Abstract
Ning, S., Budas, G. R., Churchill, E. N., Chen, C., Knox, S. J. and Mochly-Rosen, D. Mitigation of Radiation-Induced Dermatitis by Activation of Aldehyde Dehydrogenase 2 Using Topical Alda-1 in Mice.
Radiation-induced dermatitis is a debilitating clinical problem in cancer patients undergoing cancer radiation therapy. It is also a possible outcome of exposure to high levels of radiation due to accident or hostile activity. We report that activation of aldehyde dehydrogenase 2 (ALDH2) enzymatic activity using the allosteric agonist, Alda-1, significantly reduced 4-hydroxynonenal adducts accumulation, delayed the onset of radiation dermatitis and substantially reduced symptoms in a clinically-relevant model of radiation-induced dermatitis. Importantly, Alda-1 did not radioprotect tumors in mice. Rather, it increased the sensitivity of the tumors to radiation therapy. This is the first report of reactive aldehydes playing a role in the intrinsic radiosensitivity of normal and tumor tissues. Our findings suggest that ALDH2 represents a novel target for the treatment of radiation dermatitis without reducing the benefit of radiotherapy.
INTRODUCTION
Radiation-induced dermatitis is an adverse side effect commonly occurring in cancer patients undergoing radiation therapy. Clinical symptoms range from mild erythema to moist desquamation and skin ulceration, and can lead to treatment interruption, thus reducing the efficacy of the radiotherapy. Agents that reduce or delay the onset of these symptoms would therefore be of enormous therapeutic potential (1, 2). Further, the recent accident at the Fukushima Daiichi nuclear power plant (3) and the risk from nuclear threat to the population (4) highlights the need to develop novel treatments for acute radiation sickness.
Radiation-induced skin injury is primarily due to cellular oxidative stress (5). The accumulating reactive oxygen species induced by ionizing radiation lead to lipid peroxidation and the formation of highly reactive aldehydes, such as 4-hydroxynonenal (4-HNE) (6) and malondialdehyde (7). The cellular impact of such reactive aldehydes generation is not yet fully understood. However, it is known that these reactive aldehydes interact with proteins and interfere with cellular function by forming cytotoxic protein adducts (8). In humans, the multi-gene family of aldehyde dehydrogenase (EC.1.2.1.3) is the major detoxifying enzyme for cytotoxic reactive aldehydes (9). Expression of different aldehyde dehydrogenase (ALDH) isozymes has been detected in a wide range of human skin samples of chest, abdomen and foreskin (10, 11). The mitochondrial enzyme, aldehyde dehydrogenase 2 (ALDH2) is one of the principle enzymes that detoxifies these toxic aldehydes. In human and rodent skin sections, ALDH2 is predominantly localized in epidermis, sebaceous glands and hair follicles (11). Recently, we identified a small molecule activator of ALDH2, Alda-1, by using a high-throughput screening technique (12). Based on the co-crystal structure and kinetic assays, Alda-1 was shown to function as a shield to the critical cysteines in the catalytic center of ALDH2 holoenzyme against 4-HNE inactivation of ALDH2 and thereby increases the catalytic activity of ALDH2 and reduces cell death caused by oxidative stress in an in vivo model of myocardial ischemia (12–14). We hypothesized that activation of ALDH2 in skin cells by Alda-1 should prevent radiation-induced dermatitis by reducing the accumulation of oxidative stress-induced aldehydes.
