Abstract
Ru complexes with chiral diphosphines and amine-based ligands achieve high catalytic activity and enantioselectivity for the hydrogenation of ketones under neutral to slightly basic conditions. The chiral environment is controllable by changing the combination of these two ligands. A concerted six-membered transition state is proposed to be the origin of the high reactivity. The η6-arene/TsDPEN–Ru and MsDPEN–Cp*Ir catalysts effect the asymmetric reaction under slightly acidic conditions. A variety of chiral secondary alcohols are obtained in high enantiomeric excess.
Keywords: asymmetric hydrogenation, chiral alcohols, enantioselectivity, Ir catalysts, ketones, Ru catalysts
Introduction
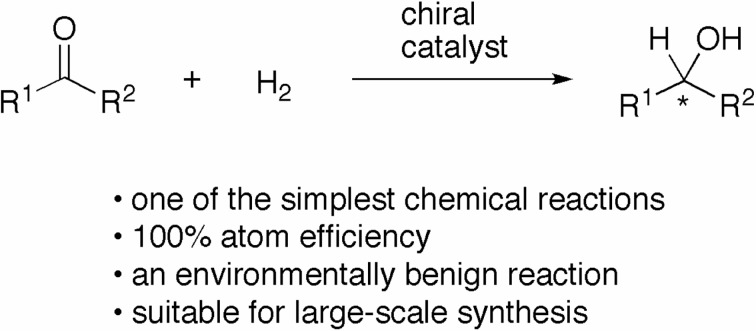
Asymmetric hydrogenation of ketones is among the simplest chemical transformations, and it affords optically active secondary alcohols that serve as useful intermediates for the synthesis of biologically active compounds such as medicines, perfumes, and agrochemicals (Scheme 1).1),2) In the presence of an appropriate chiral catalyst, molecular hydrogen (H2) is repeatedly activated and selectively added to the carbonyl moiety from one of two enantiofaces. An atomic efficiency of 100% contributes to provide an environmentally benign synthetic process. These properties indicate that the asymmetric hydrogenation of ketones is a suitable chemical process for the large-scale synthesis of chiral compounds.
Scheme 1.
The efficiency of chiral catalysts is estimated by the activity, enantioselectivity, and scope of substrates. Highly active catalysts exhibit high turnover number (TON) and/or turnover frequency (TOF = TON•h−1 or TON•s−1). The enantioselective ability of catalysts is determined by the enantiomeric excess (ee) of the products. Catalysts, which are applicable to a wide range of substrates, are desirable from the synthetic viewpoint. This review article describes our studies of the development of efficient catalysts for the asymmetric hydrogenation of ketones.3)
Early discovery of BINAP/chiral 1,2-diamine–Ru catalysts
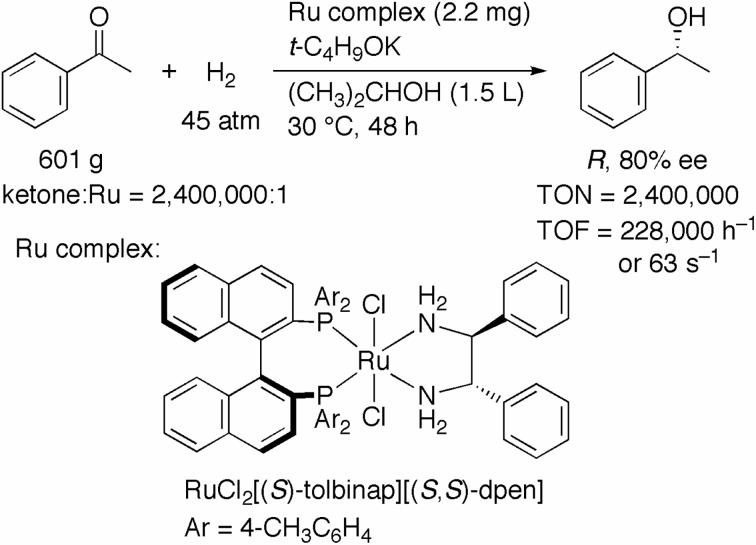
In 1995, we reported a ternary catalyst system consisting of RuCl2[(S)-binap](dmf)n [oligomeric form; BINAP = 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl], (S,S)-1,2-diphenylethylenediamine (DPEN), and KOH, which shows high activity and enantioselectivity in the hydrogenation of ketones in 2-propanol.4) This ternary system was improved to yield a more reactive catalyst system, RuCl2[(S )-tolbinap][(S,S)-dpen] [TolBINAP = 2,2′-bis(4-tolyl-phosphino)-1,1′-binaphthyl] and t-C4H9OK (Scheme 2).5) When 601 g of acetophenone, a simple aromatic ketone, was hydrogenated at 45 atm of H2 at 30 °C with only 2.2 mg of the (S)-TolBINAP/(S,S)-DPEN–Ru(II) complex for 48 h, (R)-1-phenylethanol in 80% ee was quantitatively obtained. The TON and TOF at 30% conversion were calculated to be 2,400,000 and 228,000 h−1 (or 63 s−1), respectively. Thus, the highest catalytic activity in the hydrogenation of ketones so far reported was achieved.
Scheme 2.
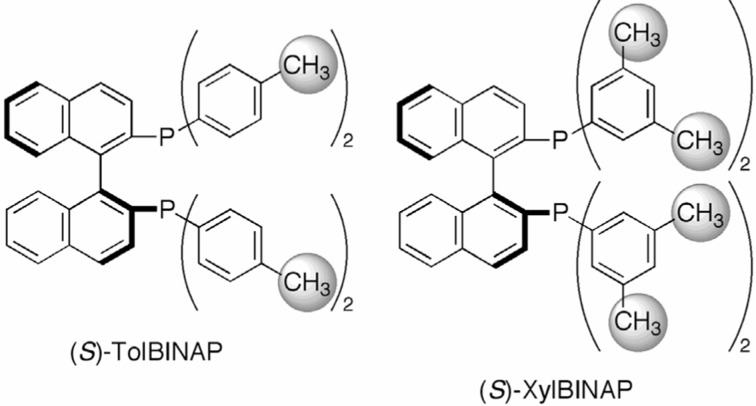
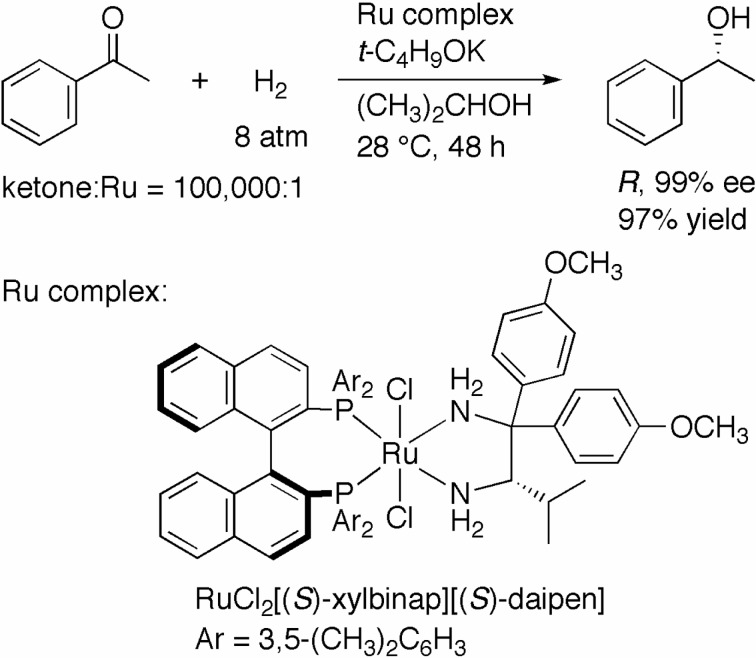
As shown in Scheme 2, we have developed a highly active catalyst for the asymmetric hydrogenation of ketones. However, from synthetic point of view, optical yield of 80% was still insufficient. Fortunately, this problem was resolved by replacing the use of TolBINAP with that of 2,2′-bis(di-3,5-xylyl-phosphino)-1,1′-binaphthyl (XylBINAP), which has sterically more demanding 3,5-xylyl moieties on the phosphorus atoms (Fig. 1). Thus, the hydrogenation of acetophenone with RuCl2[(S )-xylbinap][(S )-daipen] (DAIPEN = 1,1-di(4-anisyl)-2-isopropyl-1,2-ethylenediamine) and t-C4H9OK at a substrate-to-catalyst molar ratio (S/C) of 100,000 under 8 atm of H2 was completed in 48 h to afford (R)-1-phenylethanol in 99% ee quantitatively (Scheme 3).6)
Fig. 1.
Scheme 3.
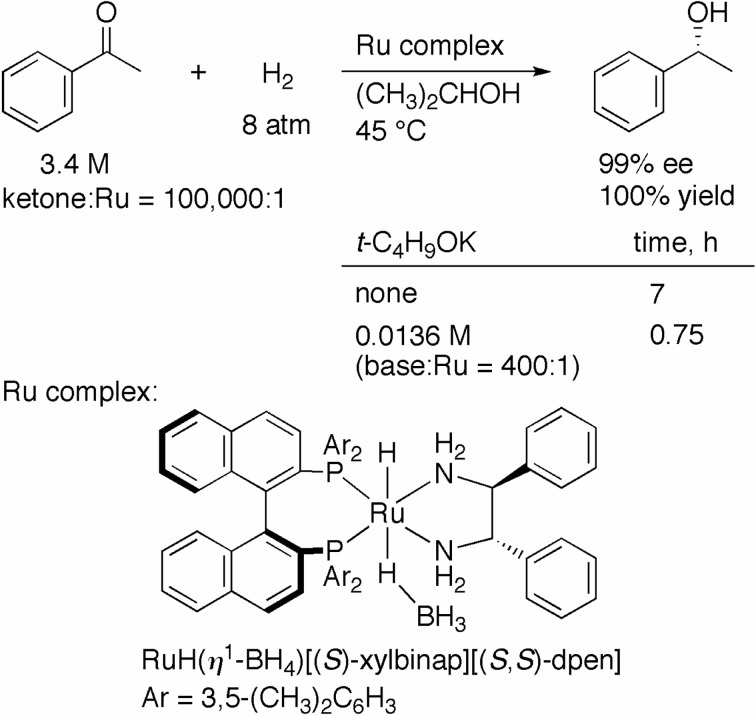
RuCl2(diphosphine)(1,2-diamine) is a catalyst precursor, and addition of base in 2-propanol (basic and reductive conditions) is required to form an active RuH species. We proceeded to develop a RuH complex with sufficient stability for use as a reagent. The desired complex, RuH(η1-BH4)[(S )-xylbi-nap][(S,S)-dpen], was readily prepared from the corresponding RuCl2 complex and an excess amount of NaBH4 (Scheme 4).7) This complex was isolated as a crystalline solid, and the structure was determined by X-ray diffraction measurement. The RuH complex shows high catalytic activity in 2-propanol without addition of a base. Acetophenone was hydrogenated with an S/C of 100,000 under 8 atm of H2 for 7 h to give the R alcohol in 99% ee quantitatively (Scheme 4). Interestingly, an even higher level of reactivity was achieved when a small amount of t-C4H9OK (400 equiv to Ru) was added to the system under otherwise identical conditions. The reaction was completed within 45 min, without a loss of en-antioselectivity. This finding suggested that base plays a positive role in the catalytic cycle.
Scheme 4.
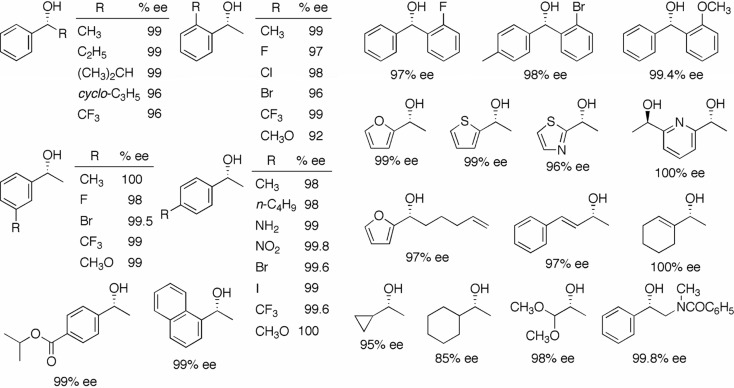
A variety of simple ketones has been hydrogenated with (S)-XylBINAP/(S)-DAIPEN–Ru and (S)-XylBINAP/(S,S)-DPEN–Ru catalysts to afford the chiral alcohols in excellent enantioselectivity quantitatively.3) Typical examples are depicted in Fig. 2. When the use of (R)-XylBINAP/(R)-DAIPEN or the (R)-XylBINAP/(R,R)-DPEN combined complex, the antipode of the chiral alcohols was obtained. Aromatic ketones with primary and secondary alkyl moieties were equally hydrogenated with excellent enantioselectivity.6),7) Interestingly, 2,2,2-trifluoroacetophenone was hydrogenated with the same sense of enantioface selection. Substitution of an electron-donating or electron-attracting group at the 2′, 3′, or 4′ position of the phenyl ring exerted little effect on stereoselectivity. The high degree of carbonyl-selectivity of this reaction was noteworthy, such that many aromatic substituents including NH2, NO2, (CH3)2CHOCO, and the halogens of substrates, were left intact.
