Abstract
BACKGROUND
The phosphoinositide 3-kinase/Akt/mammalian target of rapamycin pathway plays a critical role in the pathogenesis of hepatocellular carcinoma (HCC). We performed a single-arm, phase 1/2 study of everolimus in patients with advanced HCC.
METHODS
Patients with histologically confirmed measurable advanced HCC, 0–2 prior regimens, and adequate hematologic, hepatic, and renal functions received everolimus at 5 mg/day or 10 mg/day orally (6 weeks/cycle). The primary end points were determination of a safe dosage of everolimus (phase 1) and progression-free survival (PFS) at 24 weeks (phase 2).
RESULTS
Twenty-eight patients were enrolled and evaluable for efficacy and toxicity. No dose-limiting toxicities were observed at the 5 mg/day (n = 3) or 10 mg/day (n = 6) dosage level in phase 1. Twenty-five patients received everolimus at 10 mg/day. Grade 3–4 adverse events included lymphopenia (n = 3), aspartate transaminase (n = 3), hyponatremia (n = 2), and 1 patient each with anemia, alanine transaminase, hyperglycemia, proteinuria, rash, and hypoxia. One patient (4%) had partial response (95% confidence interval [CI], 0.9%–19.6%). The median PFS and overall survival were 3.8 months (95% CI, 2.1–4.6) and 8.4 months (95% CI, 3.9–21.1), respectively. The estimated PFS rate at 24 weeks was 28.6% (95% CI, 7.9%–49.3%).
CONCLUSION
Everolimus was well tolerated in patients with advanced HCC, and 10 mg/day was defined as the phase 2 dosage. Although the study did not proceed to the second stage of phase 2, preliminary antitumor activity was observed with everolimus in patients with advanced HCC, most of whom had prior systemic treatment.
Keywords: hepatocellular carcinoma, everolimus, mTOR inhibitors, angiogenesis, clinical trial
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third most common cause of cancer-related mortality worldwide.1 After decades of research, 2 randomized trials demonstrated that sorafenib improved survival in patients with advanced HCC and established sorafenib as a standard first-line treatment for advanced HCC.2, 3 Other molecularly targeted agents are under clinical development4; however, most of these agents also target angiogenesis pathways. Therefore, novel agents that inhibit other hepatocarcinogenesis pathways are urgently needed.
Increasing evidence supports an important role for the phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway and potential for inhibiting mTOR as a therapeutic strategy in HCC.5 Aberrant mTOR signaling as reflected by upstream activation through epidermal growth factor receptor (EGFR) and insulin-like growth factor 1 receptor is well documented, and mutations in p110alpha (PIK3CA) have been reported in HCC.6 Hepatitis B virus X-proteins activate the PI3K pathway by modulating phosphorylation of Akt.7 The mTOR pathway is involved in production of proangiogenic factors, including vascular endothelial growth factor (VEGF); therefore, inhibition of the mTOR pathway may have antiangiogenic activity in HCC.8 Preclinical data have demonstrated that mTOR inhibitors were effective in inhibiting cell growth and tumor vascularity in HCC cell lines and HCC tumor models.8, 9 HCC can be induced in a transgenic hepatocyte-specific phosphatase and tensin homolog (PTEN)-deficient mouse model.10 Phosphorylation of ribosomal protein S6 kinase (p-RPS6) downstream of PI3K/mTOR is present in approximately 50% of HCC cases. Chromosomal gains in the mTOR-associated protein RICTOR and p-RPS6–positive staining correlate with HCC recurrence following surgical resection.11
To evaluate whether inhibition of the PI3K/Akt/mTOR pathway is safe and effective in patients with advanced HCC, we initiated a phase 1/2 study of everolimus, an oral small-molecule serine-threonine kinase inhibitor of mTOR, in patients with advanced HCC.
MATERIALS AND METHODS
Patient Population
The trial was approved by the institutional review board at the Dana-Farber/Harvard Cancer Center (Boston, Mass). All patients provided written informed consent before study participation.
