Introduction
Enteric and diarrheal diseases are a major worldwide cause of death among children under the age of 5. In this age group, diarrhea occurs 2.5 billion times per year [1] and causes 15% of childhood deaths.[2] Diarrheal diseases claim 59 million disability-adjusted life years (DALYs), nearly all from children in low- and middle-income countries.[3] Despite this enormous burden, these numbers fail to capture the full impact of enteric and diarrheal diseases. Early and frequent exposure to intestinal pathogens begins a cycle (Figure 1A) that affects digestion, nutrient absorption, growth, and immunity.[4] Repeated infections, with either overt diarrhea or subclinical enteropathy, produce acute and chronic undernutrition,[5] which leads to more frequent and severe infections.[6] Undernutrition contributes to 53% of childhood deaths [7] and is the leading risk factor for poor health outcomes in childhood;[8] survivors are at risk for developmental deficits in growth, fitness, and cognition that persist into adulthood with devastating consequences.[4] These consequences have a multiplicative effect on calculations of DALYs from diarrheal disease.[9]
Figure 1. The vicious cycle of diarrhea and undernutrition in susceptible children.
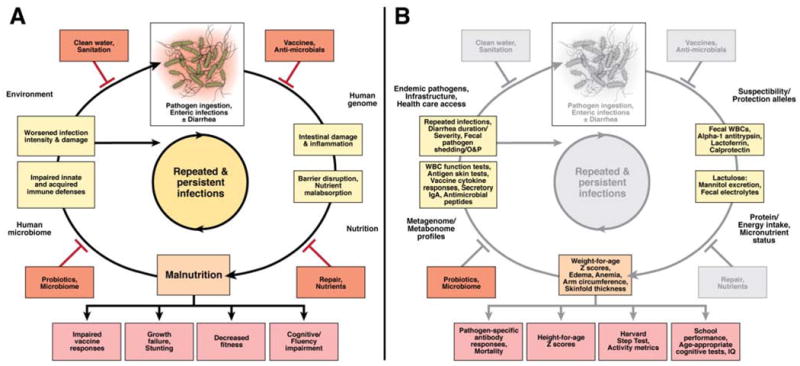
(A) The devastating synergy between enteric infections and undernutrition is influenced by the environment, the human genome, host nutrition, and the human microbiome. Various interventions (red boxes) may inhibit progression to the next step in the cycle, minimizing both acute and chronic morbidities. (B) Employing a spectrum of disease outcome measures would lend greater insight into the pathology underlying enteric and diarrheal diseases, while providing a more complete understanding of interventions targeting basic steps of enteric and diarrheal disease pathogenesis. Adapted with permission from Wiley: Nutrition Reviews,4 copyright 2008. http://www.http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1753-4887.
Fortunately, there are strategies to break this cycle, although each approach has limitations. Sustainable access to potable water and improved sanitation reduces pathogen exposure; a $70 billion annual investment would only begin to reduce the number of people without these necessities (2.5 billion) by 2015.[10] Antimicrobial agents are effective against specific pathogens but are expensive and can exacerbate toxin-mediated diseases, disrupt the human microbiome, and induce antibiotic-associated diarrhea as well as drug resistance. Immunization with enteric vaccines can reduce the burden of severe diarrhea, but vaccines must be kept in the cold, only protect against specific pathogens, and are less effective in regions of high mortality. For example, the efficacy of the live, attenuated rotavirus vaccine against severe disease is only 48.3% in southeast Asia [11] and 39.3% in sub-Saharan Africa.[12] Zinc reduces the propensity to develop recurrent diarrhea and oral rehydration solution (ORS) attenuates overt symptoms of diarrhea and dehydration.[13] However, these approaches do not adequately address broader growth and developmental processes that could yield long-term benefits. Likewise, trials of therapeutics to reduce diarrhea severity, unplanned intravenous fluid administration, or duration of hospitalization fail to address longer-term, initially subclinical, consequences of recurrent infections. Complementary outcome measures, including measures of growth and biomarkers of acute intestinal inflammation, barrier disruption, and impaired immunity, would provide greater insight into underlying pathology and therapeutic efficacy. Use of these measures could reduce acute, overt, as well as chronic, often unrecognized, intestinal diseases (Figure 1B).
No single intervention is sufficient to eliminate the global burden of enteric and diarrheal diseases. Vaccines, for example, can protect against limited infectious agents but immunization can be overwhelmed by heavily contaminated water. Multiple interventions could work synergistically, such as the combination of improved water and sanitation, vaccines, micro- and macronutrient provision, and selectively-targeted antimicrobial therapy (e.g. single-dose albendazole for intestinal helminths). Do current global health strategies use the best available interventions?
One underexplored approach, probiotics, could combine favorable safety profiles with improved nutrition and microbiome function. Probiotics are live microorganisms that confer a health benefit on the host,[14] and have been used to treat multiple gastrointestinal (GI) diseases.[15] Microbes are inexpensive to grow, and have the potential for rapid global scale-up. Is there compelling evidence to recommend developing probiotics-based strategies to complement current approaches against enteric and diarrheal diseases for children in developing countries? If so, what steps must be taken before these therapies are ready for clinical impact in the global health arena?
Clinical Evidence
In 1907, Russian Nobel laureate Elie Metchnikoff suggested that ingestion of microbes could benefit human health.[16] As we learned about multidrug-resistant pathogens and the role of the human microbiome in health and disease, numerous trials showed the safety of probiotics[17] and their beneficial outcomes in patients with various illnesses.[18] A systematic review that included 12 randomized, controlled trials (RCTs) in the Cochrane database (the majority from affluent countries) concluded that probiotics reduced the mean duration of acute diarrhea in children by 29.2 hours in a fixed-effects model and by 30.48 hours in a random-effects model.[19] Two meta-analyses that evaluated similar studies found statistically significant but modest reductions of diarrhea duration.[20,21] Although combination analyses of trials with microbes of different genera, species, strains, and doses provide limited information about specific therapeutic interventions, it is clear that many probiotics reduce the duration of acute diarrhea.
