Abstract
Transcranial Magnetic Stimulation (TMS) induces electrical currents in the brain to stimulate neural tissue. This article reviews our present understanding of TMS methodology, focusing on its biophysical foundations. We concentrate on how the laws of electromagnetic induction apply to TMS; addressing issues such as the location, area (i.e., focality), depth, and mechanism of TMS stimulation. We also present a review of the present limitations and future potential of the technique.
Keywords: TMS, brain stimulation, neuro-modulation
The use of electricity to alter neural function was first used almost 2000 years ago (Largus. 1529). Today, based on advancements in both electrophysiology and electromagnetic theory, numerous techniques have been developed that generate currents within the human nervous system to influence neural activity, cognition, and behavior (including Transcranial Magnetic Stimulation (TMS), Transcranial Direct Current Stimulation (tDCS), Deep Brain Stimulation (DBS), and others) (Wagner et al. 2007). TMS in particular has become the standard stimulation technique for the noninvasive exploration of cognitive function (Pascual-Leone et al. 2000), whereby neural tissue is stimulated by using the principles of electromagnetic induction to generate electrical currents in the brain (Barker et al. 1985). When TMS currents are applied with the appropriate pulse frequency, duration (number of pulses/bursts and inter-pulse/burst interval), and amplitude, a neuromodulatory effect is induced by which neural function and behavior are altered during (on-line) and beyond (off-line) the stimulation period. Despite the widespread use of magnetic stimulation, we are only just beginning to grasp the fundamental biophysical and electrophysiological foundations of the technique (Wagner et al. 2004; Allen et al. 2007; Ridding and Rothwell 2007; Thickbroom 2007; Bestmann 2008). However, just as a physician benefits from an understanding of the chemical reactions and subsequent physiological cascades initiated with pharmaceutical treatments, a neuroscientist must be aware of the fundamentals of how the electromagnetic fields interact with the nervous system in order to appropriately apply TMS. With such an understanding, one can accurately target parietal regions during studies of visual attention (Chambers et al. 2004), one can determine if limits in focality result in competing nodal effects during motor studies (Thielscher and Kammer 2002), or one can address how the depth of stimulation determines whether one directly or indirectly stimulates emotional circuits during affective neuroscience studies(Zangen et al. 2005). Conversely, such technical considerations are often ignored during the design and interpretation of cognitive TMS studies, considerably reducing the conclusory power and relevance of these studies. Thus, this article reviews our current understanding of TMS methodology, with the goal of highlighting such technical considerations. Overall, we present an abbreviated description of the fundamentals of TMS directed towards cognitive scientists and a focus on areas of future research for the TMS community.
Laws of induction and shaping the fields for stimulation
Magnetic stimulation is based on Faraday’s law of electromagnetic induction (d’Arsonval 1896; Faraday 1914). When a material is exposed to a time changing magnetic field an electric field is induced, which in turn drives currents in the material; see Figure 1A.
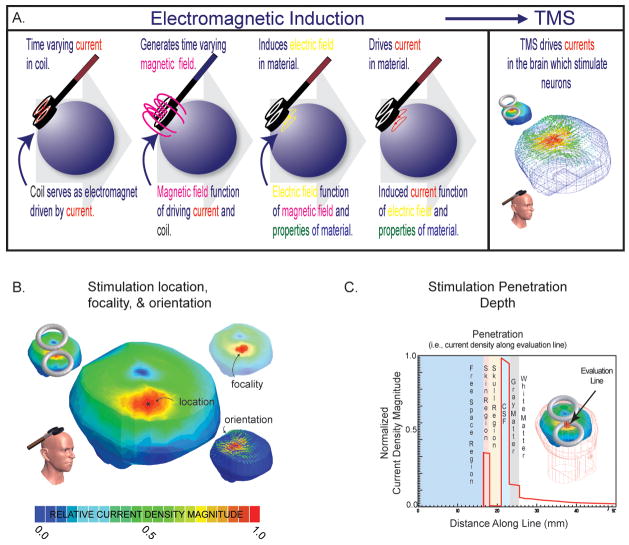
Figure 1. Foundations of stimulation.
