Abstract
Iodine deficiency disorders were prevalent in China until the introduction of universal salt iodization in 1995. Concerns have recently arisen about possible excess iodine intake in this context. To document iodine intake and the contribution from iodized salt in China, we surveyed dietary iodine intake during China’s nationally representative 2007 total diet study (TDS) and during an additional TDS in 4 coastal provinces and Beijing in 2009. Iodine intake was broken down by age and sex in 2009. Mean daily iodine and salt intake and the contribution from different food and beverage groups (and in 2009, individual items) was measured. The iodine in food cooked with iodized and noniodized salt was also assessed. The mean calculated iodine intake of a standard male in China was 425 μg/d in 2007 and 325 μg/d in coastal areas in 2009, well below the upper limit (UL) in all provinces. In 2009, iodine intake was above the UL in only 1–7% of age-sex groups, except among children (18–19%). A concerning number of individuals consumed less than the WHO-recommended daily allowance, including 31.5% of adult women. Salt contributed 63.5% of food iodine, and 24.6% of salt iodine was lost in cooking. Overall salt consumption declined between the surveys. Salt iodization assures iodine nutrition in China where environmental iodine is widely lacking. The risk of iodine excess is low, but planned decreases in salt iodization levels may increase the existing risk of inadequate intake. Regular monitoring of urinary iodine and more research on the impact of excess iodine intake is recommended.
Introduction
Iodine deficiency is common among the inhabitants of environments where naturally occurring iodine is low. It has severe implications for human health, especially during pregnancy, when a resulting lack of thyroid hormone can cause severe and irreversible damage to the brain of the developing embryo and fetus. Globally, it affects about one-third of the world’s population and was responsible for almost 3.5 million disability-affected life years in 2004 (1). China was formerly severely affected by iodine deficiency, with 720 million people at risk. In the early 1970s, surveys identified 35 million individuals with iodine deficiency disorders (IDD)10 manifesting as goiter, and an additional 250,000 with typical cretinism (2). A meta-analysis of 36 studies from the affected locations showed a mean deficit in intelligence quotient of 11 points (3).
The globally recommended strategy to prevent IDD is universal salt iodization (USI), which is safe, cost-effective, and sustainable (4). China introduced a USI policy in 1995 with all edible salt (including table, food, and animal salt) iodized according to a national standard, most recently 35 ± 15 mg/kg. This proved very effective; a national survey in 2002 found the virtual elimination of IDD. In 2010, household coverage of adequately iodized salt (35 ± 15 mg/kg) exceeded 95% and was <80% in only 33 of China’s 2831 counties, most of them in western provinces with large salt lakes. However, coverage <90% prevails in 55 counties, including 23 in coastal areas (5). Meanwhile, global progress on iodized salt consumption remains at ∼70% of households (6), although the number of countries reporting and those with household coverage >70% has increased steadily (7).
In recent years, changes in the reported spectrum and incidence of thyroid diseases have been linked to the increased iodine intake resulting from USI. One recognized consequence of introducing USI is a transient increase in iodine-induced hyperthyroidism (IIH) (8,9), although the incidence of IIH does not increase with chronic iodine excess (10) and IIH almost always disappears after a few years (8,9). Iodine-induced thyroiditis has also been observed after increasing iodine intake but has not been unequivocally associated with USI (10). These disorders seem to occur in genetically predisposed individuals (11). On the other hand, links have also been made between iodine excess and hypothyroidism. In Japan, hypothyroidism was more prevalent in thyroid autoantibody-negative participants with high urinary iodine excretion (UIE), inferring a relationship between excessive iodine intake and thyroid dysfunction (12). More recent research in China’s Liaoning province inferred that high iodine intake may drive thyroid function from a state of potential autoimmune impairment to overt hypothyroidism (13,14) and that iodine intake should be reduced. Danish scholars also demonstrated that iodine intake either below or above the recommended levels is associated with an increased population risk of thyroid disease (15). All researchers recommend careful monitoring of population iodine status.
In China, iodized salt is the main vehicle for iodine supplementation. Salt production is tightly controlled and the sale of noniodized salt is restricted. However, China was described by WHO as “at risk of IIH in susceptible groups” based on median UIE of 246 μg/L among children in 2005 (16) and more recent assessments found higher levels of UIE in some surveyed areas (17). In addition, concerns about the thyroid health of populations in coastal provinces in the context of USI have circulated in the local media (18, 19) and international medical literature (13,14). Some coastal cities have been unofficially allowing the sale of noniodized salt, which formerly required a prescription, and there were calls for liberalizing provincial control of such sales.
Threats to USI in China raise the specter of recrudescence of IDD in a nation admired for its “world’s best achievements” in this area and might influence USI in many nations at risk of IDD. On the other hand, excess iodine consumption may also have adverse public health impacts. Given these threats, and in the absence of national, population-representative, and age-disaggregated data on UIE, China’s dietary iodine intake is thus highly relevant to policy on USI. This paper reports dietary iodine intake and the contribution from iodized salt among Chinese residents, including by age and sex among coastal populations, after more than a decade of USI.
Methods
The 2007 China total diet study (TDS) and a 2009 TDS focusing on dietary iodine intake among coastal residents and inland Beijing are reported.
2007 TDS.
A TDS is recommended by WHO to identify dietary hazards by analyzing prepared foods or food groups reflecting the average dietary intake of a given population (20). It has been used to measure iodine intake elsewhere (21, 22) and includes drinking water and water used in cooking as well as salt, condiments, and cooking oil (23). TDS can also be used to quantify the intake of substances by age and sex.