Fig. 2.
Unsymmetrical 2-substituted benzophenones were also found to be good substrates for stereo-selective hydrogenation (Fig. 2).8) Even the 2-fluorinated ketone, in which the size of fluorine atom is similar to that of a hydrogen atom, was converted to the benzhydrol in 97% ee. Heteroaromatic ketones were hydrogenated with excellent enantioselectivity without injury at heteroaromatic moieties, which are relatively unstable under hydrogenation conditions.9) Conjugated and unconjugated olefinic ketones were selectively hydrogenated to the unsaturated alcohols.6),10)–13) No saturation of olefinic linkage was observed. This chemoselectivity proved to be quite unique, as olefinic moieties are very easily reduced in most traditional hydrogenation systems. It was found that cycloalkyl methyl ketones, dial-koxymethyl methyl ketones, and α-amino aromatic ketones could also be hydrogenated with high en-antioselectivity.6),14)
Mechanistic considerations
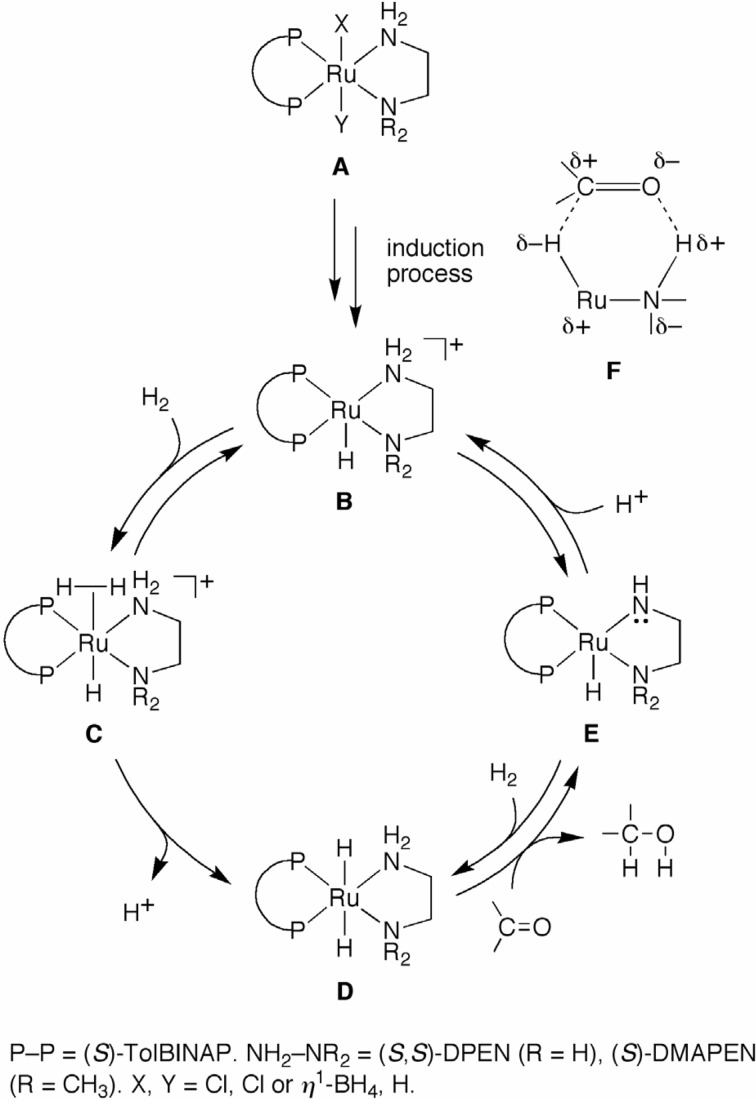
Scheme 5 shows a proposed mechanism for the hydrogenation of ketones with the (S)-TolBINAP/(S,S)-DPEN–Ru(II) catalyst (R = H).15)–18) Complex A represents a catalyst precursor in which X, Y = Cl, Cl or η1-BH4, H, respectively. The precatalyst A is converted to the cationic species B under reductive conditions with or without an additional base in an alcoholic solvent. In the presence of H2, species B reversibly gives the molecular hydrogen complex C, which is converted to the active RuH2 complex D by deprotonation. This step (C →D) is promoted by the presence of the base. A ketone immediately reacts with D, affording the alcohol and the 16-electron Ru–amide species E. The prompt protonation of E in an alcoholic medium regenerates the cationic species B, while E partially returns to D by the reaction with H2. The E → D process is significant under highly basic conditions.
Scheme 5.
The active RuH2 complex D has a fac-configuration for the hydride and two nitrogen atoms of the diamine ligand (Scheme 5). Therefore, this species smoothly reacts with the ketone via the pericyclic six-membered transition state (TS), which is schematically shown as F. The Hδ−–Ruδ+–Nδ−–Hδ+ quadropole on the catalyst fits with the Cδ+=Oδ− dipole of the ketone, thus lowering the activation energy. At least one “NH” moiety, preferably an “NH2” group for steric reasons, is necessary on the diamine ligands for the creation of highly active catalysts for the hydrogenation of ketones. Thus, the reduction of a ketone occurs in the outer coordination sphere of complex D, such that no sub-strate–Ru interaction is involved. This unique TS causes high carbonyl-selectivity over carbon–carbon multiple bonds, as described above (see Fig. 2).
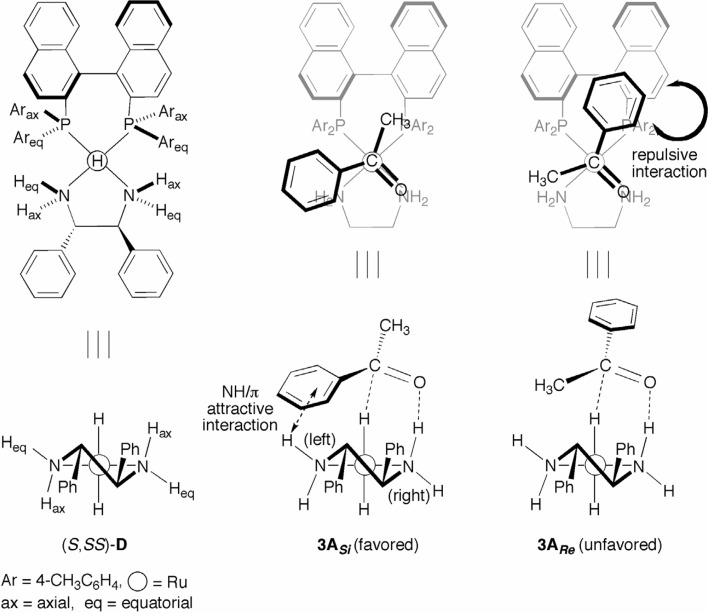
The asymmetric hydrogenation of acetophenone, catalyzed by the (S)-TolBINAP/(S,S)-DPEN–Ru(II) complex, selectively yields (R)-1-phenylethanol (see Scheme 2). The mode of enantioface selection is rationalized by the use of the molecular models shown in Fig. 3.15) The RuH2 complex with (S)-TolBINAP and (S,S)-DPEN [(S,SS)-D] (see also Scheme 5; R = H) has a C2-symmetric structure. The skewed five-membered chelate ring of (S,S)-DPEN determines the position of the respective axial and equatorial amino protons, Hax and Heq. Here, Hax is more reactive than is Heq, because the Hδ−–Ruδ+–Nδ−–Hax δ+ quadropole with a smaller dihedral angle preferably interacts with the substrate Cδ+=Oδ− dipole. Acetophenone approaches the quadropole reaction site with the si-face (3ASi) or re-face (3ARe) as the manner shown in Fig. 3. The TS 3ASi is preferable to the diastereomeric TS 3ARe, because the 3ARe suffers extreme nonbonded repulsion between the aromatic groups of the (S)-TolBINAP and the acetophenone phenyl ring, resulting in selective production of the R alcohol. The secondary attractive interaction between the NHeq and the phenyl ring of the ketone appears to further stabilize the TS 3ASi. This mechanistic interpretation is supported by experimental results showing that the Ru catalyst, with the sterically more hindered XylBINAP, exhibits higher enantioselectivity (see Schemes 2–4).
Fig. 3.
The TolBINAP/DMAPEN–Ru catalyst: Effect of n-substituents of diamine ligands
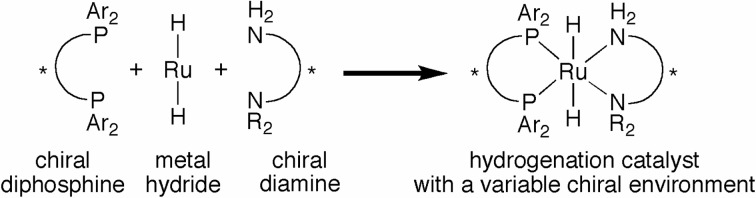
As schematically shown in Fig. 4, our hydrogenation catalysts possess chiral diphosphine and diamine ligands. Thus, a variety of chiral environment can be constructed around the center metal simply by change the combination of the two different chiral ligands. We therefore became interested in the effects of the N-substituents (R in Fig. 4) of the diamine ligands on the enantioselectivity.19)
Fig. 4.
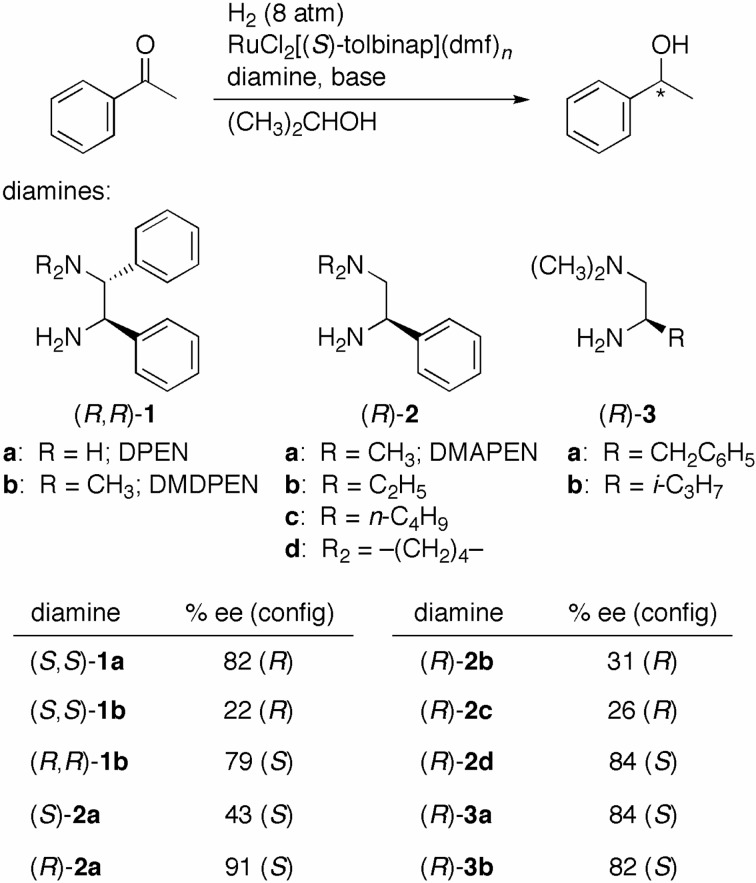
The hydrogenation of acetophenone with a catalyst system prepared in situ from RuCl2[(S)-tolbinap](dmf)n and (S,S)-DPEN [(S,S)-1a] in a base (KOH or t-C4H9OK) containing 2-propanol gave (R)-1-phenylethanol in 82% ee quantitatively (Scheme 6).5) The ee value was notably decreased to 22% when the reaction was catalyzed by the Ru complex with (S)-TolBINAP and (S,S)-N,N-dimethyl-1,2-ethylenediamine [(S,S)-DMDPEN; (S,S)-1b].19) Of note, the use of the (S)-TolBINAP/(R,R)-1b–Ru complex afforded the S alcohol in 79% ee. Thus, the replacement of an NH2 group of the diamine 1a with the N(CH3)2 moiety changed the matching combination of chiral ligands from (S)-TolBINAP/(S,S)-1a to (S)-TolBINAP/(R,R)-1b, as well as reversed the absolute configuration of the alcoholic product from R to S.
