Abstract
Patients with cancer are at high risk of developing venous thromboembolism (VTE), including deep venous thrombosis and pulmonary embolism. Compared to non-cancer patients, VTE in cancer is more frequently associated with clinical consequences, including recurrent VTE, bleeding, and an increase in the risk of death. Low-molecular-weight heparins (LMWHs) are commonly recommended for the prevention and treatment of VTE in cancer patients because of their favorable risk-to-benefit profile. Indeed, compared with vitamin K antagonists, LMWHs are characterized by a reduced need for coagulation monitoring, few major bleeding episodes, and once-daily dosing, which make these drugs more suitable in the cancer setting. Guidelines have been published recently with the aim to improve the clinical outcomes in cancer patients at risk of VTE and its complications. Coagulation activation in cancer may have a role not only in thrombosis but also in tumor growth and dissemination. Hence, inhibition of fibrin formation has been considered a possible tool against the progression of malignant disease. Clinical studies show that anticoagulant drugs may have a beneficial effect on survival in cancer patients, with a major role for LMWHs. Recently a number of prospective randomized clinical trials to test LMWHs to improve cancer survival as a primary endpoint in cancer patients have been conducted. Although the results are controversial, the interest in this research area remains high.
Keywords: venous thromboembolism, VTE, LMWH
Introduction
Tumor growth is associated with the development of a hypercoagulable state and an increased risk of thrombosis in the host. Thromboembolic disease can be the earliest clinical sign of a tumor, as originally reported by the French clinician Armand Trousseau over a century ago and recently confirmed by controlled prospective clinical trials.1,2 Conversely, patients already diagnosed with cancer have a significantly higher risk of developing “secondary” thrombosis in specific conditions.3,4 Important in this setting is the triggering role of antitumor therapies (ie, surgery, chemotherapy, radiotherapy, hormone therapy, and angiogenesis inhibitors) and of supportive therapies (ie, steroids, blood transfusion, white blood cell growth factors, and erythropoiesis-stimulating agents such as erythropoietin), which further increase the cancer-associated thrombotic risk.5,6
The interaction and relative effects of the risk factors associated with venous thromboembolism (VTE) in cancer patients is highly complex, making the pretreatment assessment of VTE difficult. Recently, a VTE risk-assessment model for patients undergoing chemotherapy was published and is based on five predictive variables in cancer patients, including cancer site, prechemotherapy platelet count, hemoglobin levels or the use of erythropoiesis-stimulating agents, prechemotherapy leukocyte count, and body mass index.7
Even in the absence of overt thrombosis, cancer patients commonly present with abnormalities in laboratory coagulation tests, underlying a subclinical hypercoagulable condition, characterized by varying degrees of blood clotting activation.5,8,9 The results of laboratory tests demonstrate that there is a continuous process of fibrin formation and lysis during the development of malignancy.10–13 The pathogenesis of thrombophilia in cancer is multifactorial. An important role is attributed to the tumor cell capacity to interact with and activate the host hemostatic system through the expression of procoagulant factors (ie, tissue factor and cancer procoagulant), the production of inflammatory cytokines (ie, interleukin-1β and tumor necrosis factor-α), and the direct adhesion to vascular cells, including platelets, endothelial cells, and monocytes.14,15
Treatment of VTE in cancer patients is challenging because of the high rates of anticoagulant-associated bleeding and treatment failures compared with patients with thrombosis and no cancer.16 According to a study of Prandoni et al, patients with cancer and VTE were approximately four times more likely to develop recurrent thromboembolic complications and twice as likely to develop major bleeding while receiving anticoagulant treatment than those without malignancy.17 Notably, cancer-associated VTE has important clinical and economic consequences, including increased morbidity consequential to hospitalization and anticoagulation use, bleeding complications, increased risk of VTE recurrences, and delays in cancer treatment.18
The standard treatment for acute VTE is an initial therapy with low-molecular-weight heparins (LMWHs), unfractionated heparin (UFH), or fondaparinux followed by long-term therapy with a vitamin K antagonist (VKA). However, this approach for long-term therapy is not highly effective in patients with cancer.17 Many aspects make problematic the administration of oral anticoagulant therapy in patients with cancer, including the frequent interruption of anticoagulant therapy due to invasive procedures and chemotherapy-induced thrombocytopenia, the difficulty of laboratory monitoring due to poor venous access, drug interactions, malnutrition, vomiting, and liver dysfunction. Altogether, these limitations can lead to unpredictable levels of anticoagulation and might be responsible for the increased risk of VTE recurrence and bleeding in cancer patients. Unlike VKA, LMWHs have predictable pharmacokinetic properties and drug interactions – the subcutaneous injection of LMWHs overcomes poor gastrointestinal absorption, and laboratory monitoring is not routinely required as the therapeutic dosage is based on the patient’s weight.19 The rapid onset of action and the predictable clearance, render LMWHs suitable for patients frequently requiring interruptions of anticoagulant therapy. For all these reasons, LMWHs have been tested versus VKA in prospective randomized clinical trials (RCTs) of efficacy in the treatment of VTE in cancer patients. The results clearly show a superiority of LMWHs, which are now the drugs of choice for initial and long-term therapy of VTE in the cancer patient.20
Overview of pharmacology and pharmacokinetics of LMWHs
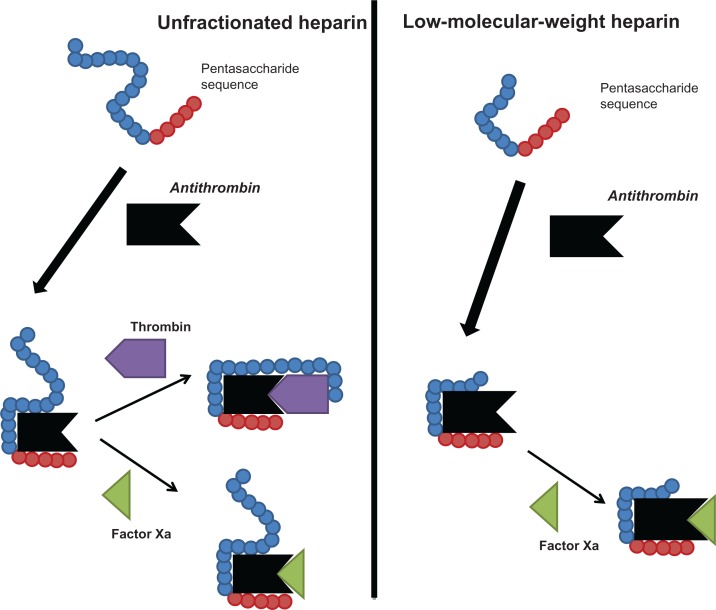
Heparin, a naturally occurring glycosaminoglycan, is a mixture of sulfated polysaccharide chains of different molecular weight. Heparins are synthesized by mast cells and distributed widely throughout the organs of mammalian species. In medicine, heparins are used as antithrombotic drugs. Heparin preparations are heterogeneous with respect to molecular size, anticoagulant activity, and pharmacokinetic properties. The molecular weight (MW) of UFH ranges from 3 to 30 kDa, with a mean MW of 15 kDa (about 45 monosaccharide chains). Heparins exert their major anticoagulant activity in blood by binding to and potentiating the activity of the natural anticoagulant antithrombin (AT) through a unique glucosamine unit contained within a pentasaccharide sequence19 (Figure 1). Only about one-third of administered UFH binds to AT, and is responsible for most of its anticoagulant effect. The heparin-AT complex inactivates a number of coagulation enzymes, including activated factor II (thrombin), factor X, factor IX, factor XI, and factor XII. Thrombin and activated factor X (FXa) are the most responsive to inhibition, and human thrombin is about tenfold more sensitive than FXa to inhibition by the heparin-AT complex. For thrombin inhibition, heparin must bind to both the coagulation enzyme and AT, whereas binding of heparin to the enzyme is not required for inhibition of FXa. The formation of a ternary complex between AT, thrombin, and heparin results in the inactivation of thrombin. For this reason, heparin’s activity against thrombin is size dependent, the ternary complex requiring at least 18 saccharide units for efficient formation. In contrast, anti-FXa activity requires only the pentasaccharide binding site. The heparin molecules with fewer than 18 saccharides lack the chain length to bridge between thrombin and AT and therefore are unable to inhibit thrombin. By inactivating thrombin, heparins not only prevent fibrin formation but also inhibit thrombin-induced activation of platelets and of factors V and VIII. In contrast, very small heparin fragments containing the high-affinity pentasaccharide sequence catalyze inhibition of FXa by AT. This size difference has led to the development of LMWHs and, more recently, to fondaparinux, a synthetic pentasaccharide, as anticoagulant drugs. LMWHs target FXa activity rather than thrombin (IIa) activity, with the aim of facilitating a more subtle regulation of coagulation and an improved therapeutic index.
