Abstract
Articaine is an intermediate-potency, short-acting amide local anesthetic with a fast metabolism due to an ester group in its structure. It is effective with local infiltration or peripheral nerve block in dentistry, when administered as a spinal, epidural, ocular, or regional nerve block, or when injected intravenously for regional anesthesia. In comparative trials, its clinical effects were not generally significantly different from those of other short-acting local anesthetics like lidocaine, prilocaine, and chloroprocaine, and there is no conclusive evidence demonstrating above-average neurotoxicity. Articaine proved to be suitable and safe for procedures requiring a short duration of action in which a fast onset of anesthesia is desired, eg, dental procedures and ambulatory spinal anesthesia, in normal and in special populations.
Keywords: articaine, regional anesthesia, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability, neurotoxicity
Introduction and historical background
Local anesthetics block peripheral nerves and are used to prevent pain, to provide motor blockade during surgical or dental procedures, for pain control during labor, or postoperatively and in the management of chronic pain.1 Cocaine was the first reported ester-type local anesthetic for clinical use, in 1886, followed by procaine in 1904. In the search for less allergic compounds with a faster onset, the amide-type local anesthetic lignocaine was synthesized by Swedish chemist Nils Löfgren in 1943 and marketed as lidocaine in 1949. Since then, other amide local anesthetics have been introduced and used clinically for their favorable onset time and duration, eg, mepivacaine, prilocaine, bupivacaine, etidocaine, and ropivacaine. Among this group, articaine, originally synthesized as carticaine, entered dentistry practice in 1973.2 Epidural administration and comparison with lidocaine started in 1974.3,4 In 1984, it was released in Canada, followed by the UK in 1998, the rest of Europe and the US in 2000, and Australia in 2005. Currently, articaine 4% with adrenaline 5 μg/mL is widely used in dentistry.
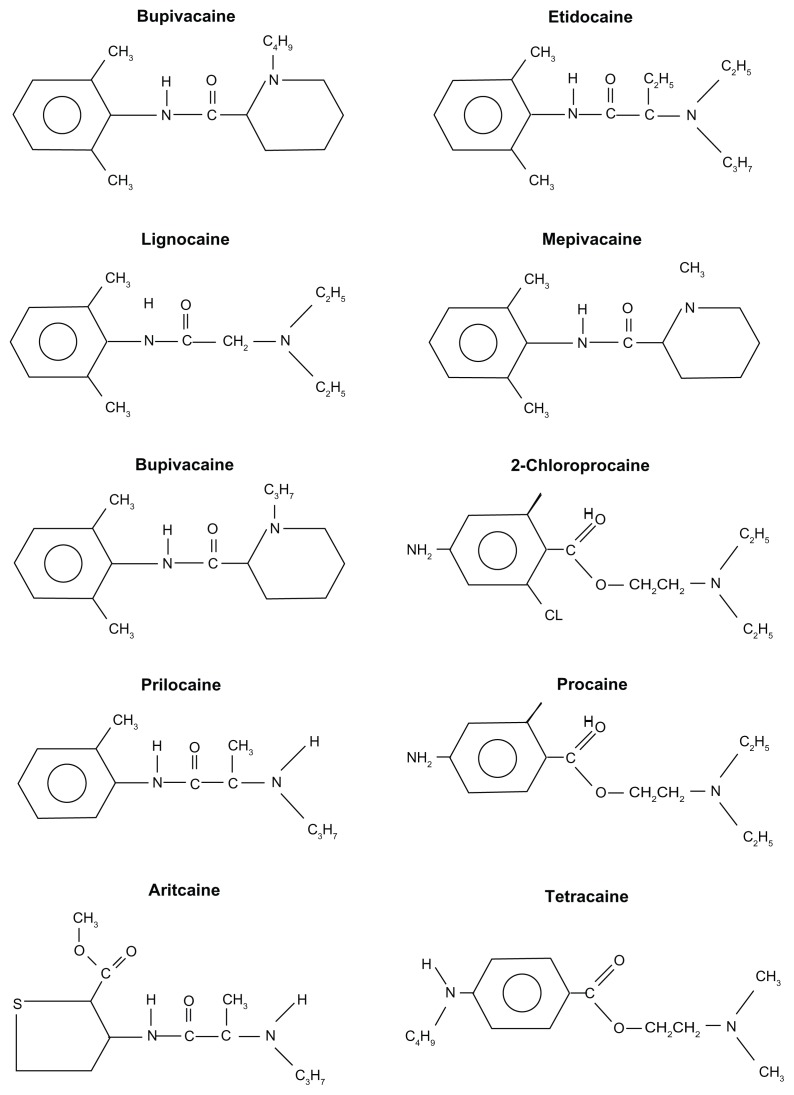
Articaine (4-methyl-3-[2-(propylamino)-propionamido]-2-thiophene-carboxylic acid, methyl ester hydrochloride) differs from the other amide local anesthetics because it contains a thiophene ring (Figures 1 and 2). The thiophene ring allows greater lipid solubility, which facilitates diffusion across the lipid-rich nerve membrane to access target receptors.1 In addition, articaine contains an ester group, so that hydrolyzation occurs in the plasma by nonspecific cholinesterases, further metabolism, and excretion, primarily in the kidneys.
Figure 1.
Structure of local anesthetics.
Figure 2.
3-D image of the articaine molecule.