A. Electromagnetic induction. A magnetic field (~1–4 Teslas pulsed over 0.125–1 ms, dependent on device parameters) induces an electric field that drives currents in the brain (of a magnitude of approximately 5.13 ×10−8 A/m2 in the cortex per 1 A/s steady state source, dependent on the tissue properties). Currents are carried by free charges and ions (called ohmic or resistive currents) or through the polarization of dipoles imbedded in the tissues or distributed in ionic layers surrounding the cells (called displacement or capacitive currents); see (Wagner et al. 2004). These currents are at the foundation of stimulation. B. Location, focality, orientations. The biophysical foundations can be used to determine the predicted maximum location of stimulation (*), the area of stimulation (usually ranging from ~100–200+ mm2 with a classic figure-of-eight coil with two 3.5 cm radius windings (herein defined as area from 90 to 100% of current maximum) dependent on relative head to coil placement), and the current density orientations relative to the neural tissue to be stimulated (see below). Note, circular and figure-of-eight coils are most common- whereby, figure-of-eight coils make use of the superposition of the individual fields generated by two coupled circular-coils, resulting in a more intense central-point; with circular-coils, the greatest intensity is expected to be maximum normal to the coil center-point distal from the coil face, but along the coil’s edge proximal to its face (Jalinous 1991). Of the two, the figure-of-eight coil is generally considered more focal (Ueno et al. 1988); however, the relative coil (size and position) to tissue distribution and anticipated stimulated distribution always needs to be considered, as there are conditions where circular-coils provide more focal stimulation (e.g. using small tilted circular-coils in animals (or children) with smaller heads(Wagner et al. 2007)). C. Depth of stimulation. The biophysical foundations can also be used to determine the depth of stimulation. Herein, we demonstrate the current density magnitude evaluated along an evaluation line in a healthy head, where the line is normal to the coil face and transecting all of the head tissues (note that the current density magnitude varies with the conductivity (and permittivity) of the tissues, and herein as CSF is the best conductor in the system, it demonstrates the highest current density). Generally with traditional coils, penetration is limited as the inductive magnetic fields are negligible less than a few centimeters from the scalp (for example see (Jalinous 1991)); and although one could increase the depth of stimulation with amplified source intensity, focality will conversely decrease as larger regions of tissue are exposed to increased current densities. Groups, such as Zangen’s and Davey’s, are actively pursuing improvements on these limitations (Zangen et al. 2005; Davey and Riehl 2006).
In the case of TMS, the stimulation coil serves as an electromagnet and generates a time changing magnetic field, the distribution of which is determined by both the current driving the electromagnet (magnitude and time course) and the physical properties of the stimulating coil (geometry and material properties). (For a detailed TMS device technology/electronics review see (Hsu et al. 2003; Davey and Riehl 2005)). When the coil is placed on the human scalp and the magnetic field is focused on the brain, an electrical field is induced in the underlying neural and non-neural tissues. This electrical field drives currents in the tissues, the characteristics of which are determined by their electrical conductivity and permittivity. Thus, the cortical current densities of TMS are determined by the stimulus waveform, the stimulating coil, and the relative coil-to-tissue distribution which is unique to each subject being stimulated.
The electromagnetic field distributions that arise during TMS are fundamental to understanding the resultant neural effects and have been studied to predict the cellular mechanism of activation (Roth and Basser 1990; Nagarajan et al. 1993), location (De Lucia et al. 2007; Wagner et al. 2007), focality (Ueno et al. 1988; Cohen and Cuffin 1991; Toschi et al. 2008), depth of penetration (Heller and Hulsteyn 1992; Zangen et al. 2005), and degree of stimulation (Bohning et al. 1997; Wagner et al. 2007); see Figure 1B and 1C. The field distributions can be also used for the quantitative analysis of the safety parameters (McCreery and Agnew 1990; McCreery et al. 1990; Wagner et al. 2007) and the technological potential of TMS (Davey and Riehl 2006; Kim et al. 2006).
Mechanism of activation and location of stimulation
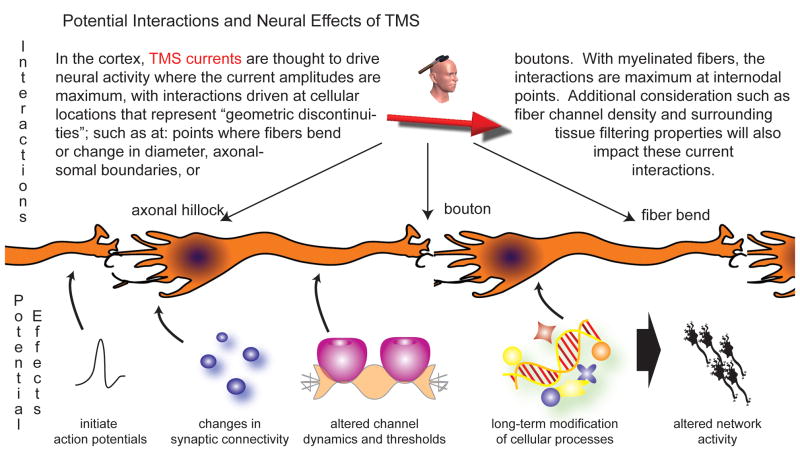
Magnetic stimulation is thought to actively initiate action potentials in neurons and/or alter the level of neural excitability during (online) and after (offline) stimulation. In addition to actively initiating action potentials, TMS may also manifest its effects through the induced modification of: membrane resting potentials and thresholds, channel properties with subsequent alterations in spontaneous activity, synaptic connectivity, timing dynamics of cellular gating components, and/or other similar mechanisms. Generally, it is thought that on-line suprathreshold TMS effects actively initiate action potentials of stimulated cells, such as for generating phosphenes during perception studies (Ramos-Estebanez et al. 2007), and that both online supra- and subthreshold TMS effects can alter integrated network activity, such as by using TMS to alter performance during working memory tasks (Luber et al. 2008). Offline TMS effects are thought to result from an alteration of the long-term excitability of neural cells and networks following stimulation (Thickbroom 2007). Yet, there is still significant debate as to what field mechanism drives active stimulation, whether it is the current’s movement and polarization of charge relative to neural structures, the electrical field’s interaction with the neuron channels at axonal boundaries, or other mechanisms (Ranck 1975; Roth and Basser 1990; Maccabee et al. 1993; Nagarajan et al. 1993; Lu et al. 2008; Rotem and Moses 2008). Even less agreement exists about the cellular-field interactions responsible for differentiating between sub-threshold vs. supra-threshold mechanisms, and between online and offline effects. However, all of the electromagnetic field parameters have an interconnected effect on the neural tissue and cells, and cortical TMS stimulation is most likely to be maximally initiated in the brain region where the currents are maximum, and specifically at axonal boundaries (such as axonal-soma and axonal-bouton boundaries) or fiber bends of individual cells that represent geometrical discontinuities at which the stimulating currents have their maximum impact (Tranchina and Nicholson 1986; Maccabee et al. 1993; Nagarajan et al. 1993); See Figure 2. Thus, even though the dynamics of offline cellular and network after-effects are influenced by the hodological (i.e., connectivity-based) features of the stimulated and neighboring brain regions, the initial stimulation targeting is guided by focusing the maximum of the stimulating currents at a single cortical target node associated with a predicted function (Valero-Cabre et al. 2005; Valero-Cabre et al. 2007); see Figure 1B.