Figure 1.
Anticoagulant mechanisms of unfractionated and low-molecular-weight heparins.
After administration by intravenous infusion or by subcutaneous injection, UFH binds to a variety of plasma proteins, including histidine-rich glycoprotein, platelet factor 4, vitronectin, and von Willebrand factor, thereby lowering its bioavailability and producing a variable anticoagulant response. Heparin exhibits complex pharmacokinetics and is cleared by two mechanisms. The rapid, saturable phase of elimination consists of receptor-mediated internalization of heparin by endothelial cells and macrophages, while the slower, nonsaturable mechanism is represented by renal elimination. The anticoagulant effect of heparin, therefore, is not linearly related to the dose when in the therapeutic range. The biologic half-life of heparin increases from 30 minutes following an intravenous bolus dose of 25 U/kg to 150 minutes following a bolus dose of 400 U/kg.
LMWHs can be obtained by controlled chemical, enzymatic, physical, and radiochemical depolymerization of UFH. They possess a mean MW of between 3 and 6 kDa and chain lengths of 12–18 saccharide units (Table 1). Different LMWHs show distinct structural differences and MW component distribution, which determine differences in their chemical and biological activities, particularly, different anti-FXa and anti-FIIa activities, which translates to a particular anti-FXa/anti-FIIa ratio.21
Table 1.
Primary prophylaxis in the cancer patient: recommendations
| Patient group | Recommended | Not recommended |
|---|---|---|
| Patients undergoing surgery | Prophylaxis with low-dose UFH or LMWH for at least 7–10 days Extended prophylaxis up to 4 weeks after discharge in patients with high-risk features |
Contraindication to anticoagulation: consider mechanical methods alone |
| Hospitalized patients | VTE prophylaxis with anticoagulants | Contraindication to anticoagulation |
| Ambulatory patients receiving chemotherapy | Only patients with multiple myeloma receiving thalidomide or lenalidomide prophylaxis with LMWH or adjusted dose warfarin | All other patient categories |
Abbreviations: LMWH, low-molecular-weight heparin; UFH, unfractionated heparin; VTE, venous thromboembolism.
Compared with UFH, LMWHs have an increased anti-Xa/anti-IIa activity ratio, reduced plasma protein binding, lower tendency to bind to endothelial cells,22 decreased interaction with platelets and platelet factor 4, a prolonged half-life, and increased bioavailability after subcutaneous administration. All these characteristics translate to a consistent and predictable absorption rate and bioavailability patterns. Bioavailability of LMWHs is different according to molecule features, ranging from 87% to 98%.21–22 The apparent volume of distribution of the anti-FXa activity of LMWHs following subcutaneous injection is close to plasma or blood volume; the volume of distribution of enoxaparin (5.3 L) has been shown to be significantly lower than dalteparin (7.7 L) and nadroparin (6.8 L).23
UFH and LMWHs are metabolized by depolymerization and desulfation.22,24 After being degraded by the liver, heparins are eliminated by the kidneys as metabolites, retaining their biologic activity.24 The clearance of LMWHs does not change as a function of administered dose (unlike that of UFH, which is dose dependent), which may be attributed to the lower cellular uptake of LMWHs compared with UFH.25 The average apparent total body clearance of enoxaparin has been shown to be lower than that of UFH,25 and further differences also have been observed between enoxaparin, dalteparin, and nadroparin.26
Management of VTE in cancer patients – focus on the use of LMWHs
Prophylaxis of VTE
The efficacy of prophylactic strategies to prevent VTE in at-risk hospitalized patients has been well demonstrated. For example, pharmacological prophylaxis reduces the risk of pulmonary embolism (PE) by 75% in general surgical patients27 and by 57% in medical patients.28 However, VTE prevention in cancer patients is more complicated compared with non-cancer patients, as they are prone to greater recurrence rates and a higher incidence of bleeding complications.16,17 It has been shown that cancer patients undergoing surgery benefit from effective pharmacological prophylaxis,29 and that extended duration of thromboprophylaxis with LMWHs is beneficial to patients undergoing major abdominal or pelvic surgery.30 This is reflected in current guidelines, which recommend that all cancer patients undergoing major surgery should receive heparin-based prophylaxis for a minimum of 7–10 days, with supportive mechanical prophylaxis in those patients at highest risk.31 Clinical trials have demonstrated the benefit of VTE prophylaxis either with low-dose UFH or LMWHs in hospitalized general medical patients, including patients with cancer. In contrast to hospitalized cancer patients, the primary prevention of thrombosis in ambulatory cancer patients is still debated. Although there is evidence that LMWHs are effective in reducing VTE in selected outpatients receiving chemotherapy, the optimal dose, duration, and specific patient populations remain to be defined.
Thromboprophylaxis in surgical cancer patients
The most commonly used thromboprophylaxis regimens in general surgery consist of a single preoperative dose of UFH or LMWH followed by postoperative doses every 8–24 hours. UFH is typically administered at a prophylactic dose of 5000 IU twice or three times daily. A meta-analysis of eight trials that included patients undergoing surgery for cancer showed no differences in asymptomatic deep-vein thrombosis (DVT), clinical PE, death, and major bleeding between patients administered LMWH or UFH.27 The results of these studies provide evidence that once-daily LMWH is as safe and effective as several injections of UFH per day for the prevention of postoperative DVT in oncological patients. However, the risk of VTE complications is increased in patients who undergo cancer-related surgery for at least two reasons: (1) cancer-related surgery tends to be more extensive and often involves venous trauma, and (2) there is a tendency for these patients to be immobilized for prolonged periods. In addition, cancer treatments, the use of central venous catheters, and the hypercoagulable state associated with malignancy also heightens the VTE risk for cancer patients undergoing surgery. In a subgroup analysis of the MC-4 randomized trial, over 6000 surgical patients with malignant disease receiving perioperative UFH or LMWH certoparin, were compared with 17,000 surgical patients without malignancy. In this trial, despite the use of thromboprophylaxis, the rate of fatal PE was 3.7 times higher in patients with cancer than in non-cancer patients.32 The hypothesis that a higher dose of LMWH would be associated with a lower incidence of postoperative thromboembolic complications was tested in a study of over 2000 patients undergoing elective general surgery for malignant and benign abdominal disease. In this trial, increasing the dose of the LMWH dalteparin sodium from 2500 to 5000 IU once daily was associated with a reduction in the frequency of postoperative DVT in cancer surgery from 14.9% to 8.5%, without significant increase in bleeding complications.33 Conversely, in patients without malignant disease, the reduction in postoperative DVT rate was associated with a significant increase in perioperative bleeding complications.33 In recent years, a number of trials have shown that LMWHs can reduce venographic DVT with extended out-of-hospital prophylaxis in patients undergoing major joint-replacement surgery. A meta-analysis of these trials has suggested that the rate of clinical DVT after hip replacement is also reduced with a longer treatment.34 On the basis of these results, the double-blind RCT ENOXACAN II evaluated the effect of extended prophylaxis in patients undergoing surgery for cancer. Particularly, patients undergoing planned curative open surgery for abdominal or pelvic cancer, received enoxaparin (40 mg subcutaneously) daily for 6–10 days and were then randomly assigned to receive either enoxaparin or placebo for another 21 days.30 Bilateral venography was performed at the end of treatment. There was a statistically significant reduction in DVT from 12% with placebo to 4.8% with extended prophylaxis, which persisted at 3 months of follow-up. There were no significant differences in the rates of bleeding or other complications. Similar results were provided by an open study of patients undergoing major abdominal surgery randomized to receive regular postoperative prophylaxis (7 days) or extended prophylaxis (28 days) with LMWH dalteparin.35 The results of the randomized, double-blind study CANBESURE have been published. In this study, patients admitted for abdominal or pelvic surgery for cancer received 3500 IU of bemiparin for 8 days and then were randomized to receive either bemiparin or placebo for 20 additional days. This trial did not find an advantage of 4 weeks compared with 1 week of prophylaxis with bemiparin in reducing the primary composite efficacy outcome (ie, DVT, nonfatal PE, and all-cause mortality). However, a significant decrease of major VTE (4.6% vs 0.8%, P = 0.010) was observed without concomitant increase in bleeding complications.36
Thromboprophylaxis in medical cancer patients
There are two main clinical situations in which to consider VTE prophylaxis in the medical patient with cancer: the first involves the patient who is hospitalized for an acute illness, and the second, the ambulatory patient who is receiving chemotherapy or radiation.