Scheme 6.
A single chiral center in the ethylenediamine suffices to achieve high enantioselectivity. In fact, when acetophenone was hydrogenated with the (S)-TolBINAP/(R)-2-dimethylamino-1-phenylethylamine [(R)-DMAPEM; (R)-2a]–Ru catalyst, the S alcohol was obtained in 91% ee.19) This ee value was higher than that obtained (i.e., 82% ee) with the use of the original (S)-TolBINAP/(S,S)-1a–Ru system. The diastereomeric (S)-TolBINAP/(S)-1b–Ru catalyst gave a lower optical yield of 43%. Interestingly, the use of Ru catalysts with the more sterically hindered diethylamino and dibutylamino ligands, (R)-2b and (R)-2c, resulted in a much lower ee with the reverse sense of enantioselection. The diamine ligand with a relatively small pyrrolidinyl moiety, (R)-2d, directed the same asymmetric sense as that obtained with (R)-2a. The structure of the substituent (R) connecting to the chiral center of diamine ligands had less of an effect on enantioselectivity. The Ru catalysts with diamine ligands (R)-3a (R = CH2C6H5) and (R)-3b (R = i-C3H7) gave the S products in 84% and 82% ee, respectively.
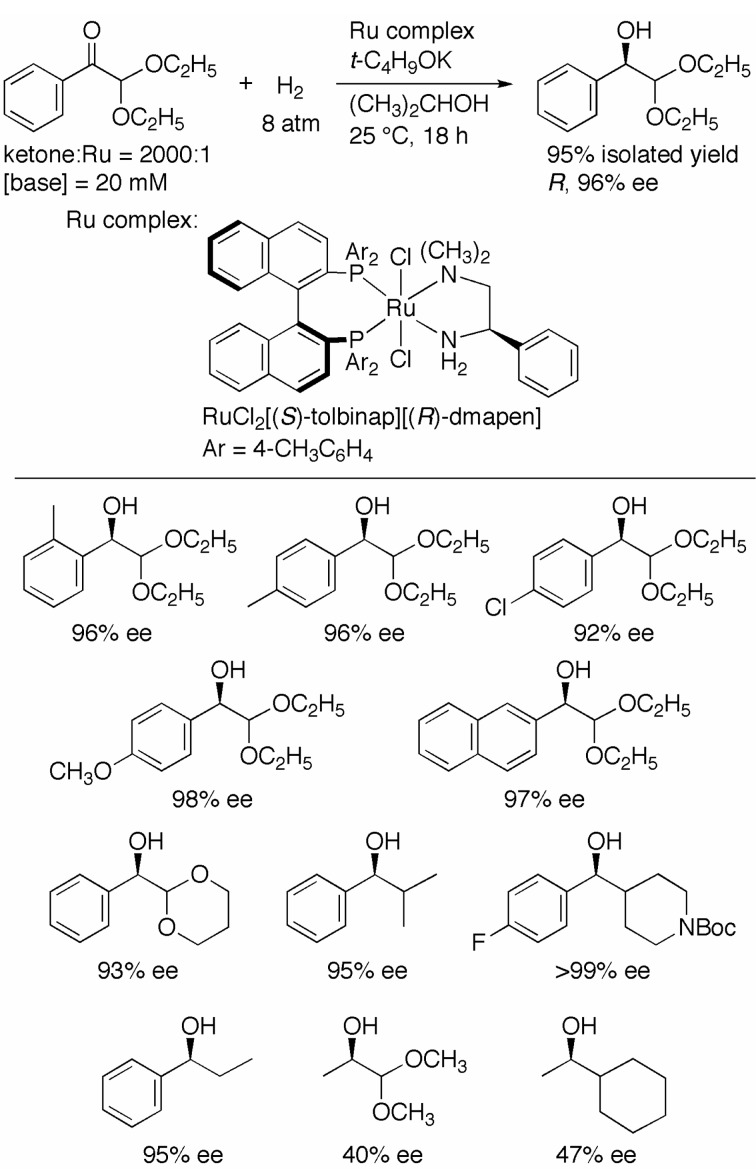
We applied the new (S)-TolBINAP/(R)-DMAPEN–Ru catalyst to the asymmetric hydrogenation of arylglyoxal dialkylacetals, a type of α-substituted functionalized aromatic ketones.19),20) As shown in Scheme 7, phenylglyoxal diethylacetal was hydrogenated in the presence of RuCl2[(S )-tolbinap][(R)-dmapen] (S/C = 2000), and t-C4H9OK in 2-propanol gave the R hydroxy acetal in 96% ee. The sense of enantioselection based on the phenyl ring was the same as that obtained in the reaction with acetophenone (see Scheme 6). The highest enantioselectivity so far reported was thus achieved. The same reaction, when catalyzed by the (R)-XylBINAP/(R)-DAIPEN–Ru complex, resulted in only 37% ee of the R alcohol. Substrates with CH3, Cl, and CH3O groups on the phenyl rings were hydrogenated in up to 98% optical yield. The naphthyl ketone and the substrate with a cyclic acetal also reacted in the same manner. Interestingly, the (S)-TolBINAP/(R)-DMAPEN–Ru catalyst exhibited the same level of activity and stereoselectivity in the reaction of aromatic ketones with simple primary and secondary alkyl groups. The hydrogenation of pyruvic aldehyde dimethylacetal and cyclohexyl methyl ketone afforded the alcoholic products with the same configuration in 40% and 47% ee, respectively. This catalyst appeared to recognize the dialkoxy methyl moieties as just simple secondary alkyl groups, and thus the aryl groups in the substrates were required to achieve high enantioselectivity.
Scheme 7.
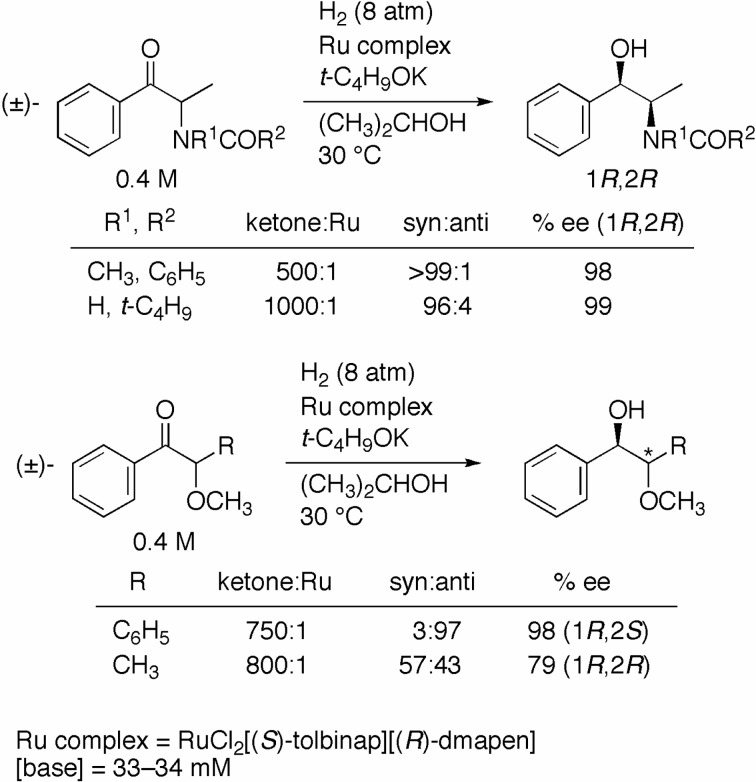
The (S)-TolBINAP/(R)-DMAPEN–Ru catalyst was also effective for the enantio- and diastereoselective hydrogenation of α-substituted aromatic ketones via dynamic kinetic resolution (Scheme 8).19),20) Racemic 2-(benzoylmethylamino)propiophenone (R1 = CH3, R2 = C6H5; 0.4 M) was hydrogenated at 8 atm of H2 with the S/R complex in basic 2-propanol ([base] = 33 mM) to afford the 1R,2R amino alcohol in 98% ee with predominant syn selectivity, and the product was readily converted to (−)-pseudoephedrine, a nasal decongestant, by the removal of a benzoyl group. The reaction of racemic 2-(pivaloylamino)propiophenone (R1 = H, R2 = t-C4H9) also gave the 1R,2R product (syn:anti = 96:4) in 99% ee. On the other hand, the hydrogenation of racemic benzoin methyl ether (R = C6H5) with the same S/R complex gave the 1R,2S methoxy alcohol (syn:anti = 3:97) in 98% ee. To the best of knowledge, this is the first example of the highly anti-selective asymmetric hydrogenation of the α-methoxy ketone. Under the same conditions, racemic 2-methoxypropiophenone (R = CH3) was converted to a 57:43 syn/anti product mixture.
Scheme 8.
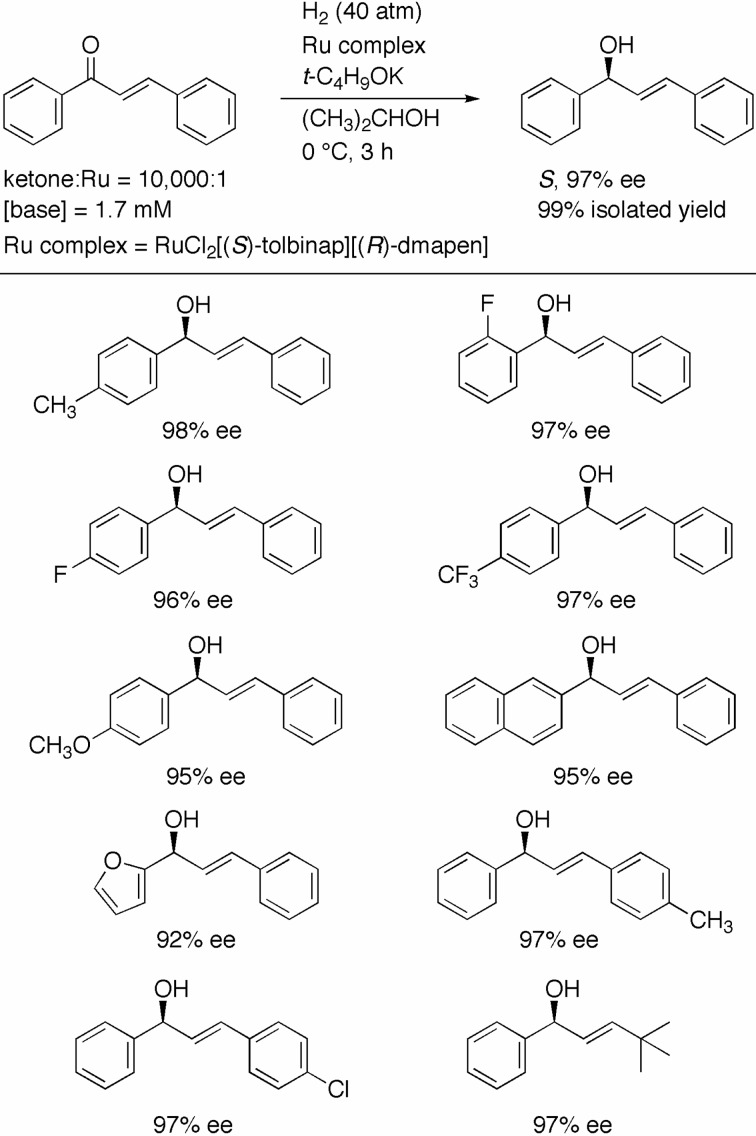
For the highly enantioselective hydrogenation of prochiral ketones (R1COR2, R1 ≠ R2), it is necessary to precisely differentiate between two substituents, R1 and R2, which connect to the carbonyl carbon. The use of XylBINAP/DAIPEN–Ru and TolBINAP/DMAPEN–Ru catalysts for the asymmetric reaction has rendered the accurate distinction between sp2-carbon groups (sp2-CG: aryl, heteroaryl, and vinyl groups) and sp3-carbon groups (sp3-CG: primary, secondary, and some hetero-substituted alkyl groups) possible (see Fig. 2 and Scheme 7). Some differentiation between sp2-CGs (2-substituted benzophenones) as well as sp3-CGs (cycloalkyl methyl ketones) has also been successfully carried out. However, to date, the discrimination of aryl groups (sp2-CG) from vinyl groups (sp2-CG) has remained difficult and therefore unrealized. Indeed, the hydrogenation of (E)-chalcone with the XylBINAP/DAIPEN–Ru catalyst gave the allylic alcohol in only 45% ee.21) The highest ee value achieved in the reduction with catecholborane catalyzed by a chiral Ga complex has been 75%.22) To date, there are still no reports in the literature of successful attempts at enzymatic reduction achieving such discrimination. The TolBINAP/DMAPEN–Ru catalyst has been used to realize the highly enantioselective hydrogenation of aryl vinyl ketones to allylic alcohols. When (E)-chalcone was hydrogenated with RuCl2[(S)-tolbinap][(R)-dmapen] and t-C4H9OK at an S/C of 10,000 in 2-propanol under 40 atm of H2 at 0 °C for 3 h, the S-allylic alcohol was obtained in 97% ee and 99% chemical yield accompanied by 1% of 1,3-diphenyl-1-propanone, a saturated ketone (Scheme 9).23) This saturated-ketone byproduct was formed by the isomerization of the allylic alcohol. The sense of enantioselection was consistent with that in the reaction of other aromatic ketones. A series of substituted chalcones with electron-donating and –attracting groups was converted to the desired products in up to 98% ee. Moreover, naphthyl and furyl derivatives were also found to react in the same manner.
