Abstract
Purpose
We measured dynamic biomechanics of loss-of-resistance (LOR) epidural placement in prone cadavers, focussing on the period immediately following LOR, to estimate forces acting on the tissue of the epidural space.
Methods
An epidural syringe with 17G Hustead needle was instrumented to track force on the plunger, pressure in the chamber, and movement of barrel and plunger. Insertions were attempted in five formalin-preserved cadavers from T2–3 to L4–5, using LOR with saline or air, and confirmed with X-ray.
Results
Sixteen insertions were successful. Soft tissues in formalin-preserved cadavers are much harder than in living humans. With continuous pressure on the plunger, fluid thrust through the needle at the point of LOR was significantly greater (P = 0.005) with saline (mean ± standard deviation [95% confidence intervals]: 19.3 ± 14.9 [8.3 to 30.3] N); than with air (0.17 ± 0.25 [0 to 0.39] N). Stress exerted on epidural tissue was similar (air = 7792 ± 920 [6986 to 8598] Pa; saline = 7378 ± 3019 [5141 to 9614] Pa); and in both cases was greater than the stress exerted by cerebrospinal fluid pushing outwardly on the dura (4800 Pa).
Conclusion
Formalin-preserved cadavers are too stiff to make them an experimental model from which we can generalize to live humans, although we were successful in entering the epidural space and testing the instrumentation for further studies on live animals or humans. Continuous pressure on the plunger while advancing the epidural needle may “blow” the dura away from the needle tip and help prevent dural puncture. Better results are seen with saline rather than air.
Keywords: epidural anesthesia, cadaver, spinal headache
Introduction
Epidural analgesia is frequently used for relief of pain. Risks include infection, hematoma, and nerve damage;1–3 systemic toxicity, intrathecal drug injection, and backache; and dural puncture (DP) resulting in a spinal headache.4 Of these, only backache (controversial) and DP are common.5–7 Avoiding DP requires having the needle enter the epidural space and advance no further. This is commonly done by various means of sensing the loss-of-resistance (LOR) to injection as the needle tip enters the epidural space. Surprisingly little is known about the biomechanics of epidural insertion, although epidural analgesia and anesthesia using LOR have been practiced since 1921.8,9 This pilot study explores the use of formalin-preserved cadavers for studying epidural biomechanics and the relationship between forces and motion of the syringe, needle, and contents at the moment of LOR.
The usual LOR technique involves attaching a syringe to the epidural needle and attempting to inject small amounts of air or saline as the needle is advanced, although other LOR techniques have been described.10 When the epidural space is entered, there is a sudden decreased resistance to injection and the air or saline is easily discharged from the syringe.
LOR techniques may be classified by the amount and pattern of pressure in the advancing syringe, with three techniques forming a continuum. Some practitioners use a “low pressure technique” that consists of repeatedly advancing the needle one or two millimetres without any force on the plunger, followed by testing LOR by pushing on the plunger. The needle tip thus enters the epidural space with the LOR fluid at atmospheric pressure. An “intermediate pressure technique” advances the needle while always keeping some positive pressure in the fluid by simultaneously pushing on the plunger with a force insufficient in itself to advance the needle. A major textbook, Miller’s Anesthesia,11 recommends this intermediate pressure technique, and it is the theme of this experiment. At the other end of the spectrum is a rarely-used “high pressure technique”, with solely the force of the thumb on the plunger used to advance the plunger, needle, syringe, and all. With this technique, when the needle breaks through the ligamentum flavum, the needle stops, but the plunger continues to advance, with rapid injection of fluid into the epidural space.
It is helpful to understand the following two terms: a) Fluid thrust – the force produced by injecting a jet of fluid into another fluid, or into empty space. This is the product of flow × velocity × density. Thrust can cause movement of liquid at a distance from the needle; and b) Stress – if the needle opening at LOR encounters an impervious solid, then the force pushing the plunger is transmitted to the solid. This stress pushing the solid tissue away from the needle tip is traditionally expressed in units of pressure and can result in strain, the term for the deformation of the material subjected to a mechanical stress that depends on the properties of that material.