Pharmacodynamic properties
Mechanism of action
Articaine blocks nerve conduction by reversibly binding to the α-subunit of the voltage-gated sodium channels within the inner cavity of the nerve, similar to other local anesthetics. Binding of articaine to the sodium channel reduces sodium influx so that the threshold potential will not be reached and impulse conduction stops. The blocking action of articaine on the sodium channel is state dependent: it has the highest affinity for the open state, an intermediate affinity for the inactivated state, and the lowest affinity for the resting state.5
The degree of neuronal block is affected by the diameter of the nerve. Larger-diameter fibers (touch/pressure/ motor) require higher concentrations of local anesthetic compared with small myelinated fibers (pain afferents).6 Articaine is lipid soluble, highly protein-bound (94%), and has a dissociation constant (pKa) of 7.8. Articaine is an intermediate-potency, short-acting local anesthetic with a fast onset of action.1
Relative potency
Ascribing local anesthetic potency is an attempt to define the sensitivity of nerves to different local anesthetics and to estimate anesthetic requirements during regional anesthesia. The potency of local anesthetics increases parallel with increasing lipid solubility. The binding ability of local anesthetics to the phospholipid membrane as a result of physicochemical features and in vivo interaction has also been found to be directly in parallel with the potency.7 In clinical practice, other factors affect the potency of a local anesthetic, including:
hydrogen ion balance
fiber size, type, and myelination
vasodilator/vasoconstrictor properties (affects rate of vascular uptake)
frequency of nerve stimulation
ambient pH (lower pH results in greater ionisation and a reduction in efficacy)
electrolyte concentrations (hypokalemia and hypercalcemia antagonizes blockade)
For assessing the potency of different local anesthetics, dose-finding, single-dose nerve blocks are studied to determine the median effective dose or the minimum local analgesic dose. The relative analgesic potency for articaine, based on early investigations and specified of its equieffective anesthetic concentration, often compared to lidocaine, is intermediate.8,9
Pharmacokinetic properties
Absorption and distribution
Local anesthetic drugs are administered to the areas around the nerves to be blocked, eg, the skin, subcutaneous tissues, retrobulbar, intrathecal, and epidural spaces. Some of the drug will be absorbed into the systemic circulation; how much will depend on the vascularity of the area to which the drug has been applied and intrinsic effects of the drug or its additives on vessel diameter. Articaine, like most local anesthetics at concentrations that are used clinically, has a vasodilatory effect, increasing its systemic absorption. This is countered in preparations with epinephrine 1:60,000, 1:100,000, and 1:200,000 (5 μg/mL).10
The distribution of the drug is influenced by the degree of tissue and plasma protein binding of the drug. The more protein-bound the agent, the longer the duration of action, as free drug is more slowly made available for metabolism. Based on its physiochemical and stereochemical properties, protein binding of articaine is 94%.11
Metabolism and elimination
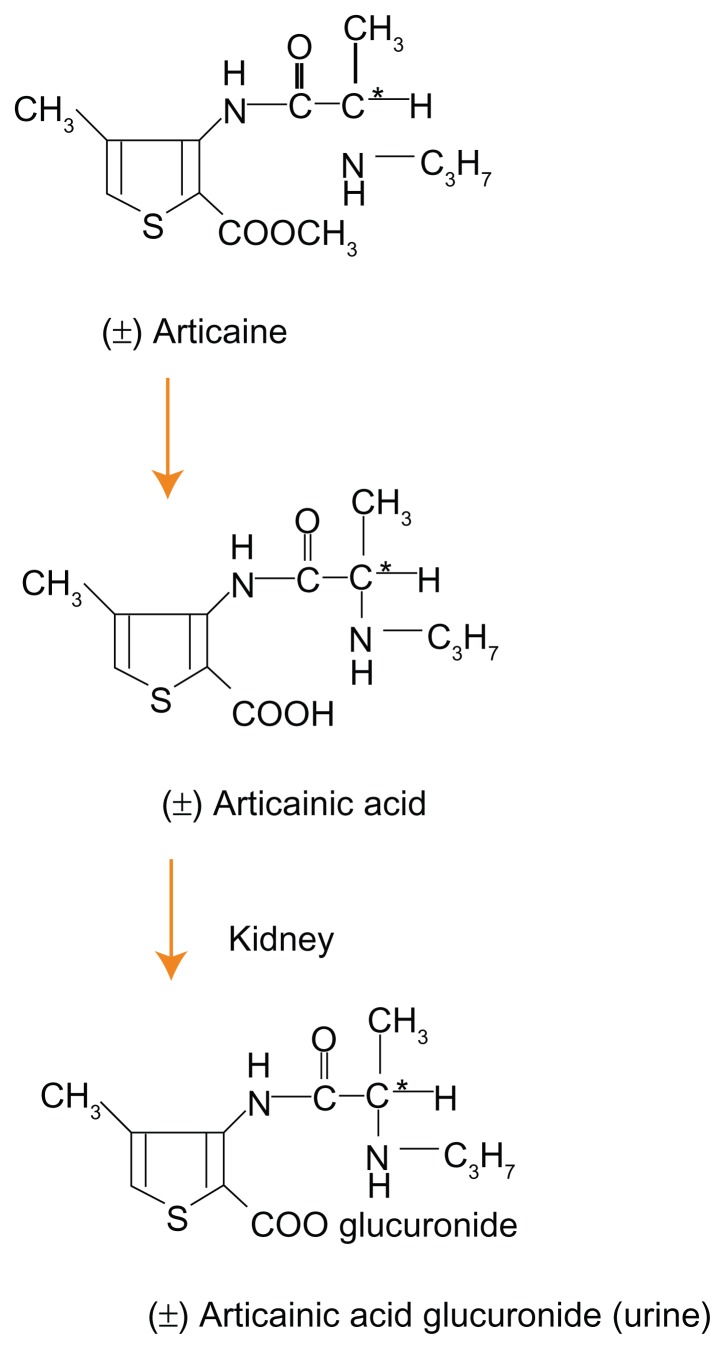
The molecular structure of articaine is characterized by having both lipophilic and hydrophilic ends connected by a hydrocarbon chain. The “CO linkage” between the hydrocarbon chain and the lipophilic aromatic ring classifies articaine as being an ester local anesthetic, in which the link is metabolized in the serum by plasma cholinesterase. Articaine is quickly metabolized via hydrolysis into its inactive metabolite articainic acid, which is partly metabolized in the kidney into articainic acid glucuronide (Figure 3).12
Figure 3.
Metabolism of articaine into articainic acid and articainic acid glucoronide.
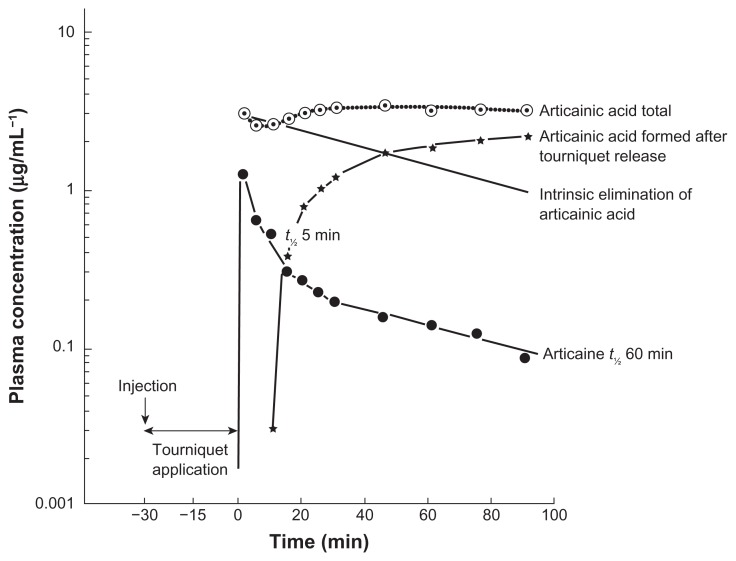
The pharmacokinetics and metabolism of articaine have been studied in ten patients undergoing intravenous regional anesthesia using 40 mL articaine 0.5% (200 mg).13 During tourniquet application and regional analgesia, 55% of the administered dose was already hydrolyzed by plasma (20%) and tissue (35%) esterase activity. After releasing the tourniquet, articaine and its metabolite articainic acid appeared in the blood; articaine was rapidly eliminated with a half-life of approximately 60 minutes (Figure 4). Low systemic concentrations and rapid metabolism of articaine also have been observed in a study during and after tumescent local anesthesia (infusion) for liposuction using dosages up to 38.2 mg/kg body weight.14 Average maximum plasma concentrations (Cmax) for articaine ranged from 136 (hips) to 264 ng/mL (abdomen); the average extent of absorption (AUC) ranged from 827 to 2203 ng · h/mL. The corresponding Cmax and AUC values for articainic acid were substantially higher, ranging from 1719 to 7292 ng/mL and from 13,464 ng · h/mL (chin) to 74,962 ng · h/mL (abdomen), respectively. In liposuction, part of the applied drug is removed in the aspirate, and around 30% of the infused dose was recovered in the plasma.