Scheme 9.
Mechanistic considerations of hydrogenation with the TolBINAP/DMAPEN–Ru catalyst
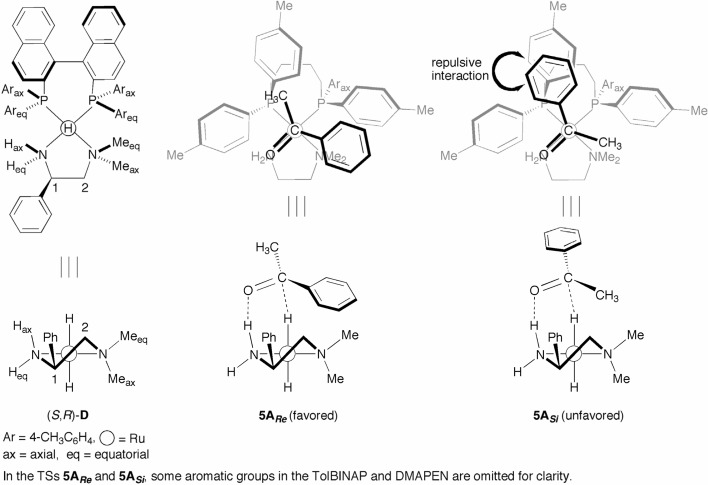
The hydrogenation of ketones catalyzed by the (S)-TolBINAP/(R)-DMAPEN–Ru complex appears to proceed through the same catalyst cycle as that observed in the reaction with the (S)-TolBINAP/(S,S)-DPEN–Ru catalyst, because the reaction conditions and chemoselective features were found to be the same in both cases.15),19) The trans-RuH2[(S )-tolbinap][(R)-dmapen] complex [species D (R = CH3) in Scheme 5] is the expected active species because of the strong σ-donating property of the hydride.24),25) Figure 5 schematically illustrates the trans-RuH2 complex (S,R)-D and two diastereomeric TSs, 5ARe and 5ASi, of the asymmetric hydrogenation of acetophenone.19) As shown in Fig. 3, hydrogenation of the ketone proceeds through the six-membered TSs formed by the Hδ−–Ruδ+–Nδ−–Hax δ+ quadropole and the substrate Cδ+=Oδ− dipole. In the Re-face selected TS 5ARe, the CH3 group of acetophenone is oriented to the “V-shape channel” of TolBINAP’s Arax–P–Areq (Ar = 4-CH3C6H4) moiety, and the planner phenyl ring of the substrate is located on the amino Meeq group. On the other hand, the wide-spread phenyl ring is directed toward the narrow TolBINAP’s V-shape channel, causing significant nonbonded repulsion in the Si-face selected TS, 5ASi. Therefore, the reaction selectively occurs via the TS 5ARe to give (S )-1-phenylethanol (91% ee). The mode of enantioselection gives a higher optical yield in the reaction of aryl secondary alkyl ketones (95–>99% ee, see Scheme 7). A secondary alkyl group fits in the V-shape channel in the TS 5ARe, while steric repulsion between the secondary alkyl group and the amino Meeq group, as well as the repulsive interaction between the aromatic ring of the substrate and TolBINAP’s V-shape channel, increases the activation energy of the TS 5ASi. These results suggest that the (S)-TolBINAP/(R)-DMAPEN–Ru catalyst differentiates between the two carbonyl substituents primarily based on their shape, and only to a lesser degree by their size.
Fig. 5.
The phenyl ring of (R)-DMAPEN bound to the diamine skeleton primarily contributes to fixation of the five-membered chelate structure in the TS 5ARe. Therefore, diamines 3a (R = CH2C6H5) and 3b (R = i-C3H7) exhibit basically the same efficiency as that of DMAPEN (see Scheme 6).
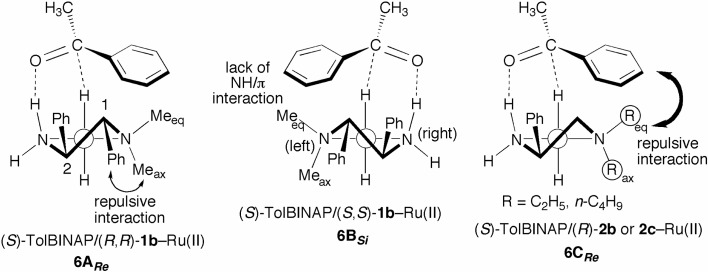
Replacement of an NH2 group of DPEN by an N(CH3)2 moiety dramatically changes enantioselection in the hydrogenation of acetophenone.19) The reaction catalyzed by the original (S)-TolBINAP/(S,S)-DPEN–Ru complex resulted in (R)-1-phenylethanol in 82% ee (see Scheme 6). On the other hand, the (S)-TolBINAP/(S,S)-DMDPEN–Ru catalyst gave the R alcohol in only 22% ee, while the diastereomeric S/R,R catalyst afforded the S alcohol in 79% ee. The latter S/R,R catalyst showed the same sense of enantioselection as that of the (S)-TolBINAP/(R)-DMAPEN–Ru catalyst, albeit the optical yield was lower (79% vs 91%). Hydrogenation with the (S)-TolBINAP/(R,R)-DMDPEN–Ru catalyst appears to proceed through the TS 6ARe shown in Fig. 6, which resembles the TS 5ARe in Fig. 5. The relatively low optical yield of 79% is derived from destabilization of the TS 6ARe by a 1,2-repulsive interaction between the C1-phenyl and the amino Meax groups in the five-membered chelate ring. The reaction with the (S)-TolBINAP/(S,S)-DMDPEN system seems to occur through the TS 6BSi, in which two CH3 groups are substituted on the N(left) atom. This TS 6BSi is not stabilized due to lack of the NH/π attractive interaction observed in the TS 3ASi illustrated in Fig. 3, causing a low level of enantio-selectivity.
Fig. 6.
The notable effect of the size of amino substituents on the enantioselectivity shown in Scheme 6 is explained by the TS model 6CRe in Fig. 6. In the reaction using the Ru catalysts with diamines 2b (R = C2H5) and 2c (R = n-C4H9), the TS is destabilized by a steric repulsive interaction between the phenyl group of acetophenone and the alkyl (R) moiety, which is bulkier than CH3 group. When a diamine, 2d, with a small pyrrolidinyl group is used, a comparable level of enantioselectivity to that achieved with DMAPEN is attainable.
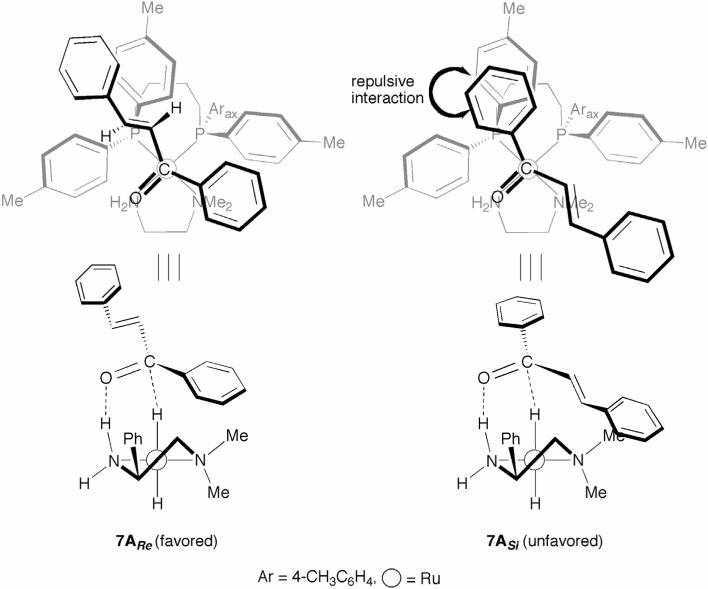
Due to its unique chiral environment, the (S)-TolBINAP/(R)-DMAPEN–Ru catalyst carried out the highly enantioselective hydrogenation of aryl vinyl ketones to the allylic alcohols (Scheme 9).23) An explanation for this high level of enantioselectivity is rationalized by the use of TS models illustrated in Fig. 7. The Re-face selective TS 7ARe is favored over the Si-face directed TS 7ASi, predominantly affording the S-alcoholic product. The “sickle-shape” vinyl group of the substrate provides a good fit for the TolBINAP’s V-shape channel; however, the “plate-shape” phenyl ring creates marked repulsion from the V-shape channel.
Fig. 7.
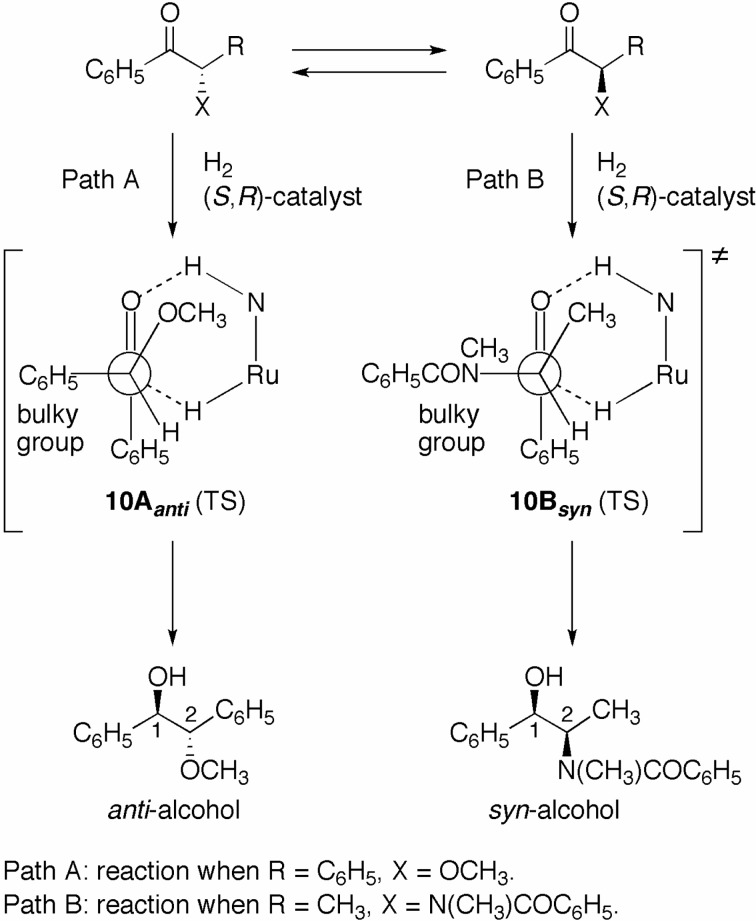
The (S)-TolBINAP/(R)-DMAPEN–Ru catalyst provides for high enantio- and diastereoselectivity in the hydrogenation of α-substituted aromatic ketones through dynamic kinetic resolution, as shown in Scheme 8.19),20) The mode of enantioselectivity is accounted for by the TS models depicted in Fig. 5. Scheme 10 summarizes the pathway that yields β-hetero-substituted alcohols in a diastereoselective manner, which is interpreted by use of the Felkin– Anh-type TS models 10Aanti and 10Bsyn.26),27) The syn/anti-selectivity is highly dependent on the α-substitution pattern of the chiral ketones. The chiral-ketone [C6H5COCH(X)R] is in rapid equilibrium under slightly basic conditions ([base] = 33–34 mM). Benzoin methyl ether (R = C6H5, X = OCH3) is hydrogenated by the RuH2 complex (S,R)-D in Fig. 5 via the six-membered TS 10Aanti, in which the bulkier phenyl ring locates antiperiplaner to the hydridic RuH moiety, predominantly affording the anti-alcohol (Path A). It was found that the reduction of 2-methoxypropiophenone (R = CH3, X =OCH3) gave the corresponding alcohol in a 57:43 syn/anti ratio, due to the small size difference between the CH3 and OCH3 groups. These results suggested that in this case, the electronegativity of the α-substituents is not a decisive factor in diastereo-selection, although electronegativity does frequently control diastereoselectivity in nucleophilic addition reactions of carbonyl compounds. The hydrogenation of 2-(benzoylmethylamino)propiophenone (R = CH3, X = N(CH3)COC6H5), catalyzed by the S/R complex, occurs through the TS 10Bsyn to exclusively afford the syn-alcohol. The bulky N(CH3)COC6H5 group is dominantly oriented antiperiplanar to the RuH moiety in this TS. Moreover, 2-(pivaloylamino)propiophenone (R = CH3, X = NHCO-t-C4H9) reacts in the same manner.