Hospitalized cancer patients
Clinical trials have demonstrated the benefit of VTE prophylaxis in hospitalized general medical patients. In particular, in the last few decades, the use of prophylaxis with LMWHs has been extensively explored. Although no studies have been designed ad hoc for cancer patients, different proportions of these subjects have been enrolled in the clinical trials conducted so far. The first important study was the MEDENOX study,37 a double-blind trial that randomly assigned 1102 hospitalized patients to receive 40 mg of enoxaparin, 20 mg of enoxaparin, or placebo once daily for 6–14 days. The incidence of VTE was significantly lower in patients randomized to 40 mg of enoxaparin compared with placebo (5.5% vs 14.9%; P < 0.001), while there were no significant differences between patients that received 20 mg enoxaparin or placebo. A post-hoc analysis of this study demonstrated a 50% risk reduction (95% confidence interval [CI]: 0.14–1.72) of objectively confirmed VTE (either symptomatic and asymptomatic) in the subgroup of cancer patients receiving 40 mg LMWH enoxaparin compared with placebo.38 These results were substantially confirmed by the PREVENT trial, which randomized acutely ill medical patients (n = 3706) to receive either LMWH dalteparin 5000 IU daily or placebo for 14 days and followed up for 90 days. Overall, the incidence of VTE was reduced from 4.96% in the placebo group to 2.77% in the group treated with dalteparin.39 A retrospective post-hoc analysis revealed that in the subset of cancer patients, the VTE rate fell from 8.3% with placebo to 3% with LMWH (63% risk reduction).40 A third study is the ARTEMIS trial on the efficacy and safety of 2.5 mg once-daily fondaparinux versus placebo in older acute medical inpatients at moderate to high risk of VTE.41 Of the 890 enrolled patients, about 15% of the patients had previous or current cancer. Fondaparinux reduced the incidence of VTE by 46.7% (P = 0.029 vs placebo), with a same frequency of major bleeding (0.2% in each group). Therefore, it would seem reasonable that patients with advanced malignancy who are bedridden should receive prophylaxis with either low dose UFH or LMWH.
Ambulatory cancer patients
The evidence on the primary prevention of thrombosis in ambulatory cancer patients is under investigation. The first evidence of the benefit of thromboprophylaxis in this setting came from a double-blind RCT, in which patients with metastatic breast cancer were given either very low-dose warfarin (1 mg for 6 weeks followed by an adjusted dose to a target prothrombin time international normalized ratio [INR]: of 1.3–1.9), or placebo, during chemotherapy.42 There was an 85% risk reduction in VTE rate in patients receiving warfarin, with no increase in bleeding. However, oncologists do not routinely use prophylaxis with oral anticoagulants in cancer patients receiving chemotherapy, for a series of reasons, including the concern for bleeding, an underestimation of the impact of the thrombotic complications, the logistics of laboratory monitoring, and dose adjustment in patients with cancer.
In the last decade, LMWHs, which possess many advantages over warfarin, have been tested in the ambulatory setting. The two most recent trials, conducted in patients with advanced pancreatic cancer who receive systemic chemotherapy, have shown positive results with LMWH prophylaxis. In particular, the CONKO-004 trial found a 87% risk reduction of VTE (9.9% vs 1.3%; P < 0.01) using the LMWH enoxaparin at 1 mg/kg once daily for 3 months, compared with no prophylaxis;43,44 while the FRAGEM study reported a 61% risk reduction of VTE (31% vs 12%; P = 0.02) using the CLOT20 study therapeutic scheme of LMWH dalteparin.45
Results from the CONKO-004 and FRAGEM trials are, however, in contrast with other studies evaluating LMWHs given at prophylactic doses in ambulatory cancer patients. In particular, TOPIC-1 and TOPIC-2 RCTs, conducted to evaluate the effect of LMWH certoparin prophylaxis in patients with advanced breast cancer or non-small cell lung cancer (NSCLC), respectively, did not show statistically significant reduction of VTE rate with the use of LMWHs compared with placebo.46 In the PRODIGE study, the VTE rate in patients with malignant glioma treated with prophylactic doses of LMWH dalteparin (9%) was lower compared with placebo (14.9%) but not statistically significant.47
On the basis of these contrasting evidences, it seems that standard prophylaxis doses of LMWHs may be insufficient in patients with cancer to prevent thrombosis. Nevertheless, another interpretation is that thromboprophylaxis is beneficial in only certain tumor types. Recently, the results of a large Italian study (ie, the PROTECHT study) on the efficacy of thromboprophylaxis with LMWH nadroparin in reducing the rate of VTE in ambulatory cancer patients receiving chemotherapy have been published.48 Patients (n = 1150) with metastatic or locally advanced lung, gastrointestinal, pancreatic, breast, ovarian, or head and neck cancer were randomly assigned to receive LMWH nadroparin (3850 anti-Xa UI/day) or placebo for the overall duration of chemotherapy or up to a maximum of 4 months. The results showed that 15/769 (2%) patients treated with nadroparin had thromboembolic events versus 15/381 (3.9%) patients treated with placebo. VTE accounted for 22 events, 14 of which occurred in patients with lung cancer.48 Overall, there is sound evidence that LMWHs are effective in reducing clinically important VTE in selected outpatients receiving chemotherapy, but the optimal dose, duration, and specific patient populations have to be further defined.
Recommendations
Based on the well-established VTE risk and emerging evidence showing the benefits of prophylaxis, a number of guidelines and consensus statements have been published on the use of VTE prophylaxis for cancer patients. Most noteworthy are those by the American College of Chest Physicians,49 the International Union of Angiology,50 the National Comprehensive Cancer Network,51 the Italian Association of Medical Oncology,52 the French National Federation of the League of Centers Against Cancer,53 the European Society of Medical Oncology,54 and the most recent guidelines from the American Society of Clinical Oncology.55 Table 2 summarizes the recommendations of the various guidelines in the different clinical settings. There is a broad agreement among the scientific panels on the importance of thromboprophylaxis in hospitalized patients with cancer, including prolonged prophylaxis in high-risk surgical patients. Prophylaxis is not currently recommended for ambulatory patients with cancer (with exceptions) or for central venous catheters. All of the panels agree that LMWHs are preferred for the long-term treatment of VTE in cancer. Areas that warrant further research include the benefit of prophylaxis in the ambulatory setting, the risk–benefit ratio of prophylaxis for hospitalized patients with cancer, an understanding of incidental VTE, and the impact of anticoagulation on survival.
Table 2.
Comparison of the main characteristics of the commercially available LMWH cited in the article
| LMWH | Method of depolymerization | Mean molecular weight (kDa) | Anti-Xa/anti-IIa ratio | Half-life (hours) |
|---|---|---|---|---|
| Dalteparin | Nitrous acid | 6.0 | 1.9–3.2 | 2.3–2.8 |
| Enoxaparin | Alkaline | 4.5 | 3.3–5.3 | 4.0–4.4 |
| Nadroparin | Nitrous acid | 4.3 | 2.5–4.0 | 3.7 |
| Tinzaparin | Enzymatic | 6.5 | 1.5–2.5 | 3.0 |
| Certoparin | Isoamyl nitrite | 5.6 | 2.0–2.4 | 3.5 |
| Bemiparin | Alkaline | 3.6 | 8.0 | 5.2–5.4 |
Abbreviation: LMWH, low-molecular-weight heparin.