Figure 4.
Plasma concentration time curve of articaine and its metabolite articainic acid after intravenous regional anesthesia using 40 mL articaine 0.5% (200 mg).
Abbreviations: t½, half-life; min, minutes.
Special populations
Patients with hepatic or renal impairment
Metabolism and elimination of exogenous substances in general depend significantly on normal function of the liver and kidney(s). Metabolism of local anesthetics produces metabolites that are more water soluble and ready to excrete than the parent compounds. Articaine is metabolized in the serum by plasma cholinesterase; although synthesis of cholinesterase is decreased in patients with liver diseases, fast hydrolysis is presumably preserved in their erythrocytes.15 Seventy-five percent of articainic acid is excreted unchanged; the rest is glucuronidated by the kidneys before excretion. In patients with severe renal failure, both metabolites can accumulate, which in theory can cause local anesthetic systemic toxicity (LAST). The pharmacokinetics of lidocaine have been studied in renal-failure patients receiving hemodialysis.16 Case reports describing other local anesthetics associate LAST with underlying cardiac, neurologic, pulmonary, renal, hepatic, or metabolic disease. The American Society of Regional Anesthesia and Pain Medicine advises that heightened vigilance may be warranted in these patients, particularly if they are at the extremes of age.
Children
Pharmacodynamics of local anesthetics in children are comparable to those in adults; pharmacokinetics, on the other hand, differ significantly. Special caution should be observed when using the amide local anesthetics because a lower intrinsic clearance or a decreased serum protein binding can easily lead to an increased risk of toxic reactions in younger patients.17 The route of administration is one of the main determinants of safety in the use of local anesthetics in neonates and children; the application of articaine in children is mainly for those undergoing dental procedures for which local anesthesia is required or as an addition to general anesthesia. The absorption of local anesthetics from mucous membrane after topical anesthesia is increased in children due to a greater local blood flow and cardiac output than in adults. In a study investigating 27 children 3–12 years of age, the authors advised the use of 2% articaine in pediatric dentistry because of the lower Cmax and the shorter half-life.18 They showed a shorter time to maximum concentration and increased clearance compared to investigations in adults. Based on their findings, they concluded there is no need to lower the articaine dose administered to adults in mg/kg for children. Vasoactive agents like epinephrine are very effective in reducing systemic uptake of local anesthetics, resulting in a longer duration and a lower Cmax.19 Articaine 4% with epinephrine 1:100,000 was also shown to be effective and safe for use in pediatric dentistry. Among patients 4–13 years of age, the only adverse event directly related to articaine was accidental lip injury; no pharmacokinetic investigation was performed.
Prolonged numbness appears to be the most frequent adverse event after articaine for dental intervention, occurring primarily in children younger than 7 years old.20 Notwithstanding manufacturers’ recommendations that articaine not be used in children under 7 years of age, 21% of 373 American dentists satisfactorily use articaine in younger children-2–3 years of age.21 The available literature on articaine use in children shows that it is safe and effective for clinical procedures in children of all ages.22
Elderly
With advancing age, peripheral nerve conduction changes: there is a decrease in the number and density of nerve fibers, a degeneration of axons as a result of a reduction in the expression and axonal transport of cytoskeletal proteins, and an increase in motor unit action potentials.23–25 Peripheral motor and sensory conduction velocities slow progressively, and the onset latencies of F-waves and somatosensory evoked potentials increase gradually with advancing age.24 These and other age-related physiologic changes concerning the peripheral nervous system most probably have a direct effect on the clinical duration of peripheral nerve blocks and might be the cause of direct local anesthetic neurotoxicity.25,26 All local anesthetics are neurotoxic in a concentration-dependent manner due to neuroapoptosis, but articaine had the lowest apoptotic potency in a study investigating amide-type local anesthetics and ester-type local anesthetics in a human neuronal cell culture model.27
Furthermore, aging is associated with loss of reserve capacity, and renal, hepatic, and cardiac diseases cause reduced clearance of local anesthetics, which requires reduction of the dose for repeated or continuous administration. The magnitude of the reduction of all local anesthetics should be related to the expected influence of the pharmacodynamic or pharmacokinetic change.28 In healthy elderly and young volunteers, it has been shown that the metabolism of articaine is age independent.29
Obstetrics
Pain management in the parturient requires knowledge of maternal and fetal physiology; the challenge is to provide fast analgesia and minimize physiologic perturbations. Epidural anesthesia with a variety of local anesthetics is by far the most used technique for this purpose. Articaine is rarely used for this indication. Two Russian investigators claimed that epidural anesthesia using a single dose of 1% articaine (Ultracaine) of 1.0–1.2 mg/kg in over 1000 healthy and high-risk parturients proved to be highly effective and safe for the mother and exerted no depressive effect on the newborn.30,31 Two German groups compared articaine (carticaine 1.5%) with bupivacaine for epidural anesthesia in cesarean sections (n = 25 and n = 15).32,33 Onset, quality of the analgesia, hemodynamic stability, and newborn Apgar scores were good. Pharmacokinetic investigation showed an articaine plasma concentration of 0.48 μg/mL at delivery, proving its rapid metabolism.33 The ratio of the unmetabolized drug to that of the metabolite found in maternal serum at that moment was 0.75. The umbilical venous-maternal arterial serum concentration ratio was 0.32. The latter is equal to early investigation after placental transfer of carticaine where the mean neonatal carticaine plasma concentration of nine newborns was found to be 32% (±7%) of the maternal after epidural anesthesia.34 Referenced in the literature for other local anesthetics are lidocaine (0.52–0.58), mepivacaine (0.64), and bupivacaine (0.23–0.26).
A special aspect that should be taken in account when articaine is used during pregnancy is its metabolism by plasma cholinesterases and the reduced hydrolysis rates in newborns and infants up to 6 months. Plasma cholinesterase activity or better butyrylcholinesterase (BChE) activity of healthy, normal infants has been found to be reduced by 50% compared to normal adults.35 This reduction seemed not of clinical importance.18 It can be of clinical importance if the newborn (and/or the mother) possesses one or more of the 58 possible known mutations in the butyrylcholinesterase gene (BCHE). BChE serves as the primary articaine hydrolase producing articainic acid; reduction or inactivity of BChE may contribute to increased toxicity after articaine use. However, articaine has an important second metabolism that prevents increasing plasma concentrations similar to cocaine toxicity in people with mutations in the BCHE gene that reduce BChE activity.36,37
Therapeutic efficacy
Spinal anesthesia
In ambulatory spinal anesthesia in day-care patients, a short-acting local anesthetic is preferred. In addition to the short duration of motor blockade and postoperative bladder dysfunction, fast onset and low toxicity are qualities of a favorable profile. Articaine has in common with lidocaine, prilocaine, and chloroprocaine that it meets these qualities. 38–40 Mutual comparison of these four local anesthetics showed no evident significant clinical advantages for any over another. The results of the articaine groups are shown in Table 1, with the caveat that the designs of the studies were quite different, as were the dosages and concentrations used: articaine (1.25 mg/kg and 80 mg, 5% with glucose 10%) versus lidocaine9,41; articaine (50 mg, 2% plain) versus prilocaine; 42 articaine (60 mg, 2% plain) versus chloroprocaine.43 Other studies have investigated different articaine solutions or articaine with glucose versus bupivacaine; spinal blockade characteristics are displayed in Table 2.38,44–46
Table 1.