There are studies of probiotics for enteric and diarrheal diseases targeting children in developing regions (Table S1). The majority of RCTs studied acute gastroenteritis and reported modest reductions in diarrhea duration. However, the effects of probiotics were statistically equivalent to those of placebo in 25% of trials. Some of the negative results might be attributable to small sample size or administration of insufficient doses;[22-24] each trial must be viewed in the context of the specific disease and probiotic strain analyzed. Two RCTs evaluated probiotics for children with persistent diarrhea and reported dramatic reductions in diarrhea duration—4.8 and 3.9 days in Argentina[25] and India,[26] respectively. Two trials evaluated probiotics for diarrhea prevention; children in Peru had 13% fewer diarrheal episodes after 15 months of Lactobacillus rhamnosus,[27] whereas diarrhea frequency was reduced by 14% among children in India who received daily doses of Lactobacillus casei for 12 weeks, with a 12-week follow-up period.[28]
Few studies have examined markers of acute or chronic immunity or underlying intestinal function. Administration of Bifidobacterium bifidum and Streptococcus thermophilus increased numbers of CD4+ T cells in HIV-infected Brazilian children.[29] A similar effect was observed following administration of L. rhamnosus to HIV-infected adults in Tanzania.[30] Tropical enteropathy was studied in Malawian children using urinary carbohydrate excretion; L. rhamnosus failed to improve ratios of lactulose:mannitol excreted,[31] despite evidence that probiotics ameliorated GI permeability defects in children with atopic dermatitis in Germany.[32] Probiotics improved growth among healthy children in Thailand[33] and Estonia,[34] but not among HIV-exposed infants in South Africa.[35] In Malawi, probiotics failed to improve nutrition status in severely malnourished children who received inpatient nutritional rehabilitation. Despite the negative primary outcome, there was a trend toward decreased mortality among children treated with probiotics on an outpatient basis.[36] Probiotics also reduced the duration of rotavirus shedding in India.[37] Strategies to reduce fecal shedding of pathogens are important for the billions of people who live without adequate sanitation.
Probiotics could also have a role in immunization programs. L. rhamnosus increased the virus-specific antibody response in children with acute rotaviral gatroenteritis,[38] so immunostimulatory probiotics might help children's immune systems increase the memory responses to vaccines. Based on initial data from studies in industrialized regions, Bifidobacterium longum and L. rhamnosus, administered during the first 6 months of life, increased vaccine-specific antibody production following vaccination against hepatitis B.[39] Infants that were given Lactobacillus paracasei from 4 to 13 months of age had increased titers of antibodies to Haemophilus influenzae type B (Hib) capsular polysaccharide, diphtheria toxin, and tetanus toxoid. [40] Concentrations of antibodies against Hib increased among infants when women were given daily doses of probiotics during the final month of pregnancy; therapy continued for infants during their first 6 months of life.[41] Taking probiotics during pregnancy and lactation appears to be safe[42] and might yield post-natal benefits. Intriguingly, maternal consumption of L. rhamnosus or B. lactis increased the amount of immunoglobulin (Ig)A detected in breast milk.[43] Increased IgA levels in breast milk might protect infants from enteric pathogens and serve as a biomarker for studies of probiosis in lactating women. It is a challenge to assimilate and analyze all the clinical evidence of the effects of probiotics. Study quality varies, randomization and blinding methods are rarely reported, and appropriate placebos are not always used. Exclusion criteria are numerous, limiting the generalization of findings to children that are very ill. Probiotic strain designations, bacterial growth phase, and variations in administration (in fermented dairy products, infant formula, solid food, ORS, water, juice, capsules) are often unreported. It is important that studies report these parameters, so findings can be reproduced—proteins and metabolites synthesized by live microorganisms are strain-specific and vary with growth conditions. Many probiotics have shown beneficial effects, but improving our knowledge of the mechanisms that mediate these effects would facilitate identification of more potent probiotics for specific applications.
Probiotic Mechanisms
There are 3 general classes of probiotic anti-pathogenic mechanisms: direct antagonism, immunomodulation, and exclusion (Figure 2).
Figure 2. Three general mechanisms of probiosis for enteric infections.
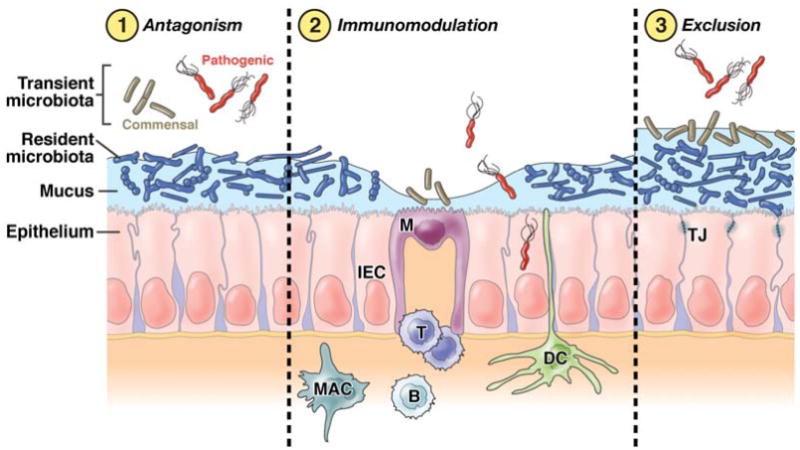
In direct antagonism, probiotics kill or inhibit the pathogen to limit infection, or they down-regulate the expression of virulence factors, such as adhesins or toxins, required for pathogenesis. Probiotics can also interact with the immune system (immunomodulation) to enhance the functionality of innate and/or adaptive immunity, or to limit the ability of the pathogen to initiate or facilitate an immune response. Through “exclusion,” probiotics can alter the microenvironment to prevent pathogens from gaining access to appropriate receptors, to limit pathogen attachment, entry, or translocation, or to improve barrier function. A beneficial microbe may use a combination of these mechanisms, and may employ different mechanisms against different pathogens. B, B cell; DC, dendritic cell; IEC, intestinal epithelial cell; M, M cell; MAC, macrophage; T, T cell; TJ, tight junction.
Direct antagonism
Many probiotics secrete small molecules or bioactive peptides that have anti-microbial activities. Lactobacillus salivarius UCC118 protects mice against infection with Listeria monocytogenes; UCC118 produces a broad-spectrum bacteriocin (antimicrobial peptide) that kills Listeria in the lumen of the GI tract, preventing translocation and systemic spread of infection.[44] Mice were not protected when they were fed a derivative of UCC118 that no longer produced the bacteriocin or when they were infected with an engineered strain of Listeria that was resistant to the bacteriocin. Listeria pathogens were eliminated within 30 minutes following oral administration of UCC118, indicating a direct effect of the probiotic on the pathogen. UCC118 also protects mice against Salmonella infection, but the bacteriocin is not involved. Thus, a single probiotic can protect mammals against different pathogens via multiple mechanisms.
