Abstract
It is commonly assumed that High Arctic lakes and ponds were not affected by direct local human activities before the arrival of Europeans, because most native peoples were primarily nomadic, maintained relatively low population densities, and practiced unintrusive hunting and gathering technologies. Our archeological and paleolimnological data show that this was not always the case. Thule Inuit whalers, whose winter settlements consisted of houses constructed from the bones of bowhead whales on Somerset Island between about anno Domini 1200 and 1600, markedly changed pond water quality and ecology. The arrival of whalers 8 centuries ago caused marked changes in water chemistry and the expansion of moss substrates. Although whalers abandoned the area >4 centuries ago, the legacy of these human disturbances is still evident in the pond's present-day limnology and is characterized by elevated nutrient concentrations and atypical biota. This is the earliest reported paleolimnological record of changes in aquatic ecology associated with local human activities in Canada or the United States, or for any circumpolar ecosystem.
The Thule Inuit were the first whalers in the High Arctic. They migrated to the Canadian Arctic and Greenland from Alaska ≈1,000 years ago, bringing with them a well developed whaling technology. This technology included umiaks (large open skin boats usually rowed by a crew of seven to eight individuals), whaling harpoons and lances, and seal-skin floats. Whaling was carried out in open water, with one or more umiaks pursuing a whale. Once adjacent to the whale, one or more harpoons were thrust into the animal. The harpoon head was detachable and toggled, so that when tension was applied to the attached harpoon line, the harpoon head rotated up to 90° and remained embedded in the animal. Several inflated seal-skin floats were attached to the harpoon lines and served to provide drag that quickly exhausted the whale. When the exhausted whale came to rest on the surface, it was approached, and lances were used to penetrate the heart or other vital organs. The dead whale was then towed to shore, where it was flensed.
The greatest concentration of Thule winter whale-bone houses and of archeological whale bone in the Canadian Arctic occurs on southeastern Somerset Island (1). Archeological sites are recognizable from aerial surveys because of the bleached bones on the tundra landscape and because local vegetation is often more lush as it continues to be fertilized by the slowly decomposing bones and other refuse (2). Most of these sites are adjacent to Arctic ponds, which provided peat for dwelling construction. The primary goal of our study was therefore to determine whether Thule Inuit whalers, who butchered, consumed, and discarded products of whales and used whale bones as architectural materials, had significantly altered local freshwater ecosystems. If so, these sites provide examples of long-term human-induced changes in Arctic aquatic ecosystems, a region widely believed to have been unaffected by local human impacts until very recently. If whaling activities affected these systems, then paleolimnological studies, such as the one reported here, might also be used to determine the initial periods of Thule whaling and to help track the spread of Thule whaling societies eastward and northward after their initial entry into the Arctic from Alaska, at present a much-debated topic (3, 4).
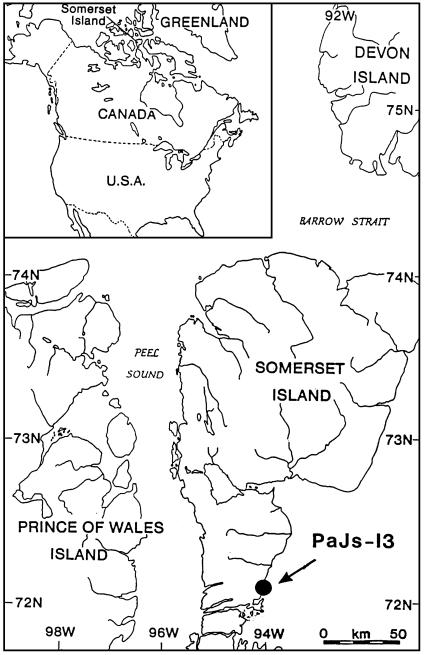
The Thule site we examined (PaJs-13; 72° 08.66′N, 94° 01.50′W) is located on the coast ≈5 km north of the entrance to Hazard Inlet (Fig. 1). It is underlain by marine beach sediments (primarily sand and gravel), in turn derived from Paleozoic limestones, dolomites, and sandstones (5). Outcrops of these rock types occur adjacent to the study area. The site was first investigated by McCartney (6) and was subsequently mapped and partly excavated by Savelle (7, 8), as was the neighboring site of PaJs-2 by Whitridge (9). Our site consists of 11 large semisubterranean whale-bone houses (Fig. 2), several smaller, shallower sod dwellings with little whale bone, and a ring of 10 bowhead crania of probable ceremonial function. The beach adjacent to the site and as far south as the entrance to Hazard Inlet is strewn with several thousand bones from the flensing and caching of bowhead whales. Remains of at least 125 bowhead whales occur within the site and on the adjacent flensing beaches. In addition to the whale products at the site (bones, baleen, and skin), other remains included bones of several hundred ringed seals and bones and other remains of lesser numbers of beluga whale, Arctic hare, Arctic fox, bird, fish, dog or wolf, caribou, and musk ox. Most tools were fashioned from the bones of these animals (primarily from whale bone and caribou antler).