Figure 2. Potential cortical interactions and effects of TMS.
This figure demonstrates locations of TMS induced current-cell interactions which can drive both immediate and long term effects in the cellular activity and integrated network behavior. Many of these concepts still need to be experimentally verified, and this represents one of the expanding frontiers of TMS research (including further areas such as potential astrocyte signaling roles in stimulation, subcortical neural stimulation mechanisms, state dependency effects, etc).
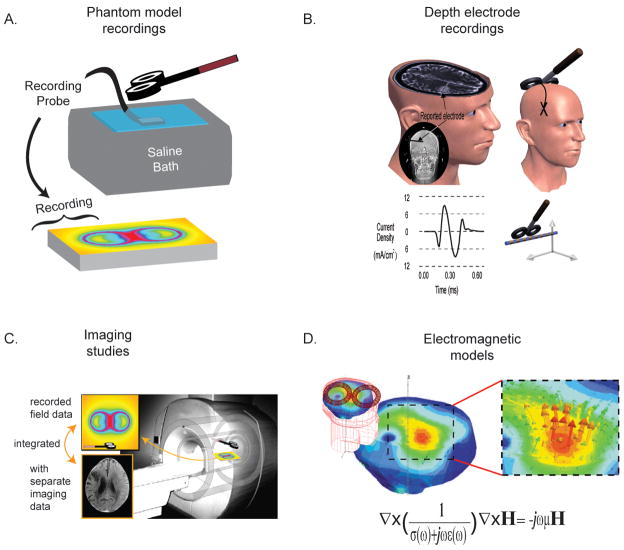
The location of the cortical current density maxima and associated fields have been predicted via phantom studies (Tay 1989; Yunokuchi et al. 1998), depth electrode recordings in humans and animals (Tay 1989; Lisanby 1998; Wagner et al. 2007), imaging studies (Bohning et al. 1997; Valero-Cabre et al. 2005; Valero-Cabre et al. 2007), and via electromagnetic modeling (Wagner et al. 2007); see Figure 3. Due to limitations in the other methods (Wagner et al. 2007), the latter is the primary method for predicting the maximum current density locations, whereby field maxima are determined by solving Maxwell’s equations in systems that model the anatomical geometry and tissue properties of the human head relative to the stimulating coil. Early models were based on simplified geometries representing the human brain, such as infinite planes or spheres, which modeled the tissues as simple homogenous conductors. With improved computational resources, models now include more realistic geometries (Nadeem et al. 2003; Lu et al. 2008; Toschi et al. 2008), tissue anisotropies (Miranda et al. 2003; De Lucia et al. 2007), and frequency dependent conductivity and permittivity (Wagner et al. 2004). Tissue anisotropies can have a significant effect on the induced current-to-neural orientations (see below), and the individual tissue anatomies and electromagnetic parameters have proven necessary in predicting the maximum current density locations. These parameters are especially important in regions of cortical inhomogeneity, such as boundaries between sulci and gyri, as they can often lead to the perturbation of the predicted location (and magnitude) of current density maxima when compared to homogenous brain regions (Miranda et al. 2003; Wagner et al. 2004). To date, maximum current density location predictions are made via MRI guided neuro-tracking systems, the basis and limitations of which are discussed below. Future work in this area will integrate our knowledge of the cellular basis of stimulation with our understanding of the electromagnetic field- tissue interactions to more effectively guide TMS targeting.
Figure 3. Multiple methods exist to evaluate the stimulating fields, including.
A. Phantom models. Recording probes are placed within containers of various geometries containing saline or other materials used to model the human head. B. Depth electrode recordings have been made of the current densities or induced voltage gradients in human and animal subjects. C. Imagining studies have been used to provide field information (adapted from (Nobel 2003)). D. Electromagnetic models are the primary method used to predict the stimulating field distributions.