Scheme 10.
The TolBINAP/IPHAN–Ru and TolBINAP/PICA–Ru catalysts: Achievement of unique enantioselection
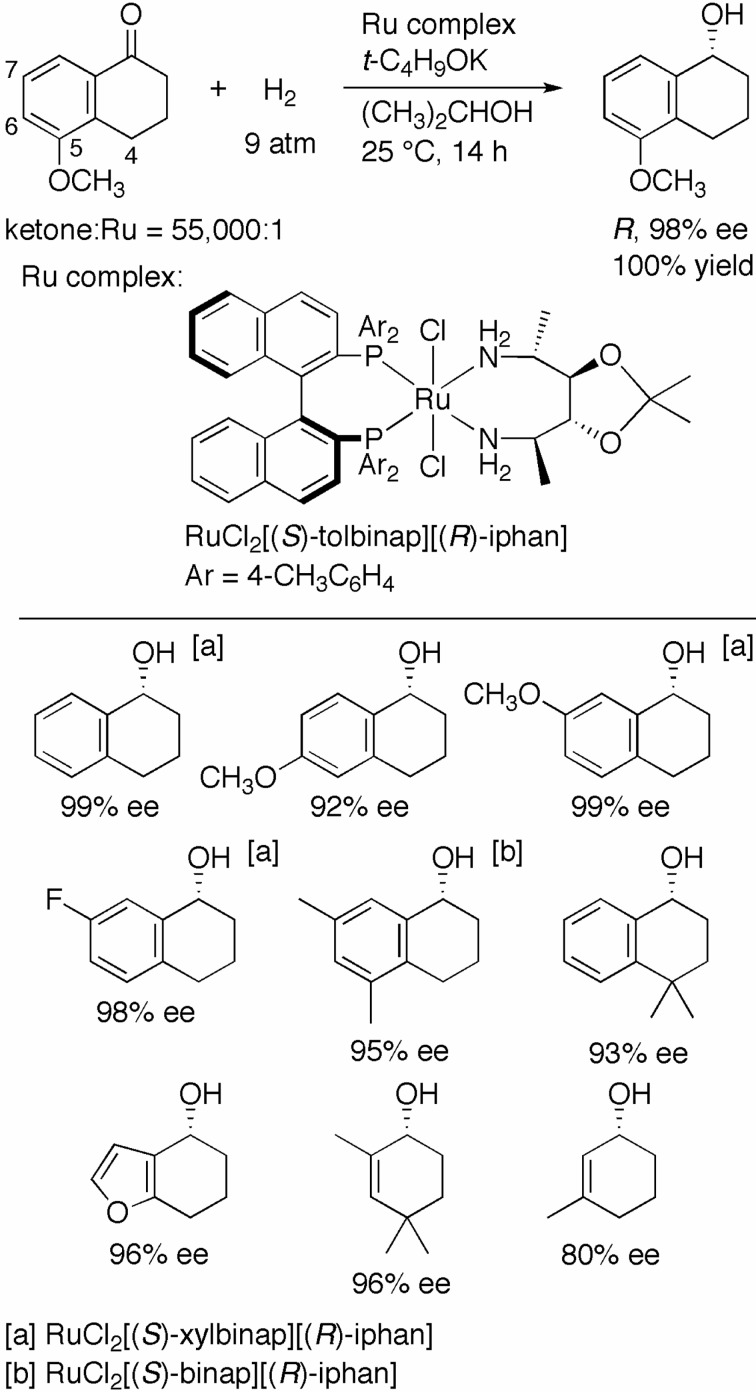
1-Tetralones, which are cyclic aromatic ketones with a fused ring system, are difficult substrates to hydrogenate with the original chiral diphosphine/1,2-diamine–Ru catalysts. This problem was resolved by the use of a chiral 1,4-diamine instead of the 1,2-diamine as a ligand. Thus, hydrogenation of 5-methoxy-1-tetralone with RuCl2[(S )-tolbinap][(R)-iphan] [(R)-IPHAN = (2R,3R,4R,5R)-3,4-O-isopropylidene-2,5-diamine] (S/C = 55,000) and t-C4H9OK in 2-propanol under 9 atm of H2 afforded the R alcohol in 98% ee quantitatively (Scheme 11).28) The flexible seven-membered chelate ring formed by the chiral 1,4-diamine and Ru center appears to fit well with the rigid structure of the fused bicycle of this ketone. Indeed, the (S)-TolBINAP/(R)-IPHAN–Ru catalyst exhibited excellent activity and enantioselectivity for the hydrogenation of 4-, 5-, or 6-substituted 1-tetralones. The (S)-XylBINAP and (R)-IPHAN-combined catalyst gave the best results in the reaction of 1-tetralone itself and the 7-substituted analogues. For the hydrogenation of 5,7-dimethyl-1-tetralone, the (S)-BINAP/(R)-IPHAN combination was most suitable. 4,5,6,7-Tetrahydrobenzofuran-4-one, a heteroaromatic analogue, was also hydrogenated with the (S)-TolBINAP/(R)-IPHAN–Ru catalyst to give the desired product in 96% ee without decomposition of the heteroaromatic moiety. Substituted 2-cyclohexenones are recognized as simplified analogues of 1-tetralone. It was therefore possible to hydrogenate 2,4,4-trimethyl-2-cyclohexenone and the 3-methyl substituted enone with the S/R catalyst to afford the cyclic allylic alcohols in 96% and 80% ee, respectively.
Scheme 11.
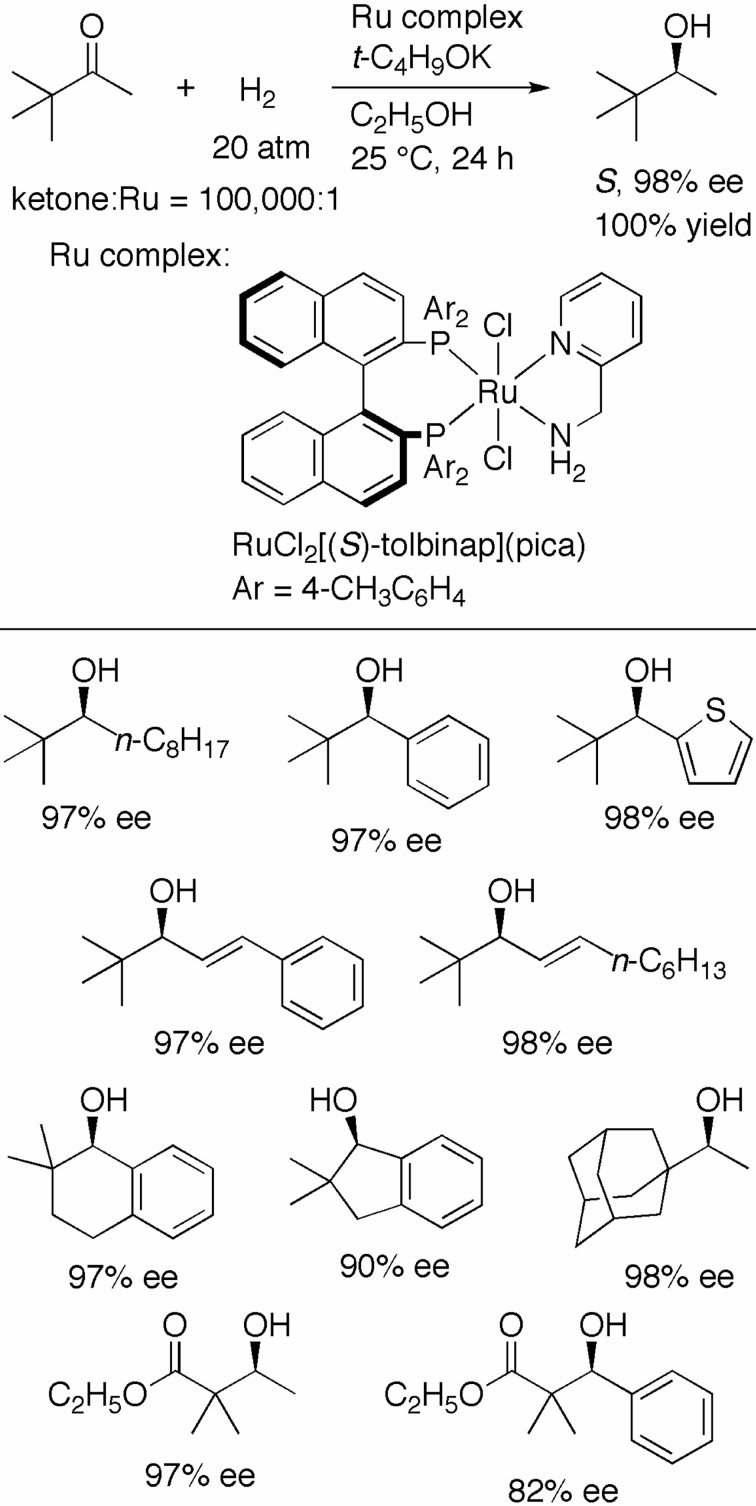
The asymmetric hydrogenation of sterically hindered tert-alkyl ketones has been a persistent problem in the field of organic synthesis. For instance, the reaction of pinacolone with the (S)-TolBINAP/(S,S)-DPEN–Ru catalyst at an S/C of 2000 under 9 atm of H2 for 24 h gave (S )-3,3-dimethyl-2-butanol in only 20% yield and 14% ee. We designed RuCl2[(S )-tolbinap](pica) (PICA = α-picolylamine; Scheme 12) to achieve high catalytic efficiency, as the PICA ligand was expected to create a large pocket over the pyridine moiety, thereby enabling the approach of the substrate with a bulky tert-alkyl group.29) Indeed, the hydrogenation of pinacolone with the (S)-TolBINAP/PICA–Ru complex at an S/C of 100,000 in a base containing ethanol quantitatively afforded the S alcohol in 98% ee (Scheme 12). The optical yield was decreased to 36% when the reaction was conducted in 2-propanol. This is the only catalyst that provides high activity and enantioselectivity for this reaction. A series of tert-butyl ketones with alkyl, aryl, heteroaryl, and vinyl groups was converted to the corresponding alcohol in excellent ee. The sense of enantioselectivity, based on the tert-butyl group, remained consistent. The high carbonyl selectivity in the reaction of α,β-unsaturated ketones suggests that this hydrogenation also proceeded through the six-membered TS F illustrated in Scheme 5. Cyclic aromatic ketones, as well as 1-adamantyl ketone, were hydrogenated in the same manner. Interestingly, α,α-disubstituted β-keto esters were successfully reduced with the same sense of enantioselectivity as that seen in the reaction of pinacolone, suggesting that the sterically hindered ester moiety resembles a simple tert-alkyl group.
Scheme 12.
