Abstract
Many procedures used in assisted reproductive technologies (ART) to treat human infertility entail culture of preimplantation embryos. Moreover, there is an increasing trend to culture embryos for longer periods of time before uterine transfer to identify the “best” embryos for transfer and to minimize multiple pregnancies. Embryo culture, however, can perturb embryo metabolism and gene expression, and the long-term consequences of culture are unknown. We have explored the behavioral consequences of embryo culture by using a 129S6/SvEvTac/C57BL/6J F1 mouse model and find that adults derived from cultured embryos exhibit specific behavioral alterations in the elevated zero maze and Morris water maze tasks.
Fetal adaptations in utero to maternal undernutrition or malnutrition can lead to specific diseases in the adult, including coronary heart disease, high blood pressure, and type II diabetes (1). This phenomenon, termed the fetal origins of adult disease or the Barker hypothesis, has been extrapolated back to preimplantation development (2). Pregnant rats fed a low-protein diet (modified so that caloric intake was unaltered) only during the period of preimplantation development gave birth to female offspring with reduced birth weight and male offspring with increased systolic blood pressure and abnormal organ-to-body weight ratios, relative to offspring derived from pregnant rats fed a higher protein diet. Thus, modest alterations in the environment experienced by the preimplantation embryo can have long-term consequences. This finding may be especially important in the context of treating human infertility by using the methods of assisted reproductive technology (ART), which inevitably entail embryo culture (3). To identify “high quality” embryos for transfer and to minimize multiple pregnancies, a recent approach is to transfer blastocysts rather than earlier cleavage-stage embryos (4), which also provides the appropriate temporal synchronization of the embryo and uterus. The assumption is that further development to the blastocyst stage permits selection of the one or two embryos with the highest developmental potential for transfer. Development to the blastocyst stage, however, requires culture for 5-6 days, almost twice as long as that required for development of cleavage-stage embryos.
Preimplantation embryos in culture exhibit metabolic changes that are likely to be in response to suboptimal culture conditions. For example, anaerobic glycolysis accounts for 100% of glucose utilization of mouse blastocysts that develop in vitro from the morula stage. In vivo, however, conversion to lactate accounts for only 40-50% of glucose used by mouse blastocysts (5). At the molecular level, patterns of gene expression also change in response to embryo culture (6). Under certain culture conditions, the imprinted H19 gene exhibits biallelic expression after embryo culture, and this loss of imprinting correlates with the loss of DNA methylation in the differentially methylated region implicated in regulating H19 expression (7). Loss of imprinting in humans can result in Angelman, Prader-Willi, and Beckwith-Wiedemann syndromes, and some of these syndromes are associated with behavioral abnormalities (8). Results of recent retrospective studies revealed that children conceived by ART display a significantly higher incidence of Angelman (9) and Beckwith-Wiedemann syndromes due to loss of imprinting (10, 11) and retinoblastoma (12). Whether ART is responsible remains unknown, but, if so, embryo culture has been raised as a possible cause/contributing factor (10). To ascertain whether early perturbations in metabolism and gene expression in response to embryo culture have long-term consequences that are manifested in the offspring, we examined the effect of culture of mouse preimplantation embryos to the blastocyst stage on development and behavior of the offspring. We find that adults derived from cultured embryos exhibit specific behavioral alterations in anxiety/locomotor activity and spatial memory.
Methods
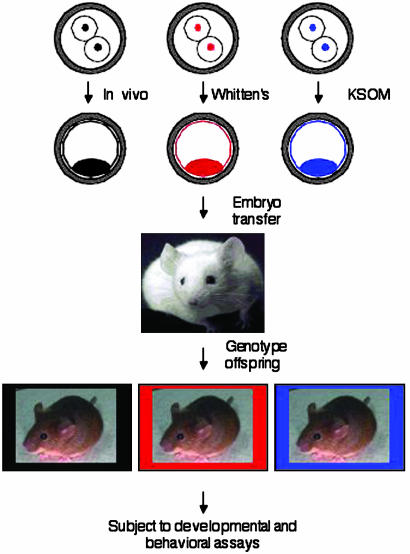
Experimental Design and Embryo Culture, Selection, and Transfer. A confounding factor in many studies examining the effects of embryo manipulation is that each experimental group experiences a different maternal environment after embryo transfer. For this reason, we used an experimental design in which genetically marked embryos from different culture conditions were transferred at the blastocyst stage to the same foster mother (Fig. 1); culture was initiated at the two-cell stage to allow maturation, fertilization, and genome activation to occur in vivo. Two-cell embryos were flushed from the oviducts with bicarbonate-free MEM supplemented with pyruvate (100 μg/ml), gentamycin (10 μg/ml), polyvinylpyrrolidone (3 mg/ml), and 25 mM Hepes, pH 7.2 (MEM/PVP). The embryos were derived by mating female 129S6/SvEvTac (129Sv) with male C57BL/6J (B6) mice that either contained or did not contain the transgene (Fig. 1). The embryos then were