Abstract
This chapter reviews the neurological phenotype of Down syndrome (DS) in early development, childhood, and aging. Neuroanatomic abnormalities in DS are manifested as aberrations in gross brain structure as well as characteristic microdysgenetic changes. As the result of these morphological abnormalities, brain circuitry is impaired. While an intellectual disability is ubiquitous in DS, there is a wide range of variation in cognitive performance and a growing understanding between aberrant brain circuitry and the cognitive phenotype. Hypotonia is most marked at birth, affecting gait and ligamentous laxity. Seizures are bimodal in presentation with infantile spasms common in infancy and generalized seizures associated with cognitive decline observed in later years. While all individuals have the characteristic neuropathology of Alzheimer's disease (AD) by age 40years, the prevalence of dementia is not universal. The tendency to develop AD is related, in part, to several genes on chromosome 21 that are overexpressed in DS. Intraneuronal accumulation of β-amyloid appears to trigger a cascade of neurodegeneration resulting in the neuropathological and clinical manifestations of dementia. Functional brain imaging has elucidated the temporal sequence of amyloid deposition and glucose metabolic rate in the development of dementia in DS. Mitochondrial abnormalities contribute to oxidative stress which is part of AD pathogenesis in DS as well as AD in the general population. A variety of medical comorbidities threaten cognitive performance including sleep apnea, abnormalities in thyroid metabolism, and behavioral disturbances. Mouse models for DS are providing a platform for the formulation of clinical trials with intervention targeted to synaptic plasticity, brain biochemistry, and morphological brain alterations.
Keywords: Down syndrome, brain development, seizures, hypotonia, dementia, clinical trials
Neurological phenotypes in Down syndrome
The neurological phenotype in Down syndrome (DS) is the product of genetic expression and environmental influences. Like the other forms of genetically determined developmental disability, the neurological phenotype in DS changes across the life span. Changes in gene expression can determine differentiation of tissue involved in development and in functional decline associated with aging. Put differently, from the moment of conception we begin to age, a process involving decay in cellular structures, gene regulation, and DNA sequencing. Biological findings indicate that individuals with DS provide a link between development and aging. This review will examine the neurological phenotype at different age epochs in DS.
Neuroanatomic abnormalities and cognitive implications
The morphology of the brain in DS is a characteristic of the disorder and includes reduced brain weight with diminished proportions in the volumes of the frontal and temporal lobes. The brains of adults with DS are about 20% smaller than typically developing brains even when the measure is corrected for reduced body size (Kemper, 1991). This reduction in brain size appears in 4–5-month fetuses and progresses during the last 3months of gestation (Engidawork and Lubec, 2003; Guihard-Costa et al., 2006). Magnetic resonance imaging (MRI) studies show an approximate 17% decrease in children with DS between 10 and 20years (Pinter et al., 2001a). MRI studies confirm a selective decrease in the volumes of the hippocampus and the temporal lobes. The brain is brachycephalic with a small cerebellum, simplified gyral appearance, and a narrow superior temporal gyrus (Coyle et al., 1986; Wisniewski, 1990). These anatomic findings have also been seen by voxel-based morphometric MRI studies of the brain in children and adults with DS (Pinter et al., 2001a,b; Teipel et al., 2003, 2004; White and Alkire, 2003). The MRI studies have shown a remarkable preservation of subcortical gray matter structures in the face of a generally diminished brain volume, suggesting that there may be a temporal disassociation in development between the cortical versus subcortical areas. This may be an example of the disruption of developmental timing and homeostasis that occurs in DS and possibly other examples of aneuploidy (Pritchard and Kola, 1999). Unexplainably, the parahippocampal gyrus may be larger than normal in DS (Kesslak et al., 1994).
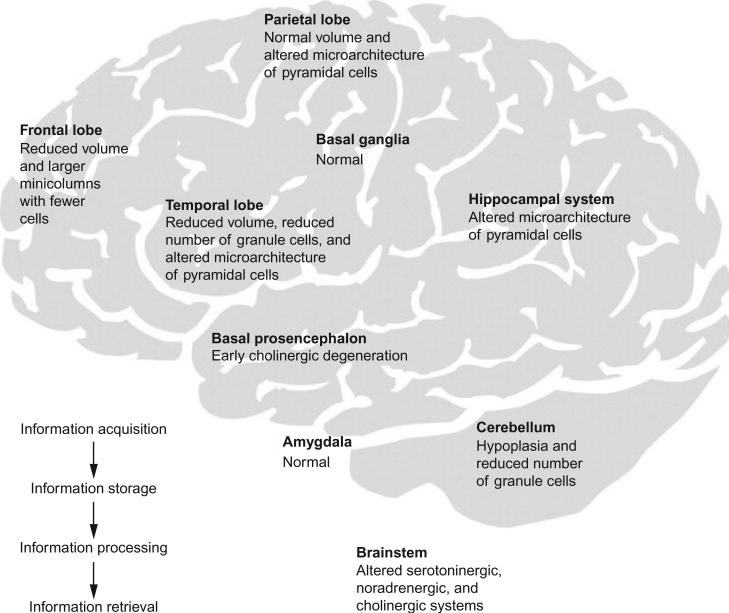
These gross anatomic abnormalities suggest that aberrations in fetal and early postnatal development may underlie the neuroanatomic abnormalities in DS. On the histological level, morphometric studies of the cortex show a reduced number of neurons, decreased neuronal densities, and abnormal neuronal distribution most marked in cortical layers II and IV. Ultra-structural studies show that synaptic density, synaptic length, and the contact zones between neurons are abnormal (Raz et al., 1995) (Fig. 1). Individuals with DS show a 20–50% reduction of neurons from birth to age 60months, suggesting that a deceleration in neurogenesis begins after 22weeks gestation (Schmidt-Sidor et al., 1990). However, neurogenetic cell differentiation is also impaired in fetuses with DS starting at 17weeks gestation as demonstrated by reduced numbers of dividing cells in the dentate gyrus and periventricular white matter (Contestabile et al., 2007). Factors cited to explain the decreased generation of neurons and their subsequent atrophy in DS have included a deleterious effect of the extra chromosome 21 on the timing of the mitotic cell cycle (Mittwoch and Wilkie, 1971). An analysis of cell proteins expressed in various stages of the cell cycle has shown a prolongation of the G2 phase of the cell cycle, the last step in inter-phase prior to mitotic division (Contestabile et al., 2007). In fetuses with DS, an analysis of cell phenotypes shows less reduction in astrocytes as compared to neurons (Guidi et al., 2008). In this study, there was an increased incidence of apoptotic cell death, suggesting that both neurogenesis and death of neurons are accounting for some of the microscopic alterations in the brain. Children with DS actually display greater dendritic branching and total dendritic length than controls up to age 6months, but then show a decrease in dendritic arborization during childhood and adult life (Becker et al., 1991). The functional consequence of these morphogenetic changes results in abnormal neuronal connectivity and limited processing of information. Smaller neurons and shorter dendritic lengths may result in shorter connections in which networks tend to be local rather than interhemispheral in scope. As recently reviewed (Lott and Dierssen, 2010), the cerebellum may be implicated in both cognition and affect in individuals with DS. The cerebellum is small. It seems plausible that neuro-psychological abnormalities in DS such as impaired attention, executive control, language learning, working memory, and emotional responses may reflect dysfunction in the cerebellar–cortical–limbic circuitry (Strick et al., 2009).
Fig. 1.
The learning circuit in DS. Structures involved in information acquisition, processing, or storage are affected in DS. The figure depicts some of the described changes that may lead to cognitive dysfunction in individuals with DS. (Reprinted from Lancet Neurology, Lott IT and Dierssen M, Cognitive deficits and associated neurological complications in individuals with Down's syndrome, 2010, June 9(6):623–33 with permission from Elsevier).
Cognitive development in DS has been described elsewhere in this volume (Edgin et al., 2010a). The precise effect of the above-described cyto-architectonic abnormalities on cognitive development in DS is not clear, in part due to individual variation in performance measures with age (Couzens et al., 2011; Tsao and Kindelberger, 2009). Weakness in language abilities of children with DS has been noted (Dykens et al., 2006; Fidler et al., 2005; Rondal et al., 2003). But even here, there is performance improvement with age as noted in lexical store, comprehension of interpersonal relations, and visual motor processing. Socialization and competence in daily living skills appear to improve through age 30years in DS (Dressler et al., 2010). Children with DS appear to present memory profiles that are distinct from other Williams and fragile-X syndromes in that DS is characterized by good immediate visual memory and rapid phonological retrieval with poor verbal working memory skills (Conners et al., 2011; Edgin et al., 2010b). As reviewed elsewhere in this volume, a battery of neuropsychological measures has been developed reflecting the functional status of the prefrontal cortex, hippocampus, and cerebellum.
Hypotonia
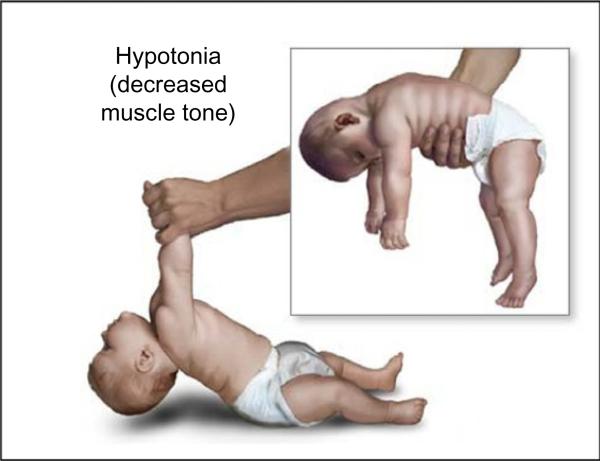
Hypotonia is ubiquitous in infants with DS and is defined as decreased resistance to passive muscle stretch (Fig. 2). The ligamentous laxity resulting from hypotonia is associated with a delay in motor development (Carr, 1970; Melyn and White, 1973). Infants with DS show a sequence of motor development similar to typically developing toddlers but a lower rate of motor milestone acquisition (Agiovlasitis et al., 2009). The hypotonia induces difficulty in postural control such that an individual with DS is focused on overcoming a lack of equilibrium compared to controls and this necessitates a different strategy in motor development (Rigoldi et al., 2011). The instability in trunk control may be linked to atypical finger grasping patterns (Jover et al., 2010). In a quantitative movement protocol measuring functional mobility, individuals with DS appeared to show longer durations of execution across all of the standardized tasks (Galli et al., 2010). As a consequence of hypotonia, individuals with DS have inherent joint laxity resulting in reduced gait stability and increased energetic costs for physical exertion (Agiovlasitis et al., 2009). These atypical gait patterns include longer stance time, decreased hip extension, and increased hip abduction during the swing phase. Hypotonia in DS is often associated with low levels of physical activity with the result of decreased bone mass accrual and predisposition to fractures (Hawli et al., 2009). The low levels of physical activity in DS result in a higher body mass index, lower levels of lean mass, and reduced bone mass-related parameters, which, in turn, may affect cardiovascular strength capacities (Gonzalez-Aguero et al., 2010).