Focality and depth of stimulation
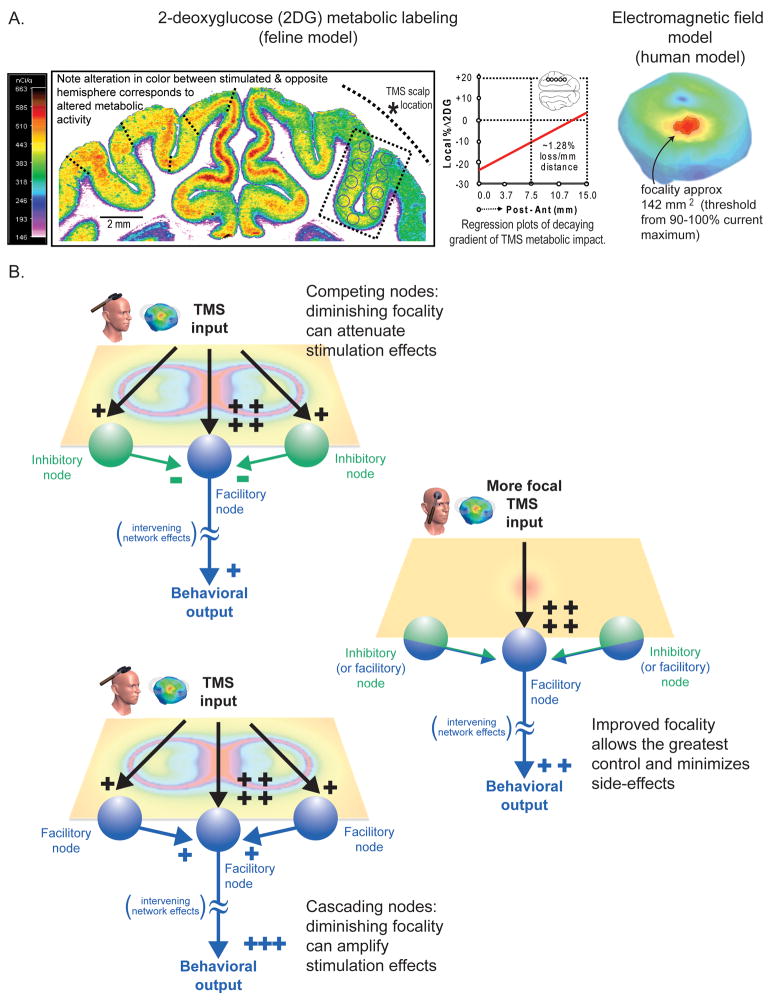
In addition to the location of stimulation, the penetration depth and focality of stimulation are key to understanding the TMS methodology. With TMS, current densities will always be maximum on the cortical surface, as the fields that comprise the TMS pulses (frequencies <10kHz) have corresponding wavelengths that are much larger than the dimensions of the human head, and thus preclude the superposition of multiple beams that could superpose for maxima below the cortical surface (Heller and Hulsteyn 1992). Therefore, even though coils and shielding mechanisms are being developed that attenuate the rate of the field decay from the scalp surface, allowing deeper structures to be stimulated (Carbunaru and Durand 2001; Zangen et al. 2005; Davey and Riehl 2006), such paradigms will always maximally affect the overlaying cortical surface. Thus, TMS focality is often estimated from a calculation of the cortical surface area where the current density magnitude (or electric field) produced by stimulation exceeds a certain threshold relative to their overall maxima or a preset value correlated to the neural stimulation threshold; see Figure 1B. This area is usually determined through model based calculations (Cohen and Cuffin 1991; Toschi et al. 2008) or from direct and indirect metabolic, electrophysiological, or behavioral measurements of the cortical effect (Ueno et al. 1990; Valero-Cabre et al. 2005). Early methods over-estimated the focality of TMS based on simplified models and metrics, predicting regions of even less than 25 mm2 with standard figure-of-eight coils, but recent studies have shown that although a small cortical region might be in the peak of the field, much broader regions of cortex are affected, easily exceeding 100–200 mm2 (dependent on the coil (size, type, and relative position), tissue distribution, and application paradigm); see Figure 4A. Additionally, while a single node might be maximally stimulated by TMS, current spread could potentially generate competing nodal effects in surrounding regions holding reciprocal inhibitory/facilitory connections in numerous and complex permutations (Fitzgerald et al. 2006; Thickbroom 2007); see Figure 4B. To address these limitations, researchers are developing innovative coils and shielding devices to improve TMS focality (Carbunaru and Durand 2001; Davey and Riehl 2006). However, because heating and internal repulsive forces become the limiting factor for coils smaller than approximately 2.5 cm in diameter (Cohen and Cuffin 1991), TMS efficiency diminishes rapidly with decreasing coil size. Thus, advancements in coil design and materials, heat dissipation techniques, and shielding mechanisms, are essential for improving the effectiveness and focality of TMS.
Figure 4. TMS focality.