Fig. 1.
Map showing the location of the Thule site PaJs-13 on Somerset Island in the Canadian Arctic (modified from ref. 7).
Fig. 2.
Unexcavated Thule whale-bone house at site PaJs-13, view north, with the cored pond in the background. Junko Habu provides scale.
Six dwellings and several other features at the site were excavated (7, 8). The recovered artifacts and radiocarbon dates (see Table 1 and discussion, discussed below) document occupation from approximately the 13th century to the end of the 16th century. House contemporaneity has traditionally been difficult to establish, but lack of dwelling overlap, similarity in artifact assemblages amongst dwellings, and refitting of broken artifact pieces amongst at least three of six excavated houses suggest that many, if not most, of the dwellings were occupied simultaneously. By using historic North Alaskan Inuit bowhead whaling societies as recent analogues (10, 11), we infer that a typical Thule whaling crew would have consisted of seven to eight adult males, and would have required four to five households to supply its members. Given that each household probably consisted of seven to eight individuals, we estimate that a minimum population of 50-60 individuals occupied the site at its peak.
Table 1. Radiocarbon dates of antler samples from dwellings at PaJs-13.
| Dwelling | Age 14C | Calibrated 2-SD age range | Laboratory code |
|---|---|---|---|
| House 1 | 467 ± 44 | A.D. 1403-1484 | AA53783 |
| House 3 | 613 ± 46 | A.D. 1287-1418 | AA53792 |
| 582 ± 46 | A.D. 1296-1432 | AA53794 | |
| House 4 | 419 ± 42 | A.D. 1422-1625 | AA53785 |
| 620 ± 66 | A.D. 1277-1432 | AA53789 | |
| House 5 | 601 ± 51 | A.D. 1288-1428 | AA53791 |
| 761 ± 47 | A.D. 1192-1298 | AA53793 |
Our main objective was to determine whether the ecology of these ponds was influenced by Thule whaling societies. Previous paleolimnological studies of undisturbed High Arctic ponds (12-14) and several unpublished investigations have provided paleolimnological records that reflect a relatively complacent environment from the times of their inceptions until the 19th century, with fossil diatom assemblages overwhelmingly dominated by small, benthic Fragilaria diatoms. After the mid-1800s, diatom assemblages typically changed, with marked increases in taxa that indicate longer growing seasons associated with a warmer climate. However, none of these previous studies dealt with ponds that were likely influenced by prehistoric human habitation.
To determine whether Thule whalers affected pond ontogeny, we used the same high-resolution paleolimnological techniques that were applied previously to undisturbed High Arctic ponds (12, 13). 210Pb dating and other geochronological tools, as described below, indicated that our sedimentary record represents about the last 1,200 years of pond history. Past environmental changes were inferred primarily from diatoms, a diverse algal group whose community composition has been used to reconstruct past ecological changes in many types of aquatic habitats (15), including High Arctic ponds (12, 13). Because bowhead whales are highly enriched in δ15N (16), stable nitrogen isotopes (δ15N) were also used to demonstrate that decaying whale carcasses were present during our recorded limnological changes.