MATERIALS AND METHODS
Study Design
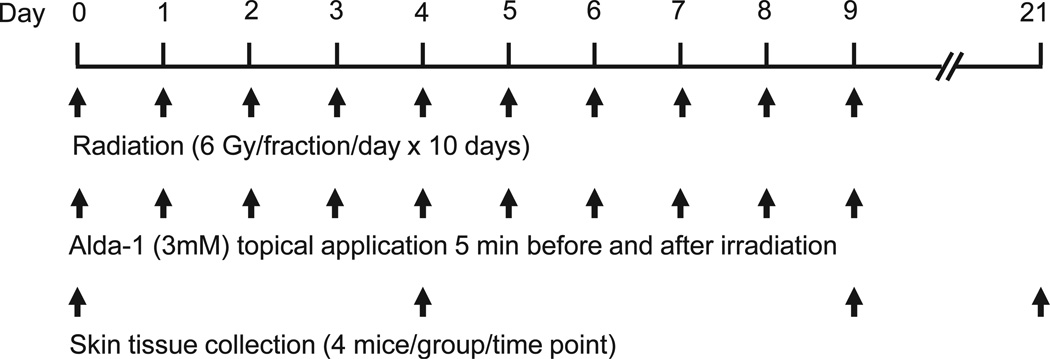
To establish an animal model of radiation-induced dermatitis, a 2 × 2 cm area of skin on the back of normal C57BL mice was irradiated locally at 6 Gy daily for 10 consecutive days. Effects of Alda-1 on radiation-induced dermatitis, ALDH enzyme activity, and 4-HNE protein adduct formation were assessed in both the irradiated and nonirradiated skin tissues. The scheme in Fig. 1 shows the schedule of irradiation, Alda-1 application and collection of skin samples for histopathological and immunohistochemical studies.
FIG. 1.
The scheme shows the schedule of irradiation, Alda-1 application and collection of skin tissues for histopathological and immunohistochemistry studies.
Animal Models
C57BL mice, C3H mice and immunodeficient nude mice (male, 7–8 weeks old and 20–25 grams in body weight) were purchased from Charles River Laboratories. Mice were tested and found to be negative for specific pathogens. Mice were normally bred and maintained under specific pathogen-free conditions, and sterilized food and water were available ad libitum. The animal experiments described herein were approved by the Stanford University Administrative Panel for Laboratory Animal Care.
External Beam Gamma Irradiation
Gamma irradiation was given using a Philips RT-250 200kVp X-ray machine (12.5 mA, half value layer, 1.0 mm Cu). To induce dermatitis, unanesthetized mice were placed in individual lead boxes with skin (2 × 2 cm on the back) protruding through a cut-out window at the rear of each box. X-ray irradiation was delivered locally to the skin at a dose rate of 130 cGy/min.
Alda-1 Administration
Alda-1 (3 mM in 95% ethanol solution in a volume of 0.25 ml) was applied locally on the skin. When combined with radiation, Alda-1 was applied on the skin over the subcutaneously inoculated tumors 5 min before and after irradiation.
Evaluation of Dermatitis
Skin reaction to radiation was assessed according to the Radiation Therapy Oncology Group (RTOG) score system (2) as shown below:
-
0
No change over baseline.
-
1
Follicular, faint or dull erythema/epilation/dry; desquamation/decreased sweating.
-
2
Tender or bright erythema, patchy moist desquamation/moderate edema.
-
3
Confluent, moist desquamation other than skin folds, pitting edema.
-
4
Ulceration, hemorrhage, necrosis.
ALDH Enzymatic Activity Assay
Enzymatic activity of ALDH was determined spectrophotometrically by monitoring the reductive reaction of NAD+ to NADH as previously described (12). Briefly, following treatment, the skin was dissected and homogenized using a polytron homogenizer in 10× volume lysis buffer (0.1 M Tris-HCl, pH 8.0, 10 mM DTT, 20% glycerol, 1% Triton, plus protease and phosphatase inhibitors). Tissue extract was centrifuged at 700g to pellet nuclei and cellular debris, followed by centrifugation at 10,000g to collect mitochondrial-enriched fractions. Protein concentration was assessed by Bradford Assay. ALDH assay was carried out at 25°C in 50 mM sodium pyrophosphate buffer (pH = 9.5) by adding 2.5 mM NAD, 10 mM acetaldehyde and 400 µg of skin protein lysate samples. The production of NADH was measured by monitoring the absorbance at 340 nm every 30 s for 5 min on a Beckman DU800 spectrophotometer. ALDH enzymatic activity was expressed as µmol NADH/min/mg protein.
Immunohistochemistry and Histopathological Analysis
The epidermal thickness, as an indicator of skin damage, and the formation of 4-HNE protein adducts were quantified with histopathological and immunohistochemistry staining. Mice were irradiated with 6 Gy daily for 5 days from day 0 to day 4 with topical use of either 95% ethanol (vehicle) or Alda-1. The skin of two normal mice was topically treated with 95% ethanol and no irradiation served as an untreated control. One hour after the last radiation dose on day 4, mice were euthanized and skin tissues in the irradiated field (2 × 0.5 cm) were removed. The skin tissues were either fixed in 4% formaldehyde and stained with hematoxylin and eosin (H&E), or were freshly frozen in optimal cutting temperature (O.C.T) compound and stained with an antibody specifically against 4-HNE adducts (Calbiochem, EMD Chemicals, Gibbstown, NJ). Slides were visualized and photographed using a Leica fluorescence/light microscope (Leica Microsystems, Germany). For quantification of the skin thickness and 4-HNE staining, 4 fields per skin section were randomly analyzed using a computer assisted morphometric system (Leica Quantimet 500, Cambridge, UK) and data are presented as a mean ± SD of each group (4 mice per group).
Tumor Models
Murine squamous cell carcinoma SCC VII cells and human melanoma M21 cells were grown and maintained in DMEM medium (Invitrogen) supplemented with 10% fetal calf serum, 100 units/ml penicillin, and 100 µg/mL streptomycin in a 37°C humidified incubator with a mixture of 95% air/5% CO2. SCC VII cells (5 × 105 cells in 0.05 mL Hank’s solution) were subcutaneously inoculated in the right flank of C3H mice. M21 cells (2 × 106 cells in 0.05 mL Hank’s solution) were subcutaneously inoculated in nude mice. One tumor per mouse was implanted. Length and width of tumors were measured with calipers before the start of treatment and 3 times a week thereafter. Tumor volume was calculated using the formula: tumor volume = π/6 × length × width2. When tumors reach an average size of 150 mm3 (approximately 10 days after implantation), mice were randomized to the different treatment groups. Fractionated radiation therapy was delivered locally to the tumor daily for 5 consecutive days. Alda-1 was applied on the skin over the subcutaneous tumors 5 min before and after radiation. Tumor volume quadrupling time and tumor growth delay time were used as the primary end points for this study to determine the significance of treatment on the reduction and/or inhibition in tumor growth. Tumor volume quadrupling time is defined as the time required for a given tumor to reach a 4-fold (4×) increase in volume when compared with the tumor volume measured before the start of treatment. Tumor volume quadrupling time (in days) was determined by a best-fit regression analysis. Tumor growth delay time is defined as the difference between the tumor volume quadrupling time of treated tumors compared to that of untreated control tumors. Both the tumor volume quadrupling time and tumor growth delay times were calculated for each individual animal and then averaged for each group. Data are presented as percentage (%) of the pretreatment volume on day 0.
Statistical Analysis of Data
Treatment effects were statistically analyzed using an unpaired two-tailed Student’s t test. The means, standard deviations, and P values were calculated.
RESULTS AND DISCUSSION
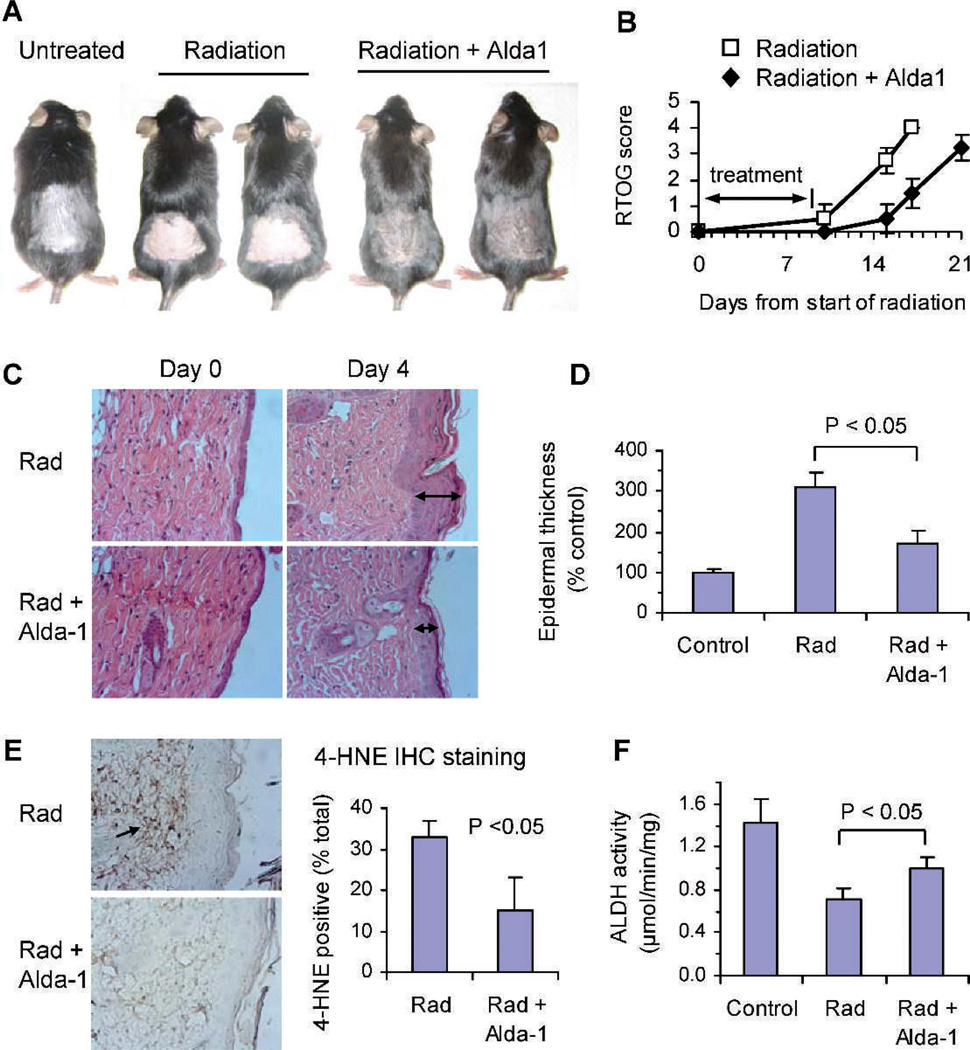
To induce radiation dermatitis, C57BL mice were γ irradiated on the back with 6 Gy for 10 consecutive days and the development of dermatitis was monitored. The skin in the radiation field developed erythema within the first 2 days after completion of 10 days of irradiation, and then gradually became patchy moist desquamation and moderate edema. Six to eight days (day 15–17) after irradiation, the skin was completely desquamated and ulceration occurred (Fig. 2A, radiation only). The ulcerated skin was eventually healed by scar formation 10–14 days after radiation.
FIG. 2.