Articaine versus other short-acting local anesthetics for spinal anesthesia
| Kaukinen9 | Timmerman41 | Förster43 | Hendriks42 | |
|---|---|---|---|---|
| Year | 1978 | 2007 | 2010 | 2008 |
| Surgery | Urology | Ambulatory surgery | Knee arthroscopy | Knee arthroscopy |
| Number of patients | 60 | 40 | 39 | 36 |
| Solution | 5% in glucose 10% | 5% in glucose 10% | 2% plain | 2% plain |
| Mg articaine | 68.8 ± 1.5 (1.25 mg/kg, 100 mg maximum) | 80 | 60 | 50 |
| Standard opioid administration | Pethidine 50 mg premedication in all patients | – | – | – |
| Rescue opioid administered (number of patients) | 1 | – | 1 | 0 |
| Assessment of level sensory blockade | Pinprick | Pinprick | Pinprick | Cold sensation |
| Block height, median (range) | T8 (L2–T4) | T7 | T10 (T11–T5) | T10 (L1–T3) |
| Time to two-dermatome regression (median, minutes) | Not assessed | Not assessed | 60 (45–75) | 60 (24–104) |
| Duration of motor block (mean, 95% confidence interval or SD) (minutes) | 90 | 118 (107–129) | 130 (28.4) | 140 (33) |
| Time to spontaneous voiding (mean, 95% confidence interval or SD) (minutes) | Not assessed | 256 (242–270) | 219 (71.6) | 184 (39) |
| TNS (number of patients) | Not assessed | 2 | 0 | 1 |
| Days of follow-up for TNS | 1 | 7 | 1 |
Abbreviations: SD, standard deviation; TNS, transient neurologic syndrome.
Table 2.
Articaine in different solutions or articaine versus bupivacaine for spinal anesthesia
| Kozlov44 | Kallio38 | Dijkstra45 | Bachmann46 | |||
|---|---|---|---|---|---|---|
| Year | 1998 | 2006 | 2008 | 2012 | ||
| Surgery | Lower part of the body, lower limbs | Lower limb | Lower limb | Inguinal herniorrhaphy | ||
| Number of patients | 50 | 30 | 30 | 30 | 39 | 40 |
| Solution | 5% in glucose 10% | 3% in glucose 7.5% | 5% in glucose 8% | 4% in glucose 10% | ||
| Mg articaine | – | 60 | 84 | 108 | 80 | 84 |
| Standard opioid administration | – | Fentanyl 0.001 mg/kg IV before puncture in all patients | – | – | ||
| Rescue opioid administered (number of patients) | – | 5 | 2 | 0 | 0 | 10 |
| Assessment of level sensory blockade | ? | Pinprick | Pinprick | Pinprick | ||
| Block height, median (range) | ? | T4 (T9–T1) | T4 (T10–C7) | T4 (T9–C3) | T6 (T9.5–T4.5) | T5 (T10–T1) |
| Time to two-dermatome regression (median, minutes) | Not assessed | 60 (30–150) | 75 (25–120) | 75 (45–120) | Not assessed | 60 (25–135) |
| Duration of motor block (median/range/mean/SD) (minutes) | Adequate analgesia 124.1 (3.4) |
120 (45–180) | 120 (75–150) | 120 (75–240) | 101 (80–129) | 105 (75–195) |
| Time to spontaneous voiding (median/range/mean/SD) (mins) | Not assessed | 249 (161–425) | 271 (192–490) | 275 (175–403) | 257 (210–293) | 273 (155–459) |
| TNS (number of patients) | Not assessed | 0 | 0 | 0 | 1 | 2 |
| Days of follow-up for TNS | – | 1 | 1 | 1 | 10 | 7 |
Abbreviations: IV, intravenous; SD, standard deviation; TNS, transient neurologic syndrome.
More randomized, double-blind, controlled trials are needed to demonstrate if articaine has advantages over other local anesthetics in ambulatory spinal anesthesia. Articaine provides rapid onset and satisfactory anesthesia for about 1 hour; recovery is fast with an acceptable time of about 3.5 hours to first spontaneous voiding (Tables 1 and 2). Lidocaine and prilocaine have a long history of use for spinal anesthesia and concerns regarding neurotoxicity. The reported incidence of transient neurological symptoms or transient radicular irritations ranges from 0.4% up to 40%.47 All local anesthetics are potentially neurotoxic, and so is articaine. The incidence of nerve damage with intrathecal articaine appears to be low.38,41–43,45,46 Articaine at low concentrations (2%–3%), with or without glucose, has been demonstrated to be very suitable for spinal anesthesia in day-care patients for lower limb surgery, wherein discharge criteria might be met rapidly.45
Epidural anesthesia
Although articaine has favorable pharmacokinetic properties, clinical trials to investigate the efficacy and safety for epidural anesthesia have been scarce. In 1977, carticaine was compared with lidocaine for epidural anesthesia in a double-blinded study. Single-shot epidurals using 10 mL of a 2% solution with adrenaline 1:200,000 were performed in 116 urologic patients.4 The results showed no statistically significant difference as regards latency, spread, duration, or motor blockade obtained with the two substances. Marked differences in predefined side effects or their frequency were not noted. Pharmacokinetic studies have been performed in six patients after epidural administration of 600 mg articaine; half-lives of elimination of articaine were 0.6 hours (first phase: distribution) and 2.5 hours (second phase: hydrolyses in plasma) and 2.5 hours for the metabolite articainic acid.48,49 Further epidural use of articaine is limited to labor-pain relief.30–33 An anecdotal study showed adequate analgesia using 4–8 mL/h articaine in continuous cervical epidural anesthesia for hand surgery.50 Articaine is not a first-choice local anesthetic for epidural anesthesia. The “spectrum” of local anesthetics contains more suitable drugs, local anesthetics, and adjuvants, for instance, to treat acute pain with close to zero motor block or postoperative pain.51
Intravenous regional anesthesia
Intravenous regional anesthesia is mainly used for procedures up to 45 minutes at most. After administration, the local anesthetic blocks peripheral nerves and larger nerves through intraneural venous distribution, whereas ischemia by the tourniquet cuff and compression of nerves under the tourniquet contribute to the surgical anesthesia. Lidocaine and prilocaine have been proved safe and effective over decades of use. In two comparative studies, articaine turned out to be equally suitable for intravenous regional anesthesia. Articaine had a somewhat faster onset, 2.5–5 minutes after injection of 200 mg (40 mL, 0.5%) compared to lidocaine and prilocaine, and no signs of local anesthetic toxicity or systemic toxicity occurred, apart from a spontaneously disappearing rash after an hour.52,53
Dentistry
Articaine has been widely used in dental surgery. Dentists started to use carticaine around 1977.54 In dentistry, articaine has been investigated extensively. Clinical trials comparing articaine mostly with lidocaine have varied in study design and site of action. The overwhelming majority of references in the literature describing the alleged neurotoxicity of articain concern paraesthesia and prolonged numbness after dental procedures. An excellent review of the dental literature was published last year.55 The authors concluded that articaine is a safe and effective local anesthetic drug to use in all aspects of clinical dentistry for patients of all ages, with properties comparable to other common local anesthetic agents. Although there may be controversy regarding its safety and advantages in comparison to other local anesthetics, there is no conclusive evidence demonstrating neurotoxicity or significantly superior anesthetic properties of articaine for dental procedures. The choice whether to use articaine or another local anesthetic is based on the personal preference and experiences of individual clinicians.56 Currently, articaine is available as a 4% solution containing 1:100,000 or 1:200,000 epinephrine. Clinical trials comparing 4% with 2% solutions show no clinical advantage of 4% over a 2% solution.57,58