Study Site and Current Limnology.
The unnamed pond that we chose for detailed paleolimnological reconstructions was the relatively large water body (≈400 m long, and varying in width from 20 m to 100 m) immediately north and west of the whale-bone houses and discarded whale bones (Figs. 2 and 3). Photographs, maps, and other details regarding the archeological site have been published (7, 17). Like most High Arctic ponds, including the other ponds that have been studied with paleolimnological techniques (12, 13), our study site was shallow, with a maximum depth of ≈0.5 m. The pond is circumneutral to alkaline (pH in 1994 and 1995 ranged from 7.7 to 8.4) and, although close to the sea (4.5 m above sea level), is of relatively low specific conductance (165-190 μS·cm-1). Although the Thule Inuit abandoned this area ≈4 centuries ago, decaying whale bones and other organic materials in and around this pond continue to influence water quality, as reflected by nutrient concentrations and other water chemistry variables that are still markedly elevated compared to nearby, undisturbed ponds. For example, detailed water chemistry analyses conducted in July of 1994 and 1995 revealed that our study site differed from almost all of the hundreds of other ponds we have surveyed throughout the High Arctic without archeological remains in their catchments (18). This pond had relatively elevated total phosphorus (8.1-17.8 μg·liter-1), high dissolved organic carbon (3.9-5.7 mg·liter-1), and somewhat higher calcium (26.2-32.8 mg·liter-1) concentrations. Such total phosphorus concentrations may not seem elevated by more southern limnological standards, but these values are uncharacteristically high for the ultraoligotrophic ponds and lakes that characterize this Arctic region. For example, on our July 21, 1995, survey of 10 ponds on Somerset Island, the Thule pond site had a total phosphorus concentration of 8.1 μg·liter-1. This value was the lowest concentration we recorded for the pond, because it was taken late in the season; nevertheless, it was the highest of the 10 ponds we surveyed that day, which ranged from 1.1 to 4.7 μg·liter-1, with a mean of 2.7 μg·liter-1. Furthermore, our July 1997 survey of 34 ponds and lakes on nearby Victoria Island (18) yielded a mean total phosphorus concentration of only 1.3 μg·liter-1. Over the last 20 years, we have sampled a few other High Arctic ponds with discarded whale bones in their catchments, and these ponds also displayed relatively high concentrations of key water quality variables, such as total phosphorus.
Fig. 3.
Aerial view of PaJs-13 showing several excavated houses and the southern part of the cored pond. View is looking northwest.
Methods
Field Methods. We used the same high-resolution sediment sampling techniques that we have used in our other High Arctic studies (13). We took several Glew sediment cores (19) from the central region of the pond (all ≈23 cm in length) in July 1996, and sectioned them at 0.5-cm intervals by using a Glew close-interval extruder (20). Probing with a metal rod revealed that the pond contained only ≈23 cm of sediment, so we are confident that our cores represent the entire sedimentary history of this site. Sedimentation rates are typically low for these High Arctic regions (13).
Geochronology. We used 210Pb dating, applying alpha spectrometry, to provide geochronological control for the last ≈200 years of sediment accumulation (21). Archeological materials (antlers) were dated by accelerator MS radiocarbon dating (22).
Diatom Analyses. Diatom samples were prepared by using standard sediment digestion protocols (23), and taxa were identified and enumerated by using the procedures we used in our previous paleolimnological studies (13). Diatom nomenclature for some taxa remains in a certain state of flux. In our counts, Fragilaria construens is synonymous with Staurosira construens, Fragilaria pinnata is synonymous with Staurosirella pinnata, and Navicula laevissima is synonymous with Sellaphora laevissima.