washed in either Whitten's medium (WM) (13) or KSOM (K+-modified simplex optimized medium) (14) and cultured to the fully expanded blastocyst stage under oil in 50 μl of either Whitten's medium or KSOM in 5% CO2/5% O2/90% N2 at 37°C as described (7); we find that typically >70% of the embryos develop in vitro to the blastocyst stage in these media. A greater fraction of the embryos that develop in KSOM are fully expanded when compared with those that develop in Whitten's medium, and hence we initiated cultures with more embryos in Whitten's medium to ensure that sufficient numbers of fully expanded Whitten's-derived blastocysts were present. Fully expanded blastocysts that developed in vivo were harvested by flushing the uteri with MEM/PVP. Immediately after collecting the in vivo-derived blastocysts, these embryos were combined with equal numbers (usually five to six) of fully expanded Whitten's medium- and KSOM-cultured blastocysts. These pooled zona pellucida-intact embryos (15-18) were then transferred to the uterine horns of a pseudopregnant foster mother according to standard procedures (15). In each experiment, the transgene was assigned to a different group to minimize the possibility that any observed effect was due to the transgene. All animal experiments were approved by the Institutional Animal Care and Use Committee and were consistent with National Institutes of Health guidelines.
Fig. 1.
Schematic of experimental design. Two lines of transgenic homozygous male mice were generated so that all of the offspring harbor the transgene. Each transgene was in a C57BL/6J (B6) background, and these mice were mated to 129S6/SvEvTac (129Sv) female mice, producing F1 offspring that were hemizygous for the transgene. This combination of strains was chosen because these transgenes were available in the C57BL/6J background, and 129Sv/B6 hybrid mice perform well in the behavioral assays (17, 35). One transgenic line carried lacZ (LN30) (36), and the other carried the tetOp DNA sequence (TETO) (37, 38). Wild-type mice were of the same genetic background, namely, 129Sv/B6 F1 hybrid. After birth, the offspring were genotyped by Southern blot analysis and subjected to a battery of developmental and behavioral assays described in the text. Studies of these transgenic mouse lines have not revealed any differences in behavior (37, 38), and none of the differences described here can be attributed to genotype (data not shown).
Behavioral Assays. Anxiety was measured by using the elevated zero maze test as described (16). Briefly, to begin each trial, mice were placed in the center of a closed quadrant of the circular maze and were allowed to explore the maze for 5 min; all mice were started in the same closed quadrant of the maze. After each trial, the maze was cleaned thoroughly with a bleach solution (0.25%), then cleaned with water, and then dried. Mice from each experimental group were tested in a counterbalanced order. Each trial was videotaped, and the videotape was analyzed by an experimenter blind to the genotype and culture conditions of the mice. The percentage of time in the open and closed quadrants, numbers of quadrant transitions, stretch attend postures, rearing, grooming, and head dips were determined from analysis of the videotapes. A binomial regression model was used to analyze the data (stata, version 7, StataCorp, College Station, TX) for the following reason. The number of seconds spent in the open is a count variable, which is commonly modeled by using Poisson regression. A common problem with Poisson regression models is greater variance than expected based on the model. When this problem occurs, as in our data, an alternative model, the binomial regression model, is used. This model specifically accommodates this extra-Poisson variation for counts, and provides an appropriate fit for the seconds in the open modeled in this study.
Spatial learning was assayed by using the hidden platform version of the Morris water maze test (17, 18). For spatial learning, mice were trained over 6 days in a Morris water maze as described (19) in a room containing extra maze cues such as posters on the wall. During learning, mice were given four trials of 60 s each to find the hidden platform, and the latency to find the platform was recorded. After the learning period, the platform was removed, and the mice were reintroduced into the pool after 1 day and 21 days. During this “probe” trial, the swim path of the mouse was recorded and analyzed by using an automated tracking system (HVS Image, San Diego). To analyze performance during the probe trial, we determined the average distance that the mice were from where the platform was located during training, as well as the amount of time that they spent in the quadrant where the platform was previously located (18, 20). Data from the water maze were analyzed by using a repeated measures ANOVA (systat for windows, version 7.0.1, Systat Software, Richmond, CA).
Fear conditioning (21) and Rota-rod (22) assays were performed as described.