Ophthalmology, ENT and dermatology
Limited diffusion of a local anesthetic is the main drawback of peribulbar anesthesia, leading to failure to achieve eyeball akinesia in up to 50% of patients. Articaine has been studied for eye surgery because it diffuses through tissues more readily than other local anesthetic agents. The efficacy of 2% articaine with 1:200,000 epinephrine has been compared with that of a mixture of 0.5% bupivacaine and 2% lidocaine for peribulbar anesthesia in cataract surgery, using a single medial canthus injection technique.59 Articaine 9.7 mL ( standard deviation [SD] 2.1) had a more rapid onset of action, resulting in better akinesia at 5 minutes; a second injection was required in 24% compared with 51% in the bupivacaine/lidocaine group. The duration of block was adequate for surgery in all cases with a mean time from block insertion to end of surgery of 44 minutes (SD 14); articaine had a faster offset. The same study design was used for peribulbar anesthesia in cataract surgery using a single inferotemporal injection of 6–7 mL.60 A rapid onset to readiness for surgery (4.2 versus 7.2 minutes), a dense block lasting for 33 minutes (SD 9.8), and a faster offset of the block in the articaine group were demonstrated. The addition of epinephrine is not recommended, mainly because of vasospasm and arrythmias following inadvertent intravascular injection. Plain articaine 2% was used for sub-Tenon’s anesthesia in 44 patients.61 Up to 5 mL of articaine was injected until the first sign of conjunctival chemosis appeared, causing a reduction in ocular movement and readiness for surgery in 3.5 minutes (SD 2.5); one failure was reported. In a comparable randomized controlled trial, 4 mL articaine 4% in all but one of the 31 patients was enough.62 None of the patients in the articaine groups developed any clinically significant ocular motility problems following surgery.59–62
Articaine has been compared with lidocaine in 70 patients undergoing a septoplasty procedure with regard to reduction in pain and related complications.63 Numeric rating scales for pain at the 1st, 2nd, 4th, 6th, 8th, 12th, 18th, and 24th postoperative hour and analgesic consumption were significantly lower in the articaine group.
Articaine hydrochloride 4% with 1:100.000 epinephrine proved very useful for infiltrative anesthesia in cutaneous surgery by dermatologists.64 Onset is between 1 and 3 minutes, and the average duration of anesthesia 71 minutes. Articaine has no negative effect on wound healing.65
Toxicity and safety
As mentioned earlier, all commonly used local anesthetics produce neurotoxicity in a concentration-dependent manner. Proposed causes for neurological deficits are neural ischemia (due to local vasoconstriction caused by the local anesthetic itself or by epinephrine) or inflammation.66 Clinical profiles of neurotoxicity of articaine have been based on the reported incidence of transient neurologic syndrome after spinal anesthesia and paresthesia in dentistry67,68 Articaine acquired from Sanofi Aventis (Paris, France) and other local anesthetics in relative experimentally effective anesthetic concentrations have been investigated for their potency to induce apoptosis in human tumor cells (SHEP neuroblastoma cells).27 The observed neurotoxicity correlates with the lipophilicity and therefore with the potency of the local anesthetic. The concentrations of local anesthetics that induced apoptosis in their model are within the same range as those observed intrathecally after single-shot spinal anesthesia in primates and in sciatic nerves of rodents during nerve blockade.69,70 Beyond the concentration, the time of exposure to a local anesthetic seems important for the development of neurotoxicity. For this reason, permanent neurotoxicity after single application to healthy nervous tissue is fortunately a rare complication clinically.
Clinical signs are perioral and tongue paresthesia, a metallic taste, and dizziness, passing into slurred speech, diplopia, tinnitus, confusion, restlessness, and muscle twitching progressing to neuronal depression and leading to convulsions and coma. The severity of cardiovascular and central nervous system toxicity is directly related to the local anesthetic potency, dose, and rate of administration. In this context, one has to distinguish acute toxicity caused by accidental intravascular administration from toxicity caused by systemically absorbed local anesthetic.71 Recommendations regarding maximum doses of local anesthetics lack scientific justification; as a consequence, the recommended highest dose of articaine, assumed to be around 4–7 mg/kg, has to be interpreted as an important guide number.72 In general, the site of local anesthetic injection in combination with patient-related factors and their expected influence on the pharmacodynamic or pharmacokinetic change are important reasons to reduce the dose, especially for repeated or continuous administration. Even if administration of articaine or another local anesthetic is done expertly and cautiously, LAST can occur. Treatment options for LAST, particularly local anesthetic cardiotoxicity, have recently improved in the form of intravenous lipid emulsion therapy (an initial bolus of 1.5 mL/kg of a 20% solution followed by an infusion of 0.25 mL/kg/minute).73,74 Lipid emulsion is a reasonably well-tolerated and effective treatment, especially when LAST is caused by a lipophilic local anesthetic like articaine.
Articaine, similar to other local anesthetics, produces a dose-dependent delay in the transmission of impulses through the cardiac conduction system by its action on the cardiac sodium and potassium channels. Articaine can modify cardiac action potentials and ion currents at concentrations higher than the therapeutic range, but may be moderate even in the case of overdose.75 The effects of articaine and ropivacaine on the components of intracellular Ca2+ handling, the cause of negative inotropic actions, were studied and compared in canine ventricular myocardium.76 Under conditions of normal application, both articaine and ropivacaine are free of cardiodepressant effects.
Place of articaine in regional anesthesia
In regional anesthesia, the choice of a local anesthetic is determined by matching the patient’s anesthetic and/or analgesic requirements with its pharmacological properties. Articaine has a favorable profile as a fast and short-acting local anesthetic, and there is no conclusive evidence demonstrating above-average neurotoxicity. Local infiltration or topical administration of articaine has proved to be suitable for dental procedures requiring anesthesia with a short to intermediate duration of action and a fast onset, and for ambulatory spinal anesthesia. In comparative trials, its clinical effects were not generally significantly different from those of lidocaine, prilocaine, and chloroprocaine. In addition to spinal anesthesia and local infiltration, articaine 0.5% might be appropriate for intravenous regional anesthesia. Results from future clinical trials comparing 2% versus 4% articaine in clinical anesthesia and dental anesthesia (with as little as possible epinephrine in this field) will set the optimal formulation with maximal efficacy and safety for routine practice.