Radiation-induced dermatitis in C57BL mice and the effects of ALDH2 activation by Alda-1. Panel A: Alda-1 reduced the severity and delayed the onset of radiation dermatitis. The photographs were taken 5 days after completion of 10 days of irradiation (day 14 from start of radiation on day 0). Panel B: RTOG score of radiation-induced dermatitis in mice topically treated with or without Alda-1. Panels C and D: Alda-1 reduced the radiation-induced epidermal thickness of the skin. Mice were irradiated locally on the back skin for 5 days and the skin tissues were collected and stained with H&E. The epidermal thickness was quantified under a light microscope. The arrowheads indicate the thickness of irradiated skin tissues. Panel E: Alda-1 significantly reduced the 4-HNE protein adduct formation in irradiated skin. Skin samples were immunochemically stained with an anti-4-HNE adduct antibody (brown staining) and the positive staining was quantified using a computer-assisted morphometric system. Panel F: Alda-1 partially restored the ALDH enzymatic activity in irradiated skin. Mice were irradiated with a single dose of 6 Gy of radiation. Alda-1 or 95% ethanol was applied on the skin 5 min before irradiation. One hour later, the skin tissues were collected for ALDH activity assay.
To study the effect of Alda-1 on radiation-induced dermatitis, two groups of mice (8 mice per group) were irradiated as above, and topically treated with either 0.25 mL of 3 mM Alda-1 or 95% ethanol (vehicle control) 5 min before and after irradiation. The skin was examined daily and scored using the RTOG score. Topical application of Alda-1 reduced the severity and delayed the onset of radiation dermatitis by 2–5 days compared to radiation plus 95% ethanol (Fig. 2A). The mean RTOG scores obtained over 21 days from the start of radiation therapy were 2.8 ± 0.24 and 1.3 ± 0.38 for radiation plus ethanol and radiation plus Alda-1, respectively (P < 0.001; Fig. 2B).
Histological analysis of skin biopsies revealed that the epidermal thickness (an indicator of skin damage) was increased after 5 days of irradiation (day 4 as shown in Fig. 2C). Concomitantly topical treatment with Alda-1 greatly reduced the epidermal thickness as compared with radiation plus 95% ethanol vehicle (Fig. 2C). The mean epidermal thickness increased 3-fold from 11 ± 1 mm in nonirradiated mice to 35 ± 4 mm in irradiated mice (n = 6, P < 0.05, Fig. 2D). Treatment with Alda-1 reduced the radiation-induced epidermal thickening by 46% to 19 ± 4 mm (n = 6; P < 0.05 compare to radiation only). A portion of the skin samples were stained with an antibody specific against 4-HNE protein adducts. Exposure to 5 days of radiation resulted in a substantial increase in 4-HNE adduct formation in the skin and topical application of Alda-1 significantly reduced the formation of 4-HNE adducts (Fig. 2E). The 4-HNE adducts positive staining was 32.7 ± 4.0% of total area in irradiated skins and 15.0 ± 8.0% in Alda-1-treated and irradiated skins (P < 0.05).
We next determined the level of ALDH activity of irradiated or nonirradiated skin tissues using skin biopsies. A single dose of 6 Gy reduced the ALDH activity by 50% from 1.4 µmol/min/mg total protein for untreated control mice to 0.7 µmol/min/mg after radiation (P < 0.01, Fig. 2F). A single dose of 3 mM Alda-1 application restored the ALDH enzymatic activity by 42% to 1.0 µmol/min/mg (P < 0.05 compared to radiation only).