Results
Effect of Culture on Development to Term and Preweaning Development. We did not find any effect of culture on the incidence of development to term. Eighty-six embryo transfers were conducted over a period of 18 months, of which 60 resulted in offspring. A total of 1,240 blastocysts were transferred, with an average of 14.4 ± 0.4 blastocysts per transfer. The total number of blastocysts that developed in vivo, in KSOM, and in Whitten's medium was 328, 434, and 478, respectively, and the genotype distribution of wild-type, TETO, and LN30 was 330, 480, and 430, respectively. The transfers resulted in 383 live births, and litters were culled to 12 pups where appropriate. The total number of offspring that developed in vivo, in KSOM, and in Whitten's medium was 82 (25%), 130 (30%), and 141 (29.5%), respectively. None of these differences was significant by χ2 analysis. The genotype distribution of wild-type, TETO, and LN30 was 116 (35%), 137 (29%), and 100 (23%), respectively; there were 30 offspring that could not be genotyped and were not analyzed. These differences are significant by χ2 analysis, and the lower incidence of transgenic mice may reflect reduced robustness. Nevertheless, the differences in behavior that we report below cannot be ascribed to differences in genotype, but only to differences in source, i.e., whether the embryos developed in vivo or in vitro. The offspring were divided into two pools, one that was analyzed for the studies reported here and another set aside to assess the effect of age on performance in the behavioral assays.
We did not find any obvious effect of culture when the offspring were subjected to a battery of preweaning developmental assays that included righting and grasp reflexes, cliff aversion, bar holding, growth rate, incisor appearance, and eyelid opening (23, 24), suggesting that sensory and motor development are not grossly altered by embryo culture (data not shown). These developmental assays were carried out from birth to weaning at 21 days after birth. The animals were weighed every 7 days over the course of 52 weeks, and no differences were observed in body weight gain (data not shown).
Effect of Culture on Motor Performance, Anxiety, and Spatial Learning. Behavioral testing for motor performance, anxiety, and spatial learning was initiated at 3-4 months of age and completed by 4-6 months of age. Tests of classical fear conditioning were completed by 7-8 months of age. No differences in motor learning as assayed by the accelerating Rota-Rod test, or in classical conditioning as assayed by contextual and cued fear conditioning tests, were found in adults derived from cultured embryos (data not shown). In tests of anxiety using the elevated zero maze and in tests of spatial memory using the hidden platform version of the water maze, however, differences were observed in culture-derived offspring.
Anxiety can be assayed by using the elevated zero maze or elevated plus maze, rodent behavioral tests that have been used successfully to isolate novel anti-anxiety drugs with actions similar to valium (16). The elevated zero maze relies on the fact that rodents are apprehensive in both open and elevated areas and, therefore, prefer to spend the majority of their time in enclosed areas (16). Culture-derived animals spent more time in the open quadrant than in vivo-derived animals, suggesting alterations in anxiety as a result of embryo culture (Fig. 2). The total time spent in the open quadrant during the 5-min test for all animals, which is a count variable, was analyzed by using a binomial regression model, which is similar to, but more flexible than, a Poisson regression for counts. This analysis revealed no effect of genotype (P = 0.86, data not shown) but did reveal an effect of culture (culture vs. in vivo, P = 0.02). In this test of anxiety, males are less anxious than females (25), and we observed an effect of sex in that males spent a total of 26.9 ± 3.0 s (mean ± SEM) in the open quadrant whereas females spent 16.5 ± 2.6 s (mean ± SEM) (P = 0.02). A main effect of culture was still observed even after adjusting for the effect of sex (P = 0.04). Because of these sex differences, we examined the effects of culture in males and females separately (Fig. 3). An analysis of the total time spent in the open quadrant revealed significant effects for males (culture vs. in vivo, P = 0.033), but not for females (culture vs. in vivo, P = 0.389) (Fig. 3A).
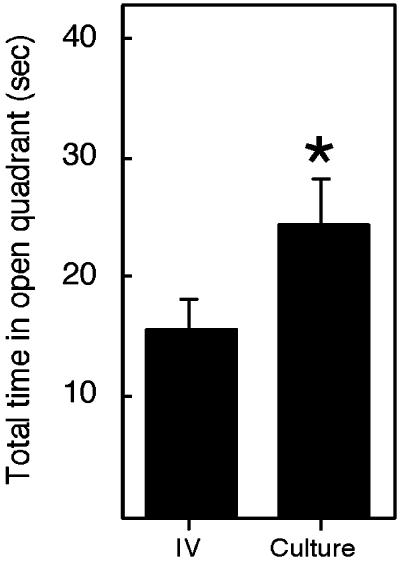
Fig. 2.