Footnotes
Disclosure
The author has no financial or personal interest in any of the products mentioned in this article; the preparation of this review was not supported by any external funding.
References
- 1.McLure HA, Rubin AP. Review of local anaesthetic agents. Minerva Anestesiol. 2005;71:59–74. [PubMed] [Google Scholar]
- 2.Ferger P, Marxkors K. Ein neues Anästhetikum in der Zahnärztlichen Prosthetik. Dtsch Zahnarztl Z. 1973;28:87–89. [PubMed] [Google Scholar]
- 3.Hendolin H, Mattila M. Hoe-40045, ein neues Lokalanësthetikum verglichen mit Lidocain bei Epiduralanästhesie. Prakt Anaesth. 1974;9:178–182. [PubMed] [Google Scholar]
- 4.Brinkløv MM. Effectivity of carticaine, a new local anesthetic. A survey and a double blind investigation comparing carticaine with lidocaine in epidural analgesia. Acta Anaesth Scand. 1977;21:5–16. doi: 10.1111/j.1399-6576.1977.tb01186.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang GK, Calderon J, Jaw SJ, Wang SY. State-dependent block of Na+ channels by articaine via the local anesthetic receptor. J Membr Biol. 2009;229:1–9. doi: 10.1007/s00232-009-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckenmaier CC, Bleckner LL. Anaesthetic agents for advanced regional anaesthesia: a North American perspective. Drugs. 2005;65:745–759. doi: 10.2165/00003495-200565060-00003. [DOI] [PubMed] [Google Scholar]
- 7.Kokubu M, Oda K, Kudo M, Machida M, Shinya N. Correlation between the anesthetic potency of local anesthetics and their binding ability to a model membrane. J Anesth. 1979;11:121–125. doi: 10.1007/BF02480073. [DOI] [PubMed] [Google Scholar]
- 8.Vähätalo K, Antila H, Lehtinen R. Articaine and lidocaine for maxillary infiltration anesthesia. Anesth Prog. 1993;40:114–116. [PMC free article] [PubMed] [Google Scholar]
- 9.Kaukinen S, Eerola R, Eerola M, Kaukinen L. Comparison of carticaine and lidocaine in spinal anaesthesia. Ann Clin Res. 1978;10:191–194. [PubMed] [Google Scholar]
- 10.Sack U, Kleemann PP. Intraoral conduction anesthesia with epinephrine- containing local anesthetics and arterial epinephrine plasma concentration. Anesth Pain Control Dent. 1992;1:77–80. [PubMed] [Google Scholar]
- 11.Mather LE, Tucker GT. Properties, absorption, and disposition of local anesthetic agents. In: Cousins MJ, Carr DB, Horlocker TT, Bridenbaugh PO, editors. Neural Blockade in Clinical Anesthesia and Pain Medicine. 4th ed. Philadelphia: Wolters Kluwer/Lippincott Williams and Wilkins; 2009. pp. 48–95. [Google Scholar]
- 12.Vree TB, Gielen MJ. Clinical pharmacology and the use of articaine for local and regional anaesthesia. Best Pract Res Clin Anaesthesiol. 2005;19:293–308. doi: 10.1016/j.bpa.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Vree TB, Simon MA, Gielen MJM, Booij LHDJ. Regional metabolism of articaine in 10 patients undergoing intravenous regional anaesthesia during day case surgery. Br J Clin Pharmacol. 1997;44:29–34. doi: 10.1046/j.1365-2125.1997.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grossmann M, Sattler G, Pistner H, et al. Pharmacokinetics of articaine hydrochloride in tumescent local anesthesia for liposuction. J Clin Pharmacol. 2004;44:1282–1289. doi: 10.1177/0091270004269014. [DOI] [PubMed] [Google Scholar]
- 15.Calvo R, Carlos R, Erill S. Effects of disease and acetazolamide on procaine hydrolysis by red cell enzymes. Clin Pharmacol Ther. 1980;27:175. doi: 10.1038/clpt.1980.27. [DOI] [PubMed] [Google Scholar]
- 16.De Martin S, Orlando R, Bertoli M. Differential effect of chronic renal failure on the pharmacokinetics of lidocaine in patients receiving hemodialysis. Clin Pharmacol Ther. 2006;80:597–606. doi: 10.1016/j.clpt.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Mazoit JX, Dalens BJ. Pharmacokinetics of local anaesthetics in infants and children. Clin Pharmacokinet. 2004;43:17–32. doi: 10.2165/00003088-200443010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Jakobs W, Ladwig B, Cichon P, Oertel R, Kirch W. Serum levels of articaine 2% and 4% in children. Anesth Prog. 1995;42:113–115. [PMC free article] [PubMed] [Google Scholar]
- 19.Malamed SF, Gagnon S, Leblanc D. Efficacy of articaine: a new amide local anesthetic. Pediatr Dent. 2000;22:307–311. doi: 10.14219/jada.archive.2000.0237. [DOI] [PubMed] [Google Scholar]
- 20.Adewumi A, Hall M, Guelmann M, Riley J. The incidence of adverse reactions following 4% septocaine (articaine) in children. Pediatr Dent. 2008;30:424–428. [PubMed] [Google Scholar]
- 21.Wright GZ, Weinberger SJ, Friedman CS, Plotzke OB. Use of articaine local anesthesia in children under 4 years of age – a retrospective report. Anesth Prog. 1989;36:268–271. [PMC free article] [PubMed] [Google Scholar]
- 22.Brickhouse TH, Unkel JH, Webb MB, Best AM, Hollowell RL. Articaine use in children among dental practitioners. Pediatr Dent. 2008;30:516–521. [PubMed] [Google Scholar]
- 23.Ochoa J, Mair WG. The normal sural nerve in man. II. Changes in the axons and Schwann cells due to ageing. Acta Neuropathol (Berl) 1969;13:217–239. doi: 10.1007/BF00690643. [DOI] [PubMed] [Google Scholar]
- 24.Dorfman LJ, Bosley TM. Age-related changes in peripheral and central nerve conduction in man. Neurology. 1979;29:38–44. doi: 10.1212/wnl.29.1.38. [DOI] [PubMed] [Google Scholar]
- 25.Hanks RK, Pietrobon R, Nielsen KC, et al. The effect of age on sciatic nerve block duration. Anesth Analg. 2006;102:588–592. doi: 10.1213/01.ane.0000189552.85175.db. [DOI] [PubMed] [Google Scholar]
- 26.Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- 27.Werdehausen R, Fazeli S, Braun S, et al. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br J Anaesth. 2009;103:711–718. doi: 10.1093/bja/aep236. [DOI] [PubMed] [Google Scholar]
- 28.Tsui BC, Wagner A, Finucane B. Regional anaesthesia in the elderly: a clinical guide. Drugs Aging. 2004;21:895–910. doi: 10.2165/00002512-200421140-00001. [DOI] [PubMed] [Google Scholar]