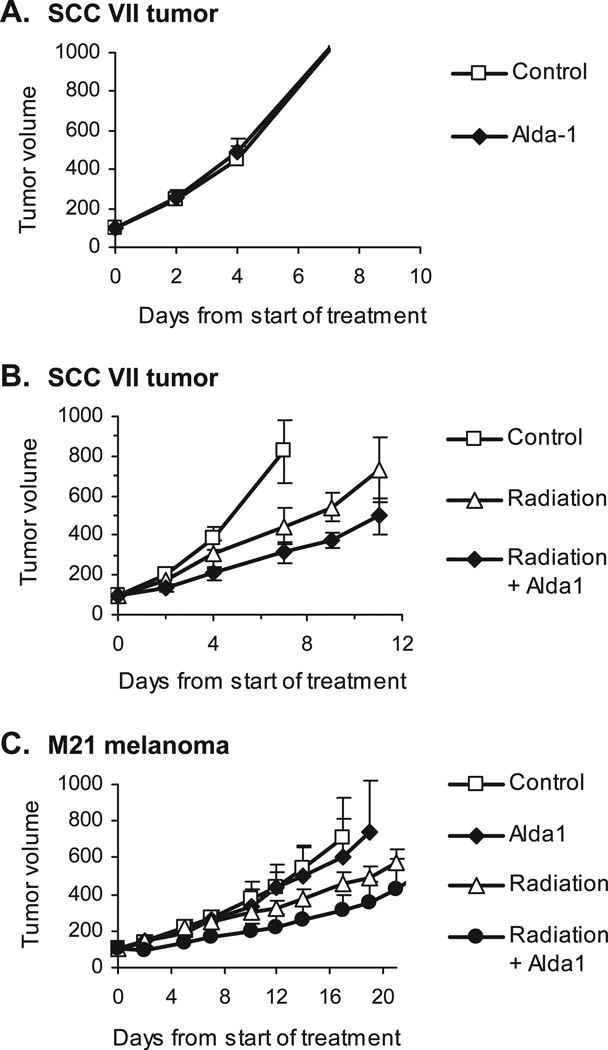
Since the radiation-induced dermatitis occurs commonly in cancer patients undergoing radiation therapy, and several studies have reported that cancer cells with higher ALDH1 activity are more resistant to radiation therapy (15, 16), we next determined whether activation of ALDH2 by Alda-1 treatment has a negative impact on the effectiveness of radiation therapy. The experiments were performed with two well-established subcutaneous tumor models of murine SCC VII squamous cell carcinoma tumors in C3H mice, and M21 human melanoma xenografts in nude mice. Mice with tumors (average tumor size: 150 mm3) were randomized into 4 groups and treated with (1) topical application of 3 mM Alda-1 daily for 5 consecutive days on the skin over the tumor, (2) 250 cGy local tumor irradiation daily for 5 days plus topical use of 95% ethanol, (3) 250 cGy local tumor irradiation daily for 5 days plus topical use of 3 mM Alda-1, and (4) topical use of 95% ethanol daily for 5 days as an untreated vehicle control. Note that 250 cGy of radiation was used because it is clinically relevant and is known to result in moderate tumor growth delay in these animal tumor models. This dose is not curative. Importantly, had we used a curative dose, it would not be possible to see either radiation protection or sensitization, because the tumor would have been eradicated. This 5-day fractionated radiation therapy regimen, while ideal for studying the effect of biological modifiers on the response of tumors to radiation, does not induce an erythematous skin reaction or desquamation.
We found that Alda-1 itself had no effect on tumor growth in mice (Fig. 3A and C). When combined with radiation therapy, Alda-1 treatment did not protect tumors from radiation. Rather, a small increase in sensitivity of tumors to fractionated radiation treatment occurred in both SCC VII and M21 tumor models (Fig. 3B and C). The combination therapy of local tumor radiation and topical application of Alda-1 significantly inhibited growth of SCC VII tumors and produced a longer tumor growth delay time of 5.0 ± 1.0 days compared with 2.0 ± 1.4 days for the group receiving radiation therapy only (P < 0.01, n = 6) (Table 1). In M21 melanoma xenografts, treatment with Alda-1 resulted in a moderate enhancement on radiation-induced tumor growth delay. The 4× tumor growth delay time was 4.1 ± 2.3 days for radiation only and 10.5 ± 5.9 days for radiation plus Alda-1, respectively, suggesting that Alda-1 might enhance the radiation sensitivity of M21 tumors in mice. However, there was no statistically significant difference (P = 0.07) in tumor growth delay time between these 2 groups. Although Alda-1-induced sensitization of radiation therapy should be further investigated, our findings that Alda-1 treatment does not interfere with radiation therapy providing support for potential benefit of ALDH2 activators, such as Alda-1, in clinical management of radiation dermatitis.
