Abstract
Background
Biological pacing performed solely via HCN2 gene transfer in vivo results in relatively slow idioventricular rates and only moderate autonomic responsiveness. We induced biological pacing using the Ca2+-stimulated adenylyl cyclase AC1 gene expressed alone or in combination with HCN2 and compared outcomes to those with single gene HCN2 transfer.
Methods and Results
We implanted adenoviral HCN2, AC1, or HCN2/AC1 constructs into the left bundle branches (LBB) of atrioventricular-blocked dogs. During steady-state gene expression (days 5-7), differences between AC1, HCN2/AC1 and HCN2 alone were evident in basal beating rate, escape time, and dependence on electronic back-up pacing. In HCN2, AC1, and HCN2/AC1, these parameters were, respectively: Basal beating rate: 50±1.5bpm, 60±5.0bpm, and 129±28.9bpm (P<0.05 for HCN2/AC1 vs. HCN2 or AC1 alone); Escape time: 2.4±0.2 sec, 1.3±0.2 sec, and 1.1±.0.4sec (P<0.05 for AC1 and HCN2/AC1 vs. HCN2); and % Electronic beats: 34±8%, 2±1%, and 6±2% (P<0.05 for AC1 and HCN2/AC1 vs. HCN2). Instantaneous (SD1) and long-term (SD2) heart rate variability (HRV) and circadian rhythm analyzed via 24h Holter recordings showed a shift toward greater sensitivity to parasympathetic modulation in animals injected with AC1 as well as a high degree of sympathetic modulation in animals injected with HCN2/AC1.
Conclusions
AC1 or HCN2/AC1 overexpression in LBB provides highly efficient biological pacing and greater sensitivity to autonomic modulation than HCN2 alone.
Keywords: Pacemakers, Gene Therapy, Adenylyl Cyclase, HCN Channels, Heart Rate Variability
Introduction
Despite ongoing technological advances, 5% of clinical pacemaker implantations have serious complications.1 These adverse events together with other limitations of electronic pacing, (e.g., limited autonomic responsiveness and battery life, difficulties associated with growth and development in pediatric care, and association with significant cardiac remodeling) have prompted development of biological alternatives.2-5 Approaches have ranged from transplanting spontaneously beating cell aggregates, e.g., derivatives of embryonic stem cells,6, 7 to delivery of pacemaker function-related genes via viral vectors or cell platforms. Although not yet implanted for biological pacing, induced pluripotent stem cells8, 9 are another potential option.
Gene therapy-based strategies reported include engaging the β-adrenergic signaling cascade via overexpression of the β2-adrenergic receptor (B2AR)10, 11 or its downstream target AC6,12 dominant negative knock-down of inward rectifier channels to eliminate the IK1 contribution to resting membrane potential,13 or overexpressing ion channels to generate inward current such as If encoded by hyperpolarization-activated cyclic nucleotide-gated (HCN) channels14-18 or mutated potassium channels.19 Each strategy has inherent advantages and shortcomings although the HCN-based approach appears currently favored for several reasons: (1) HCN channels generate de novo pacemaker function in various tissues and large animal models, whereas B2AR or AC6 generate pacemaker activity only in response to catecholamines;10-12 (2) HCN channels are activated upon hyperpolarization and remain open during diastole, thereby avoiding the prolongation of repolarization which complicates the dominant negative Ik1 strategy;20 (3), HCN channels respond to autonomic modulation via the cyclic adenosine monophosphate (cAMP) binding domain located in the carboxy terminus.21 This permits direct modulation of biological pacemaker function by cholinergic and adrenergic stimuli, a property not incorporated in potassium channel-based strategies.
Although HCN2-based biological pacemakers respond to catecholamine administration, physical activity and emotional arousal,15, 22 their basal rates are sufficiently low that electronic back-up pacing of 30-40% of beats is required. Introducing HCN-mutants has provided some improvement in the biological pacemaker profile. For example the HCN2 mutant E324A showed improved sensitivity to catecholamine stimulation as compared to wild type HCN215, the HCN1 deletion within the S3-S4 linker (235-7EVY) induced spontaneous activity in cultured guinea pig myocytes whereas wild type HCN1 overexpressing myocytes remained silent.23 Finally an HCN212 chimera incorporating the N- and C- termini of HCN2 and the transmembrane region of HCN1 increased beating rates in vivo but also induced ventricular tachycardias exceeding 200 bpm.24 Furthermore, considerable dependence on electronic back-up pacing persisted with these constructs, with pauses >2sec in response to overdrive pacing.15, 24 Hence, optimization of gene-based pacemakers is desirable. While “optimization” has not been specifically defined in the literature, we have suggested an optimally firing biological pacemaker implanted in an adult human likely would have basal rates in the 60-90 bpm range and peak catecholamine- or exercise- stimulated rates around 130-160 bpm.3
One approach to optimization might be offered by the Ca2+ stimulated adenylyl cyclase gene AC1, which is abundant in the sinoatrial node (SAN)25, 26 and shows enhanced activity in response to Ca2+.27, 28 The latter property likely contributes to the elevated baseline cAMP levels of SAN cells,26 which importantly impact not only on If but on the entire spectrum of pacemaker mechanisms in SAN cells.29 In a study of the mechanism of Ca2+-dependent β-adrenergic modulation of HCN2, AC1 overexpression in neonatal myocytes increased baseline cAMP levels, positively shifted the activation, V1/2, of overexpressed HCN2, and increased spontaneous beating.30 As expected, comparable overexpression of the working myocardial isoform AC6 did not modify these parameters.
The outcomes of these in vitro studies led us to hypothesize that AC1 overexpression in left bundle branch (LBB) will generate highly efficient biological pacemaker activity; both alone and in combination with overexpressed HCN2. We tested this in a previously reported canine model of complete heart block.15
Methods
Experiments were performed under protocols approved by the Columbia University Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication No. 85-23, revised 1996).
Adenoviral Constructs
Adenoviral constructs of green fluorescent protein (GFP), mouse HCN2, and (FLAG)-tagged AC1 (a generous gift of Dr. Ross Feldman, University of West Ontario, London, Canada), all driven by the cytomegalovirus promoter, were prepared as described previously.31 For consistency with earlier studies, we prepared 3×1010 fluorescent forming units (ffu) of one adenovirus and combined this suspension with an equal amount of another adenovirus in a total volume of 700 μL to obtain the following groups: HCN2 (n=12) comprised of 7 previously reported HCN2/GFP-treated animals22 and 5 current HCN2/GFP-treated animals. The other groups received AC1/GFP (designated AC1, n=5) or HCN2/AC1 (n=7).
Intact Canine Studies
Adult mongrel dogs (Chestnut Ridge Kennels, Chippensburg, PA) weighing 22-25 kg were anesthetized with propofol (6 mg/kg, IV) and inhalational isoflurane (1.5% to 2.5%). An electronic pacemaker (Guidant, Discovery II, Flextend lead, Guidant Corp, Indianapolis, IN) was implanted and set at VVI 35 bpm. Using a steerable catheter, we injected adenoviral vectors into three LBB sites as previously described.15 We paced each injection site transiently via the catheter electrode to facilitate pace-mapping of the origins of idioventricular rhythms electrocardiographically during follow-up. Complete atrioventricular block was induced via radiofrequency ablation. Electrocