga seals progressed rapidly and stably.
Changes in whole-cell capacitance, a measure of exocytosis (26, 27), were detected by using the software-based lock-in implementation of pulse v.8 (Heka EPC-9, ALA Scientific, Westbury, NY). The applied sine wave had a frequency of 500 Hz and peak amplitude of 20 mV, and was superimposed on a holding potential of 0 mV. Whole-cell capacitance was continuously monitored for 10–20 min.
Cell Imaging. Live osteoblasts were observed with a laser scanning confocal TCS SP2 microscope from Leica. Secretory vesicles were stained with 1 μM quinacrine for 10 min according to published protocols (28).
Results
1,25D Effects on Outward Cl- Currents in VDR WT and KO Osteoblasts. Recently, we reported the rapid potentiation of ion currents by physiological concentrations of 1,25D in osteosarcoma ROS 17/2.8 cells and modeled their role in a series of 1,25D-driven membrane events that may lead to cell secretion (2, 22, 29). In the present work, and with the aim to investigate whether a classic VDR is required for the modulation of ion channel activities by 1,25D, we comparatively studied the effects by the hormone on Cl- and Ca2+ channels in primary calvarial osteoblasts obtained from mice that express (WT) or lack (KO) a functional VDR.
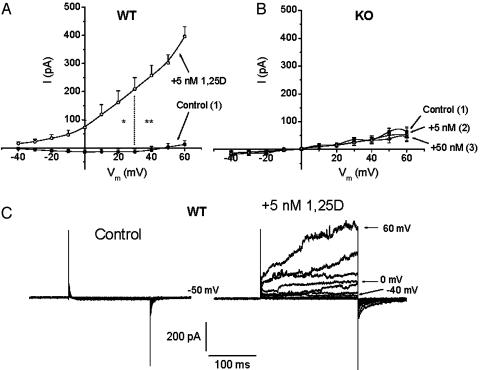
Fig. 1 shows whole-cell Cl- currents obtained from VDR WT and KO osteoblasts, before and after the addition of nanomolar concentrations of 1,25D. In the absence of the hormone, and under the recording conditions described in Materials and Methods, VDR WT and KO osteoblasts developed small (few pA), non-inactivating currents at depolarizing potentials between -40 and 60 mV (Fig. 1C). The addition of 5 nM 1,25D to the bath caused, in the case of VDR WT osteoblasts (Fig. 1 A), a rapid (1–5 min), remarkable activation of Cl- currents (Erev = -54 mV for the Cl- concentrations used) of 13.5 ± 1.6-fold, as measured at 60 mV in 12 studied cells (see Fig. 2). These outward currents were partially reduced by externally added 200 μM DIDS, a specific Cl- channel blocker (data not shown; see ref. 2). In addition, we found no significant differences in 1,25D activation of Cl- currents between cells obtained from homozygous (+/+) and heterozygous (+/-) VDR animals (data not shown). Conversely, externally added 5–50 nM 1,25D did not cause any significant increase in Cl- current amplitudes in VDR KO osteoblasts (0.96 ± 0.3-fold) in 11 cells studied, as shown in Figs. 1B and 2.
Fig. 1.
1,25D potentiation of Cl- currents in VDR WT but not in KO osteoblasts. Current to voltage (I/Vm) relations obtained for Cl- channel activities from VDR WT (A) and KO (B) osteoblasts, before (filled circles) and after the addition of 5 nM (open circles) and 50 nM (open triangles) 1,25D to the bath. To allow for volume-sensitive Cl- currents to reach a stable amplitude value, current amplitudes (I, pA) were measured 5–10 min after obtaining the whole-cell configuration (control curves) and 5–10 min after the addition of the hormone to the bath. The current was measured at the peak of maximal activation of outward currents elicited by a series of 200-ms depolarizing voltage steps between -40 and 60 mV from a holding potential of -50 mV, applied every second. Values represent the mean ± SEM of n = 12 WT and n = 11 KO osteoblasts. Numbers in parentheses indicate the sequential order of recordings obtained from the same single cell. (C) Typical raw currents recorded from a single VDR WT osteoblast. Recording solutions are described in Materials and Methods.
Fig. 2.
Cl- current potentiation in VDR WT osteoblasts is selective for 1,25D. Average fold increase of outward Cl- currents measured at 60 mV, 5–10 min after the addition of 5 nM 1,25D (n = 12 VDR WT and n = 11 KO osteoblasts), 0.1–1 μM cholesterol (Ch, n = 5), 10 nM of the synthetic analog 1β,25D by itself (n = 3) or followed by the addition of 5 μM 1,25D (n = 3), 5 nM the natural metabolite 25D (n = 4), and 0.1–1 μM17β-estradiol (17β-E, n = 4) to the bath. The magnitude of the response was compared to the fold increase obtained with 5 nM 1,25D on VDR WT osteoblasts. Values represent the mean ± SEM. *, P < 0.05; **, P < 0.01.
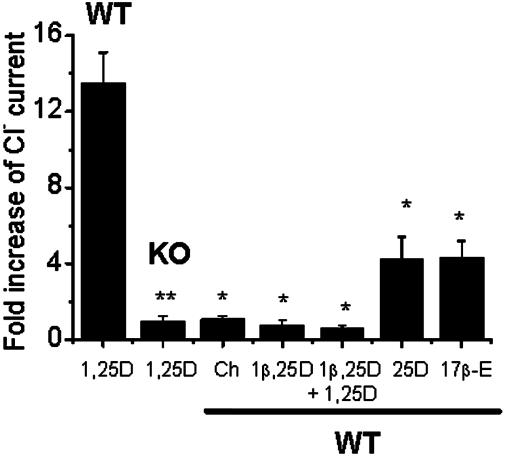
The rapid potentiation of Cl- currents in VDR WT calvarial osteoblasts was specific for 1,25D, as shown in Fig. 2. Cholesterol was inactive at concentrations of up to 1 μM (1.05 ± 0.2-fold increase, n = 5). 17β-Estradiol was only slightly effective in potentiating outward currents in VDR WT osteoblasts when added to the bath at sequential increasing concentrations of 0.01, 0.1, and 1 μM on the same cell (4.3 ± 0.9-fold increase, n = 4). Also, the stereoisomer 1β,25D, which has been shown previously to inhibit 1,25D-stimulated rapid increase of Cl- currents in ROS 17/2.8 cells (2), did not cause any significant potentiation of Cl- currents in VDR WT calvarial osteoblasts (0.74 ± 0.3 fold increase, n = 3) and inhibited any further potentiation by 1,25D in these cells. Finally, the natural metabolite 25D, which has a considerably lesser affinity than 1,25D for the VDR, did not promote any significant potentiation of Cl- currents in VDR WT osteoblasts (4.2 ± 1.2-fold increase, n = 4; Fig. 2) at a 5 nM concentration when added to the bath.
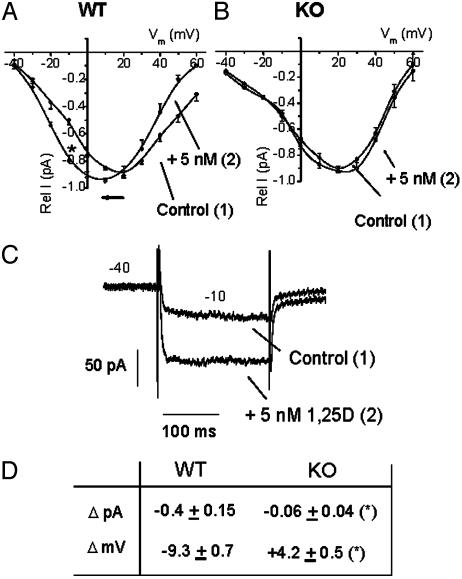
1,25D Modulation of Ca2+ Currents in VDR WT and KO Osteoblasts. 1,25D rapidly modulates the activity of L-type Ca2+ channels in ROS 17/2.8 cells by shifting the current to voltage relationships to more negative potentials, thus mimicking the effect of the dihydropyridine analog Bay K8644 (1, 2, 22). Fig. 3A shows that, in the presence of high external barium (see Materials and Methods), 5 nM 1,25D significantly shifted the peak of maximal activation of calcium channels by -9.3 ± 0.7 mV (n = 10) in VDR WT primary osteoblasts, thus facilitating the influx of the cation at low depolarizing potentials as shown with raw current traces obtained at -10 mV in the absence (control) and presence of 5 mM 1,25D (Fig. 3C). However, the effect of same and higher (50 nM) 1,25D concentrations on Ca2+ channels in VDR KO cells was not significantly different from the control (Fig. 3B). Fig. 3D summarizes the results obtained for 1,25D effects on current amplitudes (ΔpA) measured at -10 mV and voltage shift (ΔmV) in WT and KO cells. In both cell types, inward barium currents were completely blocked by 2 μM nifedipine, a specific blocker for L-type Ca2+ channels (data not shown; see ref. 2). The addition of cholesterol and 17β-estradiol at concentrations of up to 1 μM did not produce any significant changes in inward Ba2+ currents in either WT or KO cells (data not shown).
Fig. 3.
Effects of 1,25D on L-type Ca2+ channels in VDR WT and KO osteoblasts. I/Vm relations obtained for Ca2+ channel activities from VDR WT (A) and KO (B) osteoblasts, before (filled circles) and 5 min after (open circles) the addition of 5 nM 1,25D to the bath. Rel I (pA) represents the relative current value obtained for each series of 200-ms depolarizing voltage steps in relation to the maximal I (pA) value obtained for each single cell. Voltage steps were applied every second between 40 and 60 mV, from a holding potential of -40 mV. Values represent the mean ± SEM of n = 10 WT and n = 6 KO osteoblasts. Numbers in parentheses indicate the sequential order of recordings obtained from the same single cell. (C) Raw data for the typical potentiation of inward Ba2+ currents by 1,25D obtained at -10 mV from a single VDR WT osteoblast. (D) Summary of the values obtained for current amplitude increments (ΔpA) at -10 mV and shifts in I/Vm relationships (ΔmV) due to the addition of 5 nM 1,25D to VDR WT (n = 10) and KO (n = 6) osteoblasts. Data (average ± SEM) from WT and KO cells were statistically different (*, P < 0.50). Recording solutions are described in Materials and Methods.
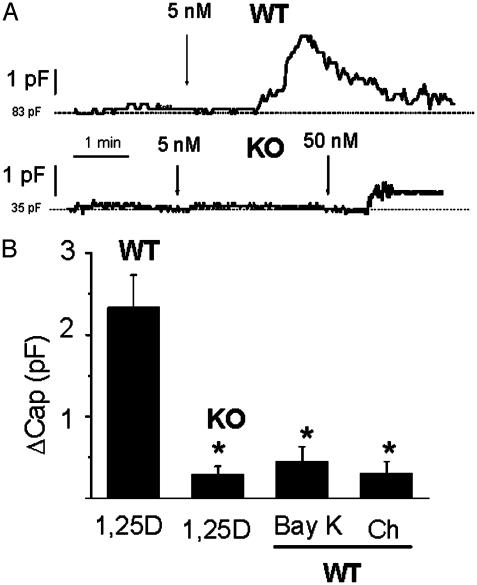
1,25D Effects on Whole-Cell Capacitance. With the purpose to investigate the physiological consequences at the single-cell level of the rapid modulation of ion channel activities by 1,25D in VDR WT primary osteoblasts, and the corresponding lack of effects in VDR KO cells, we recorded 1,25D-induced whole-cell capacitance changes as a measure of exocytosis in single osteoblasts (26, 27). Fig. 4A shows typical whole-cell capacitance traces obtained from VDR WT and KO osteoblasts during the addition of 1,25D to the bath. As depicted in Fig. 4A, 5 nM 1,25D promoted an increase in whole-cell capacitance of ≈2.4 pF from a value of 83 pF in this particular cell equivalent to a 3% increase in the cell's plasma membrane area. This capacitance response, which typically developed 1–2 min after the addition of 1,25D to the bath, took place transiently during ≈1–3 min. On the other hand, the same concentration of the hormone did not produce any significant change in whole-cell capacitance in VDR KO osteoblasts, and a higher concentration of 50 nM 1,25D on the same cell elicited a much lower response (≈0.5 pF from an initial value of 35 pF in the cell case shown in the figure) than the effect promoted by 5 nM of the hormone in VDR WT cells.
Fig. 4.
1,25D promoted an increase in whole-cell capacitance in VDR WT but not in KO osteoblasts. (A) Continuous recordings of whole-cell capacitance values obtained from a VDR WT and a KO osteoblast during the addition of 5 nM (WT) and 5–50 nM (KO) 1,25D to the bath. Cell capacitance was automatically measured every 1 sec. (B) Increments in whole-cell capacitance value (ΔCap) measured after the addition of 5 nM 1,25D (n = 8 WT and n = 8 KO cells), 1 μM (±)-Bay K8644 (n = 4), and 1 μM cholesterol (Ch, n = 3). ΔCap values (average ± SEM) were calculated as the difference between the peak of maximal capacitance value achieved after the addition of the agent and the initial basal line (dashed line) and were statistically compared to the increase produced by 5 nM 1,25D in VDR WT osteoblasts.*, P < 0.05.
Fig. 4B shows average capacitance increments (ΔCap) produced by 5 nM 1,25D obtained from VDR WT (ΔCap = 2.3 ± 0.4 pF, n = 8) and KO osteoblasts (ΔCap = 0.3 ± 0.1 pF, n = 8). ΔCap was calculated as the difference between the maximal capacitance value measured at any time during 1,25D stimulation and the basal value recorded before hormone addition (dashed lines in the recordings shown in Fig. 4A). The specificity of the response to 1,25D was studied on VDR WT cells by means of the addition of 1 μM cholesterol, which did not promote any significant change in whole-cell capacitance within 10 min.
A localized cytoplasmic calcium increase underneath the plasma membrane of secretory cells is known to play a crucial role in the fusion of secretory granules to the cell surface and the release of the granule content into the extracellular medium during the exocytotic process (30). The participation of 1,25D-sensitive Ca2+ channels in 1,25D-stimulated osteoblast secretion was studied by means of measurement of capacitance changes promoted by the addition of 1 μM (±)-Bay K8644 to the bath. The L-type Ca2+ channel agonist did not promote any significant increase in whole-cell capacitance value, as shown in Fig. 4B.
It has been established that cell membranes have a specific capacitance of 0.01 pF/μm2 (31). From this value, we calculated that an increase in the plasma membrane area of 3.14 μm2, which accounts for the fusion of a single circular area of 1 μm radius, produces a change in whole-cell capacitance of ≈31 fF. Thus, a 31-fF change can be taken as a close estimation of the capacitance change produced by the fusion of a single secretory granule to the plasma membrane surface. From this value, and the capacitance increment values in Fig. 4B, we calculated an average of 71 ± 33 and only 9 ± 6 individual secretory events at the peak of maximal 1,25D stimulation per single VDR WT and KO cell, respectively.
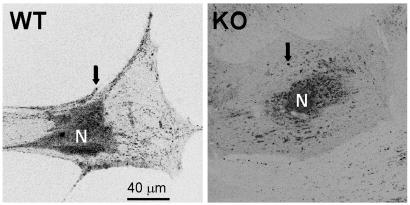
Imaging of Osteoblast Secretion. The stimulation of osteoblast secretory activities by 1,25D was observed with confocal microscopy of live cells. As shown in Fig. 5, VDR WT osteoblasts clearly differed from KO cells in the number and localization of quinacrine-loaded secretory granules. VDR WT osteoblasts typically showed a high number of granules (in the order of several hundreds) distributed both in the perinuclear area and adjacent to the plasma membrane, at all studied culture ages (between 2 and 5 weeks after isolation). Conversely, VDR KO cells showed a much lower number of secretory granules (below 100), mostly located in the perinuclear region.
Fig. 5.
Laser scanning confocal images obtained from a VDR WT and a VDR KO osteoblast in culture for 3 weeks. Secretory granules (arrows) labeled with 1 μM quinacrine (see Materials and Methods) distribute abundantly over the cytoplasm and adjacent to the cell membrane in a VDR WT osteoblast but are scarce in the cytoplasm and virtually absent from the cell periphery in a KO osteoblast. These results are typical of ≈50 VDR WT and KO cells studied.
1,25D stimulated quinacrine release from individual secretory granules into the extracellular medium when added to the external bath during confocal microscope observation of live cells. The diffusion of the dye into the bath due to the fusion of single granules to the plasma membrane occurred at a rate of about three to five events per sec in VDR WT cells and was virtually absent in KO osteoblasts. This 1,25D-stimulated exocytotic activity in WT cells typically developed 1–2 min after the addition of the hormone to the bath and lasted for a period of ≈10 sec (data not shown). These observations are in full agreement with our capacitance results shown in Fig. 4.
Discussion
Mechanisms associated with rapid, nongenomic cellular responses to steroid hormones currently are the subject of intense research by many laboratories. Although it has become broadly accepted that steroid hormones carry out membrane-related actions in a large number of target cells (15, 16), the precise molecular steps underlying nongenomic actions, as well as their physiological roles at the single-cell level, remain unclear (15, 32).
Classic steroid hormone receptors, which all belong to the same super gene family (33), have a remarkable structural homology (34) and are intracellular transcription factors that promote or suppress gene expression depending on the nature of the conformational changes induced by binding of a steroid agonist or antagonist (35–37). In the last decade there has been increasing evidence that steroid hormone actions, including 1,25D, also include biochemical interactions within the framework of the cell's cytoplasmic signaling network. However, there is no clear consensus on whether these nongenomic pathways involve a cross-talk of the steroid–nuclear receptor complex directly with signal transduction molecules or whether they become initiated by binding of the steroid to a membrane receptor possibly present in caveolae in the plasma membrane (38, 39). The purpose of this study is to investigate the requirement for the presence of a functional classic VDR to initiate the onset of 1,25D-promoted rapid responses, as well as to define the physiological significance of these membrane-related effects of the steroid hormone at the single-cell level in osteoblasts.
In their natural environment, osteoblasts, which are secretory cells, line the bone matrix in a continuous cell layer and respond to bone remodeling hormones by changing their shape, regulating their volume, and modifying the membrane potential (40). Furthermore, osteoblasts express a variety of ion channels which respond to physical and hormonal stimuli (1, 2, 22, 41). We have shown that 1,25D-sensitive Cl- and Ca2+ currents occur only in osteoblasts that express a functional classic VDR. Conversely, we found that Cl- and Ca2+ currents in VDR KO osteoblasts lack potentiation by nanomolar concentrations of 1,25D. This finding demonstrates that a functional classic steroid receptor is required for the presence of rapid response elements in this target cell.
The strain of VDR KO mice used in this article (Tokyo strain) was created by deletion of the region in the VDR gene that codes for the second zinc finger on the portion of the VDR protein that binds to the DNA (10). In this sense, any VDR remnant produced in VDR KO mice has abrogated genomic functions. This hypothesis raises different possibilities for the molecular pathways behind 1,25D-stimulated ion channel responses found only in VDR WT osteoblasts.
On one hand, 1,25D may modulate the level of gene expression of either ion channel proteins, membrane-associated ion channel modulatory proteins, and cytoplasmic transduction pathways, or the expression of a distinct membrane VDR dependent molecule. Therefore, VDR KO cells lacking normal 1,25D genomic functions could in consequence also manifest abrogated rapid electrical and secretory effects because of the lack of specific proteins associated with the cell membrane. We found that neither the natural metabolite 25D nor the synthetic analog for rapid actions, 1α,25(OH)2-lumisterol, which has been shown to potentiate Cl- currents in ROS 17/2.8 cells (2), stimulated electric responses in VDR KO osteoblasts (data not shown). 17β-Estradiol increased outward Cl- currents in the VDR WT osteoblasts, although to a significantly lesser extent than 1,25D (Fig. 2). Osteoblasts express estrogen receptors, which have been postulated to be involved in membrane-initiated rapid actions (42). The difference in the magnitude of the Cl- current responses to 17β-estradiol when compared to 1,25D may be indicative of different modulatory pathways involved in the electrical effects of each steroid.
On the other hand, the classic VDR, or a slightly modified protein, may be the receptor for rapid, membrane-associated responses by locally modulating ion channel activities. Under this hypothesis, a mutation or deletion of the VDR would result in impaired modulator actions on the electrical state of the osteoblast membrane. In separate studies, our laboratory has demonstrated in caveolae-enriched membrane fractions (CMF) the presence of a specific binding protein/receptor for [3H]1,25D (43) and immunochemical evidence in the CMF for the VDR.† Additional studies conducted in VDR WT and KO mice demonstrated that in KO animals, the CMF binding of the intestine, kidney, and lung for [3H]1,25D was greatly reduced (80%, 72%, and 85%, respectively) as compared to the corresponding WT (29). The residual binding activity in the VDR KO osteoblasts could be responsible for the detectable, although minor, stimulation of exocytosis shown in Fig. 4. Collectively, these results taken with the results of this communication suggest that in the VDR WT mouse there is a 1,25D-specific CMF receptor closely related or identical to the VDR that is not present in significant levels in VDR KO mice to regulate the membrane-initiated functions and provide a basis for the current understanding of mechanisms of rapid 1,25D actions in osteoblasts.
From a physiological perspective, our results show that the role of 1,25D membrane-related effects in osteoblasts is to regulate cell secretion within minutes. This article reports that physiological concentrations of 1,25D promote a significant increase of osteoblast whole-cell capacitance in VDR WT but not in KO cells, thus revealing that the steroid hormone rapidly facilitates the fusion to the plasma membrane of already docked secretory granules and the release of their contents into the external medium only in the presence of a functional classic VDR. Additionally, the VDR may be implicated in the synthesis and packaging of the granules content and the reduced number of granules in KO osteoblasts is a consequence of an impaired 1,25D genomic pathway.
This capacitance increase occurred typically within the first 2 min after exposure to 1,25D and was simultaneous with the potentiation of Cl- conductances promoted by the hormone. The addition of the calcium channel agonist Bay K8644 did not promote any significant capacitance increase, revealing that Ca2+ taken up through calcium channels may not be sufficient for the fusion of secretory vesicles to the osteoblast membrane and suggesting a contribution of Ca2+ from intracellular stores. Capacitance increases promoted by 1,25D developed transiently for ≈1–3 min, a value typical for the presence of a readily releasable pool of secretory granules docked to the plasma membrane as described in other cell types (44), which fuse under hormonal stimulus and are later retrieved into the cytoplasm.
From the present study, we conclude that a functional VDR is required for rapid 1,25D-stimulated electrical activities that include Cl- and Ca2+ channel activities and the fusion and discharge of secretory granules in osteoblasts.
Acknowledgments
We thank Dr. Shigeaki Kato for his generous gift of the first VDR heterozygous mouse couples to initiate our mouse colony; Dr. David Carter for his guidance in the use of the confocal microscopy facilities; Dr. Michael E. Adams for access to the pipette puller; Dr. James C. Fleet for helpful advice on the mouse genotyping; June E. Bishop for her technical contributions to the project; and Dr. Helen Henry for careful reading of the manuscript. This work was supported by U.S. Public Health Service Grant DK-09012-038 (to A.W.N.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: 1,25D, 1α,25(OH)2-vitamin D3; KO, knockout; VDR, vitamin D receptor.
Footnotes
Huhtakangas, J. A., Norman, A. W., Bishop, J. E. & Olivera, C. J. (2004) J. Steroid Biochem. Mol. Biol. (abstr.), in press.