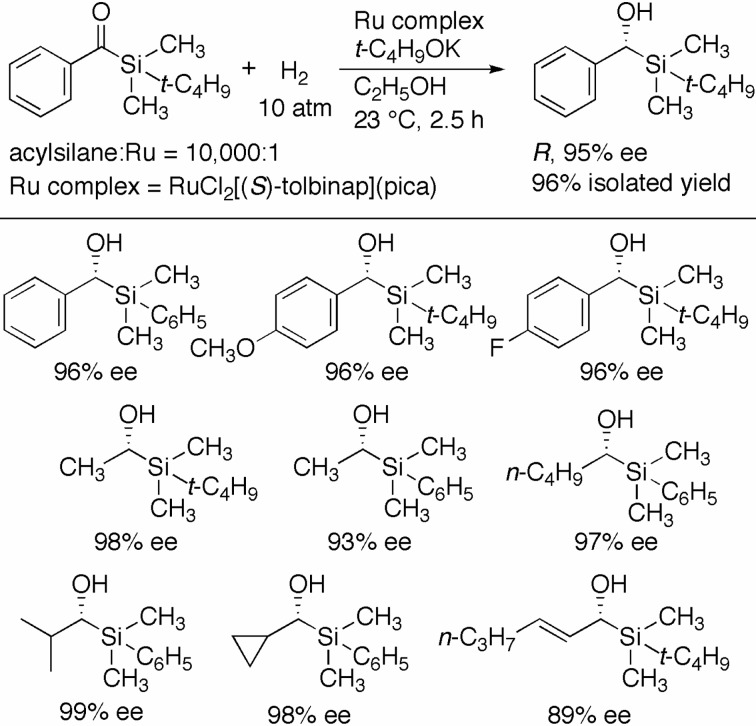
The (S)-TolBINAP/PICA–Ru catalyst has enabled the asymmetric hydrogenation of acylsilanes to chiral α-hydroxysilanes, which are chiral reagents for stereocontrolled C–C bond formation and rearrangement. When benzoyl-tert-butyldimethylsilane was hydrogenated with RuCl2[(S )-tolbinap](pica) (S/C = 10,000) and t-C4H9OK (10 mM) in ethanol under 10 atm of H2 for 2.5 h, the desired (R)-α-hydroxysilane was quantitatively obtained in 95% ee (Scheme 13).30) The concentration of t-C4H9OK was set at around 10 mM, because higher base concentration led to Si–C(OH) bond cleavage of the hydroxysilane via a Brook-type rearrangement.31) The enantioselectivity was only slightly influenced by the electronic properties of the aromatic substituents. Acylsilanes with primary, secondary, and cyclic alkyl groups were reduced with up to 99% optical yield. α,β-Unsaturated acylsilanes, which easily suffer conjugate reduction, were predominantly converted to the allylic α-hydroxysilanes. The allylic α-hydroxysilane was readily transformed to the chiral vinylsilane compounds without loss of optical purity, via the Ireland–Claisen rearrangement.32)
Scheme 13.
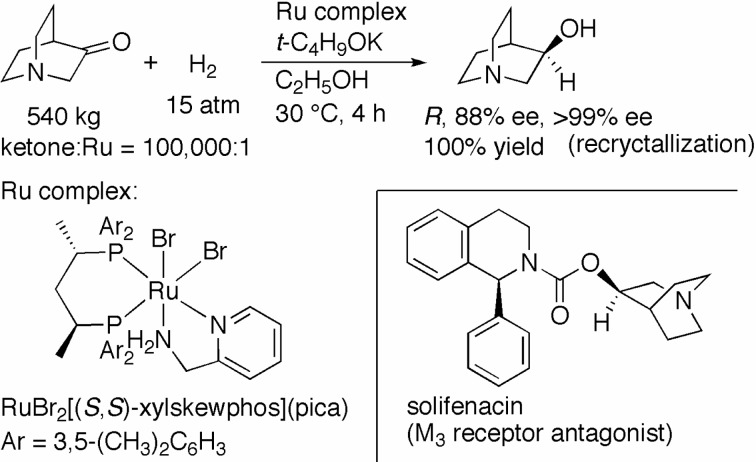
The chiral environment of the Ru catalyst with diphosphine and amine-based bidentate ligand is tunable to a specific ketonic substrate by changing the combination of these two ligands. For instance, 3-quinuclidinone, an amino ketone with a bicyclo[2.2.2] skeleton, was hydrogenated with RuBr2[(S,S)-xylskewphos](pica) at an S/C of 100,000 in a basic ethanol under 15 atm of H2 for 4 h to quantitatively afford (R)-3-quinuclidinol in 88% ee, which was readily purified to >99% ee by recrystallization (Scheme 14).33) The high catalyst efficiency, reproducibility, and operational simplicity realized 540-kg scale reactions in a chemical plant. This hydrogenation approach has been commercialized for the production of solifenacin, an M3 receptor antagonist.34)
Scheme 14.
The η6-arene/TsDPEN–Ru and MsDPEN–Cp*Ir catalysts: Beyond the border between hydrogenation and transfer hydrogenation
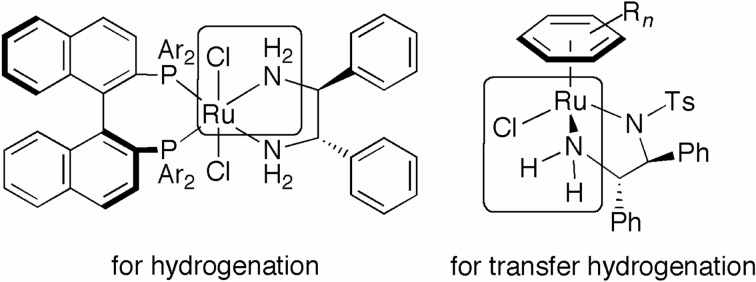
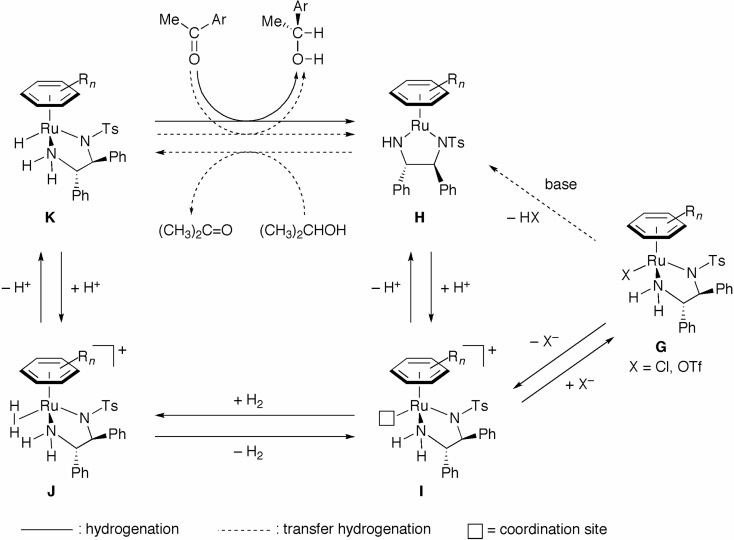
The asymmetric hydrogenation of ketones and the transfer hydrogenation using 2-propanol or formic acid as a reducing agent are complementary reactions for the synthesis of optically active alcohols. Figure 8 shows the structures of the typical catalyst precursors, RuCl2[(S )-binap][(S,S )-dpen]5) for hydrogenation, and RuCl[(S,S)-TsDpen](η6-p-cymene) [TsDPEN = N-(p-toluenesulfonyl)-1,2-diphenylethy-lenediamine]35) for transfer hydrogenation. These two complexes have a common partial structure (surrounded by lines in Fig. 8); however, the catalytic efficiencies of these two complexes differ. This difference in catalyst performance can be accounted for by the reaction mechanisms described here. As illustrated in Scheme 15, the η6-arene/TsDPEN–RuCl complex G (X = Cl) is converted to the 16-electron amide complex H by HCl elimination in basic 2-propanol, as shown by the broken arrow (depicting the transfer hydrogenation mechanism).36) The complex H then reacts with 2-propanol to give the active RuH species K and acetone. A ketonic substrate is smoothly reduced by K, resulting in the chiral alcohol and the regeneration of species H. In the catalytic cycle of hydrogenation depicted in Scheme 5, the amide complex E is readily protonated by 2-propanol and/or protic compounds to give the cationic species B, which activates H2 molecules to generate the reactive RuH2 complex D through the formation of the H2–bound compound C.15) Thus, 2-propanol is a hydride donor in the transfer hydrogenation, but it acts as a proton donor in the hydrogenation.
Fig. 8.
Scheme 15.
According to the expected mechanistic properties of the reaction, we assumed that the Ru complex G would be able to catalyze hydrogenation (not transfer hydrogenation) under the appropriate cationic conditions, shown along the unbroken line with arrow (Scheme 15).37),38) The dissociation of X− gives cationic species I with a coordination site, and it accepts the H2 molecule to give J. Heterolytic cleavage of H2 on the Ru atom forms the active RuH complex K, which reduces the ketone to afford the alcoholic product and the amide complex H. The protonation of H in protic media regenerates the cationic species I. The Ru complex G (X = OTf = OSO2CF3) shows higher reactivity than the complex (X = Cl) due to the higher capacity for dissociation of (OTf)−. The more polar protic solvent CH3OH (ɛ = 33) is favored over 2-propanol (ɛ = 20) for attaining high catalytic efficiency.
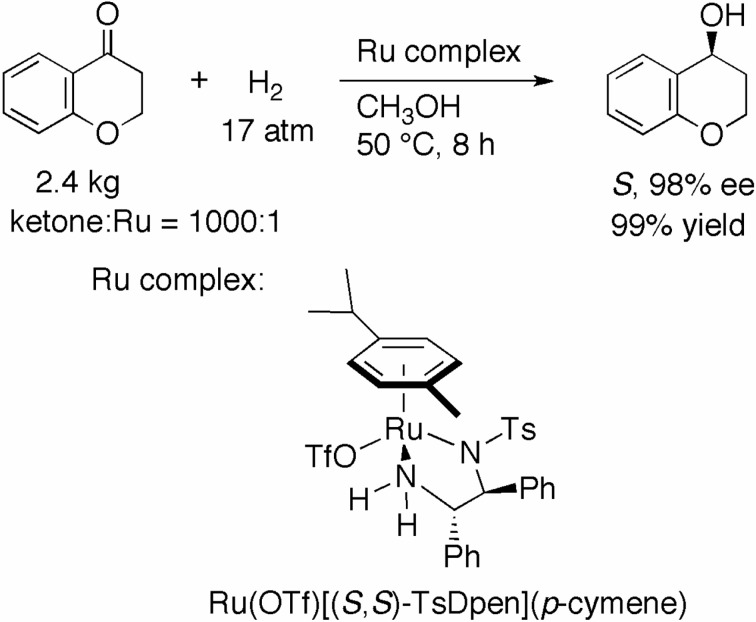
The asymmetric hydrogenation catalyzed by the η6-arene/TsDPEN–Ru complex proceeds under neutral to slightly acidic conditions. This characteristic rendered it possible to hydrogenate extremely base-labile ketonic substrates. When 2.4-kg of 4-chroma-none was reduced with Ru(OTf)[(S,S)-TsDpen](p-cymene) at an S/C of 1000 in CH3OH under 17 atm of H2 at 50 °C for 8 h, (S)-4-chromanol was quantitatively obtained in 98% ee (Scheme 16).37) The chiral alcohol is now commercially available. Due to decomposition of the substrate, the hydrogenation of 4-chromanone has only rarely been performed with the diphosphine/diamine–Ru complex in a slightly basic alcohol.
Scheme 16.