Mice derived from cultured embryos exhibit altered performance in the elevated zero maze. The elevated zero maze was used to assess anxiety as described (16), and the performance of all mice in this test is shown. IV, in vivo-derived mice; Culture, all mice obtained after culture in KSOM and WM. The data are expressed as the mean ± SEM. The number of IV-, KSOM-, and WM-derived mice analyzed was 36, 50, and 49, respectively. Transgenic and nontransgenic animals (wild-type, 42; TETO, 50; and LN30, 43) were distributed among these groups in a balanced fashion, and the observed differences cannot be attributed to genotype (data not shown). *, The difference between KSOM plus WM and IV is significant, P = 0.02. In this and subsequent figures, only the combined data of the culture-derived embryos are shown; there was little, if any, difference when the data were analyzed separately for the effect of either KSOM or WM on behavior.
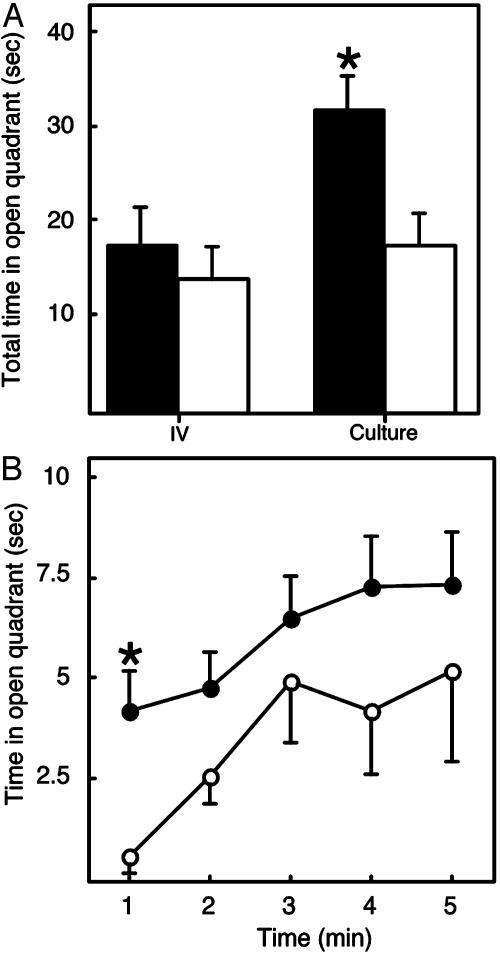
Fig. 3.
Embryo culture selectively alters performance of male mice in the elevated zero maze. The elevated zero maze was used to assess anxiety as described (16). (A) Total time spent in the open quadrant (sec, mean ± SEM) for males (filled bars) and females (open bars), respectively. *, The difference between Culture (all mice obtained after culture in KSOM and WM) and IV for the males is significant, P = 0.033. The difference between Culture and IV for the females is not significant. (B) Minute-by-minute analysis of time spent in open quadrant (sec, mean ± SEM) for males, comparing in vivo vs. culture-derived mice. Only the difference during the first minute is significant (*, P = 0.007). ○, in vivo-derived mice; •, culture-derived mice. The numbers of male mice examined for in vivo, KSOM, or WM animals were 18, 27, and 27, respectively, and the numbers of female mice were 18, 23, and 22, respectively. Transgenic and nontransgenic animals (males: wild-type, 22; TETO, 27; and LN30, 23; females: wild-type, 20; TETO, 23; and LN30, 20) were distributed among these groups in a balanced fashion, and the observed differences cannot be attributed to genotype (data not shown). No differences were observed in stretch-attend postures (data not shown), which were scored by visual analysis of videotapes (39).
In most experiments examining performance in the elevated zero maze and elevated plus maze, time in the open quadrant is analyzed over the entire 5-min trial. In our experiments, we measured the time in the open quadrant minute by minute and found that, overall, the time spent in the open quadrant was significantly different across each of the five 1-min intervals (P < 0.001). All animals spent more time in the open quadrant for minutes 2-5 compared with minute 1 (P = 0.001). The effect of culture remained significant (P = 0.03) after adjusting for the effects of sex as well as the differences across minutes. Male mice that developed in vivo spent only ≈0.5 s in the first minute in the open quadrant whereas mice derived from cultured embryos were less anxious and spent significantly more time in the open quadrant in the first minute after initial exposure to this novel environment (Fig. 3B, P = 0.007). Culture-derived females exhibited no significant differences in the time in the open quadrant during any of the time periods analyzed (data not shown). These differences seem to be specific for anxiety as measured in the elevated zero maze, and they do not extend to fear, as measured by unconditioned or conditioned freezing in fear conditioning experiments (data not shown).
We noted that culture-derived mice exhibited more quadrant transitions, a measure of locomotor activity, than their in vivo-derived counterparts (3.1 ± 0.3 vs. 1.9 ± 0.3, respectively, P = 0.021). When examined by sex, there were no differences between culture- and in vivo-derived females (2.4 ± 0.5 vs. 1.7 ± 0.4, respectively, P = 0.341), but there was a significant difference between culture- and in vivo-derived males (3.6 ± 0.5 vs. 2.0 ± 0.4, respectively, P = 0.028). As discussed below, although the increased transition number can make it difficult to ascribe the increased time spent in the open quadrant as due to reduced anxiety, this difference is another effect of embryo culture.