Nitrogen Isotopes. Sediment samples were freeze-dried and analyzed for their nitrogen stable isotope ratio (δ15N) with an automated model EA-1110 elemental C and N analyzer (CE Instruments, Milan) coupled to a Finnigan-Mat (Bremen, Germany) DELTAplus isotope ratio mass spectrometer with a conflow ii interface using International Atomic Energy Agency N1 and N2 reference standards. We attained a precision of ≈0.2%thou based on replicate analyses.
Results and Discussion
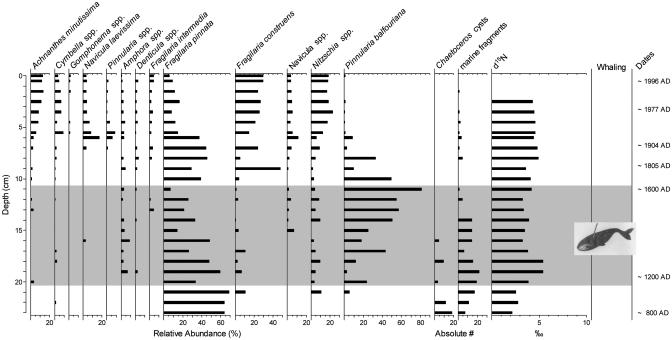
Geochronology. 210Pb dating indicated a relatively constant sedimentation rate for the uppermost 5 cm, and then a somewhat slower sedimentation rate to ≈9 cm, where unsupported 210Pb activity was beyond detection. An extrapolation of this slower sedimentation rate indicates that anno Domini (A.D.) 1600 occurs at approximately the 12-cm level (Fig. 4); however, we acknowledge that such an extrapolation is an approximation.
Fig. 4.
Summary diagram of the dominant diatom assemblage shifts and δ15N changes in the pond sediment core. The shaded region represents the time period when Thule whalers lived at this site. Diatom taxa are expressed as percentages of the total diatom sum. For clarity, some of the less common benthic taxa are grouped into generic categories. Chaetoceros cysts and marine diatom fragments are expressed as the total number of specimens counted (absolute numbers). There was insufficient sediment to measure δ15N analyses in the surface 1.5 cm.
Dating older material was more problematic, because accelerator MS radiocarbon dating from High Arctic lake sediments is often made difficult by the lack of terrestrial macrofossils in sediment cores. Moreover, because carbonates are present in this region, a hardwater effect was documented when three bulk sediment samples were dated. However, an approximate basal date for the core was obtained from an uplift curve calculated for the Hazard Inlet area, which indicates that the beach at ≈5 m emerged within the last 1,000-1,200 years, providing a maximum age for the sediment profile (A. S. Dyke, unpublished data) that is consistent with our extrapolation of the 210Pb-based chronology. Marine diatom fragments and Chaetoceros cysts near the base of the core show that we obtained a nearly complete record of this pond (Fig. 4). In addition, a simple extrapolation of the 210Pb-derived sedimentation rate, as described above, places circa (ca.) A.D. 1200 (the time of arrival of Thule whalers based on 14C dating of archeological materials; see Table 1 and ref. 3) at ≈19-20 cm, which coincides with diatom and 15N isotope changes (discussed below), indicating a pronounced shift in pond ecology at this time (Fig. 4). The 14C accelerator MS dates on antler debitage and the artifact assemblages all indicate ca. A.D. 1200-1600 (7), which closely match the ages of our paleolimnological changes. Specifically, the 14C dates on recovered archeological materials obtained from the site suggest initial occupation ca. A.D. 1200 with continued occupation until at least the early to mid-15th century (Table 1). All these dates are on antler debitage, the byproduct of tool manufacture, found within the dwellings. Although sea mammal remains are extremely common at Thule and other prehistoric sites in the Arctic, we used terrestrially derived materials because of the marine reservoir effect, which often cannot be quantified accurately for specific locations (24).