- 29.Oertel R, Ebert U, Rahn R, Kirch W. The effect of age on pharmacokinetics of the local anesthetic drug articaine. Reg Anesth Pain Med. 1999;24:524–528. doi: 10.1016/s1098-7339(99)90043-3. [DOI] [PubMed] [Google Scholar]
- 30.Semenikhin AA, Kim ED. Prolonged epidural analgesia with ultracaine for labor pain relief. Anesteziol Reanimatol. 2001;2:28–30. Russian. [PubMed] [Google Scholar]
- 31.Kim ED, Seminikhin AA. Optimal modes of epidural analgesia at labor in females at a high risk of complications. Anesteziol Reanimatol. 2006;4:59–62. Russian. [PubMed] [Google Scholar]
- 32.Hamar O, Csömör S, Jr, Tóth P, Markó J. Comparative evaluation of carticaine and bupivacaine in epidural anesthesia in cesarean section. Zentralbl Gynakol. 1986;108:739–743. German. [PubMed] [Google Scholar]
- 33.Kaukinen L, Kaukinen S, Kärkkäinen S. Epidural anesthesia with carticaine in cesarean section. A comparison with bupivacaine. Reg Anaesth. 1986;9:79–83. German. [PubMed] [Google Scholar]
- 34.Strasser K, Huch A, Huch R, Uihein M. Placental transfer of carticaine (ultracain) a new local anaesthetic agent. Z Geburtshilfe Perinatol. 1977;181:118–120. German. [PubMed] [Google Scholar]
- 35.Zsigmond EK, Downs JR. Plasma cholinesterase activity in newborns and infants. Can Anaesth Soc J. 1971;18:278–285. doi: 10.1007/BF03025463. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman RS, Henry GC, Howland MA, Weisman RS, Weil L, Goldfrank LR. Association between life-threatening cocaine toxicity and plasma cholinesterase activity. Ann Emerg Med. 1992;21:247–253. doi: 10.1016/s0196-0644(05)80883-2. [DOI] [PubMed] [Google Scholar]
- 37.Duysen EG, Lockridge O. Prolonged toxic effects after cocaine challenge in butyrylcholinesterase/plasma carboxylesterase double knockout mice: a model for butyrylcholinesterase-deficient humans. Drug Metab Dispos. 2011;39:1321–1323. doi: 10.1124/dmd.111.039917. [DOI] [PubMed] [Google Scholar]
- 38.Kallio H, Snall EV, Luode T, Rosenberg PH. Hyperbaric articaine for day-case spinal anaesthesia. Br J Anaesth. 2006;97:704–709. doi: 10.1093/bja/ael222. [DOI] [PubMed] [Google Scholar]
- 39.Ostgaard G, Hallaraker O, Ulveseth OK, Flaatten H. A randomised study of lidocaine and prilocaine for spinal anaesthesia. Acta Anaesth Scand. 2000;44:436–440. doi: 10.1034/j.1399-6576.2000.440413.x. [DOI] [PubMed] [Google Scholar]
- 40.Casati A, Danelli G, Berti M, Fioro A, Fanelli A, Benassi C. Intrathecal 2-chloroprocaine for outpatient lower limb surgery: a prospective, randomized, double-blind clinical evaluation. Anesth Analg. 2006;103:234–238. doi: 10.1213/01.ane.0000221441.44387.82. [DOI] [PubMed] [Google Scholar]
- 41.Timmerman L, van Dongen EP, Tromp E, Andriessen EJ, Kerkvliet CT, Knibbe CA. Articaine and lidocaine for spinal anaesthesia in day case surgery. Reg Anesth Pain Med. 2007;32(Suppl 1):9. [Google Scholar]
- 42.Hendriks MP, de Weert CJ, Snoeck MM, Hu H, Pluim MA, Gielen MJ. Plain articaine or prilocaine for spinal anaesthesia in day-case knee arthroscopy: a double-blind randomized trial. Br J Anaesth. 2009;102:259–263. doi: 10.1093/bja/aen357. [DOI] [PubMed] [Google Scholar]
- 43.Förster JG, Kallio H, Rosenberg PH, Harilainen A, Sandelin J, Pitkänen MT. Chloroprocaine vs articaine as spinal anaesthetics for day-case knee arthroscopy. Acta Anaesthesiol Scand. 2011;55:273–281. doi: 10.1111/j.1399-6576.2010.02325.x. [DOI] [PubMed] [Google Scholar]
- 44.Kozlov SP, Svetlov VA, Lukianov MV. Pharmacology of local anesthetics and clinical aspects of segmental blocking. II Spinal anesthesia. Anesteziol Reanimatol. 1998;5:37–42. Russian. [PubMed] [Google Scholar]
- 45.Dijkstra T, Reesink JA, Verdouw BC, Van der Pol WS, Feberwee T, Vulto AG. Spinal anaesthesia with articaine 5% vs bupivacaine 0.5% for day-case lower limb surgery: a double-blind randomized clinical trial. Br J Anaesth. 2008;100:104–108. doi: 10.1093/bja/aem332. [DOI] [PubMed] [Google Scholar]
- 46.Bachmann M, Pere P, Kairaluoma P, Rosenberg PH, Kallio H. Randomized comparison of hyperbaric articaine and hyperbaric low-dose bupivacaine along with fentanyl in spinal anaesthesia for day-case inguinal herniorrhaphy. Eur J Anaesthesiol. 2012;29:22–27. doi: 10.1097/EJA.0b013e32834a11be. [DOI] [PubMed] [Google Scholar]
- 47.De Weert K, Traksel M, Gielen M, Slappendel R, Weber E, Dirksen R. The incidence of transient neurological symptoms after spinal anaesthesia with lidocaine compared to prilocaine. Anaesthesia. 2000;55:1020–1024. doi: 10.1046/j.1365-2044.2000.01618-4.x. [DOI] [PubMed] [Google Scholar]
- 48.Oss GE, Vree TB, Baars AM, Termond EF, Booij LH. Pharmacokinetics, metabolism and renal excretion of articaine and its metabolite articainic acid in patients after epidural administration. Eur J Anaesth. 1989;6:49–56. [PubMed] [Google Scholar]
- 49.Vree TB, Van Oss GE, Gielen MJM, Booij LHDJ. Epidural metabolism of articaine to its metabolite articainic acid in five patients after epidural administration of 600 mg articaine. J Pharm Pharmacol. 1997;49:158–163. doi: 10.1111/j.2042-7158.1997.tb06772.x. [DOI] [PubMed] [Google Scholar]
- 50.Noyan A, Cepel S, Ural S, Ozel A. Continuous cervical epidural anesthesia in hand surgery. J Reconstr Microsurg. 2001;17:481–485. doi: 10.1055/s-2001-17750. [DOI] [PubMed] [Google Scholar]
- 51.Veering BT, Cousins MJ. Epidural neural blockade. In: Cousins MJ, Carr DB, Horlocker TT, Bridenbaugh PO, editors. Neural Blockade in Clinical Anesthesia and Pain Medicine. 4th ed. Philadelphia: Wolters Kluwer/Lippincott Williams and Wilkins; 2009. pp. 241–295. [Google Scholar]
- 52.Simon MA, Gielen MJ, Alberink N, Vree TB, van Egmond J. Intravenous regional anesthesia (IVRA) with 0.5% articaine, 0.5% lidocaine, or 0.5% prilocaine. A double-blind randomized clinical study. Reg Anesth. 1997;22:29–34. doi: 10.1016/s1098-7339(06)80053-2. [DOI] [PubMed] [Google Scholar]