Treatment of VTE
The standard treatment regimen for a first acute VTE episode consists of initial therapy with heparins (either UFH, LMWHs, or fondaparinux), followed by long-term therapy with a VKA agent for 3–6 months. Today, the monotherapy with LMWHs is recommended for an established VTE event in the cancer patient.56,57
Initial treatment
LMWHs are at least as efficacious as UFH in reducing recurrent thrombosis and are associated with a lower risk of major bleeding, as demonstrated by different RCTs and meta-analyses of these trials.58 Data for cancer patients are limited; however, a meta-analysis of 11 studies shows a statistically significant reduction in mortality at 3 months of follow-up with LMWH compared with UFH.59 Fondaparinux shows similar efficacy and safety as heparins for the initial treatment of VTE in the general population.60,61 However, a recently published post-hoc, subgroup analysis of the 477 cancer patients in the MATISSE DVT and PE trials suggests that fondaparinux may be less effective than LMWHs but more effective than UFH.62 There were no statistically significant differences in bleeding among these parenteral agents for initial therapy in cancer patients.59
Long-term treatment
The effects of LMWHs have been compared with those of VKAs in different RCTs of long-term VTE therapy in patients with cancer.20,63–65 The largest of these trials, the CLOT study, randomized 676 cancer patients with acute proximal DVT, PE, or both to receive treatment with the LMWH dalteparin (200 IU/kg once daily) for 5–7 days and VKA for 6 months (target INR: 2.5) or dalteparin alone for 6 months (200 IU/kg once daily for 1 month, followed by a daily dose of approximately 150 IU/kg for 5 months)20. The cumulative risk of recurrent VTE at 6 months was reduced from 17% in the VKA group to 9% in the dalteparin group, resulting in a statistically significant risk reduction of 52% (log-rank P = 0.002). Overall, there were no differences in bleeding and mortality between the two treatments. The other trials differ in design from the CLOT study, mainly in the type of LMWH (ie, enoxaparin and tinzaparin), the dose used, and/or duration of treatment (3 or 6 months). Although none of these trials, other than CLOT, demonstrated statistically significant differences between LMWHs and warfarin, there was a strong trend favoring LMWHs in all the studies. Based on published evidence, the use of LMWHs alone for the treatment of VTE in patients with cancer is endorsed by international guidelines,56,57 and the therapeutic scheme used in the CLOT study is currently the only LMWH regimen with regulatory approval for extended use in preventing recurrent VTE in cancer patients.
Treatment of recurrent thrombosis
Despite anticoagulation, up to 9% of patients with cancer-associated thrombosis treated with LMWHs or 20% treated with VKA can develop recurrent VTE. As suggested by some trials, the presence of metastasis, younger age, or a short interval between VTE and cancer diagnosis (>3 months) are predictors of recurrent thrombosis during anticoagulant treatment.66,67 Data coming from RCTs to guide optimal management in oncology patients with recurrent thrombosis are lacking. Observational data and increasing clinical experience support the use of LMWHs in this setting. In patients who developed a recurrence while on warfarin therapy, the simple increase in warfarin administration is not recommended because it is associated with an augmented bleeding risk without a benefit in reducing recurrent VTE. Therefore, the recommended practice is to switch these patients to LMWHs. In addition, dose escalation of LMWHs is often effective in patients who develop a recurrence while on therapy with this drug. In a small cohort study of cancer patients with recurrent VTE while on LMWH or warfarin, escalating the dose of LMWH by 20%–25% or switching to LMWH, respectively, was effective in preventing further thrombotic episodes.68
Impact of different LMWHs on patient-specific outcomes: focus on survival
Since the early 1980s, a beneficial effect of heparins on overall survival has been reported by several retrospective evaluations of cohorts of cancer patients enrolled in RCTs of perioperative prophylaxis with UFH versus no prophylaxis; the one conducted by Kakkar et al being the most representative study.69 Lebeau et al70 evaluated, for the first time in a prospective manner, the effect of UFH on survival in patients with small cell lung cancer (SCLC) undergoing chemotherapy. The results of this RCT showed a higher complete response rate, median survival, and survival rates in patients receiving UFH together with chemotherapy, these being statistically significant differences only in the group of patients with limited disease (P = 0.03). However, a later systematic review of all methodologically correct clinical trials in cancer patients without VTE and comparing UFH with placebo or no treatment failed to provide convincing evidence of either positive or negative effects of UFH on cancer survival.71 Meanwhile, the use of LMWHs progressively increased and therefore data about the impact of these drugs on cancer survival started to arise. In 1999, a meta-analysis of all clinical trials testing the efficacy of LMWHs versus UFH for the initial treatment of VTE showed a pooled odds ratio for 3-month mortality in cancer patients of 0.61 (95% CI: 0.40–0.93) in favor of LMWHs.72 Positive suggestions came also from the CLOT study; a post-hoc analysis of this trial found an advantage in survival in the subgroup of patients with limited disease receiving long-term LMWH dalteparin compared with warfarin.73 This indication, together with the known advantages of LMWH administration and the feasibility of long-term treatment, has prompted researchers to continue to investigate the potential role of LMWH as an antineoplastic agent. Prospective RCTs have therefore been designed to address as primary endpoint the survival of cancer patients receiving LMWHs.74 Table 3 summarizes the studies specifically designed for this purpose. The Fragmin Advanced Malignacy Outcome Study (FAMOUS)75 was a placebo-controlled RCT that examined the possible effects of the LMWH dalteparin on survival among patients with cancer without any evidence of thrombosis. Patients with different types of advanced solid malignant tumor were assigned to receive for 1 year either dalteparin (5000 IU/day, subcutaneously) or placebo along with standard cancer therapies. A statistically significant survival advantage at 2 and 3 years of randomization was found in those patients receiving dalteparin, with a relatively good prognosis at enrolment. The single-institution study by Altinbas et al revealed promising results on the benefit of a prophylactic administration of LMWH dalteparin (5000 IU/day, subcutaneously) given in combination with chemotherapy in patients with SCLC.76 The results showed an overall tumor response rate significantly higher (69.2% vs 42.5%; P = 0.07), as well as the median progression-free survival (10 months vs 6 months; P = 0.01) in patients receiving dalteparin. The randomized, placebo-controlled Malignacy and Low Molecular Weight Heparin Therapy (MALT) study evaluated the effect on survival of a therapeutic dose of the LMWH nadroparin in patients with metastatic or locally advanced solid malignancies, and no evidence of VTE.77 A modest but significant survival benefit was observed among patients treated with LMWHs, particularly in those with a life expectancy at entry of at least 6 months. In 2006, another RCT evaluated the effect on survival of the LMWH dalteparin (5000 IU) in addition to standard clinical care in patients with advanced cancer.78 No statistically significant differences were found in the median survival time for the combined standard care and placebo groups (10.5 months) compared with the combined LMWH arm (7.3 months). Kuderer et al performed the first meta-analysis and systematic review of all RCTs on the efficacy and safety of anticoagulants (LMWHs, UFH, and VKA) in the treatment of patients with cancer without VTE.79 The results obtained from eleven eligible trials show that the administration of any sort of anticoagulation significantly decreased overall 1-year mortality with a relative risk of 0.905 (95% CI: 0.85–0.97, P = 0.003 vs no anticoagulation). Interestingly, for LMWHs, the relative risk of mortality was 0.88 (95% CI: 0.79–0.98, P = 0.015), compared with a nonsignificant effect of warfarin, resulting in an absolute risk reduction of mortality of 8% for LMWHs, with also less major bleeding events in the LMWH group compared with warfarin. These findings have been confirmed in subsequent reviews.80 Since then, other studies have been published or started. In a group of patients with pancreatic carcinoma, the addition of LMWH nadroparin (2850 IU/day) to chemotherapeutic regimen (gemcitabine plus cisplatinum) significantly increased the median time to progression and survival (13.0 vs 5.5 months, P = 0.0001) for both metastatic and locally advanced carcinoma.81 However, this was a nonrandomized study performed in a very small group of patients (n = 69). In 2011, the results of the INPACT study, a multicenter, randomized, open-label study, did not show any survival benefit of nadroparin in addition to standard anticancer treatment in patients with different types of cancer (ie, NSCLC, hormone-refractory prostate cancer, or locally advanced pancreatic cancer).82 Ongoing studies on the effect of LMWHs on VTE prevention in cancer patients, including the CONKO-004,44 the FRAGEM,45 and the FRAGMATIC trials,83 incorporate as secondary objective the effect of LMWHs on cancer survival. These results are yet unknown.
Table 3.
Randomized clinical trials testing the effect of LMWHs on survival of cancer patients
| Study | Cancer type | Control | LMWH (regimen) | Effect on survivala |
|---|---|---|---|---|
| Altinbas et al76 | SCLC | None | Dalteparin (5000 IU/day, 18 weeks) | + |
| FAMOUS75 | Advanced cancer | Placebo | Dalteparin (5000 IU/day, 1 year) | +/− (+ patient with better prognosis) |
| MALT77 | Metastasized and advanced cancer | Placebo | Nadroparin (therapeutic dose 2 weeks + half dose 4 weeks) | +/− (+ patient with better prognosis) |
| Sideras et al78 | Advanced cancer | None | Dalteparin (5000 IU/day, 2 years) | + |
| INPACT82 | NSCLC, prostate, pancreatic | None | Nadroparin (therapeutic dose 2 weeks + half dose 4 weeks, weight adjusted, followed by up to six cycles) | None |
| ABEL94 | Limited SCLC | None | Bemiparin (3500 IU/day, 26 weeks) | + |
| TILT (ongoing, NCT 004775098) | NSCLC | Placebo | Tinzaparin (100 IU/kg once-daily, 12 weeks) | N/A |
Notes:
+, positive effect of LMWH on survival; N/A, results not yet available; +/−, inconclusive.