FIG. 3.
Tumor growth curves of SCC VII tumors and M21 melanoma xenografts in mice. Alda-1 (3 mM) was applied locally on the skin over the subcutaneously inoculated tumors daily until the completion of the study. Local tumor fractionated radiation therapy was delivered at 250 cGy daily for 5 days either with topical use of 95% ethanol (vehicle) or 3 mM Alda-1. Panel A: Topical application of Alda-1 had no effect on SCC VII tumor growth. Panel B: Alda-1 enhanced the antitumor effect of radiation therapy in SCC VII tumor model. Panel C: Alda-1 moderately enhanced the tumor growth inhibition of radiation therapy in M21 melanoma xenograft tumor model in nude mice. There were 5 to 6 mice per group. Data are presented as the average tumor volume of each group (mean ± SD) as a function of time from start of treatment.
TABLE 1.
4× Tumor Growth Time and Tumor Growth Delay Time of SCC VII Tumor and M21 Melanoma in Mice
| Number of mice |
4× tumor growth time (day) |
4× tumor growth delay time (day) |
P value (t test) | |||
|---|---|---|---|---|---|---|
| Control | Radiation | |||||
| SCC VII tumor | ||||||
| Control | 5 | 4.4 ± 0.6 | ||||
| Radiation | 6 | 6.4 ± 1.4 | 2.0 ± 1.4 | < 0.05 | ||
| Radiation + Alda-1 | 6 | 9.5 ± 1.0 | 5.0 ± 1.0 | < 0.01 | < 0.01 | |
| M21melanoma | Control | Alda-1 | Radiation | |||
| Control | 6 | 11.3 ± 2.0 | ||||
| Alda-1 | 5 | 12.6 ± 4.2 | 1.3 ± 4.2 | 0.5 | ||
| Radiation | 5 | 15.4 ± 2.3 | 4.1 ± 2.3 | 0.01 | 0.2 | |
| Radiation + Alda-1 | 5 | 21.8 ± 5.9 | 10.5 ± 5.9 | 0.01 | 0.02 | 0.07 |
In summary, concomitant topical application of the ALDH2 activator, Alda-1, effectively reduced the severity and delayed the onset of radiation-induced dermatitis in mice, which correlated with increased ALDH2 activity and reduced accumulation of 4-HNE adducts in the radiated skin. Therefore, ALDH2 represents a potential target for the treatment of radiation dermatitis, and ALDH2 activators, such as Alda-1, may be used as an adjunct to radiation therapy for the treatment of solid tumors in which radiation-induced dermatitis is an important clinical problem. Further, there may be benefit in using ALDH2 activators to reduce radiation sickness after accidental or warfare-induced exposure to radiation (7, 17).
ACKNOWLEDGMENT
This research is supported by a NIH grant, NIAAA11147 to DM-R. Received: November 5, 2011; accepted: January 3, 2012; published online: March 9, 2012
Footnotes
A preliminary report of the above study was provided in Proceedings of the 11th International Wolfsberg Meeting on Molecular Radiation Biology/Oncology [Budas, G.R., Ning, S., Churchill, E.N., Chen, C.H., Knox, S.J., Mochly-Rosen, D. Alda-1, a small molecule activator of aldehyde dehydrogenase 2 (ALDA2) reduced radiation dermatitis and radiosensitizes tumors in mice, Abstract P111.14, p66, 2009, June 27–29, 2009, Ermatingen, Switzerland].