The epidural space consists of loose areolar tissue. It shares some of the mechanical properties of poroelastic tissue,12 but has additional properties that are more complex because it has viscoelastic solid (fibrous bands, cells, blood vessels), semi-solid (fat stores), gel (cytosol), and liquid (interstitial fluid, blood) components. These result in a complicated mechanical materials-modeling problem that has elements of the behaviours of liquid, gel, porous matrix, elastic-deformable, and plastic-deformable materials.
We can simplify the model if we picture the epidural tissue as a three-dimensional web of viscoelastic connective tissue fibres with water-saturated spaces between them. Then the injection at the moment of LOR behaves like some combination of injecting into a liquid that absorbs and incorporates the injected fluid (air or saline), and injecting against the solid fibres. Which mechanism predominates depends upon how tightly packed are the fibres. Thus the epidural tissue response to injection lies somewhere along a spectrum that at one extreme is like a bag of liquid (very few fibres in the web), and at the other extreme like an impervious flexible solid (very closely packed fibres in the web). Injection into liquid is studied by measuring thrust of the jet of LOR fluid. Injection directly against an impervious solid is studied by measuring stress (the pressure pushing on the tissue surface) and strain (the deformation of the solid). The ratio of interstitial liquid to solid matrix determines the relative influence of thrust versus strain in moving epidural tissue.
To distinguish which of these extremes most closely models the epidural tissue, we can study the rate of pressure dissipation of injectate, comparing air with saline. Pressure dissipation after injection is inversely related to closeness of the fibres and to viscosity of the injected fluid. In mainly liquid tissue with very few fibres, air and saline both result in rapid pressure dissipation. As the fibrous matrix is more tightly woven, dissipation is slower, and the difference in dissipation rate between air (lower viscosity) and saline (higher viscosity) increases. Finally, if the epidural tissue is so tightly woven as to be impervious, a slowly-dissipating bolus of injected fluid (air faster than saline) would form at the end of the needle, producing a mechanical stress causing a deforming strain on the epidural tissue. The early post-injection pressure in this bubble would be similar for air and saline.
Clearly neither of these extremes accurately models the properties of epidural tissue. However, if both models (the thrust of injection into liquid, and the stress of injection against a solid) show that pressure on the plunger could push the dura away from the needle tip, and possibly prevent DP, then further experiments to study the properties of the tissues; the movement of the dura; and the epidemiology of dural puncture using various techniques; etc are warranted.
Hypotheses
A cadaver experiment can provide useful exploration of measurement of the mechanics of epidural needle placement using LOR and provide insights into instrumentation for further studies in living subjects;
Advancement of the epidural needle with continuous pressure during epidural placement will produce thrust and/or strain sufficient to displace the dura away from the needle tip;
The mechanics of LOR with air will differ from those using saline with respect to fluid thrust because of different densities, and with respect to the time for pressure to dissipate because of different viscosities.
Method
Subjects
With ethical permission of the University of Saskatchewan Department of Anatomy, a convenience sample of five formalin-preserved cadavers was chosen without regard to diagnosis, age at death, cause of death, or time since death.
Equipment
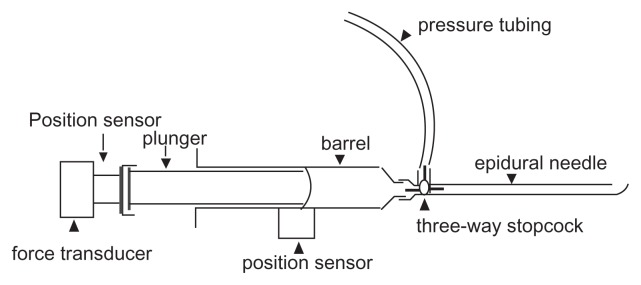
The basic setup is shown in Figure 1. Position sensors to measure motion were attached to the plunger and barrel of the syringe. A force transducer was attached to the plunger handle and the pressure at the needle tip was monitored with a pressure transducer via a three-way stopcock.
Figure 1.
Instrumented syringe.
Position sensors (Polhemus Patriot model, Polhemus Ltd, Colchester, VT) were screwed into the end of the plunger and attached to the side of the barrel with tape. Position sensor output was sampled at 60 Hz using Polhemus proprietary software.