For China’s 2007 TDS (conducted in June and July), the food composite approach was used to study the average dietary intake in 12 provinces across 4 geographical regions covering ∼50% of China’s population (10% of the world’s population). In each province, 3 survey sites (1 urban district and 2 rural counties) were randomly selected from among middle-income locations as representing the local dietary pattern. To describe the standard pattern for each province, food consumption was assessed using the household food disappearance method (23), which involves weighing and recording the intake of all residents in 90 randomly selected, middle-income households (30/survey site) over 3 consecutive days, corrected for food discarded and the actual persons consuming each meal. A standard method (23) (based on the weight, age, sex, and occupation of all those who ate meals in each household over the 3 d) was then used to calculate the average food consumption of a standard Chinese male (18–45 y, 63 kg body weight with light physical activity) in each province, as previously undertaken in China (24).
All food consumed by this standard male was clustered into 13 food groups: 1) cereals and cereal products; 2) legumes, nuts, and their products; 3) potatoes and potato products; 4) meat and meat products; 5) eggs and egg products; 6) aquatic foods and aquatic food products of animal origin; 7) milk and milk products; 8) vegetables and vegetable products; 9) fruit and fruit products; 10) sugar; 11) beverages and water; 12) alcoholic beverages; and 13) salt and other condiments and cooking oils. Items consumed at >1% by weight were included in common food lists drawn up for each food group. However, for each food group, any item comprising <1% (by weight) of the total food consumed was combined with a similar or closely related food item. Food samples collected from local markets, grocery stores, and rural households were prepared and cooked according to local consumption patterns in each province. The cooked foods were then blended to form the respective group composites, with weights proportional to the mean daily consumption for the province. These provincial food group composites were frozen and then shipped to the Key Laboratory of Chemical Safety and Health at the Chinese Center for Disease Control and Prevention (CDC), where they remained frozen at −30°C pending analysis.
The iodine intake of the standard male was assessed using the frozen food group composites of each province after apportioning intake using the method described above. In China, it is customary to add salt and other condiments not at the dinner table but during food preparation. Accordingly, after distribution of the salt, condiments, and cooking oils in food group 13 into the other 12 groups during cooking, the iodine content of the 12 food group composites was measured by the Central Laboratory of Beijing CDC using international standard practices with strict quality control. The limit of detection (LOD) was 7 × 10−4mol/L. As recommended by the WHO, samples with iodine concentration below the LOD were assigned a value of 1/2 LOD (23).
2009 Survey of dietary iodine intake among residents in Beijing and coastal areas of China.
Based on the concerns expressed in the local media, a purposive study of iodine intake was undertaken in 4 of China’s coastal provinces (ranging from north to south) and in inland Beijing for comparison in October 2009. Again, one urban and 2 rural areas were randomly sampled in each province and 30 households were selected in each.
An additional, standard TDS methodology was applied in this survey (24-h dietary recall over 3 d for each householder) (23), enabling recording of individual food consumption data and calculation of iodine intake by age and sex group in addition to again assessing the intake of a standard Chinese male. The iodine intake of 10 age-sex groups (all children aged 2–7 and 8–12 y, and males and females aged 13–17, 18–50, 51–65, and >65 y) was calculated according to the iodine concentrations of the foods consumed.
The iodine concentration of single items consumed was also analyzed, enabling the contribution of sea and other food items to be individually assessed. In consideration of the possible impact of such foods on dietary iodine intake, all aquatic foods and vegetables were tested, including those of sea origin, no matter how little they were consumed.
In addition, to evaluate the loss of the iodine in iodized salt used in cooking and the contribution of iodine from iodized salt to the iodine in cooked food, 2 sets of diet samples were analyzed based on local food samples collected in Shanghai in 2009, one cooked with commercial salt whose iodine concentration was measured during the analysis and the other with noniodized salt.
Comparison of dietary iodine intake with various standards.
The calculated iodine intake was compared with standard international indicators, including: 1) the estimated average requirement (EAR), the daily intake of a nutrient estimated to meet the requirements of one-half of the healthy individuals in a particular life stage and gender group. The EAR is the primary reference point for assessing the adequacy of nutrient intakes but is not meant to represent recommended intake levels (25). 2) The RDA, the daily dietary intake sufficient to meet the nutrient requirements of nearly all (97–98%) the healthy individuals in a particular life stage and gender group. For some authorities, whereas the RDA can be used as a guide for assessing the adequacy of intake of individuals because it exceeds the requirements of most, intakes below the RDA cannot be assumed inadequate (25). However, for others, including WHO, the RDA is considered the intake below which an individual may be at risk of IDD (26). 3) The upper limit (UL), the highest daily intake likely to pose no adverse health risk to almost all individuals in the specified age group, but consumption above which may pose a progressively increasing risk for healthy individuals (25).
The values used in this paper correspond to globally accepted levels, which differ slightly from those used in China. In particular, the internationally recommended EAR for iodine intake (95 μg/d) is lower than that used in China (120 μg/d).
Data management.
SPSS 13.0 software (IBM) was used for data handling and preparation of tables and figures.
Ethical approval.
Each survey was assessed by its donor agencies as conforming to the applicable ethical standards.
Results
Samples tested in the 2 surveys, and analytical verification.
A total of 144 samples (12 food group composites from each of 12 provinces, prepared from 663 individual food and beverage samples) from the 2007 TDS, and 60 samples (12 composites from each of 5 provinces) and 338 individual food and beverage items from the 2009 TDS were tested for iodine. The laboratory performance was verified against the results of standard reference material analyzed in the survey laboratory at the Beijing CDC. The results for the iodine intake of a standard adult male by food group are presented for the 2 surveys in Table 1. Results for the 338 individual food items in 2009 are presented in Supplemental Table 1.
TABLE 1.