A. 2DG recordings and current density model. Recordings using 2-deoxyglucose (2DG) metabolic labeling demonstrating a focal area of online effect of ~176 mm2 in a feline model; see (Valero-Cabre, Rushmore et al., 2005)). Note, smaller coils used in cats, which typically cannot be used with humans, usually lead to greater focality; but coils used in the clinic are thought to maximally stimulate regions exceeding ~100–200 mm2 (demonstrated on right with a field-model). Other studies have demonstrated that effective tissue stimulation can take place in smaller regions under certain stimulation conditions (Toschi et al. 2008). (Note- as technologies improve focality should be expressed in terms of volume.) B. Competing nodes. Even though small areas might be maximally stimulated during TMS, it is possible that nodes far from the maxima location are also affected (which might have an attenuating (or amplifying) affect on stimulation). Herein, we demonstrate the effects of a typical figure-of-eight coil with a predicted maximum effect at the coil’s center (but with competing effects at the coil boundaries) compared to a theoretical coil with greater focality (i.e., attenuated current spread) along a theoretical surface plane. Additionally when stimulation intensity is increased, side effects can potentially be induced through the coactivation of additional nodes with confounding function, which may be completely absent at lower stimulation intensities. Overall, one needs to consider all possible side-effects derived from a lack of focality, both those that might artificially boost or attenuate the magnitude the predicted behavioral effects. Fundamentally greater control in focality and depth will lead to more efficacious stimulation.
Orientation and waveform effects
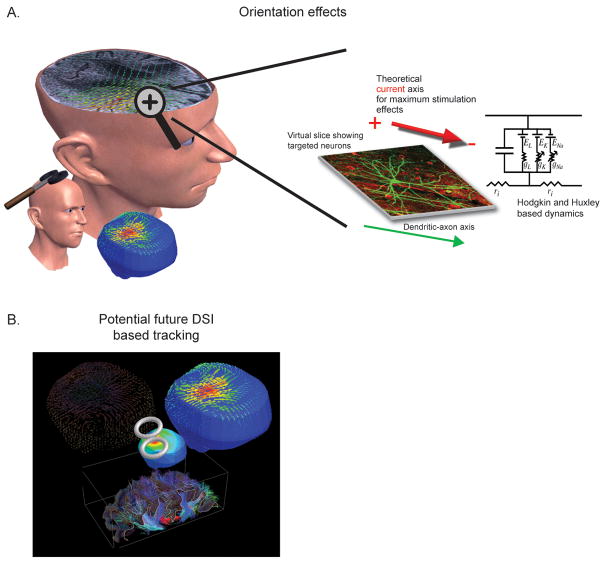
For TMS, the degree of stimulation is usually correlated with the predicted magnitude of the induced field strengths in the cortex, where it is assumed that more intense fields stimulate more cells. However, this view-point does not account for the relative induced current-to-neuron orientations or the temporal dynamics of the stimulating waveforms and their impact on stimulation (or further complicated cellular dynamics (Ranck 1975)). The orientation of individual neurons within the magnetic and induced current fields affects the efficacy of stimulation; thus, the degree to which the neuronal populations and areas are stimulated will vary depending on the morphology of the constituent neurons as well as the alignment of sulci and gyri relative to the coil placement (location, angle, and tilt). As a result, the optimal orientation of the current to the neural axonal axis will vary according to individual variability in neural architecture and current density orientations induced in individual brains; see Figure 5A. Overall, these orientation effects impact the stimulation threshold, the timing of neural response latencies, differences in evoked waveforms, and the summed network effect (Arai et al. 2005; Takahashi et al. 2005; Sommer et al. 2006; Balslev et al. 2007; Zafar et al. 2008). While there is presently no way to control directly for these effects in individual patients, Diffusion Spectrum Imaging (DSI) might provide a potential solution to this problem in the future (Wagner et al. 2006; Wedeen et al. 2008), particularly if the imaging resolution of the gray matter architecture is increased and the technique is integrated with a neurotracking/field-solver systems as discussed below; see 54B.
Figure 5. Orientation specificity.
A. Ideal stimulating current-to-neural axis orientation. An ideal axis will exist for each individual neural architecture, dependent on the individual cellular geometries (where herein the inhibitory/facilitory axis is idealized for graphical representation). B. DSI solution. Future devices could be possible where neural architecture information would be provided from DSI and integrated with a field-solver/neurotracking-system to provide stimulation guidance (part B adapted from (Wagner et al. 2006)). DSI information could be used as one of the building blocks of an electromagnetic model (providing information such as the relative neural architecture and anistropic tissue information) and integrated with both the subject anatomical data and measured electromagnetic tissue properties to more accurately solve for the TMS induced current densities. In turn, these current densities could then be projected back onto the initial DSI scans to serve as a neural architecture based predictor of stimulation.
Another factor to consider is the shape (time course) of the stimulating waveform. Today, pulse forms used for TMS include monophasic, half sine, and biphasic waveforms (other waveforms, such as near- rectangular waves are being explored, but not currently commercially available (Peterchev et al. 2008)). Each waveform shape evokes different stimulatory effects (including variations in stimulation thresholds, latencies, evoked waveform shape, etc) (Arai et al. 2005; Sommer et al. 2006; Zafar et al. 2008). The source of these differences is currently debated, but likely to be a function of the induced current distributions and a function of the individual channel response dynamics (tissues effectively filter the stimulating currents and ultimately the neural channels respond in a frequency/time dependent manner to varied stimulating waveforms (based on Hodgkin and Huxley dynamics)). Finally, by integrating an understanding of the waveform characteristics and the biophysical filtering effects of neural tissues, TMS researchers and clinicians may be able to tune stimulus waveforms to achieve optimal stimulatory efficiency. Ultimately, these waveform and orientation effects result from the physical foundations of stimulation discussed at the onset of this review, where the electromagnetic foundations of TMS provide information that needs to be integrated with the cellular electrophysiology, the connectivity-based brain features, and the cognitive outcomes of stimulation.