Abbreviations: LMWH, low-molecular-weight heparin; NCT, National Clinical Trial; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer.
Mechanisms of antitumor effect of heparins
The suggestions coming from clinical trials of the beneficial effects of LMWHs on survival from cancer have triggered basic research to investigate on the potential antitumor effects of LMWHs in vitro and in animal models. Several different and possibly interrelated biological mechanisms have been proposed, including both coagulation-dependent and -independent activities (Figure 2). Indeed it has been demonstrated that heparin can interfere with tumor progression by inhibiting: (1) heparin-binding growth factors that drive malignant cell growth; (2) tumor angiogenesis; (3) tumor cell heparanase that mediates tumor cell invasion and metastasis; (4) cell surface selectin-mediated tumor cell metastasis; and (5) blood coagulation activation that may provide an environment leading to tumor growth.
Figure 2.
Antitumor properties of low-molecular-weight heparins. The rationale for an antitumor effect of anticoagulant drugs may rely on their capacity to inhibit blood coagulation. However, these agents, particularly heparins, exhibit the capacity to block hemostatic pathways specifically implicated in tissue malignant behavior, and show a number of coagulation-independent activities against cancer.
Particularly, the inhibitory effects of heparins on angiogenesis have been actively investigated in both in vivo and in vitro systems.84 The molecular mechanisms involved in LMWH angiogenesis modulation include heparin binding to endothelial cells, induction of anti-angiogenic factors (such as tissue factor pathway inhibitor), and inhibition of proangiogenic factor release (such as tissue factor). Table 4 summarizes the most important studies conducted in vitro on the effects of LMWHs on endothelial cells exposed to angiogenic growth factors or tumor derived products.85–88 While it is well known that the anticoagulant properties of heparins are related to their chain length, the biochemical properties that could contribute to different in vitro antitumor efficacies are still not well elucidated. A study conducted by Khorana et al suggests that the different anti-proliferative and anti-angiogenic activities on endothelial cells of various commercial LMWHs, or purified heparin fractions, may be ultimately dependent on their mean MW and possibly by the sulfatation rate.86
Table 4.
In vitro studies exploring the effect of LMWH on tumor-induced endothelial cell angiogenesis
| LMWH | Experimental model | Anti-angiogenic effect | LMWH mechanism proposed | Reference |
|---|---|---|---|---|
| Dalteparin | TCM-stimulated HMEC-1 and HUVEC | Inhibition of endothelial cell tube formation | Interference with bFGF and VEGF binding to their receptors | Marchetti et al85 |
| bFGF-stimulated HUVEC | Inhibition of endothelial cell tube formation and proliferation | Interference with bFGF binding to its receptor | Khorana et al86 | |
| Enoxaparin | bFGF-stimulated HUVEC | Inhibition of endothelial cell tube formation and proliferation | Interference with bFGF binding to its receptor | Khorana et al86 |
| Tinzaparin | bFGF and TF/FVIIa stimulated HUVEC | Inhibition of endothelial cell tube formation | Increased release of TFPI | Mousa and Mohamed88 |
| bFGF-stimulated HUVEC | Inhibition of endothelial cell tube formation and proliferation | Interference with bFGF binding to its receptor | Khorana et al86 | |
| Bemiparin | TCM-stimulated HMEC-1 | Inhibition of endothelial cell tube formation, proliferation, and wound healing | Interference with angiogenic factor binding to their receptor, increased released of TFPI | Vignoli et al87 |
| Nadroparin | – | – | – | N/A |
| Certoparin | – | – | – | N/A |
Note: Selected works from the literature on the commercially available LMWH cited in the article.
Abbreviations: bFGF, basic fibroblast growth factor; HMEC-1, human microvascular endothelial cell line-1; HUVEC, human umbilical vein endothelial cells; LMWH, low-molecular-weight heparin; N/A, not applicable; TCM, tumor-conditioned medium; TF, tissue factor; FVIIa, activated coagulation factor VII; TFPI, tissue factor pathway inhibitor; VEGF, vascular endothelial growth factor.
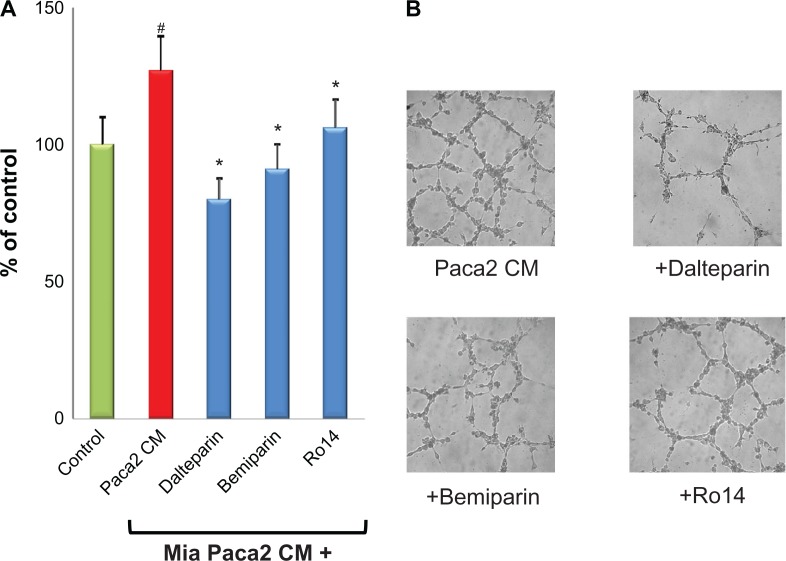
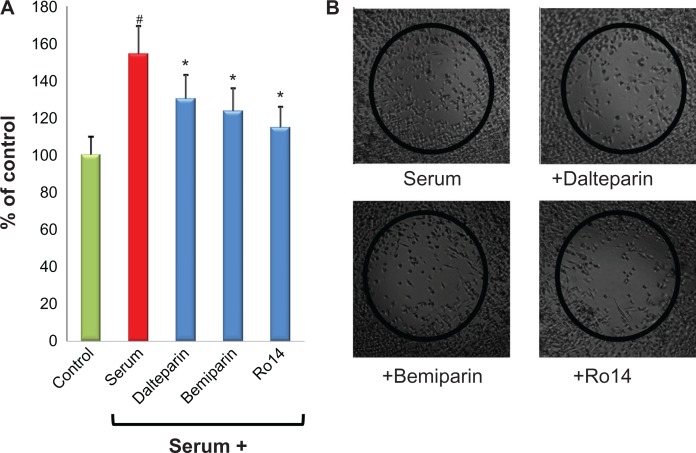
Along this line of research, we have recently demonstrated that, in an in vitro system of interaction of cancer cells with microvascular endothelial cells, two LMWHs, ie, dalteparin and enoxaparin, prevents the formation of endothelial cell capillary formation induced by breast cancer and leukemic cells, and by standard proangiogenic factors (ie, vascular endothelial growth factor and fibroblast growth factor-2).85 Very recently, a similar anti-angiogenic activity has also been described for the “second generation” LMWH bemiparin and for the ultra-LMWH RO-14.87 The authors of this present paper are currently extending their studies by exploring the in vitro antitumor effect of LMWH on pancreatic cancer. This type of tumor carries the highest risk of thrombotic events amongst any other gastrointestinal cancers, with an incidence range of 17%–57%.89 Furthermore, the diagnosis of VTE in pancreatic cancer is associated with poor overall survival.90 As shown in Figure 3, the LMWH dalteparin, bemiparin and the ultra-LMWH RO14 significantly prevented the capillary-network formation induced by a pancreatic cancer cell line. Interestingly, the anti-angiogenic effect was higher for the LMWH compared with the ultra-LMWH RO14. In addition, the same heparins showed a direct inhibitory effect on the migration of pancreatic cancer cells (Figure 4). Altogether, these in vitro data further contribute to support the evidence of a possible antitumor in vivo effect of LMWHs.
Figure 3.