Dietary iodine intake from various food groups by a standard adult male in provinces of China in 2007 and 20091
| Province and iodine intake | Cereals | Legumes | Potatoes | Meat | Eggs | Aquatic foods | Milk | Vegetables | Fruits | Sugar | Beverages and water | Alcohol beverages | Total |
| Liaoning | |||||||||||||
| 2007 μg/d | 6.7 | 16.9 | 37.1 | 24.6 | 22.7 | 14.7 | 13.9 | 239 | 3.1 | 0 | 2.8 | 0.1 | 382 |
| % of daily total | 1.8 | 4.4 | 9.7 | 6.4 | 6 | 3.9 | 3.6 | 62.6 | 0.8 | 0 | 0.7 | 0 | 100 |
| 2009 μg/d | 26.1 | 45.2 | 26.8 | 38.9 | 32.6 | 0.9 | 5.11 | 187 | 0 | 0 | 3.9 | 0 | 366 |
| % of daily total | 7.1 | 12.4 | 7.3 | 10.6 | 8.9 | 0.2 | 1.4 | 50.9 | 0 | 0 | 1.1 | 0 | 100 |
| Shanghai | |||||||||||||
| 2007 μg/d | 21.2 | 16.1 | 12.2 | 52.9 | 22.2 | 45.5 | 32.5 | 176 | 3.6 | 0 | 30.1 | 0.1 | 412 |
| % of daily total | 5.1 | 3.9 | 2.9 | 12.8 | 5.4 | 11 | 7.9 | 42.7 | 0.9 | 0 | 7.3 | 0 | 100 |
| 2009 μg/d | 2.7 | 15.7 | 2.1 | 22.9 | 11.7 | 25 | 18.5 | 124 | 0 | 0 | 3.6 | 0.3 | 226 |
| % of daily total | 1.2 | 7 | 0.9 | 10.1 | 5.2 | 11.1 | 8.2 | 54.7 | 0 | 0 | 1.6 | 0.1 | 100 |
| Fujian | |||||||||||||
| 2007 μg/d | 12.8 | 10.2 | 6.3 | 31.7 | 16.3 | 157 | 4.2 | 139 | 1.3 | 0 | 0.7 | 0 | 379 |
| % of daily total | 3.4 | 2.7 | 1.7 | 8.4 | 4.3 | 41.3 | 1.1 | 36.6 | 0.3 | 0 | 0.2 | 0 | 100 |
| 2009 μg/d | 31.6 | 5.3 | 3.9 | 36 | 7.7 | 76.2 | 3 | 107 | 1.6 | 0.3 | 14.7 | 0.8 | 288 |
| % of daily total | 11 | 1.8 | 1.3 | 12.5 | 2.7 | 26.5 | 1 | 37.1 | 0.6 | 0.1 | 5.1 | 0.3 | 100 |
| Heilongjiang | |||||||||||||
| 2007 μg/d | 16.6 | 23.8 | 15.6 | 23.8 | 24.1 | 16.2 | 9.4 | 190 | 5.3 | 0 | 4.1 | 0 | 329 |
| % of daily total | 5 | 7.2 | 4.7 | 7.2 | 7.3 | 4.9 | 2.9 | 57.8 | 1.6 | 0 | 1.2 | 0 | 100 |
| Hebei | |||||||||||||
| 2007 μg/d | 67.8 | 56.2 | 24.9 | 22 | 24.7 | 15.4 | 3.5 | 182 | 2.9 | 0 | 14.4 | 0.1 | 414 |
| % of daily total | 16.4 | 13.6 | 6 | 5.3 | 6 | 3.7 | 0.8 | 43.9 | 0.7 | 0 | 3.5 | 0 | 100 |
| Henan | |||||||||||||
| 2007 μg/d | 186 | 24.9 | 23.5 | 41 | 19.4 | 3.4 | 5.8 | 164 | 1.6 | 0 | 4.1 | 0.1 | 474 |
| % of daily total | 39.2 | 5.3 | 5 | 8.7 | 4.1 | 0.7 | 1.2 | 34.6 | 0.3 | 0 | 0.9 | 0 | 100 |
| Sha'anxi | |||||||||||||
| 2007 μg/d | 181 | 14.6 | 74 | 26.2 | 24.6 | 7.6 | 6.8 | 273 | 0.8 | 0 | 1.1 | 0 | 610 |
| % of daily total | 29.8 | 2.4 | 12.1 | 4.3 | 4 | 1.2 | 1.1 | 44.7 | 0.1 | 0 | 0.2 | 0 | 100 |
| Jiangxi | |||||||||||||
| 2007 μg/d | 5.9 | 15.9 | 7.4 | 39.3 | 17.8 | 11.1 | 0.3 | 410 | 0.7 | 0 | 1.5 | 0.4 | 511 |
| % of daily total | 1.2 | 3.1 | 1.4 | 7.7 | 3.5 | 2.2 | 0.1 | 80.4 | 0.1 | 0 | 0.3 | 0.1 | 100 |
| Ningxia | |||||||||||||
| 2007 μg/d | 4.1 | 9 | 64.8 | 12.6 | 6.1 | 6.1 | 2.6 | 166 | 4.4 | 0 | 1 | 0 | 276 |
| % of daily total | 1.5 | 3.2 | 23.4 | 4.6 | 2.2 | 2.2 | 0.9 | 60 | 1.6 | 0 | 0.4 | 0 | 100 |
| Hubei | |||||||||||||
| 2007 μg/d | 37.2 | 17.2 | 18.7 | 22 | 42.1 | 32.4 | 2.9 | 370 | 1.2 | 0 | 3.3 | 0.3 | 547 |
| % of daily total | 6.8 | 3.1 | 3.4 | 4 | 7.7 | 5.9 | 0.5 | 67.6 | 0.2 | 0 | 0.6 | 0.1 | 100 |
| Sichuan | |||||||||||||
| 2007 μg/d | 8.7 | 18.2 | 26.3 | 115.1 | 13.2 | 7.4 | 17.5 | 212 | 0.6 | 0 | 1.5 | 0 | 420 |
| % of daily total | 2.1 | 4.3 | 6.2 | 27.4 | 3.1 | 1.8 | 4.2 | 50.4 | 0.1 | 0 | 0.4 | 0 | 100 |
| Guangxi | |||||||||||||
| 2007 μg/d | 18.1 | 12.6 | 11 | 36 | 4.8 | 12.1 | 0.7 | 245 | 1.5 | 0 | 2.6 | 0 | 344 |
| % of daily total | 5.3 | 3.7 | 3.2 | 10.5 | 1.4 | 3.5 | 0.2 | 71.1 | 0.4 | 0 | 0.8 | 0 | 100 |
| Zhejiang | |||||||||||||
| 2009 μg/d | 54.6 | 16.3 | 5.5 | 21.2 | 18.5 | 35.6 | 9.3 | 241 | 1.8 | 0.2 | 15.7 | 1.3 | 421 |
| % of daily total | 13 | 3.9 | 1.3 | 5 | 4.4 | 8.4 | 2.2 | 57.2 | 0.4 | 0.1 | 3.7 | 0.3 | 100 |
| Beijing | |||||||||||||
| 2009 μg/d | 62.9 | 24.9 | 29.9 | 44.4 | 20.6 | 7.6 | 11.4 | 248 | 3.7 | 27.5 | 19.9 | 0.2 | 501 |
| % of daily total | 12.5 | 5 | 6 | 8.9 | 4.1 | 1.5 | 2.3 | 49.5 | 0.7 | 5.5 | 4 | 0 | 100 |
| Mean | |||||||||||||
| 2007 μg/d | 47.2 | 19.6 | 26.8 | 37.3 | 19.8 | 27.4 | 8.4 | 230 | 2.3 | 0 | 5.6 | 0.1 | 425 |
| % of daily total | 11.1 | 4.6 | 6.3 | 8.8 | 4.7 | 6.4 | 2 | 54.2 | 0.5 | 0 | 1.3 | 0 | 100 |
| Mean in 4 coastal provinces | |||||||||||||
| 2009 μg/d | 28.8 | 20.7 | 9.6 | 29.9 | 17.6 | 34.4 | 9.0 | 165 | 0.9 | 0.1 | 9.5 | 0.6 | 325 |
| % of daily total | 8.1 | 6.3 | 2.7 | 9.6 | 5.3 | 11.6 | 3.2 | 50.0 | 0.3 | 0 | 2.9 | 0.2 | 100 |
The 2007 and 2009 measures in 3 provinces were not directly comparable, because the surveyed locations were not the same.
Among the surveyed provinces in both 2007 and 2009, iodine intake mainly came from cooked vegetables, to which salt is added not at the table but during preparation, as is customary in China; vegetables contributed 50–60% of the overall iodine intake in both surveys. Meat was a major contributor in some provinces, particularly Sichuan, and cereals also substantively contributed to intake in Henan, Sha’anxi, and Hebei. Aquatic products (of animal origin only) were a major source of iodine in Fujian (41.3% in 2007 and 26.5% in 2009) and to a lesser extent in Shanghai (∼11% in both surveys). Although higher in iodine content by weight (Supplemental Table 1), in general, aquatic products contributed only minimally to iodine intake in coastal provinces.
The mean daily dietary iodine intake of a standard male in 2007 was calculated for each province (Table 1). Intake in 2007 was highest in Sha’anxi (609.8 μg/d), followed by Hubei, and lowest in Ningxia (276.4 μg/d). The mean iodine intake in the 12 provinces in 2007 was 425 μg/d and in 2009 it was 325 μg/d in the 4 coastal provinces and 501 μg/d in Beijing.
Three of the 5 provinces surveyed in 2007 were also surveyed in 2009. In 2, the total daily iodine intake fell substantively (by 45% in Shanghai and 24% in Fujian). The only major difference in iodine intake by food group was in Fujian residents’ intake in aquatic food, which fell by more than one-half. Interestingly, aquatic food intake there did not decrease by weight (data not shown), suggesting lower iodine content of the aquatic food consumed during the months of the 2009 survey. There were decreases across almost all food groups in Shanghai (Table 1). Data not presented indicate that salt intake declined by 35% from 2007 to 2009 in both Shanghai and Fujian. In the third province surveyed twice, Liaoning, iodine intake fell slightly, but total salt intake increased by ∼10%, with inconsistent changes across the food groups. Salt intake by weight for a standard adult male in all provinces surveyed fell from a mean of 11.5 g/d in 2007 to 9.0 g/d in 2009.
Table 2 compares the daily dietary iodine intake of a standard adult male in 12 provinces (2007) and in 4 coastal provinces and Beijing (2009) with the EAR, RDA, and UL for iodine intake for such a person. Although it declined in 2009, the calculated mean intake was well above the EAR but well below the UL in all provinces. In 2007, the mean iodine intake by province ranged from 25 to 56% and in 2009 from 21–46% of the UL. The iodine intake in coastal provinces was lower than in Beijing in 2009.
TABLE 2.