References
- 1.Caffrey, J. M. & Farach-Carson, M. C. (1989) J. Biol. Chem. 264, 20265-20274. [PubMed] [Google Scholar]
- 2.Zanello, L. P. & Norman, A. W. (1997) J. Biol. Chem. 272, 22617-22622. [DOI] [PubMed] [Google Scholar]
- 3.Song, X., Bishop, J. E., Okamura, W. H. & Norman, A. W. (1998) Endocrinology 139, 457-465. [DOI] [PubMed] [Google Scholar]
- 4.Bouillon, R., Okamura, W. H. & Norman, A. W. (1995) Endocr. Rev. 16, 200-257. [DOI] [PubMed] [Google Scholar]
- 5.Kitanaka, S., Takeyama, K., Murayama, A., Sato, T., Okumura, K., Nogami, M., Hasegawa, Y., Niimi, H., Yanagisawa, J., Tanaka, T., et al. (1998) N. Engl. J. Med. 338, 653-661. [DOI] [PubMed] [Google Scholar]
- 6.Nemere, I., Dormanen, M. C., Hammond, M. W., Okamura, W. H. & Norman, A. W. (1994) J. Biol. Chem. 269, 23750-23756. [PubMed] [Google Scholar]
- 7.Boyan, B. D., Bonewald, L. F., Sylvia, V. L., Nemere, I., Larsson, D., Norman, A. W., Rosser, J., Dean, D. D. & Schwartz, Z. (2002) Steroids 67, 235-246. [DOI] [PubMed] [Google Scholar]
- 8.Nemere, I., Schwartz, Z., Pedrozo, H. A., Sylvia, V. L., Dean, D. D. & Boyan, B. D. (1998) J. Bone Miner. Res. 13, 1353-1359. [DOI] [PubMed] [Google Scholar]
- 9.Capiati, D., Benassati, S. & Boland, R. L. (2002) J. Cell. Biochem. 86, 128-135. [DOI] [PubMed] [Google Scholar]
- 10.Yoshizawa, T., Handa, Y., Uematsu, Y., Takeda, S., Sekine, K., Yoshihara, Y., Kawakami, T., Arioka, K., Sato, H., Uchiyama, Y., et al. (1997) Nat. Genet. 16, 391-396. [DOI] [PubMed] [Google Scholar]
- 11.Li, Y. C., Pirro, A. E., Amling, M., Delling, G., Baroni, R., Bronson, R. & Demay, M. B. (1997) Proc. Natl. Acad. Sci. USA 94, 9831-9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erben, R. G., Soegiarto, D. W., Weber, K., Zeitz, U., Lieberherr, M., Gniadecki, R., Moller, G., Adamski, J. & Balling, R. (2002) Mol. Endocrinol. 16, 1524-1537. [DOI] [PubMed] [Google Scholar]
- 13.Van Cromphaut, S. J., Dewerchin, M., Hoenderop, J. G., Stockmans, I., Van Herck, E., Kato, S., Bindels, R. J., Collen, D., Carmeliet, P., Bouillon, R., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 13324-13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amling, M., Priemel, M., Holzmann, T., Chapin, K., Rueger, J. M., Baron, R. & Demay, M. B. (1999) Endocrinology 140, 4982-4987. [DOI] [PubMed] [Google Scholar]
- 15.Norman, A. W., Mizwicki, M. T. & Norman, D. P. G. (2004) Nat. Rev. Drug Discov. 3, 27-41. [DOI] [PubMed] [Google Scholar]
- 16.Losel, R. M., Falkenstein, E., Feuring, M., Schultz, A., Tillmann, H. C., Rossol-Haseroth, K. & Wehling, M. (2003) Physiol. Rev. 83, 965-1016. [DOI] [PubMed] [Google Scholar]
- 17.Norman, A. W., Bouillon, R., Farach-Carson, M. C., Bishop, J. E., Zhou, L.-X., Nemere, I., Zhao, J., Muralidharan, K. R. & Okamura, W. H. (1993) J. Biol. Chem. 268, 20022-20030. [PubMed] [Google Scholar]
- 18.Zeitz, U., Weber, K., Soegiarto, D. W., Wolf, E., Balling, R. & Erben, R. G. (2003) FASEB J. 17, 509-511. [DOI] [PubMed] [Google Scholar]
- 19.Kajikawa, M., Ishida, H., Fujimoto, S., Mukai, E., Nishimura, M., Fujita, J., Tsuura, Y., Okamoto, Y., Norman, A. W. & Seino, Y. (1999) Endocrinology 140, 4706-4712. [DOI] [PubMed] [Google Scholar]
- 20.Staal, A., Van Wijnen, A. J., Desai, R. K., Pols, H. A. P., Birkenhäger, J. C., DeLuca, H. F., Denhardt, D. T., Stein, J. L., van Leeuwen, J. P. T. M., Stein, G. S., et al. (1996) Endocrinology 137, 2001-2011. [DOI] [PubMed] [Google Scholar]