LMWHs inhibit endothelial angiogenesis induced by conditioned medium from pancreatic tumor cells. Endothelial cells were incubated with TCM from a human pancreatic carcinoma (MIA Paca2) cell line, in the presence or absence of 1 IU/mL LMWH bemiparin, dalteparin, and the ultra-LMWH Ro14. After 24 hours incubation, capillary-like tube formation was evaluated as previously described.85 (A) The three heparins significantly inhibited the increase of tube formation induced by TCM. (B) Representative pictures of selected experiments showing the inhibition by the different heparins of pancreatic tumor cell-induced endothelial cell capillary-like tube.
Notes: Data are means + standard deviations of three experiments performed in duplicate. #P < 0.05 vs control; *P < 0.05 vs Paca2 CM.
Abbreviations: LMWH, low-molecular-weight heparin; TCM, tumor-conditioned medium.
Figure 4.
LMWHs inhibit the proliferation/migration of pancreatic tumor cells. The human pancreatic carcinoma (MIA Paca2) cells were grown to confluence at 37°C in a 5% CO2 atmosphere in incubator, wounded, and grown in presence or absence of 1 IU/mL LMWH bemiparin, dalteparin, and the ultra-LMWH Ro14. After 48 hours of incubation, the rate of proliferation/migration was evaluated as previously described.87 (A) Heparins significantly affect the proliferation/migration features of pancreatic cancer cells. (B) Representative pictures of selected experiments showing the inhibition by the different heparins of proliferation/migration of pancreatic tumor cells.
Notes: Data are means + standard deviations of three different experiments performed in duplicate and are expressed as percentage of regrowth area, assuming the area occupied by cells in absence of fetal calf serum as 100% of regrowth. #P < 0.05 vs control; *P < 0.05 vs serum.
Abbreviation: LMWH, low-molecular-weight heparin.
Implications for future work and enhanced patient care
Taken together, the results of the available evidence on the beneficial effect of LMWH on the survival of cancer patients are inconclusive. The majority of the studies described above included heterogeneous populations of cancer patients. Future trials should be designed to study the effect of LMWHs in specific tumor types and stages.
Moreover, since emerging evidence shows that the use of specific angiogenesis inhibitors or erythropoiesis stimulating agents91 is associated with a further increase of VTE risk in cancer patients, it would be of interest to consider ad-hoc trials to evaluate the potential beneficial role of LMWH prophylaxis on these therapeutic regimens.
Interestingly, new LMWHs characterized by a lower mean MW, and a more defined composition of polysaccharidic chain content, have been made available. These new heparins, called ultra-LMWHs, are characterized by a high anti-FXa activity and only residual anti-FIIa activity, thus the ratio anti-FXa/anti-FIIa is much greater compared with classical LMWHs. Some ultra-LMWHs are in clinical development. One of these, semuloparin, is currently being evaluated in cancer patients in the SAVE ONCO trial.92 This is a double-blind study in patients with meta-static or locally advanced cancer of lung, colon-rectum, stomach, ovary, pancreas, or bladder, initiating a new chemotherapy course. Patients are randomized to receive semuloparin, or placebo, until change of chemotherapy. The primary efficacy outcomes are the composite of any DVT-, nonfatal PE-, and VTE-related death. The results of the 3212 patients enrolled so far demonstrate a 64% risk reduction of VTE (hazard ratio: 0.36, 95% CI: 0.21–0.60, P < 0.0001 intention-to-treat analysis) with ultra-LMWH semuloparin (VTE incidence: 1.2%) compared with placebo (3.4%), with no significant difference in major bleeding (semuloparin 1.2% vs placebo 1.1%).
Conclusion
Cancer patients present a high risk of developing VTE. All efforts must be made to prevent this potentially life-threatening complication. As in noncancer patients, the first prophylactic and therapeutic approach was based on VKA, eg, warfarin. Then, UFH was increasingly used as an alternative to VKA. However, in the last few decades, there has been a progressive shift towards the use of LMWHs, which present some advantages compared with the parental drug UFH, which are now the drugs of choice for thromboprophylaxis and VTE prevention in cancer patients. Clinical data on the comparison of LMWHs is very limited. Even if the different LMWHs may show similar therapeutic profiles, it must be noted that they possess different molecular, pharmacokinetic, and functional patterns.21
Recent studies, although partially conflicting and inconclusive, suggest a beneficial impact of LMWHs on cancer patient survival, which cannot be explained with only the prevention of VTE, but may be attributable to an anticancer action of these drugs.93 Several trials are ongoing to understand which categories of cancer patients may better benefit from an adjuvant use of LMWHs, while the very recent availability of ultra-LMWHs may open even more promising perspectives.
Footnotes
Disclosure
The authors have no financial or other conflicts of interest to declare in relation to this paper.
References
- 1.Piccioli A, Prandoni P. Venous thromboembolism as first manifestation of cancer. Acta Haematol. 2001;106:13–17. doi: 10.1159/000046584. [DOI] [PubMed] [Google Scholar]
- 2.Prandoni P, Lensing AW, Buller HR, et al. Deep-vein thrombosis and the incidence of subsequent symptomatic cancer. N Engl J Med. 1992;327:1128–1133. doi: 10.1056/NEJM199210153271604. [DOI] [PubMed] [Google Scholar]
- 3.Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NM, Lyman GH. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006;24:484–490. doi: 10.1200/JCO.2005.03.8877. [DOI] [PubMed] [Google Scholar]
- 4.Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104:2822–2829. doi: 10.1002/cncr.21496. [DOI] [PubMed] [Google Scholar]
- 5.Rickles FR, Levine MN. Epidemiology of thrombosis in cancer. Acta Haematol. 2001;106:6–12. doi: 10.1159/000046583. [DOI] [PubMed] [Google Scholar]
- 6.Falanga A, Rickles FR. Management of thrombohemorrhagic syndromes (THS) in hematologic malignancies. Hematology Am Soc Hematol Educ Program. 2007:165–171. doi: 10.1182/asheducation-2007.1.165. [DOI] [PubMed] [Google Scholar]
- 7.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falanga A, Barbui T, Rickles FR, Levine MN. Guidelines for clotting studies in cancer patients. For the Scientific and Standardization Committee of the Subcommittee on Haemostasis and Malignancy International Society of Thrombosis and Haemostasis. Thromb Haemost. 1993;70:540–542. [PubMed] [Google Scholar]
- 9.Falanga A, Donati MB. Pathogenesis of thrombosis in patients with malignancy. Int J Hematol. 2001;73:137–144. doi: 10.1007/BF02981929. [DOI] [PubMed] [Google Scholar]
- 10.Falanga A, Iacoviello L, Evangelista V, et al. Loss of blast cell procoagulant activity and improvement of hemostatic variables in patients with acute promyelocytic leukemia administered all-trans-retinoic acid. Blood. 1995;86:1072–1081. [PubMed] [Google Scholar]
- 11.Falanga A, Levine MN, Consonni R, et al. The effect of very-low-dose warfarin on markers of hypercoagulation in metastatic breast cancer: results from a randomized trial. Thromb Haemost. 1998;79:23–27. [PubMed] [Google Scholar]
- 12.Seitz R, Rappe N, Kraus M, et al. Activation of coagulation and fibrinolysis in patients with lung cancer: relation to tumour stage and prognosis. Blood Coagul Fibrinolysis. 1993;4:249–254. doi: 10.1097/00001721-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Wada H, Sase T, Yamaguchi M. Hypercoagulant states in malignant lymphoma. Exp Oncol. 2005;27:179–185. [PubMed] [Google Scholar]
- 14.Falanga A, Marchetti M, Vignoli A, Balducci D. Clotting mechanisms and cancer: implications in thrombus formation and tumor progression. Clin Adv Hematol Oncol. 2003;1:673–678. [PubMed] [Google Scholar]
- 15.Falanga A, Panova-Noeva M, Russo L. Procoagulant mechanisms in tumour cells. Best Pract Res Clin Haematol. 2009;22:49–60. doi: 10.1016/j.beha.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Hutten BA, Prins MH, Gent M, Ginsberg J, Tijssen JG, Buller HR. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: a retrospective analysis. J Clin Oncol. 2000;18:3078–3083. doi: 10.1200/JCO.2000.18.17.3078. [DOI] [PubMed] [Google Scholar]