Dietary iodine intake by a standard adult male in provinces of China in 2007 and 2009 relative to 3 intake standards1
| 2007 |
2009 |
||||||
| Province | EAR2 | RDA3 | UL4 | Province | EAR | RDA | UL |
| % | % | ||||||
| Liaoning | 402 | 255 | 35 | Liaoning | 385 | 244 | 33 |
| Shanghai | 434 | 275 | 37 | Shanghai | 238 | 151 | 21 |
| Fujian | 399 | 253 | 34 | Fujian | 303 | 192 | 26 |
| Heilongjiang | 347 | 220 | 30 | Zhejiang | 443 | 281 | 38 |
| Hebei | 435 | 276 | 38 | Beijing | 527 | 334 | 46 |
| Henan | 499 | 316 | 43 | ||||
| Shaanxi | 642 | 407 | 56 | ||||
| Jiangxi | 538 | 340 | 46 | ||||
| Ningxia | 291 | 184 | 25 | ||||
| Hubei | 576 | 365 | 50 | ||||
| Sichuan | 442 | 280 | 38 | ||||
| Guangxi | 363 | 230 | 31 | ||||
| Mean | 447 | 283 | 39 | Mean | 379 | 240 | 33 |
| Mean without Beijing | 342 | 217 | 30 | ||||
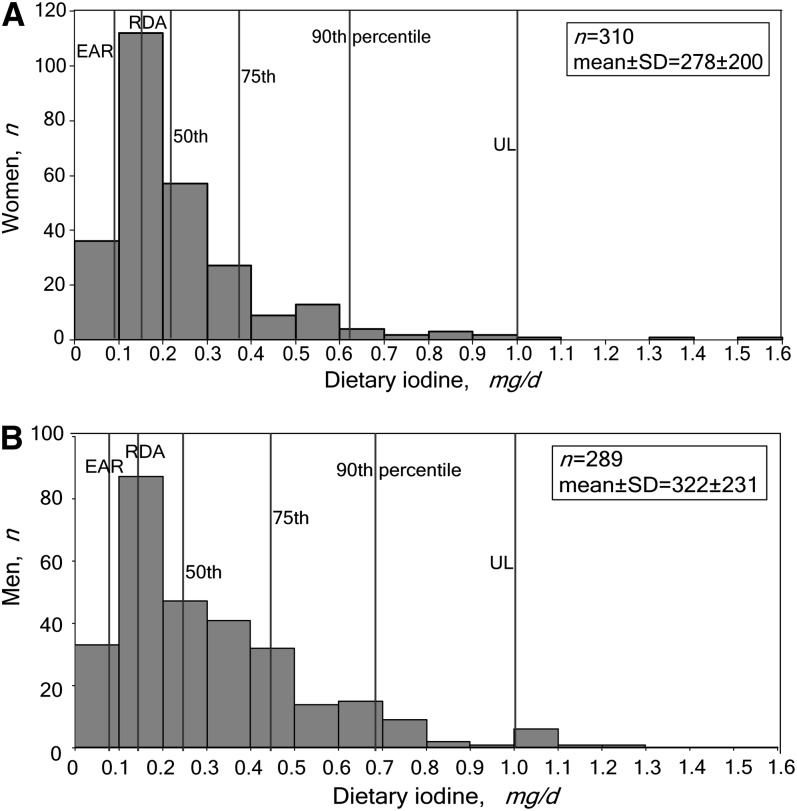
To further address concerns about iodine intake in coastal China, Table 3 presents iodine intake disaggregated by 10 age-sex groups in the 4 coastal provinces surveyed in 2009. The median daily iodine intake in each group was much lower than (mostly well below one-half) the recommended UL in all age groups. The median intakes were mostly much lower than the mean (ranging from 45 to 69% lower except among children), indicating a heavily skewed distribution of iodine intake and explaining the high calculated mean of the standard adult male (Fig. 1). Across the age-sex groups, 0–19% of individuals consumed more than the UL, although this range fell to 0–7% upon excluding the 2 child groups. Among children, although the median intake was around one-half the UL in both age groups, the means were higher at 73 and 87% of the UL in the 2–7 and 8–12 y age groups, respectively.
TABLE 3.
Dietary iodine intakes of 10 age-sex groups in 4 coastal provinces of China in 2009 and their relations to 3 intake standards1
| Iodine intake |
||||||||||
| Age, y | Sex | n | EAR2 | RDA2 | UL | Mean ± SD | Median | <EAR | <RDA | ≥UL |
| μg/d | μg/d | % | ||||||||
| 2–7 | M&F | 52 | 65 | 90 | 300 | 220 ± 238 | 152 | 21.2 | 30.8 | 19.2 |
| 8–12 | M&F | 66 | 73 | 120 | 600 | 523 ± 928 | 277 | 3.0 | 19.7 | 18.2 |
| 13–17 | M | 38 | 95 | 150 | 900 | 285 ± 165 | 285 | 10.7 | 52 | 0.0 |
| 13–17 | F | 32 | 95 | 150 | 900 | 229 ± 228 | 171 | 8.0 | 24.6 | 4.0 |
| 18–50 | M | 296 | 95 | 150 | 1100 | 531 ± 866 | 250 | 9.4 | 25.5 | 4.0 |
| 18–50 | F | 318 | 95 | 1501 | 1100 | 384 ± 754 | 212 | 10.4 | 31.5 | 3.5 |
| 51–65 | M | 173 | 95 | 150 | 1100 | 562 ± 923 | 252 | 10.4 | 29 | 6.9 |
| 51–65 | F | 196 | 95 | 150 | 1100 | 396 ± 733 | 246 | 13.2 | 30 | 5.0 |
| >65 | M | 63 | 95 | 150 | 1100 | 312 ± 215 | 260 | 15.9 | 25.4 | 1.6 |
| >65 | F | 72 | 95 | 150 | 1100 | 276 ± 289 | 189 | 20.8 | 45.8 | 2.8 |
FIGURE 1.
Dietary iodine intakes by women (A) and men (B) aged 18–50 y in 4 coastal provinces in China in 2009 by percentile and relative to 3 intake standards. Outliers (n = 7 men and 8 women) were excluded.