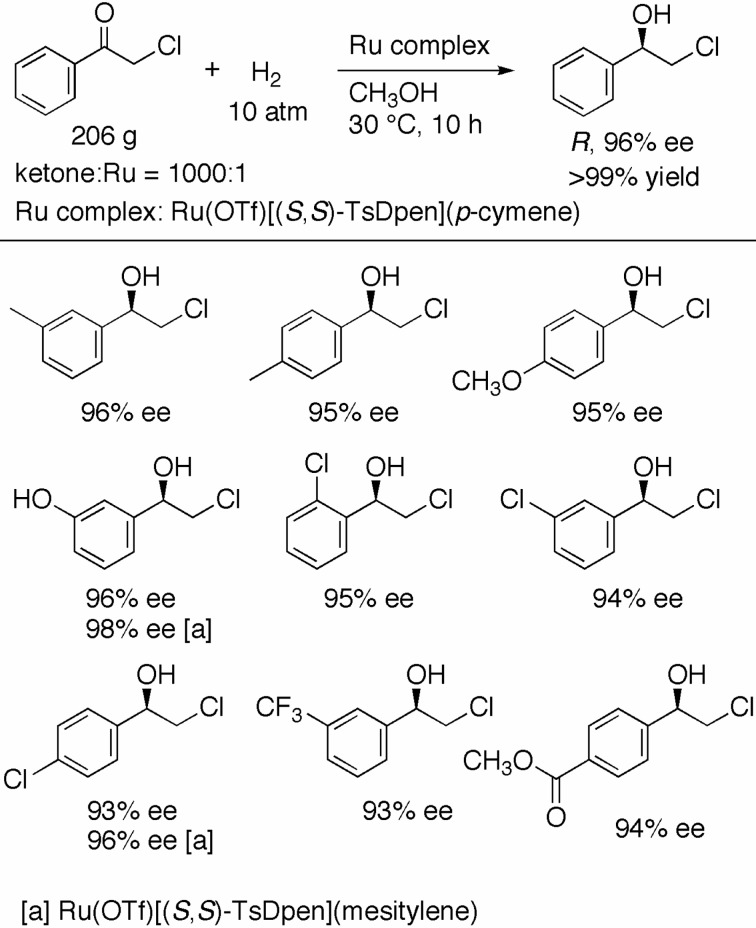
Optically active aromatic chlorohydrins are useful and versatile intermediates in the synthesis of biologically active compounds. The asymmetric hydrogenation of α-chloro ketones, which are another base-sensitive substrates, is one of the most direct and reliable methods for the preparation of this important class of compounds. We found that Ru(OTf)[(S,S)-TsDpen](p-cymene) effectively catalyzes this asymmetric transformation.39) The reaction of α-chloroacetophenone (208 g) with the S,S complex (S/C = 1000) in CH3OH under 10 atm of H2 was completed in 10 h to afford the R alcohol in 96% ee (Scheme 17). A series of chloro ketones with either an electron-attracting or -donating group on the phenyl ring was hydrogenated with high en-antioselectivity. Ru(OTf)[(S,S)-TsDpen](mesitylene) showed even higher enantioselective ability in some cases. The slightly acidic reaction conditions allowed for the hydrogenation of 2-chloro-3′-hydroxyacetophenone without protection of the phenolic hydroxy group.
Scheme 17.
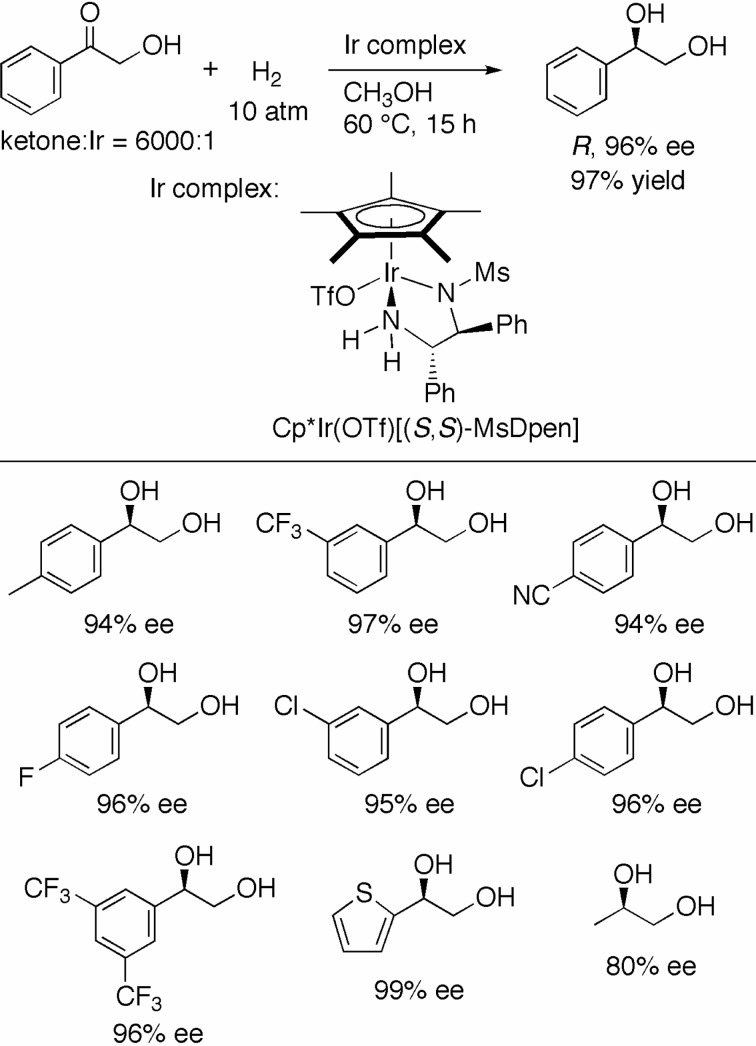
α-Hydroxy aromatic ketones are similar in structure to the above-mentioned α-chloro ketones. However, hydrogenation of the hydroxy ketones was poorly catalyzed by the Ru(OTf)(TsDpen)(p-cymene). Fortunately, this problem was resolved by the use of an isoelectronic Cp*Ir(OTf)(MsDpen) (Cp*= pentamethylcyclopentadienyl, MsDPEN = N-(methanesulfonyl)-1,2-diphenylethylenediamine) as the catalyst (Scheme 18).40) When α-hydroxyacetophenone was hydrogenated with Cp*Ir(OTf)[(S,S)-MsDpen] at an S/C of 6000 in CH3OH under 10 atm of H2 at 60 °C for 15 h, (R)-1-phenyl-1,2-ethanediol was obtained in 96% ee and 97% chemical yield. The electronic character of the catalyst notably influenced the reactivity in this case. Thus, the hydrogenation catalyzed by Cp*Ir(OTf)[(S,S)-TsDpen] (not MsDPEN) under the same reaction conditions gave the diol in only 12% yield. A series of 1-aryl-1,2-ethanediols was produced in up to 99% ee by the MsDPEN–Cp*Ir-catalyzed hydrogenation. 1-Hydroxy-2-propanone, an aliphatic ketone, was also reduced with good enantioselectivity.
Scheme 18.
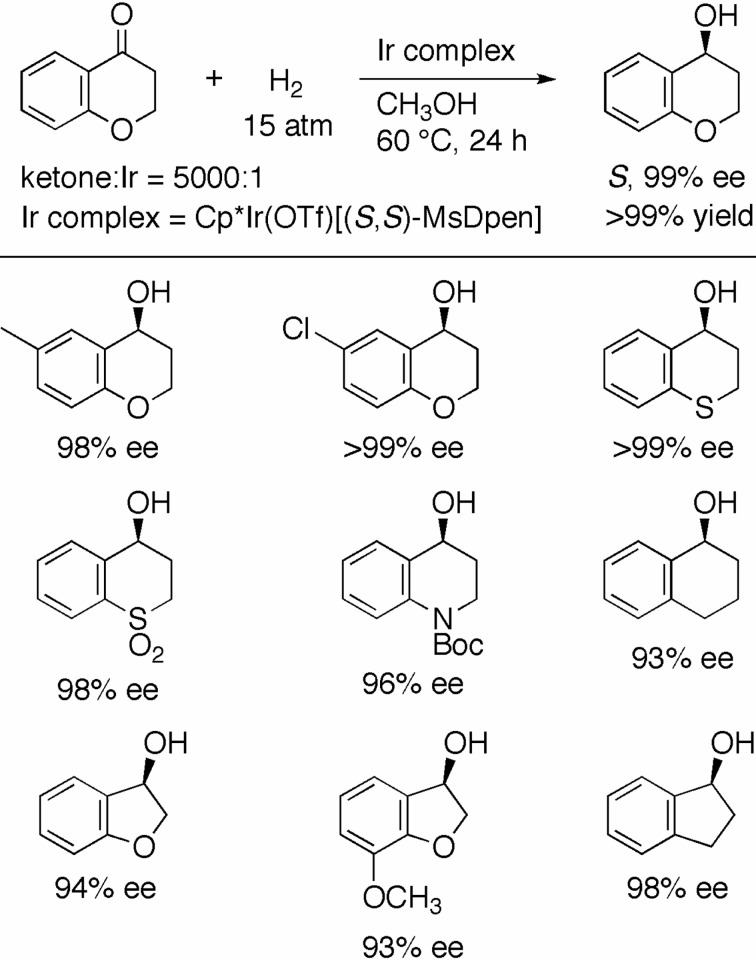
As shown in Scheme 19, the MsDPEN–Cp*Ir complex efficiently catalyzed the asymmetric hydrogenation of aromatic heterocyclic ketones. The reaction of 4-chromanone with an S/C of 5000 under 15 atm of H2 at 60 °C was completed in 24 h to afford the chiral alcohol in 99% ee.41) Thus, the enantioselectivity was even higher than that of the reaction with the p-cymene/TsDPEN–Ru complex (99% vs. 98%; see Scheme 16).37) The substituted 4-chromanones and 4-thiochromanone were hydrogenated with nearly perfect enantioselectivity. A cyclic keto sulfone and a tetrahydro-4-quinolinone were also found to be good substrates for this reaction. The hydrogenation of 1-tetralone (S/C = 500, 15 atm H2, 24 h) gave the alcohol in only 93% ee and 88% chemical yield. These results suggested that a heteroatom, especially oxygen, in the aliphatic moiety of the ketonic substrate is crucial to achieve high reactivity and enantioselectivity with this reaction. For the hydrogenation of 1-tetralone, use of the XylBINAP/IPHAN–Ru catalyst is recommended (see Scheme 11).28) 3(2H )-Benzofuranones and 1-indanone were converted to the desired alcohols in high ee by the MsDPEN–Cp*Ir–catalyzed hydrogenation.
Scheme 19.
Conclusion
High reactivity, enantioselectivity, and applicability for substrates are all desirable properties of the asymmetric hydrogenation of ketones. The rational design of chiral catalysts has been a key issue in the achievement of this goal. Four types of hydrogenation catalysts have been described in this manuscript, i.e., 1) chiral diphosphine/diamine–Ru, 2) chiral diphosphine/PICA–Ru, 3) η6-arene/TsDPEN–Ru, and 4) MsDPEN–Cp*Ir systems.
The TolBINAP/DPEN–Ru and XylBINAP/DAIPEN–Ru catalysts have achieved high catalytic activity (TON = 2,400,000, TOF = 228,000 h−1) and enantioselectivity (up to >99% ee) in the hydrogenation of simple ketones (Type 1). A concerted six-membered TS is proposed. The combination of TolBINAP and DMAPEN, an N,N-dimethyl diamine, shows high enantioselectivity for the reaction of α-branched aromatic ketones and aryl vinyl ketones by the shape-selective chiral environment (Type 1). The use of IPHAN, a chiral 1,4-diamine, instead of 1,2-diamine affords high enantioselectivity in the reaction of 1-tetralones (Type 1). The TolBINAP/PICA–Ru catalyst carries out the hydrogenation of bulky tert-alkyl ketones and acyl silanes with high stereoselectivity (Type 2). The XylSkewphos/PICA combined catalyst has commercialized the hydrogenation of 3-quinuclidinone (Type 2). The findings of mechanistic studies led to the design of the p-cymene/TsDPEN–Ru catalyst (Type 3). Slightly acidic reaction conditions allowed for the hydrogenation of base-labile 4-chromanone and α-chloro ketones. The isoelctronic MsDPEN–Cp*Ir catalyst enables the reaction of α-hydroxy ketones and cyclic aromatic ketones (Type 4).
The four types of catalyst describes here cover various regions of ketonic substrates. The custom design of catalysts is expected to generate a set of universal catalysts for the asymmetric hydrogenation of ketones.
Acknowledgements
I would like to gratefully acknowledge my collaborators at Hokkaido University, Nagoya University, Kanto Chemical Co. Inc., and Nippon Soda Co., Ltd. for their devoted efforts. Their names are given in the cited publications. This work was supported by a Grant-in-aid from JSPS, JST, and NEDO (Support program for Technology Development on the Basis of Academic Findings).
Profile
Takeshi Ohkuma was born in Gunma, Japan in 1962. He studied chemistry at Keio University, and completed his M.Sc. under the supervision of the late Professor Gen-ichi Tsuchihashi. He then moved to Nagoya University to join Professor Noyori’s research group and worked in the field of molecular catalysis. After obtaining his Ph.D. in 1991, he worked with Professor Paul A. Wender at Stanford University until 1992. In the same year, he joined the ERATO Noyori Molecular Catalysis Project. In 1996, he became an Associate Professor in the Department of Chemistry at Nagoya University, and then he was promoted to Professor in the Division of Chemical Process Engineering at Hokkaido University in 2004. His research focuses on the development of novel catalytic reactions that achieve high level of reactivity and selectivity. Professor Ohkuma received the Progress Award in Synthetic Organic Chemistry, Japan, in 1997; the N.E. ChemCat Award in Synthetic Organic Chemistry, Japan, in 1999; and the JSPS Prize (from Japan Society for the Promotion of Science) in 2007.