The force transducer was a factory-calibrated Interface SML-100 (100 lbf = 10V) powered by an SGA Strain Gauge Amplifier (Interface Inc, Scottsdale, AZ). The amplifier provides pre-filtering of the signal at 0 to 100 Hz. The pressure transducer was a clinical Transpac IV disposable transducer (Hospira Inc, Lake Forest, IL) amplified by a Hewlett Packard M1006A amplifier and M-series hospital monitor (Hewlett- Packard Co, Palo Alto, CA). The pressure transducer/recording system was calibrated against a mercury manometer (R2 = 0.99; P < 0.001).
Force and pressure were sampled at 50 Hz using a National Instruments DAQPad-6020E (National Instruments Corporation, Austin, TX) attached to a laptop computer with National Instruments Biobench V1.2 software. Digitized signals were analyzed off-line on a PC computer.
Hustead epidural needles were 17G TW (thin wall), with inside diameters of 1.2 (1.1811 to 1.2319) mm as per the standard hypodermic sizes (AISI 304 stainless steel) tubing chart (http://www.connhypo.com/pages/sub05_toc01.html [cited 2009/10/29]). The plastic syringes used for LOR (Portex “Pulsator LOR”) were those used routinely in our hospital and included in a commercial kit (Portex Continuous Epidural A3624-17; Smith Medical ASD Inc, Keene, NH). Inside diameter of the barrel is 15 mm and 1.77 mL is injected for each 1 cm movement of the plunger in the barrel.
With subjects prone on a plywood board, in order to minimize ferro-metal interference effects on the position sensors, the Polhemis Receiver was taped onto the sacrum (wrapped in a plastic glove). The X-ray machine (C-arm type) was rolled away from the body during epidural injection, also to minimize ferrometal effects. On-site calibration of the position sensors with a ruler showed no ferrometal interference using this setup. Needle placement in the epidural space was confirmed by X-rays, using 2 to 3 mL of iodinated contrast medium (Optiray 320; Tyco Health Care, Montreal, Quebec, Canada), read by the principle author, a radiologist.13,14
Procedures
Each cadaver had as many as possible of the interspinous thoracic (T) and lumbar (L) levels (T2–3 to L4–5) studied. Midline epidural insertion, randomized to LOR of 5 mL air or saline, was attempted at all thoracic and lumbar levels below T2 by two experienced practitioners (study authors). The needle was inserted only once at any site. There are no studies to determine either optimal or usual force on the plunger for the intermediate pressure technique. Therefore, rather than trying to achieve a pre-determined pressure by watching the monitor, the force that was applied to the plunger for the intermediate pressure technique was the force that felt right clinically.
Analysis
The following variables were measured or calculated: force on the plunger; pressure in the barrel; motion of the needle with respect to the cadaver; motion of the plunger with respect to the barrel; velocity of the injected fluid in the needle; and thrust and stress of the injected fluid on the epidural tissue.
Thrust acting on the epidural tissue immediately following LOR was calculated with data from relative barrel and plunger velocity knowing the diameters of barrel and needle. Approximate density of dry room-temperature air was used. At 20°C and 101.3 kPa, dry air has a density of 1.2 kg.m−3, while the density of normal saline at room temperature is 1034 kg.m.15,16
Stress against solid tissue at LOR was calculated by subtracting the epidural pressure from the pressure just prior to entry.
Formulae for plotting a) the motion of plunger displacement (the distance the plunger and barrel sensors are from each other at each sample-time [dp]); and b) the motion of displacement of the syringe and needle relative to the cadaver (dn) are presented below:
| (a) |
where 1 and 2 are the position sensors and x1n, x2n, y1n, y2n, z1n, and z2n are sample-numbers of x, y, and z axes, from 1 to n.
| (b) |
Saline velocity in the needle is equal to the velocity of the plunger relative to the barrel, multiplied by the inverse ratio of their areas. Flow was calculated by finding the rate of change in volume in the barrel, with respect to time, where 1 cm of plunger movement within the barrel is equivalent to a 1.77 mL volume change.