Using the EAR as the standard for low iodine intake, the population at risk for IDD was small in each age and sex group. On the other hand, dietary iodine intake was below the RDA in a higher proportion of those surveyed in all age-sex groups, including 31.5% of reproductive-age women, suggesting a possible ongoing risk of IDD in China with the salt iodization levels applicable at the times of these surveys and using the WHO interpretation of this indicator (26).
Comparison between cooking with iodized and noniodized salt in Shanghai.
The mean dietary iodine intake of a standard Shanghai male in 2009 using salt iodized at 28.3 mg/kg in food preparation was 226 μg/d. This figure declined to 83 μg/d when noniodized salt was used for cooking, indicating that iodized salt contributed 63.5% to the total intake of iodine in food and beverages. The loss of iodine during cooking (based on the amount added during food preparation vs. the amount measured in the cooked food) was 24.6%. This is consistent with the figure of 20% given by WHO (27).
Discussion
The iodine intake of China’s population in 2007 and that of residents of China’s coastal areas and Beijing in 2009 was generally safe; iodized salt was the main dietary source. Applying our finding that iodized salt contributes 63.5% of food and beverage iodine intake and given that iodine supplements are generally not available in China, we conclude that if salt were not iodized, iodine intake would be much lower in most of the provinces surveyed. Domestic use of noniodized salt would lower more individuals’ intake of iodine below the EAR and RDA, underscoring the importance of USI in China and elsewhere. Our findings can quite reasonably be generalized to the population of “middle China,” from whose diet a large sample and wide variety of dishes were prepared and analyzed in both surveys. They are also highly relevant to other nations where food is mostly prepared from fresh ingredients and the local environment is low in naturally occurring iodine.
On the other hand, our data suggest that iodine intake in China is high relative to that of other nations and easily high enough to avoid IDD at the population level (28). Indeed, the apparent high intake among children in coastal areas and in Beijing (data not shown) may have adverse consequences (29) and merits verification and possibly intervention to reduce salt intake among China’s children. However, we note the paucity of biological evidence used to set the dietary reference intakes for iodine in children (30) and suggest that further research should be undertaken to verify our findings and their clinical implications.
Three concerns about USI have been circulating in China for several years: population trends in thyroid illness and thyroid antibody levels in proven high-iodine intake locations; misconceptions on the risks posed by coastal residents’ diet; and the fact that people living in around 120 counties located on the former flood-plain of the Yellow River in central northern China are indeed at risk of excessive iodine intake due to high water iodine (31,32). (In these counties, noniodized salt is available for local purchase.) Our report does not address trends in thyroid disease but does identify a subpopulation of high iodine consumers among whom some may be at risk of thyroid disease as a result (14). Planned research in China on the thyroid hormone and auto-antibody status of women early in pregnancy is therefore important (33). However, the high iodine consumption of a minority of individuals should not be extrapolated to the population as a whole, e.g. by liberalizing the sale of noniodized salt, given the proven impact of USI on reducing IDD. Furthermore, this report clearly disproves fears that coastal residents are at higher risk of thyroid illness due to excessive iodine intake, and identifies vegetables, not food of sea origin, as the main source of iodine intake in these areas (with the possible exception of certain foods in Fujian). Although we assessed only the contribution of iodized salt added during cooking in one location, it seems likely that the addition of salt to vegetables and other foods during cooking is the main risk factor for iodine excess in China, not the iodine content of what is consumed. Water iodine was not a major contributor to iodine intake in surveyed locations.
At the time of our 2009 survey, in addition to debating the sale of noniodized salt, China’s government was also considering lowering the salt iodization standard from the then mandatory mean of 35 mg/kg. Indeed, it was decided that while USI remains mandatory, provinces may now iodize salt to a median within the range of 20–30 mg/kg, depending on local diet, iodized salt coverage, and UIE (34). This reduction underscores the need to monitor UIE carefully and regularly as an alternative and easy way of assessing iodine intake. UIE clearly correlates with iodized salt consumption (17, 26), but China’s most recent national UIE assessment in 2005 only reported province averages among children (16). A more recent survey in 4 provinces confirmed high UIE levels in several areas, particularly among rural residents (17). We did not separate the food from rural counties and urban districts in our laboratory analyses so cannot confirm this observation. Nonetheless, UIE monitoring is clearly recommended by global authorities (26) and should enable assessment of trends in iodine intake, including among subgroups of the population. It will be increasingly important as salt iodization levels are adjusted down and more individuals’ iodine intake even falls below the EAR, increasing the risk of inadequate intake (25). Table salt quality and iodization levels and food and animal salt should also be monitored.
Our surveys also provided interesting trend data on the consumption of salt in China, possibly demonstrating a secular decline in intake toward the WHO recommended level of 5 g/d (35). There is no official data on salt intake by age group in China since its last national nutrition survey in 2002, when mean intake was 12 g/d (36). However, our finding is in line with research data from 8 provinces surveyed 8 times since 1989 and reporting a 31% decline in salt intake in rural and urban areas (to 8.4 and 7.7 g/d, respectively) from 2000 to 2009 (J. Zhang, data presented at 11th Public Health Symposium 2010, Hangzhou, China, personal communication). Lower salt intake is a positive development, but if this represents a general trend, it will reduce iodine intake in China, particularly as salt iodization levels decrease. This again underscores the need for regular, reliable, and representative monitoring of UIE.