- 17.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 18.Khorana AA. Cancer and thrombosis: implications of published guidelines for clinical practice. Ann Oncol. 2009;20:1619–1630. doi: 10.1093/annonc/mdp068. [DOI] [PubMed] [Google Scholar]
- 19.Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119:64S–94S. doi: 10.1378/chest.119.1_suppl.64s. [DOI] [PubMed] [Google Scholar]
- 20.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 21.Fareed J, Hoppensteadt D, Schultz C, et al. Biochemical and pharmacologic heterogeneity in low molecular weight heparins. Impact on the therapeutic profile. Curr Pharm Des. 2004;10:983–999. doi: 10.2174/1381612043452811. [DOI] [PubMed] [Google Scholar]
- 22.Samama MM, Gerotziafas GT. Comparative pharmacokinetics of LMWHs. Semin Thromb Hemost. 2000;26(Suppl 1):31–38. doi: 10.1055/s-2000-9497. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson BI, Soderberg K, Widlund L, Wandeli B, Tengborn L, Risberg B. A comparative study of three low-molecular weight heparins (LMWH) and unfractionated heparin (UH) in healthy volunteers. Thromb Haemost. 1995;73:398–401. [PubMed] [Google Scholar]
- 24.Frydman A. Low-molecular-weight heparins: an overview of their pharmacodynamics, pharmacokinetics and metabolism in humans. Haemostasis. 1996;26(Suppl 2):24–38. doi: 10.1159/000217270. [DOI] [PubMed] [Google Scholar]
- 25.Bara L, Samama M. Pharmacokinetics of low molecular weight heparins. Acta Chir Scand Suppl. 1988;543:65–72. [PubMed] [Google Scholar]
- 26.Collignon F, Frydman A, Caplain H, et al. Comparison of the pharmacokinetic profiles of three low molecular mass heparins – dalteparin, enoxaparin and nadroparin – administered subcutaneously in healthy volunteers (doses for prevention of thromboembolism) Thromb Haemost. 1995;73:630–640. [PubMed] [Google Scholar]
- 27.Mismetti P, Laporte S, Darmon JY, Buchmuller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88:913–930. doi: 10.1046/j.0007-1323.2001.01800.x. [DOI] [PubMed] [Google Scholar]
- 28.Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146:278–288. doi: 10.7326/0003-4819-146-4-200702200-00007. [DOI] [PubMed] [Google Scholar]
- 29.Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double-blind randomized multicentre trial with venographic assessment. ENOXACAN Study Group. Br J Surg. 1997;84:1099–1103. [PubMed] [Google Scholar]
- 30.Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–980. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- 31.Khorana AA, Streiff MB, Farge D, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009;27:4919–4926. doi: 10.1200/JCO.2009.22.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kakkar AK, Haas S, Wolf H, Encke A. Evaluation of perioperative fatal pulmonary embolism and death in cancer surgical patients: the MC-4 cancer substudy. Thrombos Haemost. 2005;94:867–871. doi: 10.1160/TH04-03-0189. [DOI] [PubMed] [Google Scholar]
- 33.Bergqvist D, Burmark US, Flordal PA, et al. Low molecular weight heparin started before surgery as prophylaxis against deep vein thrombosis: 2500 versus 5000 XaI units in 2070 patients. Br J Surg. 1995;82:496–501. doi: 10.1002/bjs.1800820421. [DOI] [PubMed] [Google Scholar]
- 34.Hull RD, Pineo GF, Stein PD, et al. Extended out-of-hospital low-molecular-weight heparin prophylaxis against deep venous thrombosis in patients after elective hip arthroplasty: a systematic review. Ann Intern Med. 2001;135:858–869. doi: 10.7326/0003-4819-135-10-200111200-00006. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen MS, Jorgensen LN, Wille-Jorgensen P, et al. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: a multicenter randomized open-label study. J Thromb Haemost. 2006;4:2384–2390. doi: 10.1111/j.1538-7836.2006.02153.x. [DOI] [PubMed] [Google Scholar]
- 36.Kakkar VV, Balibrea JL, Martinez-Gonzalez J, Prandoni P. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost. 2010;8:1223–1229. doi: 10.1111/j.1538-7836.2010.03892.x. [DOI] [PubMed] [Google Scholar]
- 37.Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341:793–800. doi: 10.1056/NEJM199909093411103. [DOI] [PubMed] [Google Scholar]
- 38.Alikhan R, Cohen AT, Combe S, et al. Prevention of venous thromboembolism in medical patients with enoxaparin: a subgroup analysis of the MEDENOX study. Blood Coagul Fibrinolysis. 2003;14:341–346. doi: 10.1097/00001721-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874–879. doi: 10.1161/01.CIR.0000138928.83266.24. [DOI] [PubMed] [Google Scholar]
- 40.Cohen AT, Turpie AG, Leizorovicz A, Olsson CG, Vaitkus PT, Goldhaber SZ. Thromboprophylaxis with dalteparin in medical patients: which patients benefit? Vasc Med. 2007;12:123–127. doi: 10.1177/1358863X07079017. [DOI] [PubMed] [Google Scholar]
- 41.Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332:325–329. doi: 10.1136/bmj.38733.466748.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine M, Hirsh J, Gent M, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet. 1994;343:886–889. doi: 10.1016/s0140-6736(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 43.Riess H, Pelzer U, Deutschinoff G, et al. A prospective, randomized trial of chemotherapy with or without the low molecular weight heparin (LMWH) enoxaparin in patients (pts) with advanced pancreatic cancer (APC): results of the CONKO 004 trial [abstract] J Clin Oncol. 2009;27 abst LBA4506. [Google Scholar]
- 44.Riess H, Pelzer U, Hilbig A, et al. Rationale and design of PROSPECTCONKO 004: a prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy. BMC Cancer. 2008;8:361. doi: 10.1186/1471-2407-8-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maraveyas A, Waters J, Roy R, et al. Gemcitabine with or without prophylactic weight-adjusted dalteparin in patients with advanced or metastatic pancreatic cancer (APC): a multicentre, randomised phase IIB trial (the UK FRAGEM study) Eur J Cancer Suppl. 2009;7:362. [Google Scholar]
- 46.Haas SK, Kakkar AK, Kemkes-Matthes B, et al. Prevention of Venous Thromboembolism with low-molecular-weight heparin in patients with metastatic breast or lung cancer – results of the TOPIC studies [abstract] J Thromb Haemost. 2005;3 abst OR059. [Google Scholar]
- 47.Perry JR, Julian JA, Laperriere NJ, et al. PRODIGE: a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J Thromb Haemost. 2010;8:1959–1965. doi: 10.1111/j.1538-7836.2010.03973.x. [DOI] [PubMed] [Google Scholar]
- 48.Agnelli G, Gussoni G, Bianchini C, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10:943–949. doi: 10.1016/S1470-2045(09)70232-3. [DOI] [PubMed] [Google Scholar]
- 49.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 50.Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence) Int Angiol. 2006;25:101–161. [PubMed] [Google Scholar]
- 51.Streiff MB. The National Comprehensive Cancer Center Network (NCCN) guidelines on the management of venous thromboembolism in cancer patients. Thromb Res. 2010;125(Suppl 2):S128–S133. doi: 10.1016/S0049-3848(10)70030-X. [DOI] [PubMed] [Google Scholar]
- 52.Mandala M, Falanga A, Piccioli A, et al. Venous thromboembolism and cancer: guidelines of the Italian Association of Medical Oncology (AIOM) Crit Rev Oncol Hematol. 2006;59:194–204. doi: 10.1016/j.critrevonc.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Farge D, Bosquet L, Kassab-Chahmi D, et al. 2008 French national guidelines for the treatment of venous thromboembolism in patients with cancer: report from the working group. Crit Rev Oncol Hematol. 2010;73:31–46. doi: 10.1016/j.critrevonc.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Mandala M, Falanga A, Roila F. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guidelines for the management. Ann Oncol. 2010;21(Suppl 5):v274–v276. doi: 10.1093/annonc/mdq199. [DOI] [PubMed] [Google Scholar]
- 55.Lyman GH, Kuderer NM. Prevention and treatment of venous thromboembolism among patients with cancer: the American Society of Clinical Oncology Guidelines. Thromb Res. 2010;125(Suppl 2):S120–S127. doi: 10.1016/S0049-3848(10)70029-3. [DOI] [PubMed] [Google Scholar]