Statistical analysis
Continuous variables were compared with t-tests using Sigmastat version 3.11 (Systat Software, Inc. Chicago, IL). Corrections for multiple comparisons were not made because this is an exploratory study.17
Results
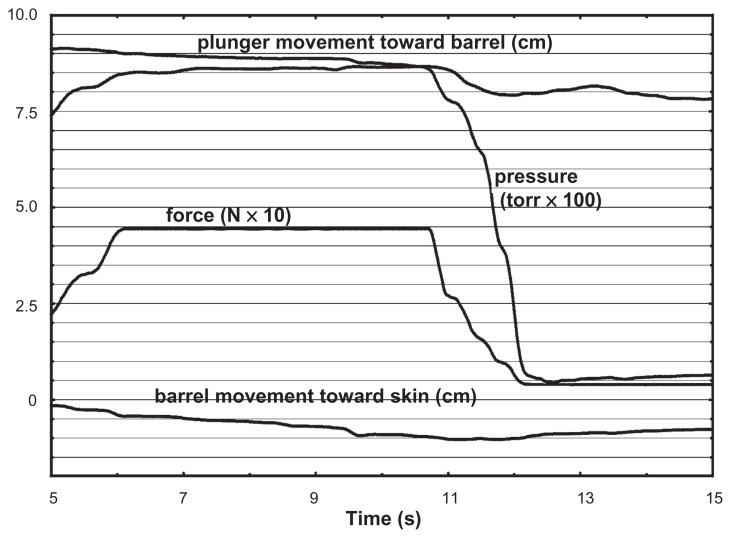
In five formalin-fixed cadavers (aged 61 to 96 years; two female, three male), X-ray proven needle insertion into the epidural space was achieved 16 times out of 70 possible insertions. For one insertion, technical problems with recording were encountered, leaving 15 satisfactory biomechanical recordings. Of these, seven used LOR of air and eight used saline. Figure 2 shows a typical recording.
Figure 2.
Typical recording: variables vs time.
Although we did not measure the force used to advance the needle, the soft tissues of the formalin-preserved cadavers were clearly much harder than those of living humans, often requiring all the force we could muster to advance the needle through skin and interspinous ligaments. This resulted in four dural punctures, far more than would be expected from experienced practitioners in living subjects.
Biomechanical measures are summarized in Table 1. The injecting force applied to the plunger; the fluid flow in the needle; and the velocity and stress of the fluid at the needle tip were not significantly different for saline compared to air. Thrust was two orders of magnitude greater for saline than for air (P = 0.005), and time for dissipation of pressure following LOR was three times greater for saline than for air (P = 0.036).
Table 1.
Biomechanical measures (mean ± standard deviation [95% confidence intervals])
| Air (n = 7) | Saline (n = 8) | P-value | |
|---|---|---|---|
| Plunger injection force applied (N) | 13.9 ± 1.6 [12.5 to 15.3] | 13.1 ± 5.4 [9.1 to 17.1] | 0.73 |
| Flow (mL.s−1) | 13.2 ± 11.4 [3.2 to 23.2] | 5.7 ± 2.3 [4.0 to 7.4] | 0.09 |
| Velocity (m.s−1) | 6.5 ± 5.6 [2.4 to 10.6] | 2.8 ± 1.1 [2.0 to 3.6] | 0.09 |
| Thrust (N) | 0.17 ± 0.25 [0.00 to 0.39] | 19.3 ±14.9 [8.3 to 30.3] | 0.005 |
| Dissipation time (ms) | 290 ± 173 [138 to 442] | 889 ± 655 [404 to 1374] | 0.036 |
| Needle tip stress (Pa) | 7792 ± 920 [6986 to 8598] | 7378 ± 3019 [5141 to 9615] | 0.73 |
Force on the plunger was recorded simultaneously with pressure at the needle tip, only to explore ease and practicality of use. Under the dynamic conditions of this experiment, it was expected that they would track each other closely with only a scalar difference. They tracked quite closely (mean R = 0.96 ± 0.02; all P < 0.001) thus either would serve in future studies. For living human experiments, it is easier to maintain sterile conditions with a standard hospital-grade pressure transduction system, attached to a sterile stopcock with sterile tubing.
Discussion
Principle findings
Are cadavers useful for studying epidural mechanics?
The soft tissues of formalin-preserved cadavers are much harder than those of the living, making them a poor model for epidural insertion with respect to the force needed to advance the needle. The properties of the epidural tissue are likely also different, although depression of the plunger at the moment of LOR did not feel clinically different from live humans. The cadavers were too stiff to be flexed, making insertion impossible at many spinal levels. There are new methods of preservation of cadavers, not yet in use in our Anatomy Department, that do not render the tissues so stiff.18,19
Can the injected fluid push the dura away from the needle tip?