- 53.Pitkanen MT, Xu M, Haasio J, Rosenberg PH. Comparison of 0.5% articaine and 0.5% prilocaine in intravenous regional anesthesia of the arm: a cross-over study in volunteers. Reg Anesth Pain Med. 1999;24:131–135. [PubMed] [Google Scholar]
- 54.Cowan A. Clinical assessment of a new local anesthetic agent – carticaine. Oral Surg Oral Med Oral Pathol. 1977;43:174–180. doi: 10.1016/0030-4220(77)90153-0. [DOI] [PubMed] [Google Scholar]
- 55.Yapp KE, Hopcraft MS, Parashos P. Articaine: a review of the literature. Br Dent J. 2011;210:323–329. doi: 10.1038/sj.bdj.2011.240. [DOI] [PubMed] [Google Scholar]
- 56.Yapp KE, Hopcraft MS, Parashos P. Dentists’ perceptions of a new local anaesthetic drug – articaine. Aust Dent J. 2012;57:18–22. doi: 10.1111/j.1834-7819.2011.01643.x. [DOI] [PubMed] [Google Scholar]
- 57.Hintze A, Paessler L. Comparative investigations on the efficacy of articaine 4% (epinephrine 1:200,000) and articaine 2% (epinephrine 1:200,000) in local infiltration anaesthesia in dentistry – a randomised double-blind study. Clin Oral Investig. 2006;10:145–150. doi: 10.1007/s00784-005-0025-0. [DOI] [PubMed] [Google Scholar]
- 58.Fritzsche C, Pässler L. Ultracain D-S und ultracain 2%-suprarenin-vergleichende untersuchungen zur lokalanästhesie in der zahnärztlichen chirurgie. Quintessenz. 2000;51:507–514. [Google Scholar]
- 59.Allman KG, McFadyen JG, Armstrong J, Sturrock GD, Wilson IH. Comparison of articaine and bupivacaine/lidocaine for single medial canthus peribulbar anaesthesia. Br J Anaesth. 2001;87:584–587. doi: 10.1093/bja/87.4.584. [DOI] [PubMed] [Google Scholar]
- 60.Allman KG, Barker LL, Werrett GC, Gouws P, Sturrock GD, Wilson IH. Comparison of articaine and bupivacaine/lidocaine for peribulbar anaesthesia by inferotemporal injection. Br J Anaesth. 2002;88:676–678. doi: 10.1093/bja/88.5.676. [DOI] [PubMed] [Google Scholar]
- 61.Gouws P, Galloway P, Jacob J, English W, Allman KG. Comparison of articaine and bupivacaine/lidocaine for sub-Tenon’s anaesthesia in cataract extraction. Br J Anaesth. 2004;92:228–230. doi: 10.1093/bja/aeh044. [DOI] [PubMed] [Google Scholar]
- 62.Raman SV, Barry JS, Murjaneh S, et al. Comparison of 4% articaine and 0.5% levobupivacaine/2% lidocaine mixture for sub-Tenon’s anaesthesia in phacoemulsification cataract surgery: a randomised controlled trial. Br J Ophthalmol. 2008;92:496–499. doi: 10.1136/bjo.2007.115576. [DOI] [PubMed] [Google Scholar]
- 63.Erkul E, Babayigit M, Kuduban O. Comparison of local anesthesia with articaine and lidocaine in septoplasty procedure. Am J Rhinol Allergy. 2010;24:123–126. doi: 10.2500/ajra.2010.24.3514. [DOI] [PubMed] [Google Scholar]
- 64.Schulze KE, Cohen PR, Nelson BR. Articaine: an effective adjunctive local anesthetic for painless surgery at the depth of the muscular fascia. Dermatol Surg. 2006;32:407–410. doi: 10.1111/j.1524-4725.2006.032082.x. [DOI] [PubMed] [Google Scholar]
- 65.Dogan N, Ucok C, Korkmaz C, Uycok O, Karasu HA. The effects of articaine hydrochloride on wound healing: an experimental study. J Oral Maxillofac Surg. 2003;61:1467–1470. doi: 10.1016/j.joms.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 66.Gerner P, Strichartz GR. Sensory and motor complications of local anesthetics. Muscle Nerve. 2008;37:421–425. doi: 10.1002/mus.20967. [DOI] [PubMed] [Google Scholar]
- 67.Zaric D, Christiansen C, Pace NL, Punjasawadwong Y. Transient neurologic symptoms (TNS) following spinal anaesthesia with lidocaine versus other local anaesthetics. Cochrane Database Syst Rev. 2005;2:CD003006. doi: 10.1002/14651858.CD003006.pub2. [DOI] [PubMed] [Google Scholar]
- 68.Pogrel MA. Permanent nerve damage from inferior alveolar nerve blocks – an update to include articaine. J Calif Dent Assoc. 2007;35:271–273. [PubMed] [Google Scholar]
- 69.Denson DD, Bridenbaugh PO, Turner PA, Phero JC. Comparison of neural blockade and pharmacokinetics after subarachnoid lidocaine in the rhesus monkey. II: Effects of volume, osmolality, and baricity. Anesth Analg. 1983;62:995–1001. [PubMed] [Google Scholar]
- 70.Nakamura T, Popitz-Bergez F, Birknes J, Strichartz GR. The critical role of concentration for lidocaine block of peripheral nerve in vivo: studies of function and drug uptake in the rat. Anesthesiology. 2003;99:1189–1197. doi: 10.1097/00000542-200311000-00028. [DOI] [PubMed] [Google Scholar]
- 71.Mather LE, Copeland SE, Ladd LA. Acute toxicity of local anesthetics: underlying pharmacokinetic and pharmacodynamic concepts. Reg Anesth Pain Med. 2005;30:553–566. doi: 10.1016/j.rapm.2005.07.186. [DOI] [PubMed] [Google Scholar]
- 72.Rosenberg PH, Veering BT, Urmey WF. Maximum recommended doses of local anesthetics: a multifactorial concept. Reg Anesth Pain Med. 2004;29:564–575. doi: 10.1016/j.rapm.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Wolf JW, Butterworth JF. Local anesthetic systemic toxicity: update on mechanisms and treatment. Curr Opin Anesthesiol. 2011;24:561–566. doi: 10.1097/ACO.0b013e32834a9394. [DOI] [PubMed] [Google Scholar]
- 74.Drasner K. Local anesthetic toxicity: optimal management to avoid neurotoxic injury and treat cardiac arrest. ASA Refresher Courses in Anesthesiology. 2011;39:33–40. [Google Scholar]
- 75.Szabó A, Szentandrássy N, Birinyi P, et al. Effects of articaine on action potential characteristics and the underlying ion currents in canine ventricular myocytes. Br J Anaesth. 2007;99:726–733. doi: 10.1093/bja/aem263. [DOI] [PubMed] [Google Scholar]
- 76.Szentandrássya N, Szabób A, Almássya J, et al. Effects of articaine and ropivacaine on calcium handling and contractility in canine ventricular myocardium. Eur J Anaesth. 2010;27:153–161. doi: 10.1097/EJA.0b013e328331a37b. [DOI] [PubMed] [Google Scholar]