Eligibility criteria were as follows: histologically proven, measurable, locally advanced, or metastatic HCC; no more than 2 prior chemotherapeutic and biologic regimens; prior chemoembolization therapy (only if performed >4 weeks before study entry and measurable disease outside of the chemoembolization field was present); age ≥18 years; Eastern Cooperative Oncology Group performance status of 0–2; Cancer of the Liver Italian Program score ≤3; and adequate bone marrow, renal, and hepatic function (absolute neutrophil count ≥1000/lL, hemoglobin level ≥9 g/dL, platelet count ≥75,000/lL, serum creatinine level ≤2.0 mg/dL, total bilirubin level <3.0 mg/dL, and aspartate aminotransferase and alanine aminotransferase levels ≤7 times the upper limit of normal).
Exclusion criteria were as follows: concurrent malignancies; significant medical comorbidities, including severely impaired lung function and uncontrolled diabetes; significant cardiovascular disease, including symptomatic congestive heart failure, myocardial infarction ≤6 months before study treatment, serious uncontrolled cardiac arrhythmia, and unstable angina; chronic use of systemic steroids or immunosuppressive therapy; active bleeding; proteinuria at baseline (>2 g/day); pregnancy or lactation; brain metastases; impairment of gastrointestinal function or gastrointestinal disease that may alter the absorption of everolimus; or prior use of mTOR inhibitors.
Study Treatment
Eligible patients received everolimus continuously daily by mouth until disease progression, unacceptable toxicity, or withdrawal of consent. Six weeks of the study drug were considered 1 cycle of treatment. The phase 1 stage of the study used the conventional 3+3 design. The starting dosage of everolimus was 5 mg/day. The planned escalated dosage level was 10 mg/day and the de-escalated dosage level was 5mg every other day.
Everolimus was interrupted and supportive management instituted for grade 3–4 hematologic or grade 3 nonhematologic toxicities. Everolimus was reinitiated with a 50% dosage reduction if resolution of the toxicity to less than grade 2 occurred within 14 days; otherwise, treatment was discontinued. Treatment was also discontinued for grade 4 nonhematologic toxicity, grade 2 or higher pneumonitis, or continued toxicity after reinstitution of everolimus at 5mg every other day. Dose-limiting toxicity (DLT) likely attributed to everolimus was evaluated during the first cycle (42 days). DLT was defined as any grade 4 neutropenia or thrombocytopenia; any grade 3 or higher nonhematologic toxicity, excluding alopecia, nausea, and vomiting (not controlled by standard antiemetic therapy); grade 3 or higher diarrhea requiring hospitalization or lasting >24 hours despite aggressive loperamide therapy; grade 3 or higher fasting hyperglycemia persisting for >1 week despite treatment with either oral antidiabetic agents or insulin; and grade 3 serum transaminases (aspartate transaminase and alanine transaminase) for patients with grade 0–1 at baseline or ≥7 times the upper limit of normal for patients with grade 2 at baseline.
On-study evaluations included toxicity assessments, peripheral blood count measurements, and a full chemistry panel every other week. Lipid panel and serum alpha-fetoprotein (AFP) levels were measured monthly. Patients were evaluated with computed tomography or magnetic resonance imaging every 6 weeks; response and progression were evaluated by independent radiologic review using RECIST criteria.12
Correlative Analyses
Genotyping was performed on formalin-fixed, paraffin-embedded tissue. Eleven of 25 patient samples from the phase 2 portion of the study were available for evaluation. Total nucleic acids were isolated using a custom platform based on the Agencourt FormaPure system (Beckman Coulter Genomics). Nucleic acids were screened for 120 somatic mutations across 13 essential cancer genes (APC, BRAF, CTNNB1, EGFR, FLT3, JAK2, KIT, KRAS, NOTCH1, NRAS, PIK3CA, PTEN, and TP53) using a high-throughput assay based on the ABI Prism SNaPshot Multiplex system.13 Mutations were confirmed by direct sequencing.
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded tissue sections using standard methods and the following polyclonal antibodies and dilutions (Cell Signaling Technology): 1:50 phospho-Akt (Ser473), 1:15 phospho-mTOR (Ser2448) and 1:75 phospho-Ribosomal Protein S6 (Ser235/236). Activation of Akt/mTOR signaling was defined as an IHC score of 2+ to 3+ (moderate to high) across each target protein on a scale of 0 to 3+.
Statistical Methods
The primary endpoint for the phase 1 portion was determination of the safety of everolimus. Two planned dose levels of everolimus were evaluated based on previous studies demonstrating that the optimum biologic dose is 10 mg. The primary endpoint for the phase 2 portion was the progression-free survival (PFS) rate at 24 weeks.