The dural sac is surrounded by the loose areolar tissue of the epidural space, in which it is able to slide upward with spinal flexion.20 The posterior dura is a flexible sheet of fibrous connective tissue held in place by a balance of the epidural tissue pressure posteriorly augmented by segmental nerve dural cuff attachments laterally, and the cerebrospinal fluid (CSF) pressure anteriorly. The lumbar anatomical features recur approximately every 36 to 40 mm in a vertical direction, and the spinal canal is 30 to 40 mm wide. Thus, a segment of posterior dura about 30 by 40 mm square is pushed by the CSF to press against the epidural loose areolar tissue at each lumbar level. Lumbar-level CSF pressure is 4.5 to 13.5 mmHg (600 to 1800 Pa) in patients lying on their sides,21 and 23.5 to 46.3 mmHg (3133 to 6173 Pa), with a mean of 36 mmHg (4800 Pa), sitting up.22
Looking first at the fluid thrust model with a sitting patient, we can translate the fluid thrust force (Table 1) into pressure on the segment of dura (air: 0.17 N ÷ 0.0012 m2 = 142 Pa = 1.06 mmHg; saline: 19.3 N ÷ 0.0012 m2 = 16,083 Pa = 121 mmHg) that could push the dura forward. This exceeds the CSF pressure of 36 mmHg (4800 Pa) that pushes the dura backward. Thus, saline could potentially generate enough fluid thrust to push dura forward away from the needle tip; air could not. Of course, this fluid thrust is dissipated as it crosses the epidural space toward the dura; both as thrust deflected in directions different from the initial injection, and as frictional heat, much more so in the complex fibrous web of the epidural tissue than would be the case when injecting into a liquid space of similar dimensions. Supporting evidence that the dura is actually pushed away from the needle tip comes from an ultrasound experiment that showed it directly in some patients.23 Addition of ultrasound to the experimental setup would be helpful.
The model of stress on impervious flexible epidural tissue seems to be of little value, since the rapid dissipation of saline pressure, and the much more rapid dissipation of air pressure show that the injected fluid does not form a stressing bolus on the surface of the epidural tissue that could result in a strain away from the needle tip, and that the epidural tissue is highly permeable. However, the stress of about 7500 Pa exceeds CSF pressure of 4800 Pa.
The different rates of pressure dissipation (air, with viscosity of 1.8 × 10−5 Pa.s−1, was 3.1 times faster than saline, with viscosity of 1.1 Pa.s−1) show that the matrix of fibrous connective tissue is an important feature of the epidural tissue; epidural tissue does not behave simply like a bag of liquid.
Air or saline?
While air would be as good as saline for pushing the dura away from the needle tip if the epidural tissue were deformable but impervious, since it behaves as a quite porous tissue, saline is more likely to move the dura.
Strengths and weaknesses of the study
The experimental model and equipment provide useful information. A weakness is that formalin-preserved cadavers are unsuitable subjects. Another issue not dealt with is the possible effects of multiple injections in the same subject, especially at adjacent spinal levels. A live human study will, of course, involve one injection per subject.
Relation to other studies
Until the report by Tran et al,24 published studies of epidural biomechanics have not incorporated the motion of plunger and needle to enable analysis of the dynamics of epidural insertion. Most investigations have simply measured injection pressures with a view to determining tissue pressure in the epidural space.25–30 Subjective testing of artificial materials and porcine cadavers,31 syringe characteristics,32,33 and the properties of free cadaver dura34 have also been studied.
Our findings support those of Tran et al.24 However, their experiment had a different position sensing system and different subjects: pork cadavers and live humans. Their purpose was to enable building better simulators, while ours posed different questions.
Implications
This experimental equipment setup is feasible and useful for the study of dynamic epidural biomechanics. Formalin-fixed cadavers are too stiff; more pliable cadavers, fresh cadavers, animals, or living humans would be suitable for further study.
Saline, but not air, injected under pressure at the moment of LOR, can potentially push the dura away from the needle tip, and help avoid dural puncture. We plan further study of the issues raised by this experiment using living humans. The data generated by such studies may also prove useful to those developing simulator based models of epidural placement.