- 21.Chen, J. J., Jin, H., Ranly, D. M., Sodek, J. & Boyan, B. D. (1999) J. Bone Miner. Res. 14, 221-229. [DOI] [PubMed] [Google Scholar]
- 22.Zanello, L. P. & Norman, A. W. (2003) Bone 33, 71-79. [DOI] [PubMed] [Google Scholar]
- 23.Redhead, C. R. & Baker, P. F. (1988) Calcif. Tissue Int. 42, 237-242. [DOI] [PubMed] [Google Scholar]
- 24.Kallay, E., Pietschmann, P., Toyokuni, S., Bajna, E., Hahn, P., Mazzucco, K., Bieglmayer, C., Kato, S. & Cross, H. S. (2001) Carcinogenesis 22, 1429-1435. [DOI] [PubMed] [Google Scholar]
- 25.Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. (1981) Pflügers Arch. 381, 85-100. [DOI] [PubMed] [Google Scholar]
- 26.Klyachko, V. A. & Jackson, M. B. (2002) Nature 418, 89-92. [DOI] [PubMed] [Google Scholar]
- 27.Moser, T. & Neher, E. (1997) Proc. Natl. Acad. Sci. USA 94, 6735-6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belan, P. V., Gerasimenko, O. V., Tepikin, A. V. & Petersen, O. H. (1996) J. Biol. Chem. 271, 7615-7619. [DOI] [PubMed] [Google Scholar]
- 29.Zanello, L. P., Huhtakangas, J. A., Olivera, C. J. & Norman, A. W. (2003) J. Bone Miner. Res. 18, Suppl. 20, S393. [Google Scholar]
- 30.Becherer, U., Moser, T., Stuhmer, W. & Oheim, M. (2003) Nat. Neurosci. 6, 846-853. [DOI] [PubMed] [Google Scholar]
- 31.Hille, B. (2001) in Ion Channels of Excitable Membranes (Sinauer, Sunderland, MA), pp. 1-22.
- 32.Coleman, K. M. & Smith, C. L. (2001) Front. Biosci. 6, D1379-D1391. [DOI] [PubMed] [Google Scholar]
- 33.Mangelsdorf, D. J., Thummel, C., Beato, M., Herrlich, P., Schütz, G., Umesono, K., Blumberg, B., Kastner, P., Mark, M., Chambon, P., et al. (1995) Cell 83, 835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weatherman, R. V., Fletterick, R. J. & Scanlon, T. S. (1999) Annu. Rev. Biochem. 68, 559-582. [DOI] [PubMed] [Google Scholar]
- 35.DeFranco, D. B. (2002) Mol. Endocrinol. 16, 1449-1455. [DOI] [PubMed] [Google Scholar]
- 36.Lee, K. C. & Lee, K. W. (2001) Trends Endocrinol. Metab. 12, 191-197. [DOI] [PubMed] [Google Scholar]
- 37.Razandi, M., Alton, G., Pedram, A., Ghonshani, S., Webb, P. & Levin, E. R. (2003) Mol. Cell. Biol. 23, 1633-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chambliss, K. L., Yuhanna, I. S., Anderson, R. G., Mendelsohn, M. E. & Shaul, P. W. (2002) Mol. Endocrinol. 16, 938-946. [DOI] [PubMed] [Google Scholar]
- 39.Cato, A. C., Nestl, A. & Mink, S. (2002) Science STKE 138, RE9. [DOI] [PubMed] [Google Scholar]
- 40.Ferrier, J., Ward-Kesthely, A., Homble, F. & Ross, S. (1987) J. Cell. Physiol. 130, 344-351. [DOI] [PubMed] [Google Scholar]
- 41.Chesnoy-Marchais, D. & Fritsch, J. (1993) Biochim. Biophys. Acta 1148, 239-248. [DOI] [PubMed] [Google Scholar]
- 42.Kousteni, S., Bellido, T., Plotkin, L. I., O'Brien, C. A., Bodenner, D. L., Han, L., Han, K., DiGregorio, G. B., Katzenellenbogen, J. A., Katzenellenbogen, B. S., et al. (2001) Cell 104, 719-730. [PubMed] [Google Scholar]
- 43.Norman, A. W., Olivera, C. J., Barreto Silva, F. R. & Bishop, J. E. (2002) Biochem. Biophys. Res. Commun. 298, 414-419. [DOI] [PubMed] [Google Scholar]
- 44.Olofsson, C. S., Gopel, S. O., Barg, S., Galvanovskis, J., Ma, X., Salehi, A., Rorsman, P. & Eliasson, L. (2002) Pflügers Arch. 444, 43-51. [DOI] [PubMed] [Google Scholar]