The trial was prespecified to include patients from the phase 1 portion (no more than 2 dose levels) into the phase 2 portion of the study. The phase 2 portion had a 2-stage design, with a target accrual of 22 evaluable patients for the first stage and a total of 40 patients for the whole study. If at least 3 of the evaluable patients included in the analysis for the first stage of the phase 2 portion were progression-free at 24 weeks, the trial would proceed to the second stage. We hypothesized that everolimus would improve the 24-week PFS rate from 10% to 25%. As designed, the overall study with 40 evaluable patients would have an 80% power at a 0.05 level of significance to detect a 15% difference of 24-week PFS rate from 10% to 25%.
Secondary endpoints included overall response rate, toxicity, time to tumor progression (TTP), median PFS, and overall survival. The Kaplan-Meier method was used to estimate the distribution of PFS and survival. All analyses were performed using SAS version 9.2 software (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Between October 2007 and June 2009, 28 patients entered into the trial (9 in phase 1, 19 in phase 2) and all were evaluable for efficacy and toxicity based on intention-to-treat analysis (Figure 1). Baseline patient characteristics are summarized in Table 1. There were 18 men (64%) and 10 women (36%) with a median age of 65 years (range, 33–81 years). Median Eastern Cooperative Oncology Group performance status was 1 (range, 0–2). Twenty-three (82%) patients had underlying Child-Pugh class A cirrhosis and 13 (47%) patients had hepatitis B virus (HBV) or hepatitis C virus (HCV) infection. The majority of patients had Barcelona Clinic Liver Cancer stage C (26 [93%]) and extrahepatic (24 [86%]) disease. Twenty patients (71%) had received prior systemic therapy, including 11 (39%) patients who had received prior sorafenib therapy and 9 (32%) patients who had received other systemic regimens, including bevacizumab alone or in combination with gemcitabine/oxaliplatin and sunitinib or ramucirumab on clinical trials. The median serum bilirubin level was 0.6 mg/dL (range, 0.6–2.9 mg/dL), and 23 (82%) patients had an elevated serum AFP level at the time of study entry.
Figure 1.
A CONSORT diagram illustrating all patients enrolled in the study is shown.
Table 1.
Patient Characteristics
| Characteristic | Value |
|---|---|
| No. of patients | 28 |
| Men/Women (%) | 18/10 (64/36) |
| Median age, y (range) | 65 (33–81) |
|
Eastern Cooperative Oncology Group performance status, no. (%) |
|
| 0 | 11 (39) |
| 1 | 16 (57) |
| 2 | 1 (4) |
| Cancer of the Liver Italian Program score, no. (%) | |
| 0 | 1 (4) |
| 1 | 9 (32) |
| 2 | 9 (32) |
| 3 | 9 (32) |
| Child-Pugh class, no. (%) | |
| A | 23 (82) |
| B | 5 (18) |
| Barcelona Clinic Liver Cancer stage, no. (%) | |
| B (intermediate) | 2 (7) |
| C (advanced) | 26 (93) |
| Macroscopic vascular invasion, no. (%) | 6 (21) |
| Extrahepatic spread, no. (%) | 24 (86) |
| Hepatocellular carcinoma etiology, no. (%) | |
| Hepatitis B virus | 5 (18) |
| Hepatitis C virus | 8 (29) |
| Alcohol | 7 (25) |
| Hemachromatosis | 2 (7) |
| Steatohepatitis | 1 (4) |
| Unknown | 7 (25) |
| Baseline laboratory values, median (range) | |
| Albumin (mg/dL) | 4.0 (2.4–4.9) |
| Aspartate transaminase (U/L) | 54.5 (25–176) |
| Total bilirubin (mg/dL) | 0.6 (0.2–2.9) |
| Alpha-fetoprotein (ng/mL) | 1729.5 (2.5–178,200.0) |
| Previous therapy, no. (%) | |
| Surgical resection | 9 (32) |
| Chemoembolization | 5 (18) |
| Radiofrequency ablation | 3 (11) |
| Radiation therapy | 1 (4) |
| Systemic therapy | 20 (71) |
| Sorafenib | 11 (39) |
| Prior systemic regimens, no. (%) | |
| 0 | 8 (29) |
| 1 | 11 (39) |
| 2 | 9 (32) |
Toxicity
In the phase 1 portion of the study, dose-finding escalation was performed with 2 planned dosages of 5 mg/day and 10 mg/day. Three patients were initially enrolled at the 5 mg/day dosage level. No DLTs were encountered during cycle 1 of treatment; therefore, an additional 3 patients were enrolled at the 10 mg/day dosage level. One patient developed grade 3 lymphopenia thought to be related to everolimus. An additional 3 patients were enrolled at the 10 mg/day dosage level. No DLTs were encountered, and the phase 2 dosage was determined to be 10 mg/day.