The surveys we report have some limitations, particularly the relatively small number of children and teenagers assessed in 2009. In addition, direct comparison of the 2007 and 2009 survey findings was not possible, because the survey sites were not identical. There are also curious differences in dietary consumption patterns across the locations and time periods; even within some provinces, there were large differences in consumption of certain foods across the 2 surveys. This may be explained by seasonal availability of certain items, but further investigation is suggested. Knowing the thyroid hormone and antibody status of those assessed in 2009 would have strengthened our conclusion that China’s iodine consumption levels are generally not excessive, but it was not possible to assess these in the context of a TDS. We did not assess other sources of iodine intake, because food and beverages constitute the only source in China. The addition of iodine to locally produced multivitamin or nutrition supplements was prohibited by law upon introduction of USI. A minority of pregnant women consumes imported, iodine-containing multivitamin supplements, but these are too expensive for the majority. Moreover, the number of individual food and beverage items assessed in 2009 by far exceeds the numbers analyzed in TDS recently conducted in other developing countries (20); the data show a reassuring internal consistency and the laboratory analysis is not doubted. These factors suggest that our findings on iodine intake are reliable. Nonetheless, we look forward to the results, hopefully available in 2013, of China’s next TDS and national nutrition survey including UIE in some of the same provinces we surveyed. The sampling in these surveys included a broader cross-section of Chinese society than ours and may enable disaggregation of iodine intake by urban-rural residence [a previous survey found higher intake in rural areas (17)] and by socio-economic group.
The calculated mean and median iodine intake levels in China appear high relative to international dietary recommendations, although these recommendations are acknowledged to be of limited value for individuals (25). Based on the international EAR, the proportion of China’s population at risk of low iodine consumption is small, but this will increase as salt iodization is adjusted down. However, based on the published, WHO-recommended daily intake levels for individuals (26), a rather high proportion of several age and sex groups are at risk. Recent evidence and opinion suggests that iodine sufficiency and normal thyroid function are especially important in the earliest stages of pregnancy (33, 37–39). However, we acknowledge research from China that also suggests risks may exist with high-normal iodine intake (14, 33).
In China and other nations at risk of IDD, it is imperative to ensure adequate iodine intake before and during pregnancy, in the same way as China now does for folic acid, to prevent neural-tube defects. Although safe iodization levels may vary according to local environmental conditions, maintaining USI at an appropriate level is an important part of this. To protect future generations of children, local iodization policy in China should be based on routine monitoring of UIE by province, certainly among women of reproductive age, and additional research on the impact and most appropriate level of salt iodization (33). More generally, there is increasing global recognition of the need to improve the knowledge of reproductive-age women on the impact of diet before and during pregnancy on the health of their children. Screening the thyroid status of women before or during early pregnancy may also be important (39). Advocacy on excess salt intake among some groups is also needed.
Supplementary Material
Acknowledgments
The authors thank Lilian Selenje, formerly of UNICEF China, for her involvement in the survey design. Y.W. participated in the design of the 2007 TDS and with X.L., S.C., D.H., and others conceived and designed the 2009 study of coastal province diet and dietary iodine intake; Y.W. and X.L. performed initial data analysis and drafted a report with equal contribution; D.H., S.C., X.L., and Y.W. performed additional data analysis and prepared the paper for publication; L.L. conducted the laboratory analysis of food samples in Beijing; S.Z. managed the processing of food samples in Shanghai; D.H., Y.W., X.L., and S.C. had primary responsibility for the paper’s final content; and X.L. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Footnotes
Supported by the National Natural Science Foundation of China (20837003; 2007 and 2009 surveys), the National Basic Research Program of China (973 Program 2012CB720804; 2009 survey), the China Ministry of Health (grant no. 200902009; 2009 survey), and UNICEF (2009 survey). The authors were entirely funded by their institutions. No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The perspectives in this article are solely those of the authors, and do not purport to represent the views of their employers
Supplemental Table I is available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Joint first authors with equal contribution.
Abbreviations used: CDC, Chinese Center for Disease Control and Prevention; EAR, estimated average requirement; IDD, iodine deficiency disorder; IIH, iodine-induced hyperthyroidism; LOD, limit of detection; TDS, total diet study; UL, upper limit; UIE, urinary iodine excretion; USI, universal salt iodization.
Literature Cited
- 1.WHO Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: Switzerland, WHO; 2009
- 2.Ma T, Lu T, Tan Y, Chen B, Zhu X. The present status of endemic goiter and endemic cretinism in China. Food Nutr Bull. 1982;4:85–9 [Google Scholar]
- 3.Qian M, Wang D, Watkins WE, Gebski V, Yan YQ, Li M, Chen ZP. The effects of iodine on intelligence in children: a meta-analysis of studies conducted in China. Asia Pac J Clin Nutr. 2005;14:32–42 [PubMed] [Google Scholar]
- 4.UNICEF-WHO Joint Committee on Health Policy. World Summit for Children mid-decade goal: iodine deficiency disorders. Geneva: UNICEF-WHO; 1994.