Fig. 2.
Hypotonia in Down syndrome. Note the head lag upon pull to sitting and the inability to support posture in ventral suspension.
Ligamentous laxity in DS provokes instability of vertebral movement at the atlanto-axial junction. The occiput, the atlas (C1), and the axis (C2) normally form a functional unit which assures a high degree of mobility of the upper cervical spine providing that strong ligaments keep these structures in place. In individuals with DS, the excessive laxity of the posterior transverse ligament which attaches the odontoid bone to C1 appears to be the most common cause of atlanto-axial subluxation although there is no universal agreement on this point (Merrick et al., 2000). In addition, C-1 hypoplasia and occult spinal canal stenosis have been linked to the increased risk of compressive cervical myelopathy in individuals with DS.
The anatomic locus for hypotonia in DS appears to be the cerebellum, a structure which shows hypocellularity due to impairment in the granular and periventricular zones during fetal development (Guidi et al., 2011). Additionally, the cerebellum plays a major role in the regulation of proprioceptive-motor control and motor learning. Beyond its effect on motor functioning, disorganized cerebellar output may impair higher order functioning such as emotion and cognition (Schmahmann, 2004; Teipel et al., 2004).
Seizures
It is estimated that 5–13% of children with DS have seizures (Arya et al., 2011; Lujic et al., 2011). The occurrence is bimodal with 40% having seizures before 1year of age—generally infantile spasms—and with 40% developing seizures after the third decade, generally tonic–clonic or myoclonic in manifestation (Pueschel et al., 1991). Infantile spasms are associated with electroencephalographic (EEG) characteristics of idiopathic rather than symptomatic epilepsy. Children with DS have better seizure control following intervention with antiepileptic drugs than typically developing children. It appears that children with DS have better seizure control compared to other patients with symptomatic infantile spasms, and early initiation of appropriate treatment may contribute to the prevention of late seizure development and better developmental outcome (Arya et al., 2011).
When Lennox–Gastaut syndrome occurs in DS, the onset is usually later than seen in the typical population and may be associated with reflex seizures (Ferlazzo et al., 2009). There is a high rate of EEG abnormalities in DS without the recognition of clinical seizures (40%); therefore, EEG findings do not correlate with outcome (Smigielska-Kuzia et al., 2009). Electroclinical characteristics of seizures in adults with DS have included progressive slowing of background activity with frontal sharp and slow waves while those with myoclonic epilepsy typically display generalized spike and slow wave discharges (Vignoli et al., 2011).
Individuals with DS over age 45 years are more likely to develop dementia than those with seizures onset at younger ages (Menendez, 2005). Up to 84% of demented individuals with DS develop seizures. Senile myoclonic epilepsy appears to be a common manifestation of dementia in DS (De Simone et al., 2010).
The cellular basis of seizures may relate to the areas of brain dysgenesis that are commonly part of the neuroanatomic presentation in DS. Areas of focal cortical dysplasia may be associated with epilepsy in the general population related to the location and size of the lesions (Andrade, 2009; Leventer et al., 2008; Sisodiya et al., 2009). These areas of dysgenesis often indicate a pattern of molecular disruption that contributes to structural disorganization of the cortex and subsequent susceptibility to epilepsy. Audiogenic seizures have been observed in the rodent model (Ts65Dn) of DS and can be attenuated with antagonists to the metabotropic glutamate receptor 5 (mGluR5). These findings suggest that the amyloid precursor protein (APP), which is over-expressed in DS, may mediate this seizure susceptibility through the glutamate receptor (Westmark et al., 2009, 2010). Seizures in the temporal lobe may damage hippocampal circuitry, a situation which is worsened by the toxic accumulation of amyloid-β peptides seen in Alzheimer's disease (AD) (Noebels, 2011). There appears to be an association between the mGluR5 and APP in that activation of the glutamate receptor elevates APP in the dendrites of primary neuronal culture as well as synaptosomes (Westmark et al., 2009). These findings suggest that APP increases seizure incidence in AD. Treatment with glutamate receptor antagonists may help repress APP and serve as a therapeutic target for AD (Sokol et al., 2011).
Seizures appear to be associated with rapid cognitive decline in demented individuals with DS (Lott et al., 2012). In a retrospective study of individuals with DS and dementia, those with seizures became untestable by standard cognitive techniques employed for individuals with DS much sooner than the group without seizures. Adjustments were made for potentially confounding covariates of age, gender, APOE4 status, baseline cognitive impairment, years since dementia onset at baseline, and treatment assignment. Like those individuals with AD in the general population who develop seizures, cognitive decline is worsened in the group with DS and dementia. Prospective studies of this association are indicated.
Dementia
The characteristic neuropathology of AD is present in the brains of individuals with DS by age 40 years (Mann and Esiri, 1989; Wisniewski et al., 1985). The findings include the accumulation of senile plaques (amyloid-β-protein) and neurofibrillary tangles (hyperphosphorylated tau protein). In the most common form of trisomy 21 in DS, there is an overexpression of the gene for APP from which the amyloid-β-protein is derived (Rumble et al., 1989). Intraneuronal accumulation of β-amyloid appears to trigger a cascade of neurodegeneration. Abnormal subcellular processing of β-amyloid leads to increased amyloid secretion and the generation of free radicals, thereby increasing the burden of oxidative stress (Zigman and Lott, 2007). Immune dysfunction in DS has been associated with the occurrence of AD (Kusters et al., 2009). The immune dysfunction begins in children with DS as reflected in abnormalities seen on the analysis of peripheral T-cell subsets, natural killer cells, and serum cytokines (Cetiner et al., 2010). DNA damage, repair, and abnormal dynamic flux have been linked to oxidative stress and AD-type neurodegeneration (Martin, 2008; Vasudevaraju et al., 2008). These observations have led to neurobiological studies of APP and the temporal events in AD pathogenesis in DS. Other genes on chromosome 21 that have been implicated in the pathogenesis of AD include superoxide dismutase, Ets-2 transcription factors, DS critical region stress-inducible factor, β-site APP cleaving enzyme, and S100β and dual-specificity tyrosine phosphorylation-regulation kinase 1A (DYRK1A) (Head and Lott, 2004).
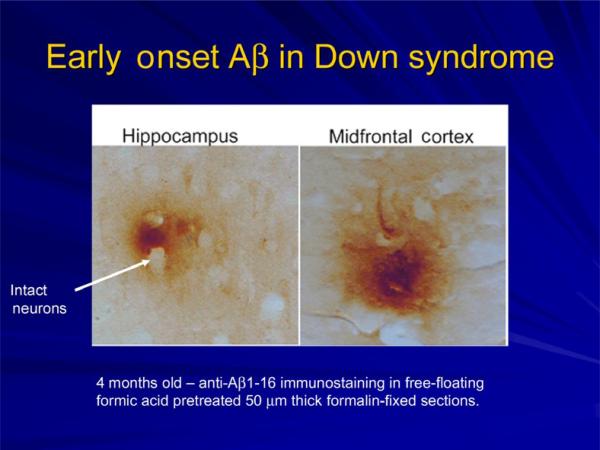
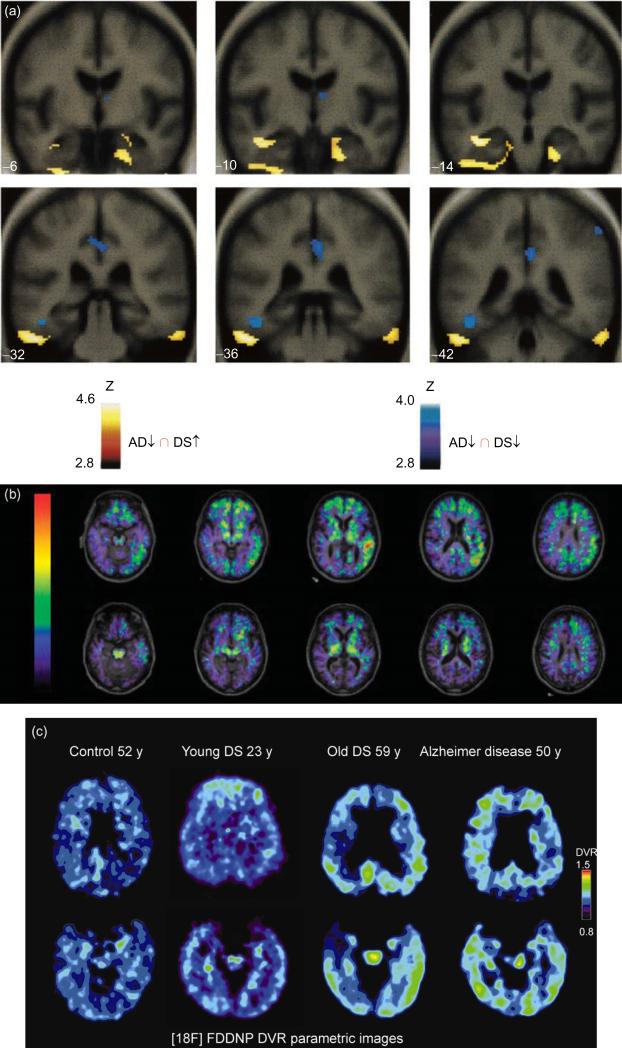
Another consequence of the gene dosage increase in APP in DS is the early deposition of β-amyloid in brain (Fig. 3). The parahippocampal and inferior temporal gyri demonstrate β-amyloid staining as young as 8years of age with subsequent accumulation in the CA-1/subiculum, dentate molecular layer, and the remainder of the hippocampal area (Leverenz and Raskind, 1998). Molecular dating techniques have shown that β-amyloid is deposited in the superficial layers of the frontal cortex in DS with subsequent transition to deeper cortical areas with age (Azizeh et al., 2000). In a report examining the distribution of intraneuronal and extracellular brain–protein aggregates associated with AD in the general population, only the individual with DS showed extracellular β-amyloid and neuritic plaques (Braak and Del Tredici, 2011). Functional neuroimaging and pathological studies have been directed toward the underlying processes of aging and dementia in DS. Positron emission imaging studies of glucose metabolic rate (GMR) in the brains of individuals with DS who are concentrating on a task show increased GMR in the medial temporal lobe in the same areas that are reduced in individuals with AD in the general population (Fig. 4a). This finding suggests that there may be a compensatory reaction occurring in the entorhinal cortex and hippo-campus in young adults with DS prior to the onset of dementia. Neurite sprouting has been seen in approximately the same areas by postmortem examination (Haier et al., 2003; Head et al., 2007). Dynamic [11C] PiB PET (11C-labeled Pittsburgh Compound B that specifically binds fibrillar β-amyloid plaques) imaging has been carried out in a small sample of adults with DS and preliminary findings suggest a correlation between amyloid deposition and age (Fig. 4b) (Landt et al., 2011). In a study of [18F] FDDNP PET (FDDNP, molecule that binds to plaques and tangles in vivo) functional brain imaging, there is high binding levels of the ligand in DS comparable to those seen in AD (Fig. 4c). Measures of behavioral dysfunction in the same study are consistent with age-related progression of AD neuropathology in this population (Nelson et al., 2011). Future functional imaging studies in DS are likely to elucidate the dynamics of amyloid plaque formation and the occurrence of neurofibrillary tangles as well as their relationship to neuropsychological measures.
Fig. 3.
Aβ deposition in brain of an infant with Down syndrome. (Image provided by Dr. Elizabeth Head, Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY).
Fig. 4.
(a) FDG imaging in Down syndrome (DS) (Reprinted from Neurology, Haier RJ, et al., Temporal cortex hypermetabolism in Down syndrome prior to the onset of dementia, 2003, Dec 23;61(12):1673–1679, with permission from Wolter Kluwer Health). Results of SPM'99 conjunction analyses. Blue areas show where glucose metabolic rate (GMR) is lower in subjects with Alzheimer's disease (AD) compared with matched controls and where GMR is lower in nondemented DS subjects compared with matched controls. Yellow areas show the conjunction of where GMR is higher in the nondemented DS group compared with matched controls and where GMR is lower in the AD subjects compared with their matched controls. Note: The yellow areas are in the inferior temporal/entorhinal cortex; blue areas are in the posterior cingulate and left fusiform gyrus. Statistical results are shown on MRI templates for six coronal slices (-6-42), which show a large section of inferiotemporal/entorhinal cortex. Talairach and Tournoux atlas coordinates of all findings (p<0.001). (b) PiB amyloid staining for dementia in DS (Reprinted from Archives of Neurology, Landt J et al., Using positron emission tomography and carbon 11-labeled Pittsburgh compound B to image brain fibrillar β-amyloid in adults with Down syndrome: Safety, acceptability, and feasibility, 2011, July; 68 (7):890–896 with permission from American Medical Association). Fused carbon 11-labeled Pittsburgh Compound B nondisplaceable binding potential and magnetic resonance images for a subject with DS and AD (top) and a control without DS (bottom). (c) FDDNP imaging in DS (Reprinted from Archives of Neurology, Nelson LD et al., Positron emission tomogrpahy of brain β-amyloid and tau levels in adults with Down syndrome, 2011, June; 68(6):768–774 with permission from American Medical Association). 18F-FDDNP PET imaging in control, young DS, old DS, and AD showing binding characteristics.
However, β-amyloid immunoreactivity has been reported in neuronal structures that have no AD pathology, suggesting that brain amyloid may not have an invariable role in the formation. An alternative explanation may relate to the DYRK1A which is located on chromosome 21 and regulates multiple genes that may contribute to deregulation of neural pathways responsible for dementia (Ji et al., 2010). Specifically, DYRK1A overexpression in the brains of individuals with DS likely contributes to early onset neurofibrillary degeneration, in part, through the hyperphosphorylation of tau. The several-fold increase in the number of DYRK1A and tau-positive neurofibrillary tangles in DS supports this hypothesis. DYRK1A also appears to enhance phosphorylation of APP, thereby elevating Aβ40 and Aβ42 levels. Thus, DYRK1A-mediated hyper-phosphorylation of tau may provide a functional link between DS and AD (Ryoo et al., 2007).
The role of plasma levels of β-amyloid as a bio-marker for AD in DS has been studied extensively. Among adults with DS, decreasing levels of plasma Aβ42, a decline in the Aβ42/Aβ40 ratio, or increasing levels of Aβ40 may be sensitive indicators of conversion to AD, possibly reflecting compartmentalization of β-amyloid peptides in the brain (Schupf et al., 2010). Plasma β-amyloid levels alone did not dissociate DS adults with and without dementia. However, in demented adults with DS, APOE4 allele was associated with higher Aβ40 but not Aβ42. After controlling for level of intellectual disability (mild, moderate, severe) and the presence or absence of dementia, there was an improved prediction of neuropsychological scores by plasma levels of β-amyloid (Head et al., 2011). There is an association of several biomarkers for AD for in the general population that are also seen in individuals with DS including APOE, SORL1, RUNX1, BACE1, and ALDH18a1 (Patel et al., 2011). However, other studies have not found a relationship between the APOE genotype and plasma levels of Aβ40 or Aβ42 (Jones et al., 2009).
Individuals with DS have an increased tendency toward oxidative stress. Lack of balance in the metabolism of free radicals may have a direct role in the development of neuropathological changes of AD in DS (Zigman and Lott, 2007). Urinary markers of oxidative stress are increased in adolescents and adults with DS (Campos et al., 2010). Children with DS have an imbalance in the plasma and urinary levels of melatonin and kynurenine, thereby enhancing susceptibility toward oxidative stress (Uberos et al., 2010). DNA damage that occurs during the process of oxidative stress has been implicated in both DS and AD, but a pathogenetic precedent has not yet been established (Martin, 2008).
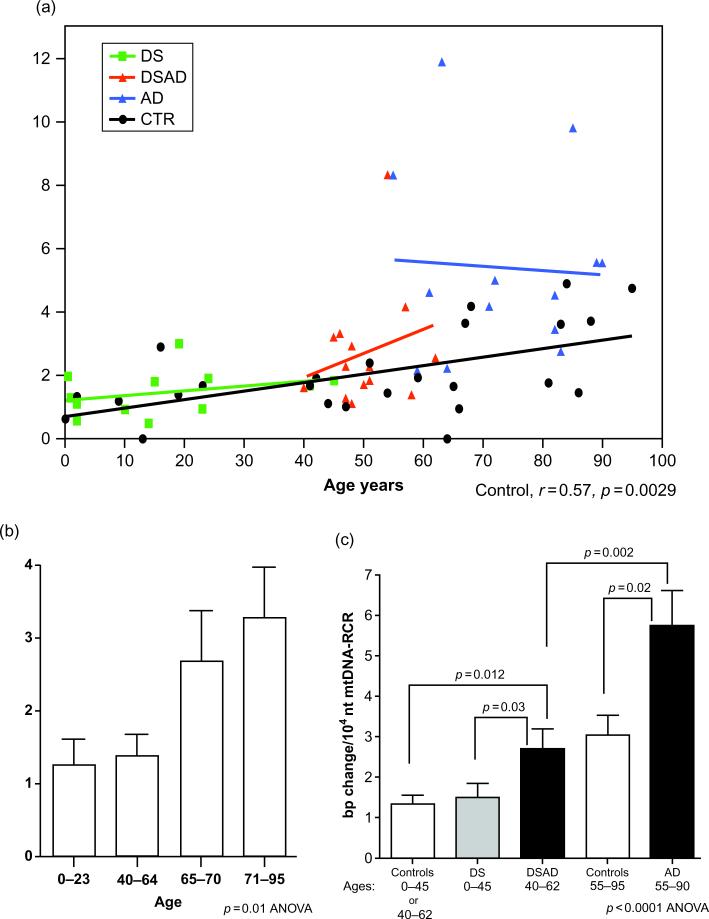
Mitochondrial control region mutations are seen in the brains of individuals with DS and dementia as well as AD (Coskun et al., 2010) (Fig. 5). These mitochondrial mutations are also seen in peripheral blood and DNA from lymphoblastoid cell lines. A complex I deficit has been described in mitochondria from skin fibroblasts in DS that produces free radicals associated with oxidative stress (Valenti et al., 2011). Methylation, which is a necessary event in mitochondrial functioning, is impaired in DS (Infantino et al., 2011). Dysregulated chromosome 21 genes in cortical neural progenitor cells from individuals with DS lead to elevated levels of S100β-induced radical oxygen species along with loss of the water channel aquaporin 4, causing an increase in programmed cell death (Lu et al., 2011). Caspase activation, another precursor of programmed cell death, occurs in association with amyloid plaques and neurofibrillary tangles in DS (Head et al., 2002). Fibroblasts in DS have a higher than normal level of apoptosis and are more susceptible to the proapoptotic effects of okadaic acid, a toxin known to induce malformations and inhibit differentiation in cell lines (Dogliotti et al., 2010). Multiple genes that are upregulated in trisomy 21 may contribute to transcriptional disruption and increase the liability to oxidative stress (Lockstone et al., 2007; Strydom et al., 2009). Oxidative stress may be a common pathogenetic mechanism across multiple genetic disorders including ataxia-telangiectasia, Fanconi anemia, Werner syndrome, and DS (Lloret et al., 2011). Clinical trials to treat oxidative stress in DS are reported elsewhere in this volume.
Fig. 5.
mtDNA Regulatory Control Region (RCR) mutation frequency in the frontal cortices of control, DS, DSAD, and AD patients. (a) The correlation of mtDNA RCR mutation frequency with aging in all four groups. (b) The comparison of mtDNA RCR mutation frequency in four different age groups: 0–23, 40–64, 65–70, and 71–95years. (c) The comparison of mtDNA RCR mutation frequencies of DSAD and AD brains to age-matched controls and DS brains. Because the control groups for DS (ages 0–40) and DSAD (ages 40–62) were not significantly different (see panel b), DS and DSAD control groups were grouped together. (Reprinted from Journal of Alzheimer's Disease, Coskun P et al., Mitochondrial dysfunction and the etiology of Alzheimer's disease and Down syndrome dementia, 2010:20, S293–S310, with permission from IOS press).
Comorbidities affecting the neurological phenotype
Obstructive sleep apnea occurs in over half of children with DS aged 2–4years and is related to otolaryngological problems associated with the disorder (Shott et al., 2006). There have been few studies of the cognitive consequences of obstructive sleep apnea in DS, but there is known to be an association of sleep apnea with lower IQ testing and behavioral difficulties (Bass et al., 2004; Melendres et al., 2004). In a small study of children with DS, sleep apnea was associated with difficulties in visual-perceptual skills involving the right hemisphere (Andreou et al., 2002). The consequence for cognition may be higher for adults with DS based upon studies of aging in the general population. Findings here show impaired working memory, attention, vigilance, and the ability to carry out simulated activities in the face of obstructive sleep apnea (Jackson et al., 2011). Volume reduction in the hippocampus has been observed in adults with chronic sleep apnea (Torelli et al., 2011). In patients with mild cognitive impairment in the general population, sleep apnea was associated with an increase in language dysfunction (Kim et al., 2011). Further studies on the neurological effect of sleep disorders in individuals with DS are indicated.
Hearing and visual disturbances can complicate the neurological evaluation of all patients with intellectual disability. Individuals with DS are prone to develop hyperopia, strabismus, blepharitis, cataracts, and glaucoma (Esbensen, 2010; van Allen et al., 1999). Hearing loss in adults in the general population may be independently associated with lower test scores of memory and executive functioning (Gates et al., 2010; Lin et al., 2011). Therefore, hearing and visual evaluation should be part of any diagnosis of cognitive dysfunction in DS.
Evaluation of thyroid functioning has been a part of health care guidelines for both children and adults with DS (Virji-Babul et al., 2007). In subclinical and overt hypothyroidism in the general population, deficits are noted in spatial, associative, and verbal memory with some amelioration by thyroid replacement therapy (Kramer et al., 2009). The impairments resulting from hypothyroidism are primarily mnemonic rather than a reflection of general cognitive slowing and suggest a disruption of normal hippocampal function or connectivity (Correia et al., 2009). However, other studies of subclinical hypothyroidism in the general population have failed to show significant neuropsychiatric problems (Baek et al., 2009). Hashimoto's encephalopathy, an autoimmune complication of hypothyroidism, which presents with myoclonus, neuropsychiatric symptoms, cognitive decline, and stroke-like episodes, has been described in DS (Brodtmann, 2009; Popova et al., 2008). The course is fluctuating in DS without a female preponderance. Histopathological brain lesions include gliosis, demyelination, spongiform transformation of neurons, and vasculitis involving venules (Vincent and Crino, 2011). There are variable abnormalities on MRI brain imaging with up to half of the studies in the normal range. There is a response in many patients to steroids or intravenous immunoglobulin.
Children with DS may be at a lower risk for significant behavioral comorbidities in that they show a lower profile of maladaptive behaviors compared to children with other intellectual disabilities (Dykens and Kasari, 1997; Stores et al., 1998). However, in comparison to typically developing age-matched peers, children with DS show higher rates of inattention, oppositional behaviors, and impulsivity (Dykens, 2007). Cleland (Cleland et al., 2010) has challenged the stereotypical perception that individuals with DS are highly social and have excellent “people” skills. Differences are cited in developing task-oriented strategies for problem solving and difficulties with goal-directed behaviors (Glenn et al., 2001; Jahromi et al., 2008; Kasari and Freeman, 2001; Wishart, 1993; Wishart and Manning, 1996). These studies provide some evidence for interpersonal difficulties experienced by individuals with DS throughout life despite general perceptions to the contrary. The curious association of DS with autistic disorder may reflect an underlying problem in social interactions in some children with DS. Recent use of screening tools and standardized diagnostic measures in 123 children with DS has yielded a prevalence of 18% for autistic spectrum disorders and 7% for the diagnosis of autism (DiGuiseppi et al., 2010). This is over a 10-fold increase compared to the general population (Lowenthal et al., 2007). Commonalities between the two conditions have been sought on several levels of research. Difficulties in word recognition, oral language, non-verbal ability, and working memory may underline reading comprehension problems in both DS and autistic spectrum disorders (Ricketts, 2011). Cortical-inhibition circuitries regulating neural plasticity has been postulated as related to the pathogenesis of brain functioning in both DS and autistic disorder (Baroncelli et al., 2011). Morphological brain studies in autism have shown dysgenetic abnormalities in the frontal lobes, amygdala, and cerebellum—all areas that have been described as dysgenetic in DS. Recent reviews have suggested that the time course of brain development may be an important determining factor (Amaral et al., 2008). Put slightly differently, deviant growth trajectories in autism may lead to developmental disruption of established patterns of functional connectivity (Lewis and Elman, 2008). Moreover, careful morphological studies of postmortem brain of individuals with autism have shown subgroup variability in neuronal density, providing a cytoarchitectonic foundation for brain heterogeneity (Simms et al., 2009). Future research is likely to highlight differences in the neuroanatomic and functional bases of the comorbidity between DS and autistic spectrum disorders. Other behavioral disorders in DS have centered on depression. While it has been thought that individuals with DS are more prone to depression than those with other forms of intellectual disability, a recent meta-review does not support the putative increased prevalence of depression in trisomy 21 (Walker et al., 2011). However, there are a number of risk factors in DS that may connote a vulnerability to depression including smaller hippocampal volumes, changes in neurotransmitter systems, deficits in attachment behaviors, and frequently occurring somatic disorders.
Altering the neurological phenotype through treatment
Understanding of the brain region affected by trisomy in mouse models has been helpful in the development of pharmacological approaches that may improve cognitive function in individuals with DS (Das and Reeves, 2011). The authors summarize the mechanisms by which pharmacological intervention appears to alter synaptic plasticity, brain biochemistry, and/or brain morphology in the mouse models for DS. The advantages of using mouse models for understanding various abnormalities in DS are numerous and include the ability to study a sufficient number of animals in a short time, genetic engineering for individual genes, control for genetic background, low cost of screening, ability to study both developing and mature subjects, modeling therapeutic interventions, and the lack of postmortem delays (Patterson, 2009; Salehi et al., 2007). Cognitive impairment may differ in various mouse models for DS and through this variation may provide a platform for mechanism-based drug design. There are many genes in the mouse that are orthologous to genes on human chromosome 21 (Das and Reeves, 2011). Studies in the mouse allow mapping of similar brain regions in the human for which there may be potential pharmacogenetic targets. However, the relationship between specific mouse targets and those in human trisomy 21 is complex. Individuals with DS show a broad spectrum of cognitive abilities and disabilities, suggesting that a single pharmacological agent may not be effective throughout the intellectual continuum in the disorder. Additionally, many of the mouse agents focus on the hippocampus while cognitive function in humans is dependent on many brain regions that interact with the hippocampus such as the entorhinal cortex, striatum, cerebellum, and cerebral cortex. The future studies of these areas in the mouse model promise to be an exciting area in the development of neurocognitive therapies for DS.
There have been a number of clinical trials in DS that are based upon trying to improve dysfunctional neuronal circuitry in the disorder. The most prominent of these has been focused on the cholinergic system in brain. Memory loss in old age is strongly correlated with a decline in the levels of acetylcholine (Mori et al., 2002; White and Ruske, 2002). The areas of the brain rich in cholinergic neurons such as the basal forebrain nuclei show a massive degeneration in cholinergic neurons. Administration of drugs that inhibit the breakdown of acetylcholine (donepezil) has reversed the memory deficits associated with aging and AD in the general population (Rusted et al., 1994; van Reekum et al., 1997).
As previously noted, individuals with DS have the characteristic neuropathology of AD by age 40years. The Ts65Dn mouse model of DS appears to parallel the cholinergic degeneration found in adults with DS and dementia which is correlated with deficient release of acetylcholine from the hippocampus (Holtzman et al., 1996). An encouraging aspect of the mouse study was the reversal of deficits in the 4-month-old Ts65Dn mouse when treated with an acetylcholinesterase inhibitor (Chang and Gold, 2008). In part, these results in the animal model led to trials of several different acetylcholinesterase inhibitors in adults with DS and dementia. However, in DS, there appears to be no consistent or compelling evidence for efficacy of acetylcholinesterase inhibitors including donepezil, galantamine, and rivastigmine in preventing the cognitive decline associated with dementia (Mohan et al., 2009a–d). Likewise, a trial of donepezil in children and adolescents with DS failed to demonstrate cognitive benefit (Kishnani et al., 2010). These results underlie the complexity of interpreting findings in animal models for human pharmacological intervention.
Basal forebrain cholinergic neuronal degeneration has been reversed by intracerebroventricular injections of nerve growth factor (Cooper et al., 2001). In evaluation of the genes within the targeted chromosomal segment in Ts65Dn mice, it became apparent that an extra copy of the gene for APP contributed to decreased transport of nerve growth factor (Salehi et al., 2006). This raises the prospect that treatment to reduce the level of APP gene expression may lessen the severity of cognitive decline in the dementia associated with DS (Megarbane et al., 2009).
Memantine is an antagonist of N-methyl d-aspartate receptor and appears to exert a positive effect on cognitive function by reducing oxidative damage and upregulating brain-derived neurotrophic factor (Marvanova et al., 2001). The compound is also a noncompetitive inhibitor of the serotonin receptor 5HT3 (Rammes et al., 2001). In the general population, the drug appears to alleviate some symptoms of AD (Ferris, 2003). In the Ts65Dn mouse, oral treatment with memantine improved spatial learning in the Morris water maze task (Rueda et al., 2008). These findings have been translated into a clinical trial of memantine for improving cognitive functioning in young adults with DS which is currently underway (Costa, 2011).
Individuals with DS and mice with partial trisomy contain an extra copy of the DYRK1A gene and this appears to be associated with changes in brain morphogenesis (Dierssen and de Lagran, 2006; Toiber et al., 2010). Learning problems similar to those seen in individuals with DS have been reported in transgenic mice using a human YAC construct that contains five genes including DYRK1A. Increased gene dosage of DYRK1A results in brain morphogenesis defects, low brain-derived neurotrophic factor levels, and mnemonic deficits in these trisomy 21 mice. Overexpression of DYRK1A may exert its effect on apoptosis and cell survival (Tejedor and Hammerle, 2011). Epigallocatechin gallate—a member of a natural polyphenols family, found in great amount in green tea leaves—is a specific and safe DYRK1A inhibitor. In a study utilizing a polyphenol diet, the major features of the transgenic phenotype were rescued (Guedj et al., 2009).
An imbalance between inhibitory and excitatory neurotransmission may contribute to altered brain function in DS. A GABA-A antagonist to the major central nervous system inhibitory transmitter appears to restore cognitive function in Ts65Dn mice but has convulsant properties. Development of a GABA-A benzodiazepine receptor inverse agonist that is selective for the α5-subtype receptor appears to eliminate the convulsant propensity while maintaining cognitive restorative properties (Braudeau et al., 2011a,b). This compound appears to have potential significance for clinical trials.
The challenge of therapeutic trials in DS is similar to those reviewed for AD in the general population for which pleiotropic interventions have been recommended (Frautschy and Cole, 2010). In this context, there is a need not only for stage-specific intervention in AD pathogenesis but also for correcting abnormal pathways that will not be affected by targeting prodromal mechanisms. In DS, these abnormal pathways include loss of synapses, aberrant compensatory responses, defects in energy metabolism, and loss of neuroprotective mechanisms. In a useful review of potential drug candidates to improve cognitive functioning in DS, agents are categorized into those that influence synaptic plasticity, brain biochemistry, or brain pathology, respectively (Das and Reeves, 2011). An ongoing conundrum for the use of agents to improve cognitive functioning is the interaction of the drug with existing medication, an issue that has been explored in the neuropsychiatric literature and which can be addressed partially through preclinical studies (Wallace et al., 2011).
Nonpharmacological interventions such as cognitive therapy and exercise have shown some improvements in individuals with AD within the general population (Buschert et al., 2010; Littbrand et al., 2011). While cognitive improvement as the result of exercise has not been proven in DS, there are improvements in work performance variables for individuals in the exercise group. Although evidence exists to support improvements in physiological and psychological aspects from strategies using mixed physical activity programs, well-conducted research examining long-term physical outcomes, adverse effects, psychosocial outcomes, and costs is required before informed practice decisions can be made (Andriolo et al., 2010; Shields and Taylor, 2010).
Medical comorbidities, some of which have been reviewed above, can effect neurological performance in individuals with DS and negatively alter the cognitive phenotype. In a study of non-psychiatric health factors leading to psychiatric decompensation in adults with DS, 40% were found to have a significant comorbid medical condition considered causal in the behavioral decline (Charlot et al., 2011). Premature onset of medical comorbidities that may affect cognitive functioning in adults with DS include visual and hearing impairments, adult onset seizures, thyroid dys-function, sleep apnea, and musculoskeletal problems (Esbensen, 2010). The quality of life in adults with DS who are at risk for AD as well as children whose intellectual development may be impacted by comorbid medical conditions can be improved considerably by systematic health care screening and adherence to health care guidelines (van Allen et al., 1999).
Concluding remarks
Many of the neurological characteristics of individuals with DS may be considered to reflect aberrations in the timing and sequence of development. Examples include systematic alterations in neural circuitry, age-specific onset of epilepsy, the evolution of amyloid deposition in brain, and interactions of genes on chromosome 21. Mouse models have been enormously helpful in providing a foundation for targets of pharmacological intervention. However, there are challenges in translating the results from mice studies to human clinical trials. It is encouraging that more trials are being developed upon specific brain mechanisms based upon detailed studies in the mouse. This approach is welcomed in an area that has been underserved in clinical trials. Understanding the basis for cognitive morbidity in DS and its potential treatment holds the additional promise of elucidating important aspects of development and aging within the general population.
Acknowledgments
This research was supported by NIH grants HD-065160, AG-21912, HD-37427. I thank Nina Movsesyan, PhD, for her assistance in the preparation of this manuscript.
References
- Agiovlasitis S, McCubbin JA, Yun J, Pavol MJ, Widrick JJ. Economy and preferred speed of walking in adults with and without Down syndrome. Adapted Physical Activity Quarterly. 2009;26:118–130. doi: 10.1123/apaq.26.2.118. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends in Neurosciences. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Andrade DM. Genetic basis in epilepsies caused by malformations of cortical development and in those with structurally normal brain. Human Genetics. 2009;126:173–193. doi: 10.1007/s00439-009-0702-1. [DOI] [PubMed] [Google Scholar]
- Andreou G, Galanopoulou C, Gourgoulianis K, Karapetsas A, Molyvdas P. Cognitive status in Down syndrome individuals with sleep disordered breathing deficits (SDB). Brain and Cognition. 2002;50:145–149. doi: 10.1016/s0278-2626(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Andriolo RB, El Dib RP, Ramos L, Atallah AN, da Silva EM. Aerobic exercise training programmes for improving physical and psychosocial health in adults with Down syndrome. Cochrane Database of Systematic Reviews. 2010:CD005176. doi: 10.1002/14651858.CD005176.pub4. [DOI] [PubMed] [Google Scholar]
- Arya R, Kabra M, Gulati S. Epilepsy in children with Down syndrome. Epileptic Disorders. 2011;13:1–7. doi: 10.1684/epd.2011.0415. [DOI] [PubMed] [Google Scholar]
- Azizeh BY, Head E, Ibrahim MA, Torp R, Tenner AJ, Kim RC, et al. Molecular dating of senile plaques in the brains of individuals with Down syndrome and in aged dogs. Experimental Neurology. 2000;163:111–122. doi: 10.1006/exnr.2000.7359. [DOI] [PubMed] [Google Scholar]
- Baek KH, Zaslavsky A, Lynch RC, Britt C, Okada Y, Siarey RJ, et al. Down's syndrome suppression of tumour growth and the role of the calcineurin inhibitor DSCR1. Nature. 2009;459:1126–1130. doi: 10.1038/nature08062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroncelli L, Braschi C, Spolidoro M, Begenisic T, Maffei L, Sale A. Brain plasticity and disease: A matter of inhibition. Neural Plasticity. 2011;2011:286073. doi: 10.1155/2011/286073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass JL, Corwin M, Gozal D, Moore C, Nishida H, Parker S, et al. The effect of chronic or intermittent hypoxia on cognition in childhood: A review of the evidence. Pediatrics. 2004;114:805–816. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- Becker L, Mito T, Takashima S, Onodera K. Growth and development of the brain in Down syndrome. Progress in Clinical and Biological Research. 1991;373:133–152. [PubMed] [Google Scholar]
- Braak H, del Tredici K. The pathological process underlying Alzheimer's disease in individuals under thirty. Acta Neuropathologica. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- Braudeau J, Dauphinot L, Duchon A, Loistron A, Dodd RH, Herault Y, et al. Chronic treatment with a promnesiant GABA-A alpha5-selective inverse agonist increases immediate early genes expression during memory processing in mice and rectifies their expression levels in a Down syndrome mouse model. Advances in Pharmacological Sciences. 2011a;2011:153218. doi: 10.1155/2011/153218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braudeau J, Delatour B, Duchon A, Pereira PL, Dauphinot L, de Chaumont F, et al. Specific targeting of the GABA-A receptor alpha5 subtype by a selective inverse agonist restores cognitive deficits in Down syndrome mice. Journal of Psychopharmacology. 2011b;25:1030–1042. doi: 10.1177/0269881111405366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodtmann A. Hashimoto encephalopathy and Down syndrome. Archives of Neurology. 2009;66:663–666. doi: 10.1001/archneurol.2009.45. [DOI] [PubMed] [Google Scholar]
- Buschert V, Bokde AL, Hampel H. Cognitive intervention in Alzheimer disease. Nature Reviews Neurology. 2010;6:508–517. doi: 10.1038/nrneurol.2010.113. [DOI] [PubMed] [Google Scholar]
- Campos C, Guzman R, Lopez-Fernandez E, Casado A. Urinary uric acid and antioxidant capacity in children and adults with Down syndrome. Clinical Biochemistry. 2010;43:228–233. doi: 10.1016/j.clinbiochem.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Carr J. Mental and motor development in young Mongol children. Journal of Mental Deficiency Research. 1970;14:205–220. doi: 10.1111/j.1365-2788.1970.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Cetiner S, Demirhan O, Inal TC, Tastemir D, Sertdemir Y. Analysis of peripheral blood T-cell subsets, natural killer cells and serum levels of cytokines in children with Down syndrome. International Journal of Immunogenetics. 2010;37:233–237. doi: 10.1111/j.1744-313X.2010.00914.x. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Age-related changes in memory and in acetylcholine functions in the hippocampus in the Ts65Dn mouse, a model of Down syndrome. Neurobiology of Learning and Memory. 2008;89:167–177. doi: 10.1016/j.nlm.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlot L, Abend S, Ravin P, Mastis K, Hunt A, Deutsch C. Non-psychiatric health problems among psychiatric inpatients with intellectual disabilities. Journal of Intellectual Disability Research. 2011;55:199–209. doi: 10.1111/j.1365-2788.2010.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland J, Wood S, Hardcastle W, Wishart J, Timmins C. Relationship between speech, oromotor, language and cognitive abilities in children with Down's syndrome. International Journal of Language & Communication Disorders. 2010;45:83–95. doi: 10.3109/13682820902745453. [DOI] [PubMed] [Google Scholar]
- Conners FA, Moore MS, Loveall SJ, Merrill EC. Memory profiles of Down, Williams, and fragile X syndromes: Implications for reading development. Journal of Developmental and Behavioral Pediatrics. 2011;32:405–417. doi: 10.1097/DBP.0b013e3182168f95. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Fila T, Ceccarelli C, Bonasoni P, Bonapace L, Santini D, et al. Cell cycle alteration and decreased cell proliferation in the hippocampal dentate gyrus and in the neocortical germinal matrix of fetuses with Down syndrome and in Ts65Dn mice. Hippocampus. 2007;17:665–678. doi: 10.1002/hipo.20308. [DOI] [PubMed] [Google Scholar]
- Cooper JD, Salehi A, Delcroix JD, Howe CL, Belichenko PV, Chua-Couzens J, et al. Failed retrograde transport of NGF in a mouse model of Down's syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10439–10444. doi: 10.1073/pnas.181219298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia N, Mullally S, Cooke G, Tun TK, Phelan N, Feeney J, et al. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. The Journal of Clinical Endocrinology and Metabolism. 2009;94:3789–3797. doi: 10.1210/jc.2008-2702. [DOI] [PubMed] [Google Scholar]
- Coskun PE, Wyrembak J, Derbereva O, Melkonian G, Doran E, Lott IT, et al. Systemic mitochondrial dysfunction and the etiology of Alzheimer's disease and Down syndrome dementia. Journal of Alzheimer's Disease. 2010;20(Suppl. 2):S293–S310. doi: 10.3233/JAD-2010-100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AC. On the promise of pharmacotherapies targeted at cognitive and neurodegenerative components of Down syndrome. Developmental Neuroscience. 2011;33:414–427. doi: 10.1159/000330861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzens D, Cuskelly M, Haynes M. Cognitive development and Down syndrome: Age-related change on the Stanford-Binet test (fourth edition). American Journal on Intellectual and Developmental Disabilities. 2011;116:181–204. doi: 10.1352/1944-7558-116.3.181. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Oster-Granite ML, Gearhart JD. The neurobiologic consequences of Down syndrome. Brain Research Bulletin. 1986;16:773–787. doi: 10.1016/0361-9230(86)90074-2. [DOI] [PubMed] [Google Scholar]
- Das I, Reeves RH. The use of mouse models to understand and improve cognitive deficits in Down syndrome. Disease Models & Mechanisms. 2011;4:596–606. doi: 10.1242/dmm.007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Simone R, Puig XS, Gélisse P, Crespel A, Genton P. Senile myoclonic epilepsy: Delineation of a common condition associated with Alzheimer's disease in Down syndrome. Seizure. 2010;19:383–389. doi: 10.1016/j.seizure.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Dierssen M, de Lagran MM. DYRK1A (dual-specificity tyrosine-phosphorylated and -regulated kinase 1A): A gene with dosage effect during development and neurogenesis. TheScientificWorldJOURNAL. 2006;6:1911–1922. doi: 10.1100/tsw.2006.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diguiseppi C, Hepburn S, Davis JM, Fidler DJ, Hartway S, Lee NR, et al. Screening for autism spectrum disorders in children with Down syndrome: Population prevalence and screening test characteristics. Journal of Developmental and Behavioral Pediatrics. 2010;31:181–191. doi: 10.1097/DBP.0b013e3181d5aa6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogliotti G, Galliera E, Dozio E, Vianello E, Villa RE, Licastro F, et al. Okadaic acid induces apoptosis in Down syndrome fibroblasts. Toxicology In Vitro. 2010;24:815–821. doi: 10.1016/j.tiv.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Dressler A, Perelli V, Feucht M, Bargagna S. Adaptive behaviour in Down syndrome: A cross-sectional study from childhood to adulthood. Wiener Klinische Wochenschrift. 2010;122:673–680. doi: 10.1007/s00508-010-1504-0. [DOI] [PubMed] [Google Scholar]
- Dykens EM. Psychiatric and behavioral disorders in persons with Down syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:272–278. doi: 10.1002/mrdd.20159. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Hodapp RM, Evans DW. Profiles and development of adaptive behavior in children with Down syndrome. Down's Syndrome, Research and Practice. 2006;9:45–50. doi: 10.3104/reprints.293. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Kasari C. Maladaptive behavior in children with Prader-Willi syndrome, Down syndrome, and nonspecific mental retardation. American Journal of Mental Retardation. 1997;102:228–237. doi: 10.1352/0895-8017(1997)102<0228:MBICWP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Edgin JO, Mason GM, Allman MJ, Capone GT, Deleon I, Maslen C, et al. Development and validation of the Arizona Cognitive Test Battery for Down syndrome. Journal of Neurodevelopmental Disorders. 2010a;2:149–164. doi: 10.1007/s11689-010-9054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgin JO, Pennington BF, Mervis CB. Neuropsychological components of intellectual disability: The contributions of immediate, working, and associative memory. Journal of Intellectual Disability Research. 2010b;54:406–417. doi: 10.1111/j.1365-2788.2010.01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engidawork E, Lubec G. Molecular changes in fetal Down syndrome brain. Journal of Neurochemistry. 2003;84:895–904. doi: 10.1046/j.1471-4159.2003.01614.x. [DOI] [PubMed] [Google Scholar]
- Esbensen AJ. Health conditions associated with aging and end of life of adults with Down syndrome. International Review of Research in Mental Retardation. 2010;39:107–126. doi: 10.1016/S0074-7750(10)39004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlazzo E, Adjien CK, Guerrini R, Calarese T, Crespel A, Elia M, et al. Lennox-Gastaut syndrome with late-onset and prominent reflex seizures in trisomy 21 patients. Epilepsia. 2009;50:1587–1595. doi: 10.1111/j.1528-1167.2008.01944.x. [DOI] [PubMed] [Google Scholar]
- Ferris SH. Evaluation of memantine for the treatment of Alzheimer's disease. Expert Opinion on Pharmacotherapy. 2003;4:2305–2313. doi: 10.1517/14656566.4.12.2305. [DOI] [PubMed] [Google Scholar]
- Fidler DJ, Most DE, Guiberson MM. Neuropsychological correlates of word identification in Down syndrome. Research in Developmental Disabilities. 2005;26:487–501. doi: 10.1016/j.ridd.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Cole GM. Why pleiotropic interventions are needed for Alzheimer's disease. Molecular Neurobiology. 2010;41:392–409. doi: 10.1007/s12035-010-8137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli M, Cimolin V, Patti P, Ferrario D, Heaney G, Albertini G, et al. Quantifying established clinical assessment measures using 3D-movement analysis in individuals with Down syndrome. Disability and Rehabilitation. 2010;32:1768–1774. doi: 10.3109/09638281003734367. [DOI] [PubMed] [Google Scholar]
- Gates GA, Gibbons LE, McCurry SM, Crane PK, Feeney MP, Larson EB. Executive dysfunction and presbycusis in older persons with and without memory loss and dementia. Cognitive and Behavioral Neurology. 2010;23:218–223. doi: 10.1097/WNN.0b013e3181d748d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn S, Dayus B, Cunningham C, Horgan M. Mastery motivation in children with Down syndrome. Down's Syndrome, Research and Practice. 2001;7:52–59. doi: 10.3104/reports.114. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Aguero A, Vicente-Rodriguez G, Moreno LA, Guerra-Balic M, Ara I, Casajus JA. Health-related physical fitness in children and adolescents with Down syndrome and response to training. Scandinavian Journal of Medicine & Science in Sports. 2010;20:716–724. doi: 10.1111/j.1600-0838.2010.01120.x. [DOI] [PubMed] [Google Scholar]
- Guedj F, Sebrie C, Rivals I, Ledru A, Paly E, Bizot JC, et al. Green tea polyphenols rescue of brain defects induced by overexpression of DYRK1A. PLoS One. 2009;4:e4606. doi: 10.1371/journal.pone.0004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi S, Bonasoni P, Ceccarelli C, Santini D, Gualtieri F, Ciani E, et al. Neurogenesis impairment and increased cell death reduce total neuron number in the hippocampal region of fetuses with Down syndrome. Brain Pathology. 2008;18:180–197. doi: 10.1111/j.1750-3639.2007.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi S, Ciani E, Bonasoni P, Santini D, Bartesaghi R. Widespread proliferation impairment and hypocellularity in the cerebellum of fetuses with Down syndrome. Brain Pathology. 2011;21:361–373. doi: 10.1111/j.1750-3639.2010.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guihard-Costa AM, Khung S, Delbecque K, Menez F, Delezoide AL. Biometry of face and brain in fetuses with trisomy 21. Pediatric Research. 2006;59:33–38. doi: 10.1203/01.pdr.0000190580.88391.9a. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Alkire MT, White NS, Uncapher MR, Head E, Lott IT, et al. Temporal cortex hypermetabolism in Down syndrome prior to the onset of dementia. Neurology. 2003;61:1673–1679. doi: 10.1212/01.wnl.0000098935.36984.25. [DOI] [PubMed] [Google Scholar]
- Hawli Y, Nasrallah M, El-Hajj Fuleihan G. Endocrine and musculoskeletal abnormalities in patients with Down syndrome. Nature Reviews Endocrinology. 2009;5:327–334. doi: 10.1038/nrendo.2009.80. [DOI] [PubMed] [Google Scholar]
- Head E, Doran E, Nistor M, Hill M, Schmitt FA, Haier RJ, et al. Plasma amyloid-beta as a function of age, level of intellectual disability, and presence of dementia in Down syndrome. Journal of Alzheimer's Disease. 2011;23:399–409. doi: 10.3233/JAD-2010-101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head E, Lott IT. Down syndrome and beta-amyloid deposition. Current Opinion in Neurology. 2004;17:95–100. doi: 10.1097/00019052-200404000-00003. [DOI] [PubMed] [Google Scholar]
- Head E, Lott IT, Cribbs DH, Cotman CW, Rohn TT. Beta-amyloid deposition and neurofibrillary tangle association with caspase activation in Down syndrome. Neuroscience Letters. 2002;330:99–103. doi: 10.1016/s0304-3940(02)00705-x. [DOI] [PubMed] [Google Scholar]
- Head E, Lott IT, Patterson D, Doran E, Haier RJ. Possible compensatory events in adult Down syndrome brain prior to the development of Alzheimer disease neuropathology: Targets for nonpharmacological intervention. Journal of Alzheimer's Disease. 2007;11:61–76. doi: 10.3233/jad-2007-11110. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Santucci D, Kilbridge J, Chua-Couzens J, Fontana DJ, Daniels SE, et al. Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13333–13338. doi: 10.1073/pnas.93.23.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantino V, Castegna A, Iacobazzi F, Spera I, Scala I, Andria G, et al. Impairment of methyl cycle affects mitochondrial methyl availability and glutathione level in Down's syndrome. Molecular Genetics and Metabolism. 2011;102:378–382. doi: 10.1016/j.ymgme.2010.11.166. [DOI] [PubMed] [Google Scholar]
- Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Progress in Brain Research. 2011;190:53–68. doi: 10.1016/B978-0-444-53817-8.00003-7. [DOI] [PubMed] [Google Scholar]
- Jahromi LB, Gulsrud A, Kasari C. Emotional competence in children with Down syndrome: Negativity and regulation. American Journal of Mental Retardation. 2008;113:32–43. doi: 10.1352/0895-8017(2008)113[32:ECICWD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ji L, Chauhan V, Mehta P, Wegiel J, Mehta S, Chauhan A. Relationship between proteolytically cleaved gelsolin and levels of amyloid-beta protein in the brains of Down syndrome subjects. Journal of Alzheimer's Disease. 2010;22:609–617. doi: 10.3233/JAD-2010-101029. [DOI] [PubMed] [Google Scholar]
- Jones EL, Hanney M, Francis PT, Ballard CG. Amyloid beta concentrations in older people with Down syndrome and dementia. Neuroscience Letters. 2009;451:162–164. doi: 10.1016/j.neulet.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Jover M, Ayoun C, Berton C, Carlier M. Specific grasp characteristics of children with trisomy 21. Developmental Psychobiology. 2010;52:782–793. doi: 10.1002/dev.20474. [DOI] [PubMed] [Google Scholar]
- Kasari C, Freeman SF. Task-related social behavior in children with Down syndrome. American Journal of Mental Retardation. 2001;106:253–264. doi: 10.1352/0895-8017(2001)106<0253:TRSBIC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kemper TL. Down syndrome. In: Peters A, Jones EJ, editors. Cerebral cortex. Plenum Press Kluwer Academic; 1991. [Google Scholar]
- Kesslak JP, Nagata SF, Lott I, Nalcioglu O. Magnetic resonance imaging analysis of age-related changes in the brains of individuals with Down's syndrome. Neurology. 1994;44:1039–1045. doi: 10.1212/wnl.44.6.1039. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lee JH, Lee DY, Jhoo JH, Woo JI. Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. The American Journal of Geriatric Psychiatry. 2011;19:374–381. doi: 10.1097/JGP.0b013e3181e9b976. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, Heller JH, Spiridigliozzi GA, Lott I, Escobar L, Richardson S, et al. Donepezil for treatment of cognitive dysfunction in children with Down syndrome aged 10–17. American Journal of Medical Genetics. Part A. 2010;152A:3028–3035. doi: 10.1002/ajmg.a.33730. [DOI] [PubMed] [Google Scholar]
- Kramer CK, von Muhlen D, Kritz-Silverstein D, Barrett-Connor E. Treated hypothyroidism, cognitive function, and depressed mood in old age: The Rancho Bernardo study. European Journal of Endocrinology. 2009;161:917–921. doi: 10.1530/EJE-09-0606. [DOI] [PubMed] [Google Scholar]
- Kusters MA, Verstegen RH, Gemen EF, de Vries E. Intrinsic defect of the immune system in children with Down syndrome: A review. Clinical and Experimental Immunology. 2009;156:189–193. doi: 10.1111/j.1365-2249.2009.03890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landt J, D'Abrera JC, Holland AJ, Aigbirhio FI, Fryer TD, Canales R, et al. Using positron emission tomography and Carbon 11-labeled Pittsburgh Compound B to image Brain Fibrillar beta-amyloid in adults with Down syndrome: Safety, acceptability, and feasibility. Archives of Neurology. 2011;68:890–896. doi: 10.1001/archneurol.2011.36. [DOI] [PubMed] [Google Scholar]
- Leventer RJ, Guerrini R, Dobyns WB. Malformations of cortical development and epilepsy. Dialogues in Clinical Neuroscience. 2008;10:47–62. doi: 10.31887/DCNS.2008.10.1/rjleventer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverenz JB, Raskind MA. Early amyloid deposition in the medial temporal lobe of young Down syndrome patients: A regional quantitative analysis. Experimental Neurology. 1998;150:296–304. doi: 10.1006/exnr.1997.6777. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Elman JL. Growth-related neural reorganization and the autism phenotype: A test of the hypothesis that altered brain growth leads to altered connectivity. Developmental Science. 2008;11:135–155. doi: 10.1111/j.1467-7687.2007.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25:763–770. doi: 10.1037/a0024238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littbrand H, Stenvall M, Rosendahl E. Applicability and effects of physical exercise on physical and cognitive functions and activities of daily living among people with dementia: A systematic review. American Journal of Physical Medicine & Rehabilitation. 2011;90:495–518. doi: 10.1097/PHM.0b013e318214de26. [DOI] [PubMed] [Google Scholar]
- Lloret A, Badia MC, Giraldo E, Ermak G, Alonso MD, Pallardo FV, et al. Amyloid-beta toxicity and tau hyperphosphorylation are linked via RCAN1 in Alzheimer's disease. Journal of Alzheimer's Disease. 2011;27:701–709. doi: 10.3233/JAD-2011-110890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockstone HE, Harris LW, Swatton JE, Wayland MT, Holland AJ, Bahn S. Gene expression profiling in the adult Down syndrome brain. Genomics. 2007;90:647–660. doi: 10.1016/j.ygeno.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lott IT, Dierssen M. Cognitive deficits and associated neurological complications in individuals with Down's syndrome. Lancet Neurology. 2010;9:623–633. doi: 10.1016/S1474-4422(10)70112-5. [DOI] [PubMed] [Google Scholar]
- Lott I, Doran E, Nguyen VQ, Tournay A, Movsesyan N, Gillen DL. Down syndrome and dementia: Seizures and cognitive decline. Journal of Alzheimer's Disease. 2012 doi: 10.3233/JAD-2012-111613. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal R, Paula CS, Schwartzman JS, Brunoni D, Mercadante MT. Prevalence of pervasive developmental disorder in Down's syndrome. Journal of Autism and Developmental Disorders. 2007;37:1394–1395. doi: 10.1007/s10803-007-0374-4. [DOI] [PubMed] [Google Scholar]
- Lu J, Esposito G, Scuderi C, Steardo L, Delli-Bovi LC, Hecht JL, et al. S100B and APP promote a gliocentric shift and impaired neurogenesis in Down syndrome neural progenitors. PLoS One. 2011;6:e22126. doi: 10.1371/journal.pone.0022126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujic L, Bosnjak VM, Delin S, Duranovic V, Krakar G. Infantile spasms in children with Down syndrome. Collegium Antropologicum. 2011;35(Suppl. 1):213–218. [PubMed] [Google Scholar]
- Mann DM, Esiri MM. The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down's syndrome. Journal of Neurological Sciences. 1989;89:169–179. doi: 10.1016/0022-510x(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Martin LJ. DNA damage and repair: Relevance to mechanisms of neurodegeneration. Journal of Neuropathology and Experimental Neurology. 2008;67:377–387. doi: 10.1097/NEN.0b013e31816ff780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvanova M, Lakso M, Pirhonen J, Nawa H, Wong G, Castren E. The neuroprotective agent memantine induces brain-derived neurotrophic factor and trkB receptor expression in rat brain. Molecular and Cellular Neurosciences. 2001;18:247–258. doi: 10.1006/mcne.2001.1027. [DOI] [PubMed] [Google Scholar]
- Megarbane A, Ravel A, Mircher C, Sturtz F, Grattau Y, Rethore MO, et al. The 50th anniversary of the discovery of trisomy 21: The past, present, and future of research and treatment of Down syndrome. Genetics in Medicine. 2009;11:611–616. doi: 10.1097/GIM.0b013e3181b2e34c. [DOI] [PubMed] [Google Scholar]
- Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114:768–775. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- Melyn MA, White DT. Mental and developmental milestones of noninstitutionalized Down's syndrome children. Pediatrics. 1973;52:542–545. [PubMed] [Google Scholar]
- Menendez M. Down syndrome, Alzheimer's disease and seizures. Brain & Development. 2005;27:246–252. doi: 10.1016/j.braindev.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Merrick J, Ezra E, Josef B, Hendel D, Steinberg DM, Wientroub S. Musculoskeletal problems in Down Syndrome European Paediatric Orthopaedic Society Survey: The Israeli sample. Journal of Pediatric Orthopaedics. Part B. 2000;9:185–192. doi: 10.1097/01202412-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Mittwoch U, Wilkie D. The effect of chlorimipramine on DNA synthesis and mitosis in cultured human cells. British Journal of Experimental Pathology. 1971;52:186–191. [PMC free article] [PubMed] [Google Scholar]
- Mohan M, Bennett C, Carpenter PK. Galantamine for dementia in people with Down syndrome. Cochrane Database of Systematic Reviews. 2009a:CD007656. doi: 10.1002/14651858.CD007656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan M, Bennett C, Carpenter PK. Memantine for dementia in people with Down syndrome. Cochrane Database of Systematic Reviews. 2009b:CD007657. doi: 10.1002/14651858.CD007657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan M, Bennett C, Carpenter PK. Rivastigmine for dementia in people with Down syndrome. Cochrane Database of Systematic Reviews. 2009c:CD007658. doi: 10.1002/14651858.CD007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan M, Carpenter PK, Bennett C. Donepezil for dementia in people with Down syndrome. Cochrane Database of Systematic Reviews. 2009d:CD007178. doi: 10.1002/14651858.CD007178.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori C, Spooner ET, Wisniewsk KE, Wisniewski TM, Yamaguch H, Saido TC, et al. Intraneuronal Abeta42 accumulation in Down syndrome brain. Amyloid. 2002;9:88–102. [PubMed] [Google Scholar]
- Nelson LD, Siddarth P, Kepe V, Scheibel KE, Huang SC, Barrio JR, et al. Positron emission tomography of brain beta-amyloid and tau levels in adults with Down syndrome. Archives of Neurology. 2011;68:768–774. doi: 10.1001/archneurol.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noebels J. A perfect storm: Converging paths of epilepsy and Alzheimer's dementia intersect in the hippocampal formation. Epilepsia. 2011;52:39–46. doi: 10.1111/j.1528-1167.2010.02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Rees SD, Kelly MA, Bain SC, Barnett AH, Thalitaya D, et al. Association of variants within APOE, SORL1, RUNX1, BACE1 and ALDH18A1 with dementia in Alzheimer's disease in subjects with Down syndrome. Neuroscience Letters. 2011;487:144–148. doi: 10.1016/j.neulet.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Patterson D. Molecular genetic analysis of Down syndrome. Human Genetics. 2009;126:195–214. doi: 10.1007/s00439-009-0696-8. [DOI] [PubMed] [Google Scholar]
- Pinter JD, Brown WE, Eliez S, Schmitt JE, Capone GT, Reiss AL. Amygdala and hippocampal volumes in children with Down syndrome: A high-resolution MRI study. Neurology. 2001a;56:972–974. doi: 10.1212/wnl.56.7.972. [DOI] [PubMed] [Google Scholar]
- Pinter JD, Eliez S, Schmitt JE, Capone GT, Reiss AL. Neuroanatomy of Down's syndrome: A high-resolution MRI study. The American Journal of Psychiatry. 2001b;158:1659–1665. doi: 10.1176/appi.ajp.158.10.1659. [DOI] [PubMed] [Google Scholar]
- Popova G, Paterson WF, Brown A, Donaldson MD. Hashimoto's thyroiditis in Down's syndrome: Clinical presentation and evolution. Hormone Research. 2008;70:278–284. doi: 10.1159/000157874. [DOI] [PubMed] [Google Scholar]
- Pritchard MA, Kola I. The “gene dosage effect” hypothesis versus the “amplified developmental instability” hypothesis in Down syndrome. Journal of Neural Transmission. Supplementum. 1999;57:293–303. [PubMed] [Google Scholar]
- Pueschel SM, Louis S, McKnight P. Seizure disorders in Down syndrome. Archives of Neurology. 1991;48:318–320. doi: 10.1001/archneur.1991.00530150088024. [DOI] [PubMed] [Google Scholar]
- Rammes G, Rupprecht R, Ferrari U, Zieglgansberger W, Parsons CG. The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neuroscience Letters. 2001;306:81–84. doi: 10.1016/s0304-3940(01)01872-9. [DOI] [PubMed] [Google Scholar]
- Raz N, Torres IJ, Briggs SD, Spencer WD, Thornton AE, Loken WJ, et al. Selective neuroanatomic abnormalities in Down's syndrome and their cognitive correlates: Evidence from MRI morphometry. Neurology. 1995;45:356–366. doi: 10.1212/wnl.45.2.356. [DOI] [PubMed] [Google Scholar]
- Ricketts J. Research review: Reading comprehension in developmental disorders of language and communication. Journal of Child Psychology and Psychiatry. 2011;52:1111–1123. doi: 10.1111/j.1469-7610.2011.02438.x. [DOI] [PubMed] [Google Scholar]