REFERENCES
- 1.Bolderston A, Lioyd NS, Wong RK, Holden L, Robb-Blenderman L. The prevention and management of acute skin reactions related to radiation therapy: a systematic review and practice guideline. Support Care Cancer. 2006;14:802–817. doi: 10.1007/s00520-006-0063-4. [DOI] [PubMed] [Google Scholar]
- 2.Salvo N, Barnes E, van Draanen J, Stacey E, Mitera G, Breen D, Giotis A, Czarnota G, Pang J, De Angelis C. Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr Oncol. 2010;17:94–112. doi: 10.3747/co.v17i4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christodouleas JP, Forrest RD, Ainsley CG, Tochner Z, Hahn SM, Glatstein E. Short-term and long-term health risks of nuclearpower- plant accidents. New Engl J Med. 2011;364:2334–2341. doi: 10.1056/NEJMra1103676. [DOI] [PubMed] [Google Scholar]
- 4.DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, et al. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep. 2011;5(suppl.1):S32–S44. doi: 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niwa Y, Sumi H, Kawahira K, Terashima T, Nakamura T, Akamatsu H. Protein oxidation damage in the stratum corneum: Evidence for a link between environmental oxidants and the changing prevalence and nature of atopic dermatitis in Japan. Br J Dermatol. 2003;149:248–254. doi: 10.1046/j.1365-2133.2003.05417.x. [DOI] [PubMed] [Google Scholar]
- 6.Aldini G, Granata P, Orioli M, Santaniello E, Carini M. Detoxification of 4-hydroxynonenal (HNE) in keratinocytes: characterization of conjugated metabolites by liquid chromatography/ electrospray ionization tandem mass spectrometry. J Mass Spectrom. 2003;38:1160–1168. doi: 10.1002/jms.533. [DOI] [PubMed] [Google Scholar]
- 7.Cui L, Pierce D, Light KE, Melchert RB, Fu Q, Kumar KS, et al. Sublethal total body irradiation leads to early cerebellar damage and oxidative stress. Curr Neurovasc Res. 2010;7:125–135. doi: 10.2174/156720210791184880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song BJ, Abdelmegeed MA, Yoo SH, Kim BJ, Jo SA, Jo I, et al. Post-translational modifications of mitochondrial aldehyde dehydrogenase and biomedical implications. J Proteomics. 2011;74:2691–2702. doi: 10.1016/j.jprot.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchitti SA, Brocker C, Stagos D, Vasiliou V. Non-P450 aldehyde oxidizing enzymes: The aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4:697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung C, Smith CK, Hoog JO, Hotchkiss SA. Expression and localization of human alcohol and aldehyde dehydrogenase enzymes in skin. Biochem Biophys Res Commun. 1999;261:100–107. doi: 10.1006/bbrc.1999.0943. [DOI] [PubMed] [Google Scholar]
- 11.Cheung C, Davies NG, Hoog JO, Hotchkiss SA, Smith Pease CK. Species variations in cutaneous alcohol dehydrogenases and aldehyde dehydrogenases may impact on toxicological assessments of alcohols and aldehydes. Toxicology. 2003;184:97–112. doi: 10.1016/s0300-483x(02)00552-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Miller S, Younus H, Vanam R, Chen CH, Mochly-Rosen D, Hurley TD. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nat Struct Mol Biol. 2010;17:159–164. doi: 10.1038/nsmb.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budas GR, Disatnik MH, Chen CH, Mochly-Rosen D. Activation of aldehyde dehydrogenase 2 (ALDH2) confers cardioprotection in protein kinase C epsilon (PKCvarepsilon) knockout mice. J Mol Cell Cardiol. 2010;48:757–764. doi: 10.1016/j.yjmcc.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YC, Chen YW, Hsu HS, Tseng LM, Huang PI, Lu KH, et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385:307–313. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 16.Croker AK, Allan AL. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDH(hi)CD44 (+) human breast cancer cells. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1692-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Aldini G, Granata P, Marinello C, Beretta G, Carini M, Facino RM. Effects of UVB radiation on 4-hydroxy-2-trans-nonenal metabolism and toxicity in human keratinocytes. Chem Res Toxicol. 2007;20:416–423. doi: 10.1021/tx0601657. [DOI] [PubMed] [Google Scholar]