Footnotes
Disclosure
The study was funded by the Departments of Anesthesia and Radiology at the University of Saskatchewan. No author has any commercial or other affiliations that are, or may be perceived to be, a conflict of interest with the work, or any other associations such as consultancies.
A note on terminology: We have attempted to use mechanically correct terminology throughout. Thus, for example, while it is commonly said that one puts pressure on a syringe plunger with the thumb, in fact it is the force on the plunger that is of interest, and that force generates pressure within the syringe.
References
- 1.Baer E. Post-dural puncture bacterial meningitis. Anesthesiology. 2006;105:381–393. doi: 10.1097/00000542-200608000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Brull R, McCartney CJL, Chan VWS, El-Beheiry H. Neurological complications after regional anesthesia: contemporary estimates of risk. Anesth Analg. 2007;104:965–974. doi: 10.1213/01.ane.0000258740.17193.ec. [DOI] [PubMed] [Google Scholar]
- 3.Ruppen W, Derry S, McQuay H, Moore A. Incidence of epidural hematoma, infection, and neurologic injury in obstetric patients with epidural analgesia/anesthesia. Anesthesiology. 2006;105:394–399. doi: 10.1097/00000542-200608000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Mulroy MF. Systemic toxicity and cardiotoxicity from local anesthetics: incidence and preventive measures. Reg Anesth Pain Med. 2002;27:556–561. doi: 10.1053/rapm.2002.37127. [DOI] [PubMed] [Google Scholar]
- 5.Chestnut DH. Obstetric Anesthesia. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2004. [Google Scholar]
- 6.Gaiser R. Postdural puncture headache. Curr Opin Anaesthesiol. 2006;19:249–253. doi: 10.1097/01.aco.0000192809.71408.ba. [DOI] [PubMed] [Google Scholar]
- 7.Munnur U, Suresh MS. Backache, headache, and neurologic deficit after regional anesthesia. Anesthesiol Clin North America. 2003;21:71–86. doi: 10.1016/s0889-8537(02)00031-7. [DOI] [PubMed] [Google Scholar]
- 8.Pages F. Anestesia metamerica. Revista de Sanidad Militar. 1921;11:351–365. [Google Scholar]
- 9.Dogliotti AM. Un promettente metodo di anestesia tronculare in studio: la rachianestesia peridurale segmentaria. Bollettino della Società Piemontese di Chirurgia. 1931;1:9. [PubMed] [Google Scholar]
- 10.Yamashita M, Tsuji M. Identification of the epidural space in children. The application of a micro-drip infusion set. Anaesthesia. 1991;46:872–874. doi: 10.1111/j.1365-2044.1991.tb09607.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown DL. Spinal, epidural, and caudal anesthesia. In: Miller RD, editor. Miller’s Anesthesia. 6th ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2005. pp. 1617–1683. [Google Scholar]
- 12.Cowin SC, Doty SB. Tissue Mechanics. New York, NY: Springer; 2007. [Google Scholar]
- 13.Bartynski WS, Grahovac SZ, Rothfus WE. Incorrect needle position during lumbar epidural steroid administration: inaccuracy of loss of air pressure resistance and requirement of fluoroscopy and epidurography during needle insertion. Am J Neuroradiol. 2005;26:502–505. [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson BA, Schellhas KP, Pollei SR. Epidurography and therapeutic epidural injections: technical considerations and experience with 5334 cases. Am J Neuroradiol. 1999;20:697–705. [PMC free article] [PubMed] [Google Scholar]
- 15.Duffin J. Physics for Anaesthetists. Springfield, CT: Charles C Thomas; 1976. [Google Scholar]
- 16.Lide DR. CRC Handbook of Chemistry and Physics. 89th ed. London, UK: Taylor and Francis; 2008. [Google Scholar]
- 17.Saville DJ. Multiple comparison procedures: the practical solution. The American Statistician. 1990;44:174–180. [Google Scholar]