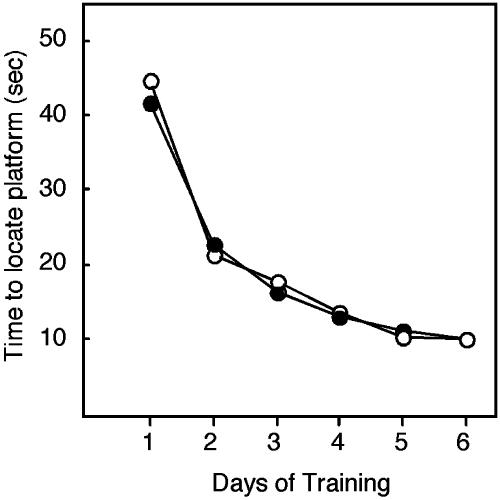
In humans, the hippocampal system is critical for episodic memory: memory for people, places, and things (26). Thus, patients with damage to their hippocampal system are unable to remember what happens to them in daily life. In rodents, lesion studies have indicated that the hippocampus is crucial for spatial and contextual learning. Spatial learning can be assayed by using the hidden platform version of the Morris water maze test (17, 18). In this analysis, we first compared the performance of mice derived from cultured embryos (combining those cultured in KSOM and WM) to that of in vivo-derived embryos and observed that embryo culture had no apparent effect on learning (Fig. 4; repeated measures ANOVA, main effect of treatment: F(1,125) = 0.22; P = 0.64). Similarly, no effect of culture was observed in the visible platform version of the water maze (data not shown). These results indicate that embryo culture does not significantly alter the ability of the resulting adult animals to swim, climb onto the platform, observe visual cues, or be motivated to escape from the water.
Fig. 4.
Mice derived from embryo culture exhibit normal spatial learning. Adult mice derived from embryos that developed either in vivo or in vitro were examined by using the hidden platform version of the Morris water maze (17, 18). ○, in vivo-derived mice; •, culture-(all mice obtained after culture in KSOM and WM)-derived mice. Error bars are omitted for clarity. Overall, when the performance of cultured embryos was compared with those that developed in vivo, there were no significant differences in learning (P > 0.05). The numbers of mice examined for in vivo, KSOM, or WM animals were 34, 47, and 46, respectively. Transgenic and nontransgenic animals (wild-type, 39; TETO, 48; and LN30, 40) were distributed among these groups in a balanced fashion, and the observed differences cannot be attributed to genotype (data not shown).
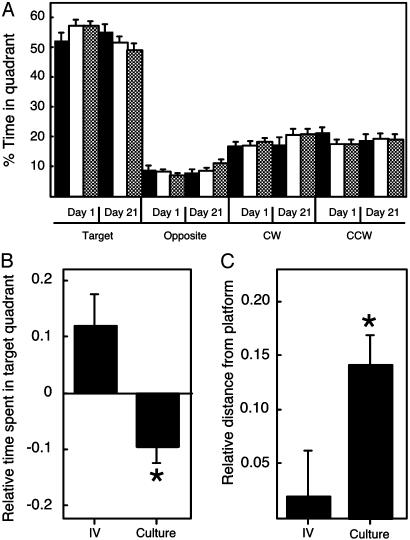
To determine whether animals were using a spatial strategy in the water maze, we performed probe trials (27) after the learning period in which the platform was removed and the mice were reintroduced into the pool after 1 day and 21 days. During these “probe” trials, the swim path of the mouse was recorded and analyzed by using an automated tracking system to determine the amount of time spent in each quadrant of the pool (Fig. 5A). This analysis revealed that, overall, mice clearly exhibited a preference for the quadrant containing the platform during training. An analysis of the probe trial data on day 1 and day 21 after training by using an ANOVA with repeated measures revealed no main effect of culture (F(2,124) = 0.20; P = 0.82), a main effect of day (F(1,124) = 5.95; P = 0.02), and a significant interaction between culture and day (F(2,125) = 4.75; P = 0.01). Because of the significant interaction between culture and day, we developed a measure of retention of spatial information on a per subject basis, by comparing an animal's performance on day 21 with its performance on day 1. The ability of mice to retain spatial information over 21 days was compromised in offspring derived from cultured embryos as reflected by reductions in the time spent in the training quadrant (Fig. 5B) (F(2,124) = 5.75; P = 0.004) and increases in the distance from the platform location (Fig. 5C) (F(2,124) = 3.61; P = 0.03) in culture-derived animals. Analysis of the performance of individual animals revealed that the differences in retention of spatial information, as measured by quadrant occupancy time and platform proximity, could not be ascribed to the anomalous performance of a few animals (data not shown). Further, these differences were not due to differences in search strategy for the platform (e.g., thigmotaxis), differences in swimming speed, or differences between different genotypes (data not shown).