The 2-stage phase 2 portion of the study proceeded with an additional 19 patients enrolled, for a total of 25 patients treated with the 10 mg/day dosage and included in stage 1 analysis of the phase 2 portion of the study. Per protocol specification, 3 additional patients were enrolled to have 22 patients evaluable for efficacy for the first stage of the phase 2 study, as 1 withdrew consent during the first cycle of treatment and 2 patients were removed for unacceptable toxicities before completing the first set of restaging scans. A total of 60 cycles were administered, with a mean dosage of 373 mg per 6-week cycle (6 weeks); 88.8% of the targeted dosage was delivered to the 25 patients included in the phase 2 analysis.
Everolimus was generally well tolerated at the 10 mg/day dosage. The most common all-grades adverse events thought to be at least possibly related to everolimus included fatigue, hyperglycemia, diarrhea, myelosuppression, transient elevation of transaminase levels, hyponatremia, anorexia, stomatitis, and rash (Table 2).
Table 2.
Toxicity of Everolimus at 10 mg/day (n=25)
| Toxicity | Any Grade |
Grade 3 |
Grade 4 |
|---|---|---|---|
| Fatigue | 13 (52) | – | – |
| Hyperglycemia | 12 (48) | 1 (4) | – |
| Anemia | 11 (44) | – | 1 (4) |
| Diarrhea | 11 (44) | – | – |
| Leukopenia | 10 (40) | – | – |
| Platelets | 10 (40) | – | – |
| Aspartate transaminase | 9 (36) | 3 (12) | – |
| Hyponatremia | 9 (36) | 2 (8) | – |
| Anorexia | 9 (36) | – | – |
| Lymphopenia | 8 (32) | 3 (12) | – |
| Stomatitis | 7 (28) | – | – |
| Rash/Desquamation | 7 (28) | 1 (4) | – |
| Alanine transaminase | 6 (24) | 1 (4) | – |
| Neutropenia | 5 (20) | – | – |
| Alkaline phosphatase | 4 (16) | – | – |
| Proteinuria | 4 (16) | 1 (4) | – |
| Vomiting | 4 (16) | – | – |
| Nausea | 4 (16) | – | – |
| Dyspnea | 4 (16) | – | – |
| Weight loss | 3 (12) | – | – |
| Acne rash | 3 (12) | – | – |
| Edema | 3 (12) | – | – |
| Taste disturbance | 3 (12) | – | – |
| Hypoalbuminemia | 2 (8) | – | – |
| Hypokalemia | 2 (8) | – | – |
| Hypertriglyceridemia | 2 (8) | – | – |
| Fever without neutropenia | 2 (8) | – | – |
| Constipation | 2 (8) | – | – |
| Nose bleeds | 2 (8) | – | – |
| Cough | 2 (8) | – | – |
| Insomnia | 2 (8) | – | – |
| Pruritis/Itching | 2 (8) | – | – |
| Pain | 2 (8) | – | – |
| Total bilirubin | 1 (4) | – | – |
| Hypernatremia | 1 (4) | – | – |
| Hypocalcemia | 1 (4) | – | – |
| Hypercholesterolemia | 1 (4) | – | – |
| Bicarbonate | 1 (4) | – | – |
| Hypoxia | 1 (4) | – | 1 (4) |
| Upper airway infection | 1 (4) | – | – |
| Allergic rhinitis | 1 (4) | – | – |
| Ascites | 1 (4) | – | – |
| Distention/Bloating | 1 (4) | – | – |
| Central nervous system | 1 (4) | – | – |
| cerebrovascular ischemia | |||
| Dehydration | 1 (4) | – | – |
| Dry mouth | 1 (4) | – | – |
| Flatulence | 1 (4) | – | – |
| Photosensitivity | 1 (4) | – | – |
| Pneumonitis/Pulmonary infiltrates | 1 (4) | – | – |
Data are presented as no. (%).