Biophysical and technological based safety considerations
The safety and dosing protocols of TMS have been established for some time, primarily related to preventing seizure induction (Wassermann 1998; Machii et al. 2006) (note the safety standards are currently being readdressed by Simone Rossi et al. to be published in Clinical Neurophysiology). However, many topics of biophysical origin should be further considered as TMS technologies evolve. For example, as TMS stimulation depths increase, it remains to be seen what cognitive side effects will result from such overlying surface stimulation. Furthermore, as different waveforms are adapted for stimulation, the mechanisms by which charges are carried in tissues will need to be further explored in terms of TMS neurohistotoxicity (McCreery and Agnew 1990; McCreery et al. 1990; Wagner et al. 2004). Another safety related area to consider during TMS studies is the use of non-translatable metrics, such as machine output power (alone or as a relative MEP threshold), to quantify stimulation efficiency. As made clear from the preceding discussion, it is difficult to apply such metrics across different patients, TMS devices, or brain regions in individual patients to describe variations in the biophysical or electrophysiological stimulatory effect. Until we have an objective measure that integrates the neural architecture, gauged cellular excitability, and induced current density distributions to be used with all studies, these metrics should be used cautiously. In the meantime while such predictive measures are unavailable, relative machine output values should at least be replaced with objective measures, such as pick-up probe captured field dynamics measured at the coil interface, to account for device variability, and correlated with simplified field calculations, to account for variability between patients.
Presently, many of these concerns are addressed in part with TMS guided MRI neurotracking systems, which predict the location of stimulation by localizing the relative projection of the stimulating coil to the underlying brain anatomy (these systems can be further integrated with field-solver systems to make generalized predictions of the induced current magnitude, focality, and penetration depth). However, the majority of commercially available MRI based neurotracking devices are based on simplified models which ignore subject specific electromagnetic field-tissue interactions (or implement over-simplified approximations of the cortical current densities) (Wagner et al. 2007). Thus, these technologies provide little if any patient specific information, no information about the predicted neural effect, and can in fact produce inaccurate predictions in regions of cortical inhomogeneity. This serves as an important area for future development, especially as technologies push the boundaries of what is possible with noninvasive stimulation.
Conclusions and future directions
TMS is a technique with considerable power for investigating and altering brain function. Nevertheless, many limitations need to be considered when designing and interpreting the outcomes of TMS studies. Biophysical limitations exist in TMS focality, depth of penetration, and targeting control. Furthermore, significant uncertainty still abounds related to the electrophysiological and biophysical basis of stimulation. However, as we improve our understanding of the TMS methodology and its supporting technologies, these limitations will be overcome, leading to improved TMS technologies or possibly entirely new noninvasive methodologies which can selectively provide controlled cortical and/or deep brain stimulation.
Footnotes
Conflict of interest statement: In addition to Dr. Timothy Wagner’s position at Harvard Medical School and MIT, he is the Chief Science Officer of Highland Instruments, a medical device company. He has multiple patents pending related to imaging, brain stimulation, and wound healing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science. 2007;317:1918–21. doi: 10.1126/science.1146426. [DOI] [PubMed] [Google Scholar]
- Arai N, Okabe S, Furubayashi T, Terao Y, Yuasa K, Ugawa Y. Comparison between short train, monophasic and biphasic repetitive TMS of the human motor cortex. Clin Neurophysiol. 2005;116:605–13. doi: 10.1016/j.clinph.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Balslev D, Braet W, McAllister C, Miall RC. Inter-individual variability in optimal current direction for TMS of the motor cortex. J Neurosci Methods. 2007;162:309–13. doi: 10.1016/j.jneumeth.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Barker AT, Freeston IL, Jalinous R, Merton PA, Morton HB. Magnetic stimulation of the human brain. Journal of Physiology (London) 1985;369:3P. [Google Scholar]