- 18.Coleman R, Kogan I. An improved low-formaldehyde embalming fluid to preserve cadavers for anatomy teaching. J Ana. 1998;192:443–446. doi: 10.1046/j.1469-7580.1998.19230443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guimaraes da Silva RM, Matera JM, Ribeiro AA. Preservation of cadavers for surgical technique training. Vet Surg. 2004;33:606–608. doi: 10.1111/j.1532-950x.2004.04083.x. [DOI] [PubMed] [Google Scholar]
- 20.Hogan QH. Epidural anatomy: new observations. Can J Anaesth. 1998;45:R40–R48. doi: 10.1007/BF03019206. [DOI] [PubMed] [Google Scholar]
- 21.Artru AA. Cerebrospinal fluid. In: Cottrell JE, Smith DS, editors. Anesthesia and Neurosurgery. 3rd ed. St Louis, MO: Mosby; 2009. pp. 93–116. [Google Scholar]
- 22.Magnaes B. Body position and cerebrospinal fluid pressure. Part 2: clinical studies on orthostatic pressure and the hydrostatic indifferent point. J Neurosurg. 1976;44:698–705. doi: 10.3171/jns.1976.44.6.0698. [DOI] [PubMed] [Google Scholar]
- 23.Karmakar MK, Li X, Ho AMH, Kwok WH, Chui PT. Real-time ultrasound-guided paramedian epidural access: evaluation of a novel in-plane technique. Br J Anaesth. 2009;102:845–854. doi: 10.1093/bja/aep079. [DOI] [PubMed] [Google Scholar]
- 24.Tran D, Hor KW, Kamani A. Instrumentation of the loss-of-resistance technique for epidural needle insertion. IEEE Trans Biomed Eng. 2009;56:820–827. doi: 10.1109/TBME.2008.2011475. [DOI] [PubMed] [Google Scholar]
- 25.Vas L, Raghavendran S, Hosalkar H, Patil B. A study of epidural pressures in infants. Paediatr Anaesth. 2001;11:575–583. doi: 10.1046/j.1460-9592.2001.00680.x. [DOI] [PubMed] [Google Scholar]
- 26.Husemeyer RP, White DC. Lumbar extradural injection pressures in pregnant women. An investigation of relationships between rate of injection, injection pressures and extent of analgesia. Br J Anaesth. 1980;52:55–60. doi: 10.1093/bja/52.1.55. [DOI] [PubMed] [Google Scholar]
- 27.Paul DL, Wildsmith JA. Extradural pressure following the injection of two volumes of bupivacaine. Br J Anaesth. 1989;62:368–372. doi: 10.1093/bja/62.4.368. [DOI] [PubMed] [Google Scholar]
- 28.Usubiaga JE, Wikinski JA, Usubiaga LE. Epidural pressure and its relation to spread of anesthetic solutions in epidural space. Anesth Analg. 1967;46:440–446. [PubMed] [Google Scholar]
- 29.Hirabayashi Y, Shimizu R, Matsuda I, Inoue S. Effect of extradural compliance and resistance on spread of extradural analgesia. Br J Anaesth. 1990;65:508–513. doi: 10.1093/bja/65.4.508. [DOI] [PubMed] [Google Scholar]
- 30.Cardoso MM, Carvalho JC. Epidural pressures and spread of 2% lidocaine in the epidural space: influence of volume and speed of injection of the local anesthetic solution. Reg Anesth Pain Med. 1998;23:14–19. doi: 10.1016/s1098-7339(98)90105-5. [DOI] [PubMed] [Google Scholar]
- 31.Dang T, Annaswamy TM, Srinivasan MA. Development and evaluation of an epidural injection simulator with force feedback for medical training. Stud Health Technol Inform. 2001;81:97–102. [PubMed] [Google Scholar]
- 32.Bricker SR, Coleman P. Variations in the gliding characteristics of 10-ml plastic syringes used to locate the extradural space by “loss of resistance” techniques. Br J Anaesth. 1988;61:776–781. doi: 10.1093/bja/61.6.776. [DOI] [PubMed] [Google Scholar]
- 33.Leiman BC, Katz J, Salzarulo H, Warters RD, Butler BD. A comparison of different methods of lubrication of glass syringes used to identify the epidural space. Anaesthesia. 1988;43:397–398. doi: 10.1111/j.1365-2044.1988.tb09023.x. [DOI] [PubMed] [Google Scholar]
- 34.Lewis MC, Lafferty JP, Sacks MS, Pallares VS, TerRiet M. How much work is required to puncture dura with Tuohy needles? Br J Anaesth. 2000;85:238–241. doi: 10.1093/bja/85.2.238. [DOI] [PubMed] [Google Scholar]