- 5.Ministry of Health Iodized salt monitoring report, 2010. Ministry of Health People's Republic of China; 2011
- 6.UNICEF The state of the world's children. New York: UNICEF; 2012
- 7.Andersson M KV, Zimmermann MB. Global iodine status in 2011 and trends over the past decade. J Nutr., e-publication, 29 Feb, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Stanbury JB, Ermans AE, Bourdoux P, Todd C, Oken E, Tonglet R, Vidor G, Braverman LE, Medeiros-Neto G. Iodine-induced hyperthyriodism: occurrence and epidemiology. Thyroid. 1998;8:83–100 [DOI] [PubMed] [Google Scholar]
- 9.Delange F, de Benoist B, Alnwick D. Risks of iodine-induced hyperthyroidism after correction of iodine deficiency by iodized salt. Thyroid. 1999;9:545–56 [DOI] [PubMed] [Google Scholar]
- 10.Yang F, Shan Z, Teng X, Li Y, Guan H, Chong W, Teng D, Yu X, Fan C, Dai H, et al. Chronic iodine excess does not increase the incidence of hyperthyroidism: a prospective community-based epidemiological survey in China. Eur J Endocrinol. 2007;156:403–8 [DOI] [PubMed] [Google Scholar]
- 11.Duntas LH. Environmental factors and autoimmune thyroiditis. Nat Clin Pract Endocrinol Metab. 2008;4:454–60 [DOI] [PubMed] [Google Scholar]
- 12.Konno N, Makita H, Yuri K, Iizuka N, Kawasaki K. Association between dietary iodine intake and prevalence of subclinical hypothyroidism in the coastal regions of Japan. J Clin Endocrinol Metab. 1994;78:393–7 [DOI] [PubMed] [Google Scholar]
- 13.Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, Jin Y, Yu X, Fan C, Chong W, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354:2783–93 [DOI] [PubMed] [Google Scholar]
- 14.Teng X, Shan Z, Chen Y, Lai Y, Yu J, Shan L, Bai X, Li Y, Li N, Li Z, et al. More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol. 2011;164:943–50 [DOI] [PubMed] [Google Scholar]
- 15.Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, Pedersen IB, Carle A. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab. 2010;24:13–27 [DOI] [PubMed] [Google Scholar]
- 16.Andersson M, Takkouche B, Egli I, Allen HE, de Benoist B. Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bull World Health Organ. 2005;83:518–25 [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Fan Y, Chen H, Li X, Wang J, Gu Y, Li M, Shu Z. Is the current iodine content in edible salt appropriate for eliminating iodine deficiency in China. Asia Pac J Clin Nutr. 2010;19:231–5 [PubMed] [Google Scholar]
- 18.Juan S. Nation sees more thyroid problems. China Daily. 3 September, 2010 [cited 2012 Mar 15]. Available from: http://www.chinadaily.com.cn/usa/2010–09/03/content_11253269.htm
- 19.Bai J. Health experts to respond to increased cancer caused by iodine excess claims. China Daily. 29 Jul 2010 [cited 2012 Mar 15]. Available from: http://www.china-daily.org/China-News/Health-experts-to-respond-to-increased-cancer-caused-by-iodine-excess-claims/
- 20.WHO Fourth International Workshop on Total Diet Studies, 2006, Beijing, China. Geneva: WHO; 2007
- 21.Rose M, Miller P, Baxter M, Appleton G, Crews H, Croasdale M. Bromine and iodine in 1997 UK total diet study samples. J Environ Monit. 2001;3:361–5 [DOI] [PubMed] [Google Scholar]
- 22.Murray CW, Egan SK, Kim H, Beru N, Bolger PM. US Food and Drug Administration's Total Diet Study: dietary intake of perchlorate and iodine. J Expo Sci Environ Epidemiol. 2008;18:571–80 [DOI] [PubMed] [Google Scholar]
- 23.WHO Guidelines for the study of dietary intakes of chemical contaminants. Geneva: WHO; 1985 [PubMed]
- 24.Chen J, Gao J. The Chinese total diet study in 1990. Part I. Chemical contaminants. J AOAC Int. 1993;76:1193–205 [PubMed] [Google Scholar]
- 25.Institute of Medicine of the National Academies of Science Dietary Reference Intakes: the essential guide to nutrient requirements. Washington, DC: The National Academies Press; 2006
- 26.WHO UNICEF ICCIDD Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. Geneva: WHO UNICEF ICCIDD; 2007
- 27.WHO Recommended iodine levels in salt and guidelines for monitoring their adequacy and effectiveness. Geneva: WHO; 1996
- 28.Flynn A, Hirvonen T, Mensink GB, Ocke MC, Serra-Majem L, Stos K, Szponar L, Tetens I, Turrini A, Fletcher R, et al. Intake of selected nutrients from foods, from fortification and from supplements in various European countries. Food Nutr Res. 2009;53 Suppl 1:1–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmermann MB, Ito Y, Hess SY, Fujieda K, Molinari L. High thyroid volume in children with excess dietary iodine intakes. Am J Clin Nutr. 2005;81:840–4 [DOI] [PubMed] [Google Scholar]
- 30.Institute of Medicine of the National Academies of Science Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington, DC: The National Academies Press; 2001 [PubMed]
- 31.Li M, Liu DR, Qu CY, Zhang PY, Qian QD, Zhang CD, Jia QZ, Wang HX, Eastman CJ, Boyages SC. Endemic goitre in central China caused by excessive iodine intake. Lancet. 1987;2:257–9 [PubMed] [Google Scholar]
- 32.Zhao J, Chen Z, Maberly G. Iodine-rich drinking water of natural origin in China. Lancet. 1998;352:2024. [DOI] [PubMed] [Google Scholar]
- 33.Teng W, Shan Z. Pregnancy and thyroid diseases in China. Thyroid. 2011;21:1053–5 [DOI] [PubMed] [Google Scholar]
- 34.Ministry of Health Announcement on food safety national standard “iodine content in edible salt;” 2011. [cited 2012 Mar 15]. Available from: http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohwsjdj/s7891/201109/53064.htm
- 35.WHO Creating an enabling environment for population-based salt reduction. Geneva: WHO; 2010
- 36.Zhai FY, He YN, Ma GS, Li YP, Wang ZH, Hu YS, Zhou LY, Cui ZH, Li Y, Yang XG. Study on the current status and trend of food consumption among Chinese population. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26:485–8 [PubMed] [Google Scholar]
- 37.Costeira MJ, Oliveira P, Santos NC, Ares S, Saenz-Rico B, de Escobar GM, Palha JA. Psychomotor development of children from an iodine-deficient region. J Pediatr. 2011;159:447–53 [DOI] [PubMed] [Google Scholar]
- 38.Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T, Xu YH, Tao FB. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab. 2011;96:3234–41 [DOI] [PubMed] [Google Scholar]
- 39.Brent GA. The debate over thyroid-function screening in pregnancy. N Engl J Med. 2012;366:562–3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.