Fig. 5.
Mice derived from embryo culture exhibit impaired spatial memory. (A) Average percentage of time spent in each quadrant of the pool during probe trials on the first and twenty-first day (day 1 and day 21) after training. The platform was located in the target quadrant, and the percentage of time in this quadrant, as well as in the opposite, adjacent clockwise (CW), and adjacent counterclockwise (CCW) quadrants, is shown. Filled bars, in vivo-derived mice; open bars, KSOM-derived mice; hatched bars, WM-derived mice. (B) Relative percentage of time in the quadrant that contained the platform during retention (probe) trials. To measure the retention of spatial information on a per subject basis, an animal's performance on day 21 was compared with its performance on day 1, and the data are expressed as this ratio minus 1 so that identical performance on day 21 and day 1 results in a value of 0. The data here and in C are both calculated in this way and are expressed as the mean ± SEM. *, The differences between animals derived from cultured embryos are statistically different from animals derived from embryos that develop in vivo; P < 0.004 for relative time spent in target quadrant. The observation that the control mice spend more time in the quadrant after 21 days than after 1 day is a phenomenon called consolidation of memory (40). (C) Relative distance from the platform location during retention (probe) trials. *, The differences between animals derived from cultured embryos are statistically different from animals derived from embryos that develop in vivo; P < 0.03 for relative distance from platform position. The numbers of mice examined are described in the legend to Fig. 4, and the observed differences cannot be attributed to genotype (data not shown).
Discussion
We report here that culture of preimplantation mouse embryos can result in small (10-20% change in magnitude) but significant long-term alterations in behavior: namely, anxiety, locomotor activity, and spatial memory. Although the hippocampus has been implicated in spatial and contextual memory in rodents (17, 18), the differences in spatial memory observed as a result of culture are not accompanied by changes in contextual memory as measured by using contextual fear conditioning (data not shown). In the study of some transgenic mice, a similar dissociation between spatial and contextual memory is observed (28). The impairment in spatial memory after culture suggests that embryo culture may selectively alter aspects of hippocampal function. Alternatively, because culture-derived animals exhibited impairments in retaining spatial information, embryo culture may alter interactions between the hippocampus and other brain regions, such as the cortex, that are required for long-term memory storage. Our battery of behavioral tests examined tasks mediated by other brain systems. These tests reveal that culture-derived mice exhibit normal fear conditioning, Rota-Rod performance, and performance on the visible platform version of the Morris water maze (data not shown). The finding that hippocampal lesions cause rats to spend more time in the open arms of an elevated plus maze (29) suggests that hippocampal dysfunction may provide a unifying explanation for our observations of altered spatial memory and performance in the elevated zero maze in culture-derived animals. Alternatively, the decreased anxiety found in males derived from cultured embryos may reflect alterations in the amygdaloid or extended amygdaloid systems (30). As mentioned above, the increased number of quadrant transitions exhibited by the culture-derived male mice when compared with in vivo-derived males can make it difficult to ascribe this difference to a decrease in anxiety, because the increased activity may be the basis for the increased amount of time spent in the open quadrant. It is also possible that, because the male mice are less anxious, they exhibit more quadrant transitions. Future experiments using alternative behavioral assays for anxiety may shed light on this issue. Nevertheless, in either case, this difference can be attributed to embryo culture. Further, it is of note that hippocampal lesions can increase activity (31).
The differences reported here cannot be attributed to delays in normal development because we did not observe any such changes in a variety of measures of physical, neurological, and behavioral development. The variances observed in behavior between individuals are not different in different groups, and the observed differences are not the consequence of a few individual “aberrant” mice. Moreover, our experimental design controls for differences in maternal environment both pre- and postnatally. Furthermore, we observed these differences throughout the course of our studies, which spanned 2 yr and were carried out in a blind fashion by several different personnel.
How perturbations in metabolism and gene expression as a result of embryo culture during the preimplantation stage could affect behavior in the adult is unknown. Subtle perturbations in placental function may be responsible, as suggested by H19 expression after embryo transfer. H19, which is expressed from the maternal allele (32), is first observed in the blastocyst only in the trophectoderm (7), which gives rise to extraembryonic tissue, and not the inner cell mass, which gives rise to the embryo proper. After implantation, H19 is expressed in the embryo. Culture in Whitten's medium results in biallelic expression of H19 in the blastocyst. After implantation, the biallelic expression of H19 that occurs as a result of culture in Whitten's medium persists within extraembryonic tissue, but not within the embryo (M. R. Mann, A. S. Doherty, R.M.S., and M. S. Bartolomei, unpublished observations). This altered H19 expression within the placenta likely reflects more global perturbations in placental gene expression (M. R. Mann, A. S. Doherty, R.M.S., and M. S. Bartolomei, unpublished observations). Such potential alterations in placental function could provide the mechanistic link between the effects of embryo culture and the development of a properly functioning nervous system.