Several in vitro studies have reported down-regulation of virulence factors in pathogens exposed to probiotics or their cell-free supernatants.[45-47] Lactobacillus acidophilus LA-5 suppresses transcription of Escherichia coli O157:H7 genes involved in adherence; this corresponds with reduced colonization in mice.[48] Probiotics can also interfere with toxin production or directly antagonize enterotoxins. Saccharomyces cerevisiae var. boulardii (S. boulardii), which reduces Clostridium difficile -associated diarrhea,[49] secretes a 54 kDa serine protease that hydrolyzes toxin A (a C. difficile virulence factor) and its receptor, which is present in the intestinal brush border.[50,51]
Immunomodulation
Probiotics elicit a variety of responses from immune cells in vitro and in vivo, through mostly unknown mechanisms.[52] The responses of specific immune cells to particular microbes result from complex interactions between surface-bound and secreted ligands (e.g. pathogen-associated molecular patterns) and host Toll-like receptors (TLRs). [53] Reductionist approaches to studying these interactions, such as analyses of knockout or transgenic mice, have provided limited information about probiotic immunomodulation. In one successful example, the presence of D-alanines on teichoic acids in the cell wall of Lactobacillus plantarum elicited production of pro-inflammatory cytokines by peripheral blood mononuclear cells.[54] Co-culture of dendritic cells with polysaccharide A (PSA) derived from Bacteroides fragilis induced naïve T cells to generate an IL-10-producing regulatory T cell population.[55] Purified PSA prevents intestinal inflammation in multiple mouse models.[56]
The immunomodulatory effects of probiotics can be species-[57] and strain-specific [58,59] and involve multiple mammalian signaling pathways that affect immune cell phenotypes. Some probiotic Lactobacillus strains increase production of tumor necrosis factor (TNF) through activation of the transcription factors NF-κB and STAT, [60] whereas others suppress TNF production by inactivating NF-κB [61,62] or mitogen-activated protein kinase and c-Jun signaling.[58] Differential immune regulation might prime the immune system to limit infections, inflammation, and pathogen-mediated damage. Probiotic signaling molecules that regulate immunity have not been identified.
Exclusion
Exclusion is used as a “catch-all” term for probiotic mechanisms that make the GI environment less hospitable for pathogens. These mechanisms include altering the resident microbiota, decreasing luminal pH, improving epithelial barrier function, interfering with pathogen binding by down-regulating specific host receptors, and stimulating production of defense-associated factors, including mucins and defensins. Multiple probiotics have been implicated in each of these functions, but clear links between individual bacterial compounds and specific responses have been difficult to establish.[63]
Rats that consumed the probiotic mixture VSL#3 increased their luminal mucin content by 60% through an unidentified, heat-resistant secreted soluble compound.[64] Some bacterial products, including short-chain fatty acids produced by fermentation, can stimulate epithelial cell differentiation and improve barrier function[65,66]—this protects against pathogens that cause disease through loss of tight junction integrity, increased paracellular transport, fluid loss, and invasion of the submucosa.[67,68] Indole, an aromatic compound secreted by commensal E. coli and detected in human feces, increases expression of genes whose products regulate production of mucins and organization of the cytoskeleton, tight junctions, and adherens junctions. Indole increases transepithelial resistance in enterocyte cultures.[69] The quorum-sensing molecule CSF, a 3 kDa heat-stabile, pepsin-sensitive pentapeptide from the probiotic Bacillus subtilis, activates the heat shock protein (Hsp27) after CSF is internalized by the enterocyte oligopeptide transporter OCTN2; Hsp27 activation protects epithelial cells from oxidant-induced stress.[70] The in vivo roles of these molecules in preventing infections have not been established.
Probiotics can also stimulate defensins, cationic antimicrobial peptides produced by cells of the intestinal epithelium.[71] The probiotic E. coli Nissle 1917 increases synthesis of human β-defensin 2 by activating NF-κB and AP-1[72] via secretion of flagellin.[73] Increased β-defensin 2 levels were detected in stool samples from healthy volunteers 9 weeks after administration of nonpathogenic E. coli.[74] Resistance to host-derived antimicrobials may be another important probiotic property. [75] Finally, some probiotics promote class switching to increase IgA production, by inducing enterocytes to secrete B-cell stimulatory factors such as APRIL. Lipopolysaccharide- and flagellin-stimulated secretion of APRIL occurs in human enterocytes via TLR4 and TLR5 signaling.[76]
In addition to anti-microbial mechanisms, probiotics benefit host physiology, nutrition, and the ability to counteract pathogenesis. In mouse models, weight gain and adiposity are influenced by the intestinal microbiome. [77-79] Metabolism could be regulated by individual microbes; for example, S.boulardii increases activities of brush border enzymes including sucrase, maltase, trehalase, lactase, aminopeptidase, and alkaline phosphatase.[80] Intestinal bacteria also synthesize niacin, pantothenic acid, biotin, folic acid, and vitamins K, C, and B12.[81] These functions of probiotics have not been correlated with pathogen resistance.
Probiotics may also interact with the enteric nervous system to attenuate secretory diarrhea.[82] In mice, Lactobacillus inhibited post-infective intestinal hypercontractility through an unidentified, heat-labile fermentation product.[83] In rats, lactobacilli reduced hypercontractility by blocking calcium-dependent potassium channels.[84,85] Lactobacilli can also blunt visceral pain responses by increasing expression of enterocyte opioid and cannabinoid receptors[86] or by inhibiting sodium channels.[87] Further studies are needed to identify microbe molecular signatures associated with specific responses against pathogens.
Using Probiotics Worldwide
Step 1: Identify Molecular Mechanisms of Probiosis and New Therapeutics
The first step to realizing the full potential of probiotics is to define the specific microbial genes, small molecules, and host–microbe interactions that mediate their beneficial functions. Basic scientists must identify, isolate, and characterize bacterial fermentation products, immunomodulating factors, antimicrobial agents, and cell-wall components that produce discrete physiological effects through specific host interactions. These types of studies will improve our understanding of probiotic function and allow microbial libraries to be screened to identify new probiotics.
The Human Microbiome Project (HMP), MetaHit, and the International Human Microbiome Consortium (IHMC) published a partial catalog of microbial reference genomes to help identify new probiotic species; [88] studies to associate changes in microbial populations[89] or microbial gene content [90] with states of health and disease are underway. For example, the anti-inflammatory effects of Faecalibacterium prausnitzii were identified after reductions in this bacterium were associated with recurrence of ileal Crohn disease.[91] However, HMP and IHMC studies have been limited to subjects in affluent, developed countries. Diarrhea disrupts the microbiota,[92] and environmental and lifestyle variations yield microbiomes that are specific to geographic regions, cultures, or ethnic groups.[93,94] The composition and function of GI microbiomes of undernourished children in disease-endemic regions must be studied separately from the microbiomes of people in developed regions.