- Bestmann S. The physiological basis of transcranial magnetic stimulation. Trends Cogn Sci. 2008;12:81–3. doi: 10.1016/j.tics.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Bohning DE, Pecheny AP, Epstein CM, Speer AM, Vincent DJ, Dannels W, George MS. Mapping TMS fields in vivo with mri. Neuroreport. 1997;8:2535–8. doi: 10.1097/00001756-199707280-00023. [DOI] [PubMed] [Google Scholar]
- Carbunaru R, Durand DM. Toroidal coil models for transcutaneous magnetic stimulation of nerves. IEEE Trans Biomed Eng. 2001;48:434–41. doi: 10.1109/10.915709. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Payne JM, Stokes MG, Mattingley JB. Fast and slow parietal pathways mediate spatial attention. Nat Neurosci. 2004;7:217–8. doi: 10.1038/nn1203. [DOI] [PubMed] [Google Scholar]
- Cohen D, Cuffin BN. Developing a more focal magnetic stimulator. Journal of Clinical Neurophysiology. 1991;8:102–111. doi: 10.1097/00004691-199101000-00013. [DOI] [PubMed] [Google Scholar]
- d’Arsonval A. Dispositifs pour la mesure des courants alternatifs de toutes fréquences. C R Soc Biol (Paris) 1896;3:450–457. [Google Scholar]
- Davey K, Riehl M. Designing TMS systems. IEEE Transactions on Magnetics. 2005;41:1142–1148. [Google Scholar]
- Davey KR, Riehl M. Suppressing the surface field during TMS. IEEE Trans Biomed Eng. 2006;53:190–194. doi: 10.1109/TBME.2005.862545. [DOI] [PubMed] [Google Scholar]
- De Lucia M, Parker GJ, Embleton K, Newton JM, Walsh V. Diffusion tensor mri-based estimation of the influence of brain tissue anisotropy on the effects of TMS. Neuroimage. 2007;36:1159–70. doi: 10.1016/j.neuroimage.2007.03.062. [DOI] [PubMed] [Google Scholar]
- Faraday M. Experimental researches in electricity. London: JM Dent; 1914. [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rtms on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–96. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Heller L, Hulsteyn DBv. Brain stimulation using electromagnetic sources: Theoretical aspects. Biophysical Journal. 1992;63:129–138. doi: 10.1016/S0006-3495(92)81587-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KH, Nagarajan SS, Durand DM. Analysis of efficiency of magnetic stimulation. IEEE Trans Biomed Eng. 2003;50:1276–85. doi: 10.1109/TBME.2003.818473. [DOI] [PubMed] [Google Scholar]
- Jalinous R. Technical and practical aspects of magnetic nerve stimulation. Journal of Clinical Neurophysiology. 1991;8:10–25. doi: 10.1097/00004691-199101000-00004. [DOI] [PubMed] [Google Scholar]
- Kim D-H, Georghiou GE, Won C. Improved field localization in TMS of the brain with the utilization of a conductive shield plate in the stimulator. IEEE Trans Biomed Eng. 2006;53:720–725. doi: 10.1109/TBME.2006.870244. [DOI] [PubMed] [Google Scholar]
- Largus S. De compositionibus medicamentorum. Paris: Wechel; 1529. [Google Scholar]
- Lisanby S. Intercerebral measurements of rtms and ecs induced voltage in vivo. Biol Psychiatry. 1998;43:100s. [Google Scholar]
- Lu M, Ueno S, Thorlin T, Persson M. Calculating the activating function in the human brain by transcranial magnetic stimulation. IEEE Trans Magnetics. 2008;44:1438–1441. [Google Scholar]
- Luber B, Stanford AD, Bulow P, Nguyen T, Rakitin BC, Habeck C, Basner R, Stern Y, Lisanby SH. Remediation of sleep-deprivation-induced working memory impairment with fmri-guided TMS. Cereb Cortex. 2008;18:2077–85. doi: 10.1093/cercor/bhm231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccabee P, Amassian V, Eberle L, Cracco R. Magnetic coil stimulation of straight and bent amphibian and mammallian peripheral nerve in vitro: Locus of excitation. J Physiol. 1993;460:201–219. doi: 10.1113/jphysiol.1993.sp019467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machii K, Cohen D, Ramos-Estebanez C, Pascual-Leone A. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clin Neurophysiol. 2006;117:455–71. doi: 10.1016/j.clinph.2005.10.014. [DOI] [PubMed] [Google Scholar]
- McCreery D, Agnew W. Neuronal and axonal injury during functional electrical stimulation; a review of the possible mechanisms. IEEE. 1990;12:1489. [Google Scholar]
- McCreery D, Agnew W, Yuen TG, Bullara L. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans Biomed Eng. 1990;37:996–1001. doi: 10.1109/10.102812. [DOI] [PubMed] [Google Scholar]
- Miranda PC, Hallett M, Basser PJ. The electric field induced in the brain by magnetic stimulation: A 3-d finite-element analysis of the effect of tissue heterogeneity and anisotropy. IEEE Trans Biomed Eng. 2003;50:1074–85. doi: 10.1109/TBME.2003.816079. [DOI] [PubMed] [Google Scholar]
- Nadeem M, Thorlin T, Gandhi O, Persson M. Computation of electric and magnetic stimulation in human head using the 3-d impedance method. IEEE Transactions on Biomedical Engineering. 2003;50:900–907. doi: 10.1109/TBME.2003.813548. [DOI] [PubMed] [Google Scholar]
- Nagarajan S, Durand DM, Warman EN. Effects of induced electric fields on finite neuronal structures: A simulation study. IEEE Transactions on Biomedical Engineering. 1993;40:1175–1188. doi: 10.1109/10.245636. [DOI] [PubMed] [Google Scholar]
- Nobel Site. 2003 http://nobelprize.org/nobel_prizes/medicine/laureates/2003/mri_press1.jpg.