Grade 3 or 4 events were relatively infrequent. Transient elevation of transaminase levels was observed, with 3 (12%) patients experiencing grade 3 elevations of aspartate transaminase and 1 (4%) patient experiencing grade 3 elevations of alanine transaminase. Grade 3 lymphopenia was seen in 3 (12%) patients, and 2 (8%) patients had grade 3 hyponatremia; 3 other grade 3 events (hyperglycemia, rash, and proteinuria), were seen in 1 patient each. One patient had grade 4 anemia secondary to gastrointestinal bleeding. This patient was found to have gastric antral vascular ectasia on endoscopy and was treated with argon plasma photocoagulation; treatment was resumed without recurrent bleeding episodes. Grade 4 hypoxia occurred in 1 patient with underlying chronic obstructive pulmonary disease and pneumonia without evidence of pneumonitis.
Clinical Efficacy
Clinical efficacy was analyzed in all 25 patients treated at the 10 mg/day dosage level. Everolimus induced a partial response in 1 (4%) patient (95% confidence interval [CI], 0.9%–19.6%) and 10 (40%) patients (95% CI, 23%–59%) achieved stable disease (SD) for at least 12 weeks with a disease control rate of 44%. Seven patients (25%) had a >50% decrease in serum AFP level from baseline. A waterfall plot of the percent change in the sum of diameters of tumor lesions from baseline in 22 patients with available repeat scans is shown in Figure 2A.
Figure 2.
(A) A waterfall plot shows the percent change from baseline in the sum of diameters of tumor lesions in 22 patients with repeat scans. (B) A Kaplan-Meier estimate of progression-free survival is shown. (C) A Kaplan-Meier estimate of overall survival is shown.
With a median follow-up time of 7.2 months, the median PFS of this cohort was 3.8 months (95% CI, 2.1–4.6 months) (Figure 2B), the median TTP was 3.9 months (95% CI, 2.1–5.5 months), and the median overall survival was 8.4 months (95% CI, 3.9–21.1 months) (Figure 2C). Because only 2 (8%) patients were progression-free at 24 weeks, the study did not proceed to the second stage of the phase 2 portion of the study. The estimated PFS rates at 24 weeks and 12 weeks, based on the Kaplan-Meier method, were 28.6% (95% CI, 7.9%–49.3%) and 57.1% (95% CI, 35.7%–78.6%), respectively.