Although our findings reveal long-term behavioral consequences of extended embryo culture, it is not known whether the mouse is a suitable model system for humans conceived by ART. For example, although even the best culture conditions for preimplantation mouse embryos are still suboptimal, it is possible, although unlikely, that the culture conditions for human embryos are optimal. Similar studies to examine this issue in humans or other primates are lacking, and such studies are clearly indicated by our results in mice. What also remains unresolved is whether the effects of culture on behavior are also observed after short-term culture of mouse embryos, e.g., to the 4- to 8-cell stage before embryo transfer. Nevertheless, the findings reported here suggest that a special effort should be made to minimize the effect of culture on the human preimplantation embryo. This finding may involve altering culture conditions because such changes can positively affect embryo metabolism and gene expression. For example, including amino acids in the culture medium enhances the embryo's ability to implant and develop after transfer (33, 34). This effect correlates with the observation that amino acids can reduce the enhanced glycolytic rate that is observed in early cleavage-stage mouse embryos in culture (34). Amino acids may also influence gene expression in the embryo because appropriate maternal monoallelic H19 expression is observed after culture in KSOM containing amino acids (7). Future studies will determine whether inclusion of amino acids or shorter periods in culture can ameliorate the impact of culture on the behavior of the resulting offspring. Whether other clinical procedures used in ART, e.g., intracytoplasmic sperm injection (ICSI), have long term consequences on behavior in this model remains to be seen.
Acknowledgments
We thank K. Matthew Lattal for his input to this project and Dick Tasca for comments on the manuscript. This research was supported by National Institutes of Health Grants HD 37032 (to R.M.S., G.S.K., and T.A.) and P50 MH 64045 (to R. E. Gur). T.A. is a John Merck Scholar and a Packard Foundation Fellow.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: ART, assisted reproductive technologies.
References
- 1.Hales, C. N. & Barker, D. J. (2001) Br. Med. Bull. 60, 5-20. [DOI] [PubMed] [Google Scholar]
- 2.Kwong, W. Y., Wild, A. E., Roberts, P., Willis, A. C. & Fleming, T. P. (2000) Development (Cambridge, U.K.) 127, 4195-4202. [DOI] [PubMed] [Google Scholar]
- 3.Schultz, R. M. & Williams, C. J. (2002) Science 296, 2188-2190. [DOI] [PubMed] [Google Scholar]
- 4.Gardner, D. K., Lane, M. & Schoolcraft, W. B. (2000) Hum. Reprod. 15, Suppl. 6, 9-23. [PubMed] [Google Scholar]
- 5.Gardner, D. K. (1998) Theriogenology 49, 83-102. [DOI] [PubMed] [Google Scholar]
- 6.Ho, Y., Doherty, A. S. & Schultz, R. M. (1994) Mol. Reprod. Dev. 38, 131-141. [DOI] [PubMed] [Google Scholar]
- 7.Doherty, A. S., Mann, M. R., Tremblay, K. D., Bartolomei, M. S. & Schultz, R. M. (2000) Biol. Reprod. 62, 1526-1535. [DOI] [PubMed] [Google Scholar]
- 8.Nicholls, R. D. & Knepper, J. L. (2001) Annu. Rev. Genomics Hum. Genet. 2, 153-175. [DOI] [PubMed] [Google Scholar]
- 9.Cox, G. F., Burger, J., Lip, V., Mau, U. A., Sperling, K., Wu, B. L. & Horsthemke, B. (2002) Am. J. Hum. Genet. 71, 162-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeBaun, M. R., Niemitz, E. L. & Feinberg, A. P. (2003) Am. J. Hum. Genet. 72, 156-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maher, E. R., Brueton, L. A., Bowdin, S. C., Luharia, A., Cooper, W., Cole, T. R., Macdonald, F., Sampson, J. R., Barratt, C. L., Reik, W. & Hawkins, M. M. (2003) J. Med. Genet. 40, 62-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moll, A. C., Imhof, S. M., Cruysbert, J. R. M., Schouten-van Meeteren, A. Y. N., Boers, M. & Van Leeuwen, F. E. (2003) Lancet 361, 309-310. [DOI] [PubMed] [Google Scholar]
- 13.Whitten, W. K. (1971) Adv. Biosci. 6, 129-139. [Google Scholar]
- 14.Erbach, G. T., Lawitts, J. A., Papaioannou, V. E. & Biggers, J. D. (1994) Biol. Reprod. 50, 1027-1033. [DOI] [PubMed] [Google Scholar]
- 15.Hogan, B., Costantini, F. & Lacy, E. (1986) Manipulating the Mouse Embryo: A Laboratory Manual. (Cold Spring Harbor Lab. Press, Plainview, NY).