The radiocarbon antler dates represent initial through to late occupation, but not necessarily final abandonment. Hence, final abandonment toward the end of the 16th century is likely, and the effects of the whale detritus would likely have continued well after abandonment. The adjacent PaJs-2 Thule village appears to have undergone a similar occupation-abandonment scenario (9). In short, all of these independent dating approaches are consistent with the chronology and interpretations we suggest for this sediment core.
Paleolimnological Indicators. Thirty-five diatom taxa were identified in the sediment core, with the relative frequencies of the dominant taxa shown in Fig. 4. The pond emerged from the sea ≈1,000-1,200 years ago, but would have been subjected to marine influences (through storms, etc.) for at least several hundred years. Not surprisingly, the oldest part of the sediment core contains marine diatom fragments, reflecting marine influences. The earliest assemblage is dominated by small benthic F. pinnata, a diatom that dominates most High Arctic alkaline sites in sediments predating recent warming trends (12-14, 25). However, unlike paleolimnological studies from other alkaline High Arctic ponds, a previously unrecorded assemblage for this time period, dominated by the moss epiphyte Pinnularia balfouriana, characterized the flora beginning at about the 19-cm level (ca. A.D. 1200). This diatom taxon peaked at a 11- to 12-cm depth (ca. A.D. 1600) and then returned to low levels, after which F. pinnata regained some of its former prominence.
The timing of these marked and atypical species changes characterized by the moss epiphyte P. balfouriana coincides with the time period of Thule settlement at the pond site, as documented by the parallel archeological study (7), and indicates that pond ecology was dramatically influenced by the Thule whalers. Nutrients were released from decaying whale bones, which, along with the other activities of the whalers, fertilized the pondwater and the surrounding catchment, thus promoting extensive moss growth (26, 27). Increasing amounts of pondwater nutrients would directly affect diatom assemblage composition, and moss proliferation would provide an important substrate for epiphytic diatom growth (12). Decomposed moss fragments are common in the sediment matrix.
Further evidence that the above diatom changes are related to the decaying whale bones and other Thule-derived organic materials can be gleaned from the stable isotope data (Fig. 4). Marine biomass is highly enriched in 15N, with δ15N averaging ≈13%thou for bowhead whales in the eastern Arctic (16, 28). These values greatly exceed those normally found in organic matter from most freshwater systems in, for example, phytoplankton and terrestrial vegetation (≈1-3%thou), which constitute the bulk of organic freshwater sediments. δ15N values in other Arctic lake and pond sediments typically fall within the range 0 to +3%thou (29). The pronounced rise in δ15N from 2.5%thou to 5.4%thou at ≈20 cm in our core is not typical of sediment cores from Arctic ponds and confirms a marine input signal from decomposition of bowhead remains, for which there is abundant archeological evidence (8). We are confident that our paleolimnological measurements of δ15N are reliably tracking inputs from the whale carcasses, because these relatively shallow ponds do not go anoxic, precluding δ15N enrichment by denitrification. Moreover, δ15N values are lowest in the basal part of the core (and before the arrival of whalers), which has the strongest marine influences (as also confirmed by the diatom profile). Because marine waters are typically enriched in δ15N relative to freshwaters, one would expect higher values in the oldest sediments. Instead, we recorded a marked increase in δ15N at the ≈20-cm level only after the period of Thule settlement.
Near the 11-cm level (ca. A.D. 1600), the P. balfouriana assemblage waned and F. pinnata regained its dominance along with the closely related benthic diatom F. construens (Fig. 4). Archeological data suggest that the Thule whalers abandoned the area about this time (9, 30). Not surprisingly, δ15N values continue to be relatively high, because decaying whale bones are still a prominent component of this system. However, with the whalers gone, eutrophication would have slowed, because no new carcasses were butchered on the site.