Step 2: Develop New Biomarkers for Acute and Chronic Intestinal Disease
To more accurately assess intestinal pathology and therapeutic efficacy, new host and microbial biomarkers must be validated. Fecal samples are easily obtained, but their microbial composition primarily reflects that of the large bowel, a self-regulating community that can resist introduction of probiotics by virtue of niche exclusion.[95] Lactobacilli, administered daily, comprise only 0.001% of the fecal microbiota and quickly disappear once they are no longer ingested—they have only a minor presence in the large bowel biome.[96,97] Many enteric pathogens infect the small bowel, overgrowth of which is a significant feature of tropical enteropathy.[98] Thus, probiotics are likely to mediate their greatest effects against enteric and diarrheal diseases in the small bowel, where they comprise a substantial proportion of the biomass and functionally alter the proximal GI tract.[99] Ideal biomarkers would reflect the health status of these sites.
Metabolomics is a systematic, quantitative analysis of changes in the complete set of low molecular weight metabolites produced by cells in response to environmental or cellular changes.[100] Bacterial products are absorbed from the bowel lumen into lymph and blood circulations; body fluids therefore contain many bacterial and host metabolites that could serve as biomarkers of relationships between food, bacteria and host cells and indicate health or disease.[101] Infection of mice with the nematode Schistosoma mansoni can be diagnosed based on alterations to the urinary metabolome, which reflect disruption of the bowel ecosystem by the intestinal parasite.[102] Urine, saliva or bowel fluid, which are abundant and easily collected, could be sources of biomarkers for small bowel function.
Analysis of metabolomic profiles lags behind advances in detection methods, and new pattern recognition systems must be developed. Comprehensive reference sets of identified metabolites must be assembled to improve yield from metabolomic comparisons.[103] Linking metabolomics to probiotics research could lead to new ways to identify biomarkers and have practical applications in developing countries. Initial studies should compare metabolomes between healthy children and those with defined enteric infections, controlling for ethnicity, age, sex, socioeconomic status, and nutritional state. Biomarkers are likely to be identified that are associated with overt and subclinical intestinal disease.
Step 3: Optimize Therapeutic Regimens for Specific Populations
High-impact interventions must be developed for target populations—namely, children under 2 years of age in disease-endemic areas. Core and variable components of their intestinal microbiomes should be catalogued. Children should be characterized with respect to overall health status, disease susceptibility, nutritional (macro and micro) status, and common enteric pathogens. Dietary evaluations must consider microbiomes and glycomes of breast milk and seek to identify natural substrates for probiotics that optimize their metabolic activities. This detailed picture of the microbiome and its interactions will guide selection of specific probiotic-based therapies for the pathogens relevant to each population.
Prospective RCTs should aim to reduce short-term pathologies associated with acute diarrhea, prevent long-term morbidities from recurrent or persistent infections, and increase vaccine efficacy. Specific strain and dose recommendations should be made, with the long-term goals of improving survival, growth, and development during childhood. With trials in multiple geographic locations and ethnic groups, patterns will emerge to guide selection of specific microbial-based therapies for specific regions of the world.
This plan has risks—although probiotics are assumed to be safe, undernourished children with immune and GI permeability defects could be more prone to bacterial translocation and sepsis. Safety monitoring will be critical. Immunostimulatory probiotics might not affect children whose immune systems have been highly stimulated by contaminated environments; in this case, other mechanistic bases of probiosis must be pursued. Finally, global application of probiotic therapies requires development of technologies to make freeze-drying, or other preparative methods of preserving probiotic viability, feasible under challenging conditions. Strategies must also be developed to deliver probiotics through local distribution networks, and the products must be acceptable to diverse cultures.
Will Probiotics Be Ready for Worldwide Use in the Near Future?
Beyond the acute effects of severe diarrhea and dehydration, repeated and persistent infections yield devastating long-term consequences. For children in less-developed settings, many probiotics are effective for acute gastroenteritis, persistent diarrhea, and diarrhea prevention; their potential roles in growth, immunity, and vaccine efficacy must be further evaluated. Mechanisms that mediate their beneficial effects are being elucidated. Characterization of specific molecular interactions between probiotics and the host or microbiome will enable selection of more potent therapeutic microbes. Biomarkers of intestinal pathology must be developed to determine therapeutic efficacy of existing and new probiotics. Ultimately, clinical studies that test specific therapies in well-defined populations must be performed to determine the overt and hidden consequences of enteric infections.
Basic scientists, clinical researchers, and industrial leaders have the opportunity to work together against one of the most pressing health problems facing the world today. If we accept the challenge, probiotic-based therapies might be incorporated into global health strategies, to reduce the burden of enteric and diarrheal diseases borne by millions of children worldwide.
Supplementary Material
Acknowledgments
The authors wish to thank the Bill & Melinda Gates Foundation, especially Thomas Brewer and Gretchen Meller, for sponsoring the meeting, “Evaluating probiotics for the prevention and treatment of enteric and diarrheal diseases for children in developing countries, ” on January 24-26, 2010 in London. G.P. is supported by the National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) F30DK081269. Work in the C.H. laboratory is supported by Science Foundation Ireland. Work in the R.L.G. laboratory is supported by the National Institute of Allergy and Infectious Diseases (ICIDR U01AI26512) and the Foundation for the National Institutes of Health/Bill & Melinda Gates Foundation MAL-ED. Work in the J. V. laboratory is supported by the NIDDK (R01 DK065075 and P30 DK065075) and National Center for Complementary and Alternative Medicine (R01 DK065075).