- Pascual-Leone A, Walsh V, Rothwell J. TMS in cognitive neuroscience--virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol. 2000;10:232–7. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Peterchev AV, Jalinous R, Lisanby SH. A transcranial magnetic stimulator inducing near-rectangular pulses with controllable pulse width. IEEE Trans Biomed Eng. 2008;55:257–66. doi: 10.1109/TBME.2007.900540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Estebanez C, Merabet LB, Machii K, Fregni F, Thut G, Wagner TA, Romei V, Amedi A, Pascual-Leone A. Visual phosphene perception modulated by subthreshold crossmodal sensory stimulation. J Neurosci. 2007;27:4178–81. doi: 10.1523/JNEUROSCI.5468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system. Brain Res. 1975;98:417–40. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007;8:559–67. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- Rotem A, Moses E. Magnetic stimulation of one-dimensional neuronal cultures. Biophys J. 2008;94:5065–78. doi: 10.1529/biophysj.107.125708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth B, Basser P. A model of stimulation of a nerve fiber by electromagnetic induction. IEEE Transactions on Biomedical Engineering. 1990;37:588–597. doi: 10.1109/10.55662. [DOI] [PubMed] [Google Scholar]
- Sommer M, Alfaro A, Rummel M, Speck S, Lang N, Tings T, Paulus W. Half-sine, monophasic and biphasic TMS of the human motor cortex. Clin Neurophysiol. 2006;117:838–44. doi: 10.1016/j.clinph.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ni Z, Yamashita T, Liang N, Sugawara K, Yahagi S, Kasai T. Differential modulations of intracortical neural circuits between two intrinsic hand muscles. Clin Neurophysiol. 2005;116:2757–64. doi: 10.1016/j.clinph.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Tay GCM, Battocletti J, Sances A, Jr, Swiontek T, Kurakami C. Measurement of magnetically induced current density in saline and in vivo. Proceedings of the Annual International Conference of the IEEE. 1989;4:1167–1168. [Google Scholar]
- Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: Experimental framework and human models. Exp Brain Res. 2007;180:583–93. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- Thielscher A, Kammer T. Linking physics with physiology in tms: A sphere field model to determine the cortical stimulation site in tms. Neuroimage. 2002;17:1117–30. doi: 10.1006/nimg.2002.1282. [DOI] [PubMed] [Google Scholar]
- Toschi N, Welt T, Guerrisi M, Keck ME. TMS in heterogeneous brain tissue: Clinical impact on focality, reproducibility and true sham stimulation. J Psychiatr Res. 2008 doi: 10.1016/j.jpsychires.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Tranchina D, Nicholoson C. A model for the polarization of neurons by extrinsically applied electric fields. Biophys J. 1986;50:1139–56. doi: 10.1016/S0006-3495(86)83558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Matsuda T, Fujiki M. Functional mapping of the human motor cortex obtained by focal and vectorial magnetic stimulation of the brain. IEEE Transactions on Magnetics. 1990;26:1539–1544. [Google Scholar]
- Ueno S, Tashiro T, Harada K. Localized stimulation of neural tissues in the brain by means of a paired configuration of time-varying magnetic fields. J Appl Phys. 1988;64:5862–5864. [Google Scholar]
- Valero-Cabre A, Payne BR, Pascual-Leone A. Opposite impact on (14)c-2-deoxyglucose brain metabolism following patterns of high and low frequency rTMS in the posterior parietal cortex. Exp Brain Res. 2007;176:603–15. doi: 10.1007/s00221-006-0639-8. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Rushmore R, Lomber SG, Payne BR, Pascual-Leone A. Impact of rTMS of the parietal cortex on metabolic brain activity: A 14c-2dg tracing study in the cat. Exp Brain Res. 2005;163:1–12. doi: 10.1007/s00221-004-2140-6. [DOI] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007 doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Wagner T, Zahn M, Wedeen VJ, Grodzinsky A, Pascual-Leone A. TMS: High resolution tracking of the induced current density in the individual human brain. Florence, Italy: OHBM; 2006. [Google Scholar]
- Wagner TA, Zahn M, Grodzinsky AJ, Pascual-Leone A. Three-dimensional head model simulation of TMS. IEEE Trans Biomed Eng. 2004;51:1586–98. doi: 10.1109/TBME.2004.827925. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of rTMS, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D’Arceuil H, de Crespigny AJ. Diffusion spectrum MRI tractography of crossing fibers. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Yunokuchi K, Kato R, Yoshida H, Tamari Y, Saito M. Study on the distributions of induced electric field in an inhomogeneous medium exposed a pulsed magnetic field. 1998;6:3294–3297. [Google Scholar]
- Zafar N, Paulus W, Sommer M. Comparative assessment of best conventional with best theta burst repetitive TMS protocols on human motor cortex excitability. Clin Neurophysiol. 2008;119:1393–9. doi: 10.1016/j.clinph.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Zangen A, Roth Y, Voller B, Hallett M. TMS of deep brain regions: Evidence for efficacy of the h-coil. Clin Neurophysiol. 2005;116:775–9. doi: 10.1016/j.clinph.2004.11.008. [DOI] [PubMed] [Google Scholar]