As noted above, the diatom flora recorded another assemblage shift over the last ≈100-150 years, with F. pinnata replaced by a diverse assemblage of other benthic taxa (Fig. 4). Similar diatom species changes have been recorded in other High Arctic paleolimnological studies and have been interpreted as warming (13, 14), although other factors may be involved. This Somerset site also records these recent assemblage shifts, although the taxa that increase at this time (e.g., the Nitzschia spp.) indicate somewhat higher nutrient conditions [including taxa that were more common with recent sewage inputs in High Arctic lakes (31)] than those recorded in the other High Arctic oligotrophic ponds that have not been influenced by Thule settlements. With warming occurring after the mid-19th century, transport of nutrients from the remaining whale bones may have been further accelerated, increasing the input of nutrients to the pond.
Nonetheless, the most striking assemblage shift that characterizes the diatom profile is the increase in moss epiphytes between ca. A.D. 1200 and A.D. 1600, coupled with the increase in δ15N, which coincides with the archeological record of the Thule settlement and whaling at the site. These diatom species changes document a marked shift in ecological conditions in the pond, indicating the development of extensive moss growth and other substrates that flourished during this period of nutrient enrichment. This pattern has not been observed in any other paleolimnological study of High Arctic ponds, in which profiles are typically dominated by small benthic Fragilaria diatoms until the period of post-19th-century warming (12-14).
Until now, High Arctic water bodies were considered to have been unaffected by local human influences, except perhaps over the last few decades with the deposition of long-range transported pollutants (32). However, our paleolimnological data show that pond ontogeny was markedly altered by the arrival and subsequent activities of Thule whalers eight centuries ago, and, thereafter, this pond followed a markedly different trajectory in its development. It is ironic that the High Arctic, generally considered to be the last refuge from local human disturbances, contains the oldest record thus far obtained in the United States or Canada of a human population affecting freshwater ecology. The legacy of this prehistoric intervention is still evident in the pond's current limnology and biology.
Acknowledgments
We thank W. Blake, Jr., W. M. Last, and colleagues from our labs for helpful comments. Additional comments provided by Drs. D. Livingstone and M. Brenner greatly improved this paper. The G. G. Hatch Stable Isotope Laboratories provided isotope analyses. This work was supported by the Natural Sciences and Engineering Research Council of Canada, the Polar Continental Shelf Project, and the Social Sciences and Humanities Research Council of Canada.
Abbreviations: A.D., anno Domini; ca., circa; MS, mass spectrometry.
References
- 1.McCartney, A. P., ed. (1979) Archaeological Whale Bone: A Northern Resource (Univ. of Arkansas, Fayetteville).
- 2.Derry, A. M., Kevan, P. G. & Rowley, D. M. (1999) Arctic 52, 204-221. [Google Scholar]
- 3.McGhee, R. (2000) in Identities and Cultural Contacts in the Arctic, eds. Appelt, M., Berglund, J. & Gullov, H. C. (Danish Natl. Mus. Danish Polar Center, Copenhagen), pp. 181-191.
- 4.Morrison, D. (2000) in Identities and Cultural Contacts in the Arctic, eds. Appelt, M., Berglund, J. & Gullov, H. C. (Danish Natl. Mus. Danish Polar Center, Copenhagen), pp. 221-228.
- 5.Dyke, A. S. (1983) Geol. Surv. Can. Mem. 404, 1-32. [Google Scholar]
- 6.McCartney, A. P. (1978) Study of Whale Bones for the Reconstruction of Canadian Arctic Bowhead Whale Stocks and Whale Use by Prehistoric Inuit (Department of Indian and Northern Affairs, Ottawa).
- 7.Habu, J. & Savelle, J. M. (1994) Quat. Res. (Japan) 33, 1-18. [Google Scholar]
- 8.Savelle, J. (1997) J. Archaeol. Sci. 24, 869-885. [Google Scholar]
- 9.Whitridge, P. G. (1999) Ph.D. thesis (Arizona State Univ., Tempe).
- 10.Spencer, R. (1959) The North Alaskan Eskimo (Bur. of Am. Ethnol., Washington, DC).
- 11.Burch, E. S. (1981) The Traditional Hunters of Point Hope, Alaska: 1800-1875 (North Slope Borough, Barrow, AK).