NIH Grant Numbers: UH3DK083990; R01 ATO04326; R01DK065075
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The United Nations Children's Fund (UNICEF)/World Health Organization(WHO) Diarrhoea: Why children are still dying and what can be done. New York: UNICEF; 2009. [Google Scholar]
- 2.Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 3.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 4.Guerrant RL, Oria RB, Moore SR, Oria MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66:487–505. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Checkley W, Buckley G, Gilman RH, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 2008;37:816–830. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schorling JB, McAuliffe JF, de Souza MA, Guerrant RL. Malnutrition is associated with increased diarrhoea incidence and duration among children in an urban Brazilian slum. Int J Epidemiol. 1990;19:728–735. doi: 10.1093/ije/19.3.728. [DOI] [PubMed] [Google Scholar]
- 7.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 8.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 9.Guerrant RL, Kosek M, Lima AA, Lorntz B, Guyatt HL. Updating the DALYs for diarrhoeal disease. Trends Parasitol. 2002;18:191–193. doi: 10.1016/s1471-4922(02)02253-5. [DOI] [PubMed] [Google Scholar]
- 10.Hutton G, Bartram J. Global costs of attaining the Millennium Development Goal for water supply and sanitation. Bull World Health Organ. 2008;86:13–19. doi: 10.2471/BLT.07.046045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaman K, Anh DD, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010 doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 12.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010 doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 13.Walker CL, Black RE. Zinc for the treatment of diarrhoea: effect on diarrhoea morbidity, mortality and incidence of future episodes. Int J Epidemiol. 2010;39(1):i63–69. doi: 10.1093/ije/dyq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Food and Agriculture Organization of the United Nations (FAO)/World Health Organization(WHO) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; Basel, Switzerland. 2001.FAO/WHO; [Google Scholar]
- 15.Preidis GA, Versalovic J. Targeting the human microbiome with antibiotics, robiotics, and prebiotics: gastroenterology enters the metagenomics era. astroenterology. 2009;136:2015–2031. doi: 10.1053/j.gastro.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metchnikoff E. The prolongation of life: optimistic studies. New York: Springer Publishing Company, Inc.; 2004. [Google Scholar]
- 17.Snydman DR. The safety of probiotics. Clin Infect Dis. 2008;46(2):S104–111. doi: 10.1086/523331. discussion S144-151. [DOI] [PubMed] [Google Scholar]
- 18.Shanahan F. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.10.025. in press. [DOI] [PubMed] [Google Scholar]
- 19.Allen SJ, Okoko B, Martinez E, Gregorio G, Dans LF. Probiotics for treating infectious diarrhoea. Cochrane Database Syst Rev. 2004:CD003048. doi: 10.1002/14651858.CD003048.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Huang JS, Bousvaros A, Lee JW, Diaz A, Davidson EJ. Efficacy of probiotic use in acute diarrhea in children: a meta-analysis. Dig Dis Sci. 2002;47:2625–2634. doi: 10.1023/a:1020501202369. [DOI] [PubMed] [Google Scholar]
- 21.Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr. 2001;33(2):S17–25. doi: 10.1097/00005176-200110002-00004. [DOI] [PubMed] [Google Scholar]
- 22.Basu S, Chatterjee M, Ganguly S, Chandra PK. Efficacy of Lactobacillus rhamnosus GG in acute watery diarrhoea of Indian children: a randomised controlled trial. J Paediatr Child Health. 2007;43:837–842. doi: 10.1111/j.1440-1754.2007.01201.x. [DOI] [PubMed] [Google Scholar]
- 23.Basu S, Paul DK, Ganguly S, Chatterjee M, Chandra PK. Efficacy of high-dose Lactobacillus rhamnosus GG in controlling acute watery diarrhea in Indian children: a randomized controlled trial. J Clin Gastroenterol. 2009;43:208–213. doi: 10.1097/MCG.0b013e31815a5780. [DOI] [PubMed] [Google Scholar]
- 24.Fang SB, Lee HC, Hu JJ, et al. Dose-dependent effect of Lactobacillus rhamnosus on quantitative reduction of faecal rotavirus shedding in children. J Trop Pediatr. 2009;55:297–301. doi: 10.1093/tropej/fmp001. [DOI] [PubMed] [Google Scholar]
- 25.Gaon D, Garcia H, Winter L, et al. Effect of Lactobacillus strains and Saccharomyces boulardii on persistent diarrhea in children. Medicina (B Aires) 2003;63:293–298. [PubMed] [Google Scholar]
- 26.Basu S, Chatterjee M, Ganguly S, Chandra PK. Effect of Lactobacillus rhamnosus GG in persistent diarrhea in Indian children: a randomized controlled trial. J Clin Gastroenterol. 2007;41:756–760. doi: 10.1097/01.mcg.0000248009.47526.ea. [DOI] [PubMed] [Google Scholar]
- 27.Oberhelman RA, Gilman RH, Sheen P, et al. A placebo-controlled trial of Lactobacillus GG to prevent diarrhea in undernourished Peruvian children. J Pediatr. 1999;134:15–20. doi: 10.1016/s0022-3476(99)70366-5. [DOI] [PubMed] [Google Scholar]
- 28.Sur D, Manna B, Niyogi SK, et al. Role of probiotic in preventing acute diarrhoea in children: a community-based, randomized, double-blind placebo-controlled field trial in an urban slum. Epidemiol Infect. 2010:1–8. doi: 10.1017/S0950268810001780. [DOI] [PubMed] [Google Scholar]
- 29.Trois L, Cardoso EM, Miura E. Use of probiotics in HIV-infected children: a randomized double-blind controlled study. J Trop Pediatr. 2008;54:19–24. doi: 10.1093/tropej/fmm066. [DOI] [PubMed] [Google Scholar]
- 30.Irvine SL, Hummelen R, Hekmat S, et al. Probiotic Yogurt Consumption is Associated With an Increase of CD4 Count Among People Living With HIV/AIDS. J Clin Gastroenterol. doi: 10.1097/MCG.0b013e3181d8fba8. [DOI] [PubMed] [Google Scholar]
- 31.Galpin L, Manary MJ, Fleming K, et al. Effect of Lactobacillus GG on intestinal integrity in Malawian children at risk of tropical enteropathy. Am J Clin Nutr. 2005;82:1040–1045. doi: 10.1093/ajcn/82.5.1040. [DOI] [PubMed] [Google Scholar]
- 32.Rosenfeldt V, Benfeldt E, Valerius NH, Paerregaard A, Michaelsen KF. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J Pediatr. 2004;145:612–616. doi: 10.1016/j.jpeds.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 33.Nopchinda S, Varavithya W, Phuapradit P, et al. Effect of bifidobacterium Bb12 with or without Streptococcus thermophilus supplemented formula on nutritional status. J Med Assoc Thai. 2002;85(4):S1225–1231. [PubMed] [Google Scholar]
- 34.Vendt N, Grunberg H, Tuure T, et al. Growth during the first 6 months of life in infants using formula enriched with Lactobacillus rhamnosus GG: double-blind, randomized trial. J Hum Nutr Diet. 2006;19:51–58. doi: 10.1111/j.1365-277X.2006.00660.x. [DOI] [PubMed] [Google Scholar]
- 35.Velaphi SC, Cooper PA, Bolton KD, et al. Growth and metabolism of infants born to women infected with human immunodeficiency virus and fed acidified whey-adapted starter formulas. Nutrition. 2008;24:203–211. doi: 10.1016/j.nut.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Kerac M, Bunn J, Seal A, et al. Probiotics and prebiotics for severe acute malnutrition (PRONUT study): a double-blind efficacy randomised controlled trial in Malawi. Lancet. 2009;374:136–144. doi: 10.1016/S0140-6736(09)60884-9. [DOI] [PubMed] [Google Scholar]
- 37.Narayanappa D. Randomized double blinded controlled trial to evaluate the efficacy and safety of Bifilac in patients with acute viral diarrhea. Indian J Pediatr. 2008;75:709–713. doi: 10.1007/s12098-008-0134-2. [DOI] [PubMed] [Google Scholar]
- 38.Kaila M, Isolauri E, Soppi E, et al. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatr Res. 1992;32:141–144. doi: 10.1203/00006450-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Soh SE, Ong DQ, Gerez I, et al. Effect of probiotic supplementation in the first 6 months of life on specific antibody responses to infant Hepatitis B vaccination. Vaccine. 2010;28:2577–2579. doi: 10.1016/j.vaccine.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 40.West CE, Gothefors L, Granstrom M, et al. Effects of feeding probiotics during weaning on infections and antibody responses to diphtheria, tetanus and Hib vaccines. Pediatr Allergy Immunol. 2008;19:53–60. doi: 10.1111/j.1399-3038.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 41.Kukkonen K, Nieminen T, Poussa T, Savilahti E, Kuitunen M. Effect of probiotics on vaccine antibody responses in infancy--a randomized placebo-controlled double-blind trial. Pediatr Allergy Immunol. 2006;17:416–421. doi: 10.1111/j.1399-3038.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 42.Dugoua JJ, Machado M, Zhu X, et al. Probiotic safety in pregnancy: a systematic review and meta-analysis of randomized controlled trials of Lactobacillus, Bifidobacterium, and Saccharomyces spp. J Obstet Gynaecol Can. 2009;31:542–552. doi: 10.1016/S1701-2163(16)34218-9. [DOI] [PubMed] [Google Scholar]
- 43.Prescott SL, Wickens K, Westcott L, et al. Supplementation with Lactobacillus rhamnosus or Bifidobacterium lactis probiotics in pregnancy increases cord blood interferon-gamma and breast milk transforming growth factor-beta and immunoglobin A detection. Clin Exp Allergy. 2008;38:1606–1614. doi: 10.1111/j.1365-2222.2008.03061.x. [DOI] [PubMed] [Google Scholar]
- 44.Corr SC, Li Y, Riedel CU, et al. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci U S A. 2007;104:7617–7621. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medellin-Pena MJ, Wang H, Johnson R, Anand S, Griffiths MW. Probiotics affect virulence-related gene expression in Escherichia coli O157:H7. Appl Environ Microbiol. 2007;73:4259–4267. doi: 10.1128/AEM.00159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carey CM, Kostrzynska M, Ojha S, Thompson S. The effect of probiotics and organic acids on Shiga-toxin 2 gene expression in enterohemorrhagic Escherichia coli O157:H7. J Microbiol Methods. 2008;73:125–132. doi: 10.1016/j.mimet.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 47.de Sablet T, Chassard C, Bernalier-Donadille A, et al. Human microbiota-secreted factors inhibit shiga toxin synthesis by enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 2009;77:783–790. doi: 10.1128/IAI.01048-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Medellin-Pena MJ, Griffiths MW. Effect of molecules secreted by Lactobacillus acidophilus strain La-5 on Escherichia coli O157:H7 colonization. Appl Environ Microbiol. 2009;75:1165–1172. doi: 10.1128/AEM.01651-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. 16:2202–2222. doi: 10.3748/wjg.v16.i18.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pothoulakis C, Kelly CP, Joshi MA, et al. Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology. 1993;104:1108–1115. doi: 10.1016/0016-5085(93)90280-p. [DOI] [PubMed] [Google Scholar]
- 51.Castagliuolo I, LaMont JT, Nikulasson ST, Pothoulakis C. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect Immun. 1996;64:5225–5232. doi: 10.1128/iai.64.12.5225-5232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Forsythe P, Bienenstock J. Immunomodulation by commensal and probiotic bacteria. Immunol Invest. 2010;39:429–448. doi: 10.3109/08820131003667978. [DOI] [PubMed] [Google Scholar]
- 53.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 54.Grangette C, Nutten S, Palumbo E, et al. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci U S A. 2005;102:10321–10326. doi: 10.1073/pnas.0504084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 57.Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168:171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 58.Lin YP, Thibodeaux CH, Pena JA, Ferry GD, Versalovic J. Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm Bowel Dis. 2008;14:1068–1083. doi: 10.1002/ibd.20448. [DOI] [PubMed] [Google Scholar]
- 59.Thomas CM, Versalovic J. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes. 2010;1:1–16. doi: 10.4161/gmic.1.3.11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miettinen M, Lehtonen A, Julkunen I, Matikainen S. Lactobacilli and Streptococci activate NF-kappa B and STAT signaling pathways in human macrophages. J Immunol. 2000;164:3733–3740. doi: 10.4049/jimmunol.164.7.3733. [DOI] [PubMed] [Google Scholar]
- 61.Petrof EO, Kojima K, Ropeleski MJ, et al. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127:1474–1487. doi: 10.1053/j.gastro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 62.Iyer C, Kosters A, Sethi G, et al. Probiotic Lactobacillus reuteri promotes TNF-induced apoptosis in human myeloid leukemia-derived cells by modulation of NF-kappaB and MAPK signalling. Cell Microbiol. 2008;10:1442–1452. doi: 10.1111/j.1462-5822.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- 63.Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2010;298:G807–819. doi: 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- 64.Caballero-Franco C, Keller K, De Simone C, Chadee K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G315–322. doi: 10.1152/ajpgi.00265.2006. [DOI] [PubMed] [Google Scholar]
- 65.Cook SI, Sellin JH. Review article: short chain fatty acids in health and disease. Aliment Pharmacol Ther. 1998;12:499–507. doi: 10.1046/j.1365-2036.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- 66.Johnson-Henry KC, Donato KA, Shen-Tu G, Gordanpour M, Sherman PM. Lactobacillus rhamnosus strain GG prevents enterohemorrhagic Escherichia coli O157:H7-induced changes in epithelial barrier function. Infect Immun. 2008;76:1340–1348. doi: 10.1128/IAI.00778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hecht G. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. VII. Enteropathogenic Escherichia coli: physiological alterations from an extracellular position. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1–7. doi: 10.1152/ajpgi.2001.281.1.G1. [DOI] [PubMed] [Google Scholar]
- 68.Guttman JA, Li Y, Wickham ME, et al. Attaching and effacing pathogen-induced tight junction disruption in vivo. Cell Microbiol. 2006;8:634–645. doi: 10.1111/j.1462-5822.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- 69.Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A. 107:228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fujiya M, Musch MW, Nakagawa Y, et al. The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe. 2007;1:299–308. doi: 10.1016/j.chom.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 72.Wehkamp J, Harder J, Wehkamp K, et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun. 2004;72:5750–5758. doi: 10.1128/IAI.72.10.5750-5758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schlee M, Wehkamp J, Altenhoefer A, et al. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect Immun. 2007;75:2399–2407. doi: 10.1128/IAI.01563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mondel M, Schroeder BO, Zimmermann K, et al. Probiotic E. coli treatment mediates antimicrobial human beta-defensin synthesis and fecal excretion in humans. Mucosal Immunol. 2009;2:166–172. doi: 10.1038/mi.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hugo AA, De Antoni GL, Perez PF. Lactobacillus delbrueckii subsp lactis (strain CIDCA 133) resists the antimicrobial activity triggered by molecules derived from enterocyte-like Caco-2 cells. Lett Appl Microbiol. 50:335–340. doi: 10.1111/j.1472-765X.2010.02796.x. [DOI] [PubMed] [Google Scholar]
- 76.He B, Xu W, Santini PA, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 77.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 78.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turnbaugh PJ, Ridaura VK, Faith JJ, et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buts JP, De Keyser N. Interaction of Saccharomyces boulardii With Intestinal Brush Border Membranes: Key to Probiotic Effects? J Pediatr Gastroenterol Nutr. doi: 10.1097/MPG.0b013e3181e23271. [DOI] [PubMed] [Google Scholar]
- 81.Resta SC. Effects of probiotics and commensals on intestinal epithelial physiology: implications for nutrient handling. J Physiol. 2009;587:4169–4174. doi: 10.1113/jphysiol.2009.176370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lundgren O, Peregrin AT, Persson K, et al. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science. 2000;287:491–495. doi: 10.1126/science.287.5452.491. [DOI] [PubMed] [Google Scholar]
- 83.Verdu EF, Bercik P, Bergonzelli GE, et al. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology. 2004;127:826–837. doi: 10.1053/j.gastro.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 84.Kunze WA, Mao YK, Wang B, et al. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med. 2009;13:2261–2270. doi: 10.1111/j.1582-4934.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang B, Mao YK, Diorio C, et al. Lactobacillus reuteri ingestion and IK(Ca) channel blockade have similar effects on rat colon motility and myenteric neurones. Neurogastroenterol Motil. 22:98–107. e133. doi: 10.1111/j.1365-2982.2009.01384.x. [DOI] [PubMed] [Google Scholar]
- 86.Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 87.Ma X, Mao YK, Wang B, et al. Lactobacillus reuteri ingestion prevents hyperexcitability of colonic DRG neurons induced by noxious stimuli. Am J Physiol Gastrointest Liver Physiol. 2009;296:G868–875. doi: 10.1152/ajpgi.90511.2008. [DOI] [PubMed] [Google Scholar]
- 88.Nelson KE, Weinstock GM, Highlander SK, et al. A catalog of reference genomes from the human microbiome. Science. 2010;328:994–999. doi: 10.1126/science.1183605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peterson J, Garges S, Giovanni M, et al. The NIH Human Microbiome Project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an antiinflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gorbach SL, Banwell JG, Jacobs B, et al. Intestinal microflora in Asiatic cholera. I. “Rice-water” stool. J Infect Dis. 1970;121:32–37. doi: 10.1093/infdis/121.1.32. [DOI] [PubMed] [Google Scholar]
- 93.Bibiloni R, Tandon P, Vargas-Voracka F, et al. Differential clustering of bowel biopsy-associated bacterial profiles of specimens collected in Mexico and Canada: what do these profiles represent? J Med Microbiol. 2008;57:111–117. doi: 10.1099/jmm.0.47321-0. [DOI] [PubMed] [Google Scholar]
- 94.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural. Africa Proc Natl Acad Sci U S A. 107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hardin G. The competitive exclusion principle. Science. 1960;131:1292–1297. doi: 10.1126/science.131.3409.1292. [DOI] [PubMed] [Google Scholar]
- 96.Tannock GW. Probiotics: time for a dose of realism. Curr Issues Intest Microbiol. 2003;4:33–42. [PubMed] [Google Scholar]
- 97.Tannock GW. Can the gut microflora of infants be modified by giving probiotics to mothers? J Pediatr Gastroenterol Nutr. 2004;38:244–246. doi: 10.1097/00005176-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 98.Tomkins AM, Wright S, James WP, Drasar BS. Proceedings: Jejunal colonization with enterobacteria in symptomatci tropical residents without acute sprue. Gut. 1975;16:392. [PMC free article] [PubMed] [Google Scholar]
- 99.van Baarlen P, Troost FJ, van Hemert S, et al. Differential NF-kappaB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc Natl Acad Sci U S A. 2009;106:2371–2376. doi: 10.1073/pnas.0809919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van der Werf MJ, Jellema RH, Hankemeier T. Microbial metabolomics: replacing trial-and-error by the unbiased selection and ranking of targets. J Ind Microbiol Biotechnol. 2005;32:234–252. doi: 10.1007/s10295-005-0231-4. [DOI] [PubMed] [Google Scholar]
- 101.Nicholson JK, Holmes E, Lindon JC, Wilson ID. The challenges of modeling mammalian biocomplexity. Nat Biotechnol. 2004;22:1268–1274. doi: 10.1038/nbt1015. [DOI] [PubMed] [Google Scholar]
- 102.Wang Y, Holmes E, Nicholson JK, et al. Metabonomic investigations in mice infected with Schistosoma mansoni: an approach for biomarker identification. Proc Natl Acad Sci U S A. 2004;101:12676–12681. doi: 10.1073/pnas.0404878101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gibney MJ, Walsh M, Brennan L, et al. Metabolomics in human nutrition: opportunities and challenges. Am J Clin Nutr. 2005;82:497–503. doi: 10.1093/ajcn.82.3.497. [DOI] [PubMed] [Google Scholar]
- 104.Raza S, Graham SM, Allen SJ, et al. Lactobacillus GG promotes recovery from acute nonbloody diarrhea in Pakistan. Pediatr Infect Dis J. 1995;14:107–111. doi: 10.1097/00006454-199502000-00005. [DOI] [PubMed] [Google Scholar]
- 105.Pant AR, Graham SM, Allen SJ, et al. Lactobacillus GG and acute diarrhoea in young children in the tropics. J Trop Pediatr. 1996;42:162–165. doi: 10.1093/tropej/42.3.162. [DOI] [PubMed] [Google Scholar]
- 106.Shornikova AV, Isolauri E, Burkanova L, Lukovnikova S, Vesikari T. A trial in the Karelian Republic of oral rehydration and Lactobacillus GG for treatment of acute diarrhoea. Acta Paediatr. 1997;86:460–465. doi: 10.1111/j.1651-2227.1997.tb08913.x. [DOI] [PubMed] [Google Scholar]
- 107.Bhatnagar S, Singh KD, Sazawal S, Saxena SK, Bhan MK. Efficacy of milk versus yogurt offered as part of a mixed diet in acute noncholera diarrhea among malnourished children. J Pediatr. 1998;132:999–1003. doi: 10.1016/s0022-3476(98)70398-1. [DOI] [PubMed] [Google Scholar]
- 108.Simakachorn N, Pichaipat V, Rithipornpaisarn P, et al. Clinical evaluation of the addition of lyophilized, heat-killed Lactobacillus acidophilus LB to oral rehydration therapy in the treatment of acute diarrhea in children. J Pediatr Gastroenterol Nutr. 2000;30:68–72. doi: 10.1097/00005176-200001000-00020. [DOI] [PubMed] [Google Scholar]
- 109.Costa-Ribeiro H, Ribeiro TC, Mattos AP, et al. Limitations of probiotic therapy in acute, severe dehydrating diarrhea. J Pediatr Gastroenterol Nutr. 2003;36:112–115. doi: 10.1097/00005176-200301000-00021. [DOI] [PubMed] [Google Scholar]
- 110.Salazar-Lindo E, Miranda-Langschwager P, Campos-Sanchez M, Chea-Woo E, Sack RB. Lactobacillus casei strain GG in the treatment of infants with acute watery diarrhea: a randomized, double-blind, placebo controlled clinical trial [ISRCTN67363048] BMC Pediatr. 2004;4:18. doi: 10.1186/1471-2431-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kurugol Z, Koturoglu G. Effects of Saccharomyces boulardii in children with acute diarrhoea. Acta Paediatr. 2005;94:44–47. doi: 10.1111/j.1651-2227.2005.tb01786.x. [DOI] [PubMed] [Google Scholar]
- 112.Sarker SA, Sultana S, Fuchs GJ, et al. Lactobacillus paracasei strain ST11 has no effect on rotavirus but ameliorates the outcome of nonrotavirus diarrhea in children from Bangladesh. Pediatrics. 2005;116:e221–228. doi: 10.1542/peds.2004-2334. [DOI] [PubMed] [Google Scholar]
- 113.Khanna V, Alam S, Malik A. Efficacy of tyndalized Lactobacillus acidophilus in acute diarrhea. Indian J Pediatr. 2005;72:935–938. doi: 10.1007/BF02731667. [DOI] [PubMed] [Google Scholar]
- 114.Szymanski H, Pejcz J, Jawien M, et al. Treatment of acute infectious diarrhoea in infants and children with a mixture of three Lactobacillus rhamnosus strains--a randomized, double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006;23:247–253. doi: 10.1111/j.1365-2036.2006.02740.x. [DOI] [PubMed] [Google Scholar]
- 115.Agustina R, Lukito W, Firmansyah A, et al. The effect of early nutritional supplementation with a mixture of probiotic, prebiotic, fiber and micronutrients in infants with acute diarrhea in Indonesia. Asia Pac J Clin Nutr. 2007;16:435–442. [PubMed] [Google Scholar]
- 116.Salazar-Lindo E, Figueroa-Quintanilla D, Caciano MI, et al. Effectiveness and safety of Lactobacillus LB in the treatment of mild acute diarrhea in children. J Pediatr Gastroenterol Nutr. 2007;44:571–576. doi: 10.1097/MPG.0b013e3180375594. [DOI] [PubMed] [Google Scholar]
- 117.Villarruel G, Rubio DM, Lopez F, et al. Saccharomyces boulardii in acute childhood diarrhoea: a randomized, placebo-controlled study. Acta Paediatr. 2007;96:538–541. doi: 10.1111/j.1651-2227.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- 118.Htwe K, Yee KS, Tin M, Vandenplas Y. Effect of Saccharomyces boulardii in the treatment of acute watery diarrhea in Myanmar children: a randomized controlled study. Am J Trop Med Hyg. 2008;78:214–216. [PubMed] [Google Scholar]
- 119.Mao M, Yu T, Xiong Y, et al. Effect of a lactose-free milk formula supplemented with bifidobacteria and streptococci on the recovery from acute diarrhoea. Asia Pac J Clin Nutr. 2008;17:30–34. [PubMed] [Google Scholar]
- 120.Dubey AP, Rajeshwari K, Chakravarty A, Famularo G. Use of VSL[sharp]3 in the treatment of rotavirus diarrhea in children: preliminary results. J Clin Gastroenterol. 2008;42(3)(Pt 1):S126–129. doi: 10.1097/MCG.0b013e31816fc2f6. [DOI] [PubMed] [Google Scholar]
- 121.Ritchie BK, Brewster DR, Tran CD, et al. Efficacy of Lactobacillus GG in aboriginal children with acute diarrhoeal disease: a randomised clinical trial. J Pediatr Gastroenterol Nutr. 2010;50:619–624. doi: 10.1097/MPG.0b013e3181bbf53d. [DOI] [PubMed] [Google Scholar]
- 122.Grandy G, Medina M, Soria R, Teran C, Araya M. Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children. BMC Infect Dis. 10:253. doi: 10.1186/1471-2334-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sazawal S, Dhingra U, Hiremath G, et al. Effects of Bifidobacterium lactis HN019 and Prebiotic Oligosaccharide Added to Milk on Iron Status, Anemia, and Growth Among Children 1 to 4 Years Old. J Pediatr Gastroenterol Nutr. 2010 doi: 10.1097/MPG.0b013e3181d98e45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