- 16.Kash, S. F., Tecott, L. H., Hodge, C. & Baekkeskov, S. (1999) Proc. Natl. Acad. Sci. USA 96, 1698-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logue, S. F., Paylor, R. & Wehner, J. M. (1997) Behav. Neurosci. 111, 104-113. [DOI] [PubMed] [Google Scholar]
- 18.Morris, R. G., Garrud, P., Rawlins, J. N. & O'Keefe, J. (1982) Nature 297, 681-683. [DOI] [PubMed] [Google Scholar]
- 19.Lattal, K. M. & Abel, T. (2001) J. Neurosci. 21, 5773-5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallagher, M., Burwell, R. & Burchinal, M. (1993) Behav. Neurosci. 107, 618-626. [DOI] [PubMed] [Google Scholar]
- 21.Nie, T. & Abel, T. (2001) Behav. Neurosci. 115, 951-956. [DOI] [PubMed] [Google Scholar]
- 22.Chennathukuzhi, V., Stein, J. M., Abel, T., Donlon, S., Yang, S., Miller, J. P., Allman, D. M., Simmons, R. A. & Hecht, N. B. (2003) Mol. Cell. Biol. 23, 6419-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dulioust, E., Toyama, K., Busnel, M. C., Moutier, R., Carlier, M., Marchaland, C., Ducot, B., Roubertoux, P. & Auroux, M. (1995) Proc. Natl. Acad. Sci. USA 92, 589-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox, W. M. (1965) Anim. Behav. 13, 234-241. [DOI] [PubMed] [Google Scholar]
- 25.Palanza, P. (2001) Neurosci. Biobehav. Rev. 25, 219-233. [DOI] [PubMed] [Google Scholar]
- 26.Stefanacci, L., Buffalo, E. A., Schmolck, H. & Squire, L. R. (2000) J. Neurosci. 20, 7024-7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenk, F. & Morris, R. G. (1985) Exp. Brain Res. 58, 11-28. [DOI] [PubMed] [Google Scholar]
- 28.Pittenger, C., Huang, Y. Y., Paletzki, R. F., Bourtchouladze, R., Scanlin, H., Vronskaya, S. & Kandel, E. R. (2002) Neuron 34, 447-462. [DOI] [PubMed] [Google Scholar]
- 29.Kjelstrup, K. G., Tuvnes, F. A., Steffenach, H. A., Murison, R., Moser, E. I. & Moser, M. B. (2002) Proc. Natl. Acad. Sci. USA 99, 10825-10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aggleton, J. P. (2000) The Amygdala: A Functional Analysis (Oxford Univ. Press, Oxford).
- 31.O'Keefe, J. & Nadel, L. (1978) The Hippocampus as a Cognitive Map (Clarendon Press, Oxford).
- 32.Bartolomei, M. S. & Tilghman, S. M. (1997) Annu. Rev. Genet. 31, 493-525. [DOI] [PubMed] [Google Scholar]
- 33.Lane, M. & Gardner, D. K. (1994) J. Reprod. Fertil. 102, 305-312. [DOI] [PubMed] [Google Scholar]
- 34.Lane, M. & Gardner, D. K. (1998) Hum. Reprod. 13, 991-997. [DOI] [PubMed] [Google Scholar]
- 35.Owen, E. H., Logue, S. F., Rasmussen, D. L. & Wehner, J. M. (1997) Neuroscience 80, 1087-1099. [DOI] [PubMed] [Google Scholar]
- 36.Dolle, M. E., Martus, H. J., Gossen, J. A., Boerrigter, M. E. & Vijg, J. (1996) Mutagenesis 11, 111-118. [DOI] [PubMed] [Google Scholar]
- 37.Mayford, M., Bach, M. E., Huang, Y.-Y., Wang, L., Hawkins, R. D. & Kandel, E. R. (1996) Science 274, 1678-1683. [DOI] [PubMed] [Google Scholar]
- 38.Mayford, M., Baranes, D., Podsypanina, K. & Kandel, E. R. (1996) Proc. Natl. Acad. Sci. USA 93, 13250-13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heisler, L. K., Chu, H. M., Brennan, T. J., Danao, J. A., Bajwa, P., Parsons, L. H. & Tecott, L. H. (1998) Proc. Natl. Acad. Sci. USA 95, 15049-15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abel, T. & Lattal, K. M. (2001) Curr. Opin. Neurobiol. 11, 180-187. [DOI] [PubMed] [Google Scholar]