- 12.Douglas, M. S. V. & Smol, J. P. (1999) in The Diatoms: Applications for the Environmental and Earth Sciences, eds. Stoermer, E. F. & Smol, J. P. (Cambridge Univ. Press, Cambridge, U.K.), pp. 227-244.
- 13.Douglas, M. S. V., Smol, J. P. & Blake, W., Jr. (1994) Science 266, 416-419. [DOI] [PubMed] [Google Scholar]
- 14.Overpeck, J., Hughen, K., Hardy, D., Bradley, R., Case, R., Douglas, M., Finney, B., Gajewski, K., Jacoby, G., Jennings, A., et al. (1997) Science 278, 1251-1256. [Google Scholar]
- 15.Stoermer, E. F. & Smol, J. P., eds. (1999) The Diatoms: Applications for the Environmental and Earth Sciences (Cambridge Univ. Press, Cambridge, U.K.).
- 16.Hobson, K. & Schell, D. (1998) Can. J. Fish. Aquat. Sci. 55, 2601-2607. [Google Scholar]
- 17.Savelle, J. M. (2002) Bull. Natl. Mus. Ethnol. (Osaka) 27, 159-188. [Google Scholar]
- 18.Michelutti, N., Holtham, A. J., Douglas, M. S. V. & Smol, J. P. (2003) J. Phycol. 39, 465-480. [Google Scholar]
- 19.Glew, J. R. (1989) J. Paleolimnol. 2, 241-243. [Google Scholar]
- 20.Glew, J. R. (1988) J. Paleolimnol. 1, 235-239. [Google Scholar]
- 21.Appleby, P. G. (2001) in Tracking Environmental Change Using Lake Sediments: Basin Analysis, Coring, and Chronological Techniques, eds. Last, W. M. & Smol, J. P. (Kluwer Academic, Dordrecht, The Netherlands), Vol. 1, pp. 171-203. [Google Scholar]
- 22.Svante, B. & Wohlfarth, B. (2001) in Tracking Environmental Change Using Lake Sediments: Basin Analysis, Coring, and Chronological Techniques, eds. Last, W. M. & Smol, J. P. (Kluwer Academic, Dordrecht, The Netherlands), Vol. 1, pp. 205-245. [Google Scholar]
- 23.Battarbee, R. W., et al. (2001) in Tracking Environmental Change Using Lake Sediments: Terrestrial, Algal, and Siliceous Indicators, eds. Smol, J. P., Birks, H. J. B. & Last, W. M. (Kluwer Academic, Dordrecht, The Netherlands), Vol. 3, pp. 155-202. [Google Scholar]
- 24.Bowman, S. (1990) Radiocarbon Dating (Univ. of California Press, Berkeley).
- 25.Smith, I. R. (2002) J. Paleolimnol. 27, 9-28. [Google Scholar]
- 26.Bowden, W. B., Finlay, J. C. & Maloney, P. E. (1994) Freshwater Biol. 32, 445-454. [Google Scholar]
- 27.Vincent, W. F. & Hobbie, J. E. (2000) in The Arctic: Environment, People, Policy, eds. Nuttall, M. & Callaghan, T. V. (Harwood, Amsterdam), pp. 197-232.
- 28.Hirons, A. C., Schell, D. M. & Finney, B. P. (2001) Oecologia 129, 591-601. [DOI] [PubMed] [Google Scholar]
- 29.Wolfe, B. B., Edwards, T. W. D. & Aravena, R. (1999) The Holocene 9, 215-222. [Google Scholar]
- 30.Maxell, M. S. (1985) Prehistory of the Eastern Arctic (Academic, Orlando, FL).
- 31.Michelutti, N., Douglas, M. S. V. & Smol, J. P. (2002) Verh. Int. Ver. Theor. Angew. Limnol. 28, 1533-1537. [Google Scholar]
- 32.Arctic Monitoring and Assessment Programme. (1998) AMAP Assessment Report: Arctic Pollution Issues (Arctic Monitoring and Assessment Programme, Oslo).