References
- 1).Ohkuma T, Noyori R. (2004) Carbonyl hydrogenation. InTransition Metals for Organic Synthesis, 2nd ed (eds. Beller M., Bolm C.). Wiley-VCH, Weinheim, pp. 29–113 [Google Scholar]
- 2).Ohkuma T, Noyori R. (2007) Enantioselective ketone and β-keto ester hydrogenations (including mechanisms). InThe Handbook of Homogeneous Hydrogenation (eds. de Vries J. G., Elsevier C. J.). Wiley-VCH, Weinheim, 3, pp. 1105–1163 [Google Scholar]
- 3).Ohkuma T. (2007) Tailor-made catalysts for asymmetric hydrogenation of ketones. J. Synth. Org. Chem. Jpn. 65, 1070–1080 [Google Scholar]
- 4).Ohkuma T., Ooka H., Hashiguchi S., Ikariya T., Noyori R. (1995) Practical enantioselective hydrogenation of aromatic ketones. J. Am. Chem. Soc. 117, 2675–2676 [Google Scholar]
- 5).Doucet H., Ohkuma T., Murata K., Yokozawa T., Kozawa M., Katayama E., et al. (1998) trans- [RuCl2(phosphane)2(1,2-diamine)] and chiral trans-[RuCl2(1,2-diphosphane)(1,2-diamine)]: shelf-stable precatalysts for the rapid, productive, and stereoselective hydrogenation of ketones. Angew. Chem. Int. Ed. 37, 1703–1707 [DOI] [PubMed] [Google Scholar]
- 6).Ohkuma T., Koizumi M., Doucet H., Pham T., Kozawa M., Murata K., et al. (1998) Asymmetric hydrogenation of alkenyl, cyclopropyl, and aryl ketones. RuCl2(xylbinap)(1,2-diamine) as a precatalyst exhibiting a wide scope. J. Am. Chem. Soc. 120, 13529–13530 [Google Scholar]
- 7).Ohkuma T., Koizumi M., Muñiz K., Hilt G., Kabuto C., Noyori R. (2002) trans-RuH(η1- BH4)(binap)(1,2-diamine): a catalyst for asymmetric hydrogenation of simple ketones under base-free conditions. J. Am. Chem. Soc. 124, 6508–6509 [DOI] [PubMed] [Google Scholar]
- 8).Ohkuma T., Koizumi M., Ikehira H., Yokozawa T., Noyori R. (2000) Selective hydrogenation of benzophenones to benzhydrols. Asymmetric synthesis of unsymmetrical diarylmethanols. Org. Lett. 2, 659–662 [DOI] [PubMed] [Google Scholar]
- 9).Ohkuma T., Koizumi M., Yoshida M., Noyori R. (2000) General asymmetric hydrogenation of hetero-aromatic ketones. Org. Lett. 2, 1749–1751 [DOI] [PubMed] [Google Scholar]
- 10).Ohkuma T., Ooka H., Ikariya T., Noyori R. (1995) Preferential hydrogenation of aldehydes and ketones. J. Am. Chem. Soc. 117, 10417–10418 [Google Scholar]
- 11).Ohkuma T., Ikehira H., Ikariya T, Noyori R. (1997) Asymmetric hydrogenation of cyclic α,β-unsaturated ketones to chiral allylic alcohols. Synlett, 467–468 [Google Scholar]
- 12).Ohkuma T., Doucet H., Pham T., Mikami K., Korenaga T., Terada M., Noyori R. (1998) Asymmetric activation of racemic ruthenium(II) complexes for enantioselective hydrogenation. J. Am. Chem. Soc. 120, 1086–1087 [Google Scholar]
- 13).Ohkuma T., Takeno H., Honda Y., Noyori R. (2001) Asymmetric hydrogenation of ketones with polymer-bound BINAP/diamine ruthenium catalysts. Adv. Synth. Catal. 343, 369–375 [Google Scholar]
- 14).Ohkuma T., Ishii D., Takeno H., Noyori R. (2000) Asymmetric hydrogenation of amino ketones using chiral RuCl2(diphosphine)(1,2-diamine) complexes. J. Am. Chem. Soc. 122, 6510–6511 [Google Scholar]
- 15).Sandoval C. A., Ohkuma T., Muñiz K., Noyori R. (2003) Mechanism of asymmetric hydrogenation of ketones catalyzed by BINAP/1,2-diamine– ruthenium(II) complexes. J. Am. Chem. Soc. 125, 13490–13503 [DOI] [PubMed] [Google Scholar]
- 16).Noyori R., Kitamura M., Ohkuma T. (2004) Toward efficient asymmetric hydrogenation: architectural and functional engineering of chiral molecular catalysts. Proc. Natl. Acad. Sci. USA 101, 5356–5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Noyori R., Sandoval C. A., Muñiz K., Ohkuma T. (2005) Metal–ligand bifunctional catalysis for asymmetric hydrogenation. Phil. Trans. R. Soc. A 363, 901–912 [DOI] [PubMed] [Google Scholar]
- 18).Abdur-Rashid K., Clapham S. E., Hadzovic A., Harvey J. N., Lough A. J., Morris R. H. (2002) Mechanism of the hydrogenation of ketones catalyzed by trans-dihydrido(diamine)ruthenium(II) complexes. J. Am. Chem. Soc. 124, 15104–15118 [DOI] [PubMed] [Google Scholar]
- 19).Ooka H., Arai N., Azuma K., Kurono N., Ohkuma T. (2008) Asymmetric hydrogenation of aromatic ketones catalyzed by the TolBINAP/DMAPEN–ruthenium(II) complex: a significant effect of N-substituents of chiral 1,2-diamine ligands on enantioselectivity. J. Org, Chem. 73, 9084–9093 [DOI] [PubMed] [Google Scholar]
- 20).Arai N., Ooka H., Azuma K., Yabuuchi T., Kurono N., Inoue T., Ohkuma T. (2007) General asymmetric hydrogenation of α-branched aromatic ketones catalyzed by TolBINAP/DMAPEN– ruthenium(II) complex. Org. Lett. 9, 939–941 [DOI] [PubMed] [Google Scholar]
- 21).Noyori R., Ohkuma T. (2001) Asymmetric catalysis by architectural and functional molecular engineering: practical chemo- and stereoselective hydrogenation of ketones. Angew. Chem. Int. Ed. 40, 40–73 [PubMed] [Google Scholar]
- 22).Woodward S, Ford A. (1998) Enantioselective reduction of ketones. UK-GB 2339780A. [DOI] [PubMed] [Google Scholar]
- 23).Arai N., Azuma K., Nii N., Ohkuma T. (2008) Highly enantioselective hydrogenation of aryl vinyl ketones to allylic alcohols catalyzed by the Tol-Binap/Dmapen ruthenium(II) complex. Angew. Chem. Int. Ed. 47, 7457–7460 [DOI] [PubMed] [Google Scholar]
- 24).Meseras F., Lledós A., Clot E., Eisenstein O. (2000) Transition metal polyhydrides: from qualitative ideas to reliable computational studies. Chem. Rev. 100, 601–636 [DOI] [PubMed] [Google Scholar]
- 25).Kubas G. J. (2001) Metal–dihydrogen and σ-bond coordination: the consummate extension of the Dewar–Chatt–Duncanson model for metal–olefin π bonding. J. Organomet. Chem. 635, 37–68 [Google Scholar]
- 26).Chérest M., Felkin H., Prudent N. (1968) Torsional strain involving partial bonds. The stereochemistry of the lithium aluminum hydride reduction of some simple open-chain ketones. Tetrahedron Lett. 9, 2199–2204 [Google Scholar]
- 27).Anh N. T. (1980) Regio- and stereo-selectivities in some nucleophilic reactions. Top. Curr. Chem. 88, 145–162 [Google Scholar]
- 28).Ohkuma T., Hattori T., Ooka H., Inoue T., Noyori R. (2004) BINAP/1,4-diamine–ruthenium(II) complexes for efficient asymmetric hydrogenation of 1-tetralones and analogues. Org. Lett. 6, 2681–2683 [DOI] [PubMed] [Google Scholar]
- 29).Ohkuma T., Sandoval C. A., Srinivasan R., Lin Q., Wei Y., Muñiz K., Noyori R. (2005) Asymmetric hydrogenation of tert-alkyl ketones. J. Am. Chem. Soc. 127, 8288–8289 [DOI] [PubMed] [Google Scholar]
- 30).Arai N., Suzuki K., Sugizaki S., Sorimachi H., Ohkuma T. (2008) Asymmetric hydrogenation of aromatic, aliphatic, and α,β-unsaturated acylsilanes catalyzed by Tol-binap/Pica ruthenium(II) complexes: practical synthesis of optically active α-hydroxysilanes. Angew. Chem. Int. Ed. 47, 1770–1773 [DOI] [PubMed] [Google Scholar]
- 31).Brook A. G. (1974) Molecular rearrangements of organosilicon compounds. Acc. Chem. Res. 7, 77–84 [Google Scholar]
- 32).Ireland R. E., Varney M. D. (1984) A chiral primary alcohol equivalent: silyl-assisted asymmetric induction in the ester enolate Claisen rearrangement. J. Am. Chem. Soc. 106, 3668–3670 [Google Scholar]
- 33).Tsutsumi K., Katayama T., Utsumi N., Murata K., Arai N., Kurono N., Ohkuma T. (2009) Practical asymmetric hydrogenation of 3-quinuclidinone catalyzed by the XylSkewphos/PICA– ruthenium(II) complex. Org. Process Res. Dev. 13, 625–628 [Google Scholar]
- 34).Naito R., Yonetoku Y., Okamoto Y., Toyoshima A., Ikeda K., Takeuchi M. (2005) Synthesis and antimuscarinic properties of quinuclidin-3-yl 1,2,3,4-tetrahydroisoquinoline-2-carboxylate derivatives as novel muscarinic receptor antagonists. J. Med. Chem. 48, 6597–6606 [DOI] [PubMed] [Google Scholar]
- 35).Noyori R., Hashiguchi S. (1997) Asymmetric transfer hydrogenation catalyzed by chiral ruthenium complexes. Acc. Chem. Res. 30, 97–102 [Google Scholar]
- 36).Haack K.–J., Hashiguchi S., Fujii A., Ikariya T., Noyori R. (1997) The catalyst precursor, catalyst and intermediate in the RuII-promoted asymmetric hydrogen transfer between alcohols and ketones. Angew. Chem. Int. Ed. Engl. 36, 285–288 [Google Scholar]
- 37).Ohkuma T., Utsumi N., Tsutsumi K., Murata K., Sandoval C., Noyori R. (2006) The hydrogenation/transfer hydrogenation network: asymmetric hydrogenation of ketones with chiral η6-arene/N-tosylethylenediamine–ruthenium(II) catalysts. J. Am. Chem. Soc. 128, 8724–8725 [DOI] [PubMed] [Google Scholar]
- 38).Sandoval C. A., Ohkuma T., Utsumi N., Tsutsumi K., Murata K, Noyori R. (2006) Mechanism of asymmetric hydrogenation of acetophenone catalyzed by chiral η6-arene–N-tosylethylenediamine– ruthenium(II) complexes. Chem. Asian J. 1–2, 102–110 [DOI] [PubMed] [Google Scholar]
- 39).Ohkuma T., Tsutsumi K., Utsumi N., Arai N., Noyori R., Murata K. (2007) Asymmetric hydrogenation of α-chloro aromatic ketones catalyzed by η6-arene/TsDPEN–ruthenium(II) complexes. Org. Lett. 9, 255–257 [DOI] [PubMed] [Google Scholar]
- 40).Ohkuma T., Utsumi N., Watanabe M., Tsutsumi K., Arai N., Murata K. (2007) Asymmetric hydrogenation of α-hydroxy ketones catalyzed by MsDPEN–Cp*Ir(III) complex. Org. Lett. 9, 2565– 2567 [DOI] [PubMed] [Google Scholar]
- 41).Utsumi N., Tsutsumi K., Watanabe M., Murata K., Arai N., Kurono N., Ohkuma T. (2010) Asymmetric hydrogenation of aromatic heterocyclic ketones catalyzed by the MsDPEN–Cp*Ir(III) complex. HETEROCYCLES 80, 141–147 [Google Scholar]