- 56.Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–5505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 57.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed) Chest. 2008;133:454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 58.Van Dongen CJ, van den Belt AG, Prins MH, Lensing AW. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev. 2004;(4):CD001100. doi: 10.1002/14651858.CD001100.pub2. [DOI] [PubMed] [Google Scholar]
- 59.Akl EA, Vasireddi SR, Gunukula S, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2011;(6):CD006649. doi: 10.1002/14651858.CD006649.pub4. [DOI] [PubMed] [Google Scholar]
- 60.Buller HR, Davidson BL, Decousus H, et al. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. New Engl J Med. 2003;349:1695–1702. doi: 10.1056/NEJMoa035451. [DOI] [PubMed] [Google Scholar]
- 61.Buller HR, Davidson BL, Decousus H, et al. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med. 2004;140:867–873. doi: 10.7326/0003-4819-140-11-200406010-00007. [DOI] [PubMed] [Google Scholar]
- 62.Van Doormaal FF, Raskob GE, Davidson BL, et al. Treatment of venous thromboembolism in patients with cancer: subgroup analysis of the Matisse clinical trials. Thromb Haemost. 2009;101:762–769. [PubMed] [Google Scholar]
- 63.Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729–1735. doi: 10.1001/archinte.162.15.1729. [DOI] [PubMed] [Google Scholar]
- 64.Deitcher SR, Kessler CM, Merli G, Rigas JR, Lyons RM, Fareed J. Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clin Appl Thromb Hemost. 2006;12:389–396. doi: 10.1177/1076029606293692. [DOI] [PubMed] [Google Scholar]
- 65.Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119:1062–1072. doi: 10.1016/j.amjmed.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 66.Trujillo-Santos J, Nieto JA, Tiberio G, et al. Predicting recurrences or major bleeding in cancer patients with venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008;100:435–439. [PubMed] [Google Scholar]
- 67.Lee AY, Parpia S, Julian J, Rickles FR, Prins M, Levine M. Risk factors for recurrent thrombosis and anticoagulant – related bleeding in cancer patients [abstract] J Thromb Haemost. 2009;7 abst 107. [Google Scholar]
- 68.Carrier M, Le Gal G, Cho R, Tierney S, Rodger M, Lee AY. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost. 2009;7:760–765. doi: 10.1111/j.1538-7836.2009.03326.x. [DOI] [PubMed] [Google Scholar]
- 69.Kakkar A, Hedges R, Williamson R, Kakkar V. Perioperative heparin-therapy inhibits late death from metastatic cancer. Int J Oncol. 1995;6:885–888. doi: 10.3892/ijo.6.4.885. [DOI] [PubMed] [Google Scholar]
- 70.Lebeau B, Chastang C, Brechot JM, et al. Subcutaneous heparin treatment increases survival in small cell lung cancer. “Petites Cellules” Group. Cancer. 1994;74:38–45. doi: 10.1002/1097-0142(19940701)74:1<38::aid-cncr2820740108>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 71.Smorenburg SM, Hettiarachchi RJ, Vink R, Buller HR. The effects of unfractionated heparin on survival in patients with malignancy – a systematic review. Thromb Haemost. 1999;82:1600–1604. [PubMed] [Google Scholar]
- 72.Hettiarachchi RJ, Smorenburg SM, Ginsberg J, Levine M, Prins MH, Buller HR. Do heparins do more than just treat thrombosis? The influence of heparins on cancer spread. Thromb Haemost. 1999;82:947–952. [PubMed] [Google Scholar]
- 73.Lee AY, Rickles FR, Julian JA, et al. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol. 2005;23:2123–2129. doi: 10.1200/JCO.2005.03.133. [DOI] [PubMed] [Google Scholar]
- 74.Falanga A. The effect of anticoagulant drugs on cancer. J Thromb Haemost. 2004;2:1263–1265. doi: 10.1111/j.1538-7836.2004.00868.x. [DOI] [PubMed] [Google Scholar]
- 75.Kakkar AK, Levine MN, Kadziola Z, et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS) J Clin Oncol. 2004;22:1944–1948. doi: 10.1200/JCO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 76.Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266–1271. doi: 10.1111/j.1538-7836.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- 77.Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130–2135. doi: 10.1200/JCO.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 78.Sideras K, Schaefer PL, Okuno SH, et al. Low-molecular-weight heparin in patients with advanced cancer: a phase 3 clinical trial. Mayo Clin Proc. 2006;81:758–767. doi: 10.4065/81.6.758. [DOI] [PubMed] [Google Scholar]
- 79.Kuderer NM, Khorana AA, Lyman GH, Francis CW. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment: impact on survival and bleeding complications. Cancer. 2007;110:1149–1161. doi: 10.1002/cncr.22892. [DOI] [PubMed] [Google Scholar]
- 80.Lazo-Langner A, Goss GD, Spaans JN, Rodger MA. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J Thromb Haemost. 2007;5:729–737. doi: 10.1111/j.1538-7836.2007.02427.x. [DOI] [PubMed] [Google Scholar]
- 81.Icli F, Akbulut H, Utkan G, et al. Low molecular weight heparin (LMWH) increases the efficacy of cisplatinum plus gemcitabine combination in advanced pancreatic cancer. J Surg Oncol. 2007;95:507–512. doi: 10.1002/jso.20728. [DOI] [PubMed] [Google Scholar]
- 82.Van Doormaal FF, Di Nisio M, Otten HM, Richel DJ, Prins M, Buller HR. Randomized trial of the effect of the low molecular weight heparin nadroparin on survival in patients with cancer. J Clin Oncol. 2011;29:2071–2076. doi: 10.1200/JCO.2010.31.9293. [DOI] [PubMed] [Google Scholar]
- 83.Griffiths GO, Burns S, Noble SI, Macbeth FR, Cohen D, Maughan TS. FRAGMATIC: a randomised phase III clinical trial investigating the effect of fragmin added to standard therapy in patients with lung cancer. BMC Cancer. 2009;9:355. doi: 10.1186/1471-2407-9-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Norrby K. Low-molecular-weight heparins and angiogenesis. APMIS. 2006;114:79–102. doi: 10.1111/j.1600-0463.2006.apm_235.x. [DOI] [PubMed] [Google Scholar]
- 85.Marchetti M, Vignoli A, Russo L, et al. Endothelial capillary tube formation and cell proliferation induced by tumor cells are affected by low molecular weight heparins and unfractionated heparin. Thromb Res. 2008;121:637–645. doi: 10.1016/j.thromres.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 86.Khorana AA, Sahni A, Altland OD, Francis CW. Heparin inhibition of endothelial cell proliferation and organization is dependent on molecular weight. Arterioscler Thromb Vasc Biol. 2003;23:2110–2115. doi: 10.1161/01.ATV.0000090671.56682.D7. [DOI] [PubMed] [Google Scholar]
- 87.Vignoli A, Marchetti M, Russo L, et al. LMWH bemiparin and ULMWH RO-14 reduce the endothelial angiogenic features elicited by leukemia, lung cancer, or breast cancer cells. Cancer Invest. 2011;29:153–161. doi: 10.3109/07357907.2010.543217. [DOI] [PubMed] [Google Scholar]
- 88.Mousa SA, Mohamed S. Inhibition of endothelial cell tube formation by the low molecular weight heparin, tinzaparin, is mediated by tissue factor pathway inhibitor. Thromb Haemost. 2004;92:627–633. doi: 10.1160/TH04-02-0069. [DOI] [PubMed] [Google Scholar]
- 89.Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004;5:655–663. doi: 10.1016/S1470-2045(04)01606-7. [DOI] [PubMed] [Google Scholar]
- 90.Chew HK, Wun T, Harvey DJ, Zhou H, White RH. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007;25:70–76. doi: 10.1200/JCO.2006.07.4393. [DOI] [PubMed] [Google Scholar]
- 91.Barbera L, Thomas G. Erythropoiesis stimulating agents, thrombosis and cancer. Radiother Oncol. 2010;95:269–276. doi: 10.1016/j.radonc.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 92.Agnelli G, George DJ, Fisher W, et al. The ultra-low molecualr weight heparin (ULMWH) semuloparin for prevention of venous thromboembolism (VTE) in patients with cancer receiving chemotherapy : SAVE ONCO study [abstract] J Clin Oncol. 2011;29 abst LBA9014. [Google Scholar]
- 93.Falanga A, Marchetti M. Heparin in tumor progression and metastatic dissemination. Semin Thromb Hemost. 2007;33:688–694. doi: 10.1055/s-2007-991536. [DOI] [PubMed] [Google Scholar]
- 94.Lecumberri R, Massuti B, Lopez Vivanco G, Font A, Gonzalez Billalabeitia E, Rocha Eobot AI. Adjuvant bemiparin in small cell lung cancer: results from the ABEL study [abstract] Thromb Res. 2010;125(Suppl 2) doi: 10.1016/j.thromres.2013.09.026. abst S163. [DOI] [PubMed] [Google Scholar]