Unplanned exploratory analyses of 25 patients treated at the 10 mg/day dosage level revealed that patients who had received prior sorafenib treatment (n = 10) had similar PFS, TTP, and overall survival compared with those who had not received prior sorafenib treatment (n = 15). In addition, patients with no viral etiology (n = 14) had longer TTP compared with those with HBV (n = 6) or HCV infection (n = 5) (data not shown).
Correlative Analyses
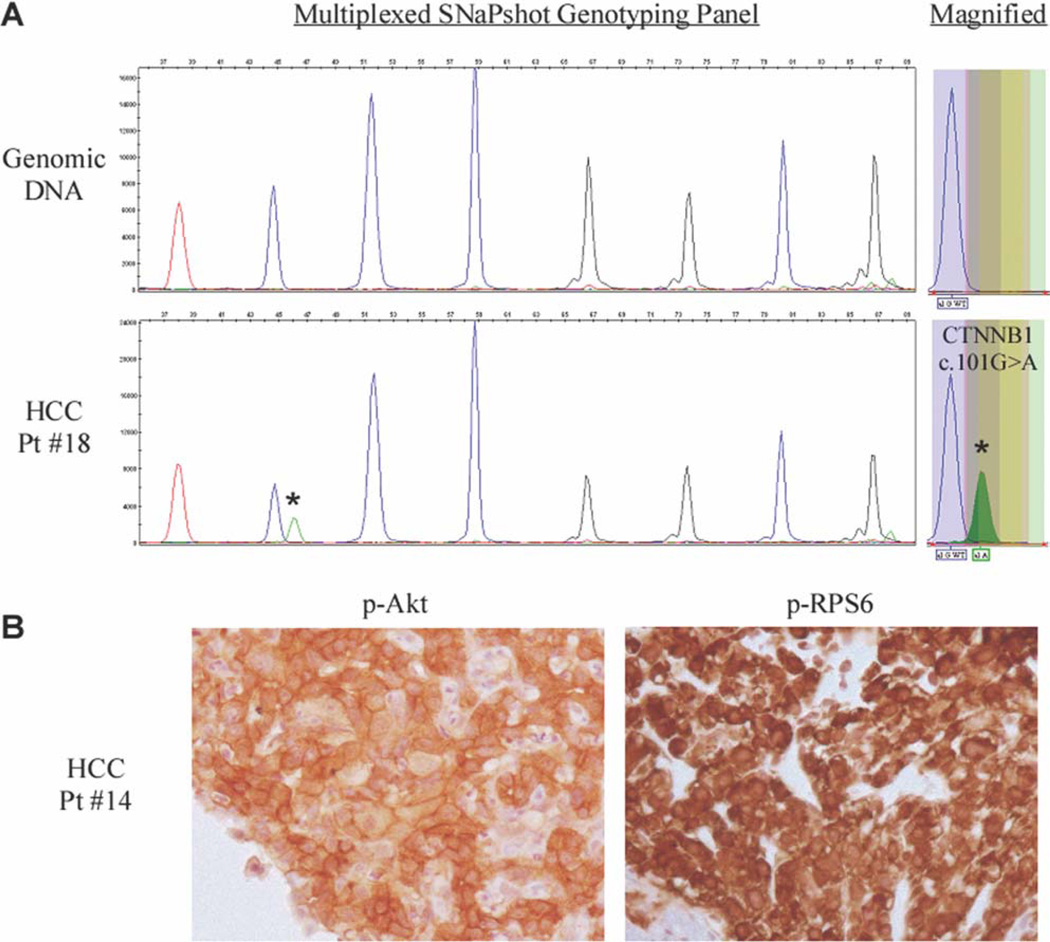
Key cancer gene mutations were queried using a previously published testing platform that was established in our institution.13 Of 11 available samples tested, a mutation in CTNNB1 (G34E; 101G>A) was identified in 1 patient (Figure 3A and Table 3). This patient experienced SD during the first 2 cycles of everolimus treatment. No other gene mutations were identified.
Figure 3.
Genotype and immunohistochemical analysis results are shown. (A) A CTNNB1 101G>A (G34E) somatic mutation was identified in the tumor of a patient exhibiting stable disease to everolimus treatment. The mutation is indicated by an asterisk (*) in the multiplexed SNaPshot panel and is magnified in the box on the right. The genomic DNA peak represents the wild-type allele. (B) Immunohistochemical analysis demonstrated activation of the Akt/mammalian target of rapamycin pathway in the tumor of a patient exhibiting a partial response to everolimus treatment.
Table 3.
Molecular Profiles
| Patient No. | Hepatitis Status |
Mutational Profiling |
Immunohistochemistry | Best Tumor Response |
||
|---|---|---|---|---|---|---|
| p-Akt | p-mTOR | p-RPS6 | ||||
| 5 | HCV | NMI | Mod | Low | High | SD |
| 9 | HBV | NMI | Mod | Low | Low | PD |
| 11 | Negative | NMI | Low | Low | Mod | SD |
| 14 | HCV | NMI | Mod | Mod | High | PR |
| 18 | Negative | CTNNB1 (G34E) | Mod | Mod | High | SD |
| 22 | Negative | NMI | Low | Low | Low | SD |
| 23 | HBV | NMI | Mod | Low | Mod | PD |
| 24 | Negative | NMI | Low | Low | Mod | PD |
| 25 | HCV | NMI | ND | ND | ND | PD |
| 26 | Steatohepatitis | NMI | High | High | High | SD |
| 28 | Negative | NMI | Mod | Low | Mod | SD |
HBV indicates hepatitis B virus; HCV, hepatitis C virus; Mod, moderate; ND, not determined; NMI, no mutation identified; p-Akt, phosphorylated Akt; p-mTOR, phosphorylated mammalian target of rapamycin; p-RPS6, phosphorylated ribosomal protein S6 kinase; PD, progressive disease; PR, partial response; SD, stable disease.