- Rigoldi C, Galli M, Mainardi L, Crivellini M, Albertini G. Postural control in children, teenagers and adults with Down syndrome. Research in Developmental Disabilities. 2011;32:170–175. doi: 10.1016/j.ridd.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Rondal JA, Elbouz M, Ylieff M, Docquier L. Francoise, a fifteen-year follow up. Down's Syndrome, Research and Practice. 2003;8:89–99. doi: 10.3104/case-studies.135. [DOI] [PubMed] [Google Scholar]
- Rueda N, Florez J, Martinez-Cue C. Effects of chronic administration of SGS-111 during adulthood and during the pre- and post-natal periods on the cognitive deficits of Ts65Dn mice, a model of Down syndrome. Behavioural Brain Research. 2008;188:355–367. doi: 10.1016/j.bbr.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Rumble B, Retallack R, Hilbich C, Simms G, Multhaup G, Martins R, et al. Amyloid A4 protein and its precursor in Down's syndrome and Alzheimer's disease. The New England Journal of Medicine. 1989;320:1446–1452. doi: 10.1056/NEJM198906013202203. [DOI] [PubMed] [Google Scholar]
- Rusted J, Graupner L, O'Connell N, Nicholls C. Does nicotine improve cognitive function? Psychopharmacology. 1994;115:547–549. doi: 10.1007/BF02245580. [DOI] [PubMed] [Google Scholar]
- Ryoo SR, Jeong HK, Radnaabazar C, Yoo JJ, Cho HJ, Lee HW, et al. DYRK1A-mediated hyperphosphorylation of Tau. A functional link between Down syndrome and Alzheimer disease. The Journal of Biological Chemistry. 2007;282:34850–34857. doi: 10.1074/jbc.M707358200. [DOI] [PubMed] [Google Scholar]
- Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, et al. Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Salehi A, Faizi M, Belichenko PV, Mobley WC. Using mouse models to explore genotype-phenotype relationship in Down syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:207–214. doi: 10.1002/mrdd.20164. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. The Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmidt-Sidor B, Wisniewski KE, Shepard TH, Sersen EA. Brain growth in Down syndrome subjects 15 to 22 weeks of gestational age and birth to 60 months. Clinical Neuropathology. 1990;9:181–190. [PubMed] [Google Scholar]
- Schupf N, Zigman WB, Tang MX, Pang D, Mayeux R, Mehta P, et al. Change in plasma Aß peptides and onset of dementia in adults with Down syndrome. Neurology. 2010;75:1639–1644. doi: 10.1212/WNL.0b013e3181fb448b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields N, Taylor NF. A student-led progressive resistance training program increases lower limb muscle strength in adolescents with Down syndrome: A randomised controlled trial. Journal of Physiotherapy. 2010;56:187–193. doi: 10.1016/s1836-9553(10)70024-2. [DOI] [PubMed] [Google Scholar]
- Shott SR, Amin R, Chini B, Heubi C, Hotze S, Akers R. Obstructive sleep apnea: Should all children with Down syndrome be tested? Archives of Otolaryngology—Head & Neck Surgery. 2006;132:432–436. doi: 10.1001/archotol.132.4.432. [DOI] [PubMed] [Google Scholar]
- Simms ML, Kemper TL, Timbie CM, Bauman ML, Blatt GJ. The anterior cingulate cortex in autism: Heterogeneity of qualitative and quantitative cytoarchitectonic features suggests possible subgroups. Acta Neuropathologica. 2009;118:673–684. doi: 10.1007/s00401-009-0568-2. [DOI] [PubMed] [Google Scholar]
- Sisodiya SM, Fauser S, Cross JH, Thom M. Focal cortical dysplasia type II: Biological features and clinical perspectives. Lancet Neurology. 2009;8:830–843. doi: 10.1016/S1474-4422(09)70201-7. [DOI] [PubMed] [Google Scholar]
- Smigielska-Kuzia J, Sobaniec W, Kulak W, Bockowski L. Clinical and EEG features of epilepsy in children and adolescents in Down syndrome. Journal of Child Neurology. 2009;24:416–420. doi: 10.1177/0883073808324542. [DOI] [PubMed] [Google Scholar]
- Sokol DK, Maloney B, Long JM, Ray B, Lahiri DK. Autism, Alzheimer disease, and fragile X: APP, FMRP, and mGluR5 are molecular links. Neurology. 2011;76:1344–1352. doi: 10.1212/WNL.0b013e3182166dc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stores R, Stores G, Fellows B, Buckley S. Daytime behaviour problems and maternal stress in children with Down's syndrome, their siblings, and non-intellectually disabled and other intellectually disabled peers. Journal of Intellectual Disability Research. 1998;42(Pt. 3):228–237. doi: 10.1046/j.1365-2788.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annual Review of Neuroscience. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Strydom A, Dickinson MJ, Shende S, Pratico D, Walker Z. Oxidative stress and cognitive ability in adults with Down syndrome. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009;33:76–80. doi: 10.1016/j.pnpbp.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Alexander GE, Schapiro MB, Moller HJ, Rapoport SI, Hampel H. Age-related cortical grey matter reductions in non-demented Down's syndrome adults determined by MRI with voxel-based morphometry. Brain. 2004;127:811–824. doi: 10.1093/brain/awh101. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Schapiro MB, Alexander GE, Krasuski JS, Horwitz B, Hoehne C, et al. Relation of corpus callosum and hippocampal size to age in nondemented adults with Down's syndrome. The American Journal of Psychiatry. 2003;160:1870–1878. doi: 10.1176/appi.ajp.160.10.1870. [DOI] [PubMed] [Google Scholar]
- Tejedor FH, Hammerle B. MNB/DRYK1A as a multiple regulator of neuronal development. FEBS. 2011;278(2):225–235. doi: 10.1111/j.1742-4658.2010.07954.x. [DOI] [PubMed] [Google Scholar]
- Toiber D, Azkona G, Ben-Ari S, Toran N, Soreq H, Dierssen M. Engineering DYRK1A overdosage yields Down syndrome-characteristic cortical splicing aberrations. Neurobiology of Disease. 2010;40:348–359. doi: 10.1016/j.nbd.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Torelli F, Moscufo N, Garreffa G, Placidi F, Romigi A, Zannino S, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. NeuroImage. 2011;54:787–793. doi: 10.1016/j.neuroimage.2010.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao R, Kindelberger C. Variability of cognitive development in children with Down syndrome: Relevance of good reasons for using the cluster procedure. Research in Developmental Disabilities. 2009;30:426–432. doi: 10.1016/j.ridd.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Uberos J, Romero J, Molina-Carballo A, Munoz-Hoyos A. Melatonin and elimination of kynurenines in children with Down's syndrome. Journal of Pediatric Endocrinology & Metabolism. 2010;23:277–282. doi: 10.1515/jpem.2010.23.3.277. [DOI] [PubMed] [Google Scholar]
- Valenti D, Manente GA, Moro L, Marra E, Vacca RA. Deficit of complex I activity in human skin fibroblasts with chromosome 21 trisomy and overproduction of reactive oxygen species by mitochondria: Involvement of the cAMP/PKA signalling pathway. The Biochemical Journal. 2011;435:679–688. doi: 10.1042/BJ20101908. [DOI] [PubMed] [Google Scholar]
- van Allen MI, Fung J, Jurenka SB. Health care concerns and guidelines for adults with Down syndrome. American Journal of Medical Genetics. 1999;89:100–110. [PubMed] [Google Scholar]
- van Reekum R, Black SE, Conn D, Clarke D. Cognition-enhancing drugs in dementia: A guide to the near future. Canadian Journal of Psychiatry. 1997;42(Suppl. 1):35S–50S. [PubMed] [Google Scholar]
- Vasudevaraju P, Bharathi, Garruto RM, Sambamurti K, Rao KS. Role of DNA dynamics in Alzheimer's disease. Brain Research Reviews. 2008;58:136–148. doi: 10.1016/j.brainresrev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Vignoli A, Zambrelli E, Chiesa V, Savini M, la Briola F, Gardella E, et al. Epilepsy in adult patients with Down syndrome: A clinical-video EEG study. Epileptic Disorders. 2011;13:125–132. doi: 10.1684/epd.2011.0426. [DOI] [PubMed] [Google Scholar]
- Vincent A, Crino PB. Systemic and neurologic autoimmune disorders associated with seizures or epilepsy. Epilepsia. 2011;52(Suppl. 3):12–17. doi: 10.1111/j.1528-1167.2011.03030.x. [DOI] [PubMed] [Google Scholar]
- Virji-Babul N, Eichmann A, Kisly D, Down J, Haslam RH. Use of health care guidelines in patients with Down syndrome by family physicians across Canada. Paediatrics & Child Health. 2007;12:179–183. [PMC free article] [PubMed] [Google Scholar]
- Walker JC, Dosen A, Buitelaar JK, Janzing JG. Depression in Down syndrome: A review of the literature. Research in Developmental Disabilities. 2011;32:1432–1440. doi: 10.1016/j.ridd.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Ballard TM, Pouzet B, Riedel WJ, Wettstein JG. Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacology, Biochemistry, and Behavior. 2011;99:130–145. doi: 10.1016/j.pbb.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Westmark CJ, Westmark PR, Malter JS. MPEP reduces seizure severity in Fmr-1 KO mice over expressing human Abeta. International Journal of Clinical and Experimental Pathology. 2009;3:56–68. [PMC free article] [PubMed] [Google Scholar]
- Westmark CJ, Westmark PR, Malter JS. Alzheimer's disease and Down syndrome rodent models exhibit audiogenic seizures. Journal of Alzheimer's Disease. 2010;20:1009–1013. doi: 10.3233/JAD-2010-100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NS, Alkire MT. Impaired thalamocortical connectivity in humans during general-anesthetic-induced unconsciousness. NeuroImage. 2003;19:402–411. doi: 10.1016/s1053-8119(03)00103-4. [DOI] [PubMed] [Google Scholar]
- White KG, Ruske AC. Memory deficits in Alzheimer's disease: The encoding hypothesis and cholinergic function. Psychonomic Bulletin and Review. 2002;9:426–437. doi: 10.3758/bf03196301. [DOI] [PubMed] [Google Scholar]
- Wishart JG . The development of learning difficulties in children with Down's syndrome. Journal of Intellectual Disability Research. 1993;37(Pt. 4):389–403. doi: 10.1111/j.1365-2788.1993.tb00882.x. [DOI] [PubMed] [Google Scholar]
- Wishart JG, Manning G. Trainee teachers’ attitudes to inclusive education for children with Down's syndrome. Journal of Intellectual Disability Research. 1996;40(Pt. 1):56–65. doi: 10.1111/j.1365-2788.1996.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Wisniewski KE. Down syndrome children often have brain with maturation delay, retardation of growth, and cortical dysgenesis. American Journal of Medical Genetics. Supplement. 1990;7:274–281. doi: 10.1002/ajmg.1320370755. [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Annals of Neurology. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- Zigman WB, Lott IT. Alzheimer's disease in Down syndrome: Neurobiology and risk. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:237–246. doi: 10.1002/mrdd.20163. [DOI] [PubMed] [Google Scholar]