Activation of the Akt/mTOR pathway was identified through phosphorylation of Akt at Ser473 (p-Akt), phosphorylation of mTOR at Ser2448 (p-mTOR), and phosphorylation of the downstream mTOR complex 1 target, ribosomal protein S6 at Ser235/236 (p-RPS6). Robust pathway activation in tumor tissue was observed in 3 out of 11 patient samples (Table 3). Moderate (2+) to high (3+) levels of p-Akt, p-mTOR, and p-RPS6 were noted in a patient demonstrating a partial response to everolimus as well as the CTNNB1 mutant–positive patient experiencing SD (Figure 3B and Table 3). Strong signaling (3+) was observed in a patient with steatohepatitis experiencing 18 weeks of SD. Statistical significance was not reached, possibly due to limited sample size.
DISCUSSION
Approval of sorafenib has stimulated drug development research in advanced HCC. However, most current efforts are focused on antiangiogenic agents, and several VEGF receptor–targeted agents are being evaluated in phase 3 clinical trials. For patients who have progressed or cannot tolerate sorafenib, there are currently no standard therapies. Therefore, there is an urgent unmet need to develop additional effective agents, particularly those with novel mechanisms of action that target hepatocarcinogenesis. In this single-arm phase 1/2 study, 10 mg/day everolimus was defined as the phase 2 dosage and demonstrated preliminary activity in advanced HCC patients.
Daily and weekly dosing of everolimus have both been explored in other phase 1 studies. Everolimus given at 10 mg/day has inhibited mTOR activity in peripheral mononuclear cells, skin cells, and tumors, as measured by abrogated phosphorylation of downstream target proteins. 14–16 In addition, these studies have suggested that daily dosing may result in more profound and persistent inhibition of mTOR activity than weekly administration. Chen et al17 reported their early experience of a randomized phase 1 and pharmacokinetic study of everolimus in advanced HCC. Two different schedules were tested in this randomized phase 1 study: continuous daily dosing and weekly dosing. DLTs observed included hyperbilirubinemia, ALT elevation, thrombocytopenia, infection, diarrhea, and cardiac ischemia. The MTD for the weekly and daily dosing schedule was determined to be 70 mg and 7.5 mg, respectively. Interestingly, reactivation of HBV was observed in 4 patients, and flaring of HCV was observed in 1 patient.
Given prior extensive experience of everolimus dosing in previous studies in other tumors, we chose a daily schedule and only examined 2 planned dosage levels at 5 mg/day and 10 mg/day instead of using the full conventional dose escalation scheme. In the phase 1 portion of our study, only 1 patient developed grade 3 lymphopenia at the 10 mg/day dosage level. The phase 2 portion of the study, with an additional 19 patients, confirmed the tolerability of the 10 mg/day dosage level. Consistent with the reported toxicity profile of everolimus, the most common adverse events included fatigue, hyperglycemia, diarrhea, myelosuppression, transient elevation of transaminases, hyponatremia, anorexia, stomatitis, and rash. Grade 3 events were relatively infrequent and included elevation of aspartate transaminase levels (12%), lymphopenia (12%), and hyponatremia (8%). Grade 4 events were rare and included 1 patient each with anemia and hypoxia. All patients with HBV in our study received suppressive anti-HBV therapy while on everolimus and no reactivation of HBV or HCV flaring were seen. Given the known immunosuppressive activity of mTOR inhibitors, patients with HBV and HCV infection should have their liver function tests and viral load closely monitored.
Although the first stage of the phase 2 portion of the study did not reach the criteria to proceed with the second stage, it provided initial evidence of antitumor activity of single agent everolimus at 10 mg/day in advanced HCC. Despite inclusion of a heavily pretreated population in our study (71% received prior systemic therapy, including 39% with prior sorafenib use and 32% with 2 prior systemic regimens), we observed a disease control rate, median PFS, and median TTP of 44%, 3.8 months, and 3.9 months, respectively. However, most patients had SD of short duration, which contributed to only 2 patients being progression-free at 24 weeks in the phase 2 portion of the study. Due to the limitations of our study (small sample size, lack of a randomized control arm, potential for patient selection bias, and heterogeneity of the patient population), clinical activity of everolimus should be further defined in advanced HCC in future trials. Our study also underscores the importance and challenges of optimally designing phase 2 trials in HCC and the value of including a randomized control arm.18
No common somatic mutations in cancer driver genes known to regulate the PI3K/Akt/mTOR signaling pathway were identified in a small cohort of patients with archived tissue samples available. However, immunohistochemical analysis identified activation of Akt/mTOR signaling in more than 25% of samples evaluated. The complexity of the PI3K/Akt/mTOR pathway has become increasingly recognized, particularly in relation to its potential as a therapeutic target in cancer.19, 20 Prior data suggest the presence of a negative feedback loop, whereby increased activation of mTOR leads to a physiologic brake on further stimulation of this pathway.21–24 In some tumor types, mTOR inhibitors may interfere with this inhibitory feedback, resulting in a paradoxical increase in signaling by PI3K and increased activation of other Akt target proteins that promote cell survival. Although the loss of this negative feedback from mTOR inhibition may limit the efficacy of single-agent mTOR inhibitors in these tumor types, it supports investigation of treatment regimens that combine mTOR inhibitors with other agents, such as direct inhibitors of PI3K, Akt, or upstream receptor tyrosine kinases.
In conclusion, everolimus given at 10 mg/day as a single agent was well tolerated in patients with advanced HCC. Although the study did not meet the predefined criteria to proceed to the second stage of phase 2, everolimus demonstrated preliminary antitumor activity in patients with advanced HCC, most of whom had received prior systemic therapy. Given substantial data implicating activation of the PI3K/Akt/mTOR pathway in HCC, the unique mechanism of action of mTOR inhibitors, and preliminary clinical experience, additional studies are needed in patients with HCC to further define its efficacy either alone or in combination with sorafenib or other agents that might have additive or synergistic activity by modulating additional components of the PI3K/Akt/mTOR or other signaling pathways.
Acknowledgments
FUNDING SOURCES
This study was supported by Novartis.
We thank Megan Berg for data collection, Susan Sheehan and Eileen Regan for study coordination, Vanessa Scialabba for nucleic acid extraction, Hector Lopez for CTNNB1 sequencing, Breton Roussel for SNaPshot genotyping, and Anna Kreshock for immunohistochemical analyses.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 4.Zhu AX. Development of sorafenib and other molecularly targeted agents in hepatocellular carcinoma. Cancer. 2008;112:250–259. doi: 10.1002/cncr.23175. [DOI] [PubMed] [Google Scholar]
- 5.Treiber G. mTOR inhibitors for hepatocellular cancer: a forward-moving target. Expert Rev Anticancer Ther. 2009;9:247–261. doi: 10.1586/14737140.9.2.247. [DOI] [PubMed] [Google Scholar]
- 6.Lee JW, Soung YH, Kim SY, et al. PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene. 2005;24:1477–1480. doi: 10.1038/sj.onc.1208304. [DOI] [PubMed] [Google Scholar]
- 7.Lee YI, Kang-Park S, Do SI. The hepatitis B virus-X protein activates a phosphatidylinositol 3-kinase-dependent survival signaling cascade. J Biol Chem. 2001;276:16969–16977. doi: 10.1074/jbc.M011263200. [DOI] [PubMed] [Google Scholar]
- 8.Huynh H, Chow KH, Soo KC, et al. RAD001 (everolimus) inhibits tumour growth in xenograft models of human hepatocellular carcinoma. J Cell Mol Med. 2009;13:1371–1380. doi: 10.1111/j.1582-4934.2008.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semela D, Piguet AC, Kolev M, et al. Vascular remodeling and antitumoral effects of mTOR inhibition in a rat model of hepatocellular carcinoma. J Hepatol. 2007;46:840–848. doi: 10.1016/j.jhep.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe S, Horie Y, Suzuki A. Hepatocyte-specific Ptendeficient mice as a novel model for nonalcoholic steatohepatitis and hepatocellular carcinoma. Hepatol Res. 2005;33:161–166. doi: 10.1016/j.hepres.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Villanueva A, Chiang DY, Newell P, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972–1983. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: a clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2:146–158. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donnell A, Faivre S, Burris HA, 3rd, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–1595. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 15.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka C, O’Reilly T, Kovarik JM, et al. Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol. 2008;26:1596–1602. doi: 10.1200/JCO.2007.14.1127. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Shiah HS, Chen CY, et al. Randomized, phase I, and pharmacokinetic (PK) study of RAD001, an mTOR inhibitor, in patients (pts) with advanced hepatocellular carcinoma (HCC) [abstract] J Clin Oncol. 2009;27(suppl):15s. Abstract 4587. [Google Scholar]
- 18.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 19.Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- 20.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Yan H, Frost P, et al. Mammalian target of rapamycin inhibitors activate the Akt kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/ insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4:1533–1540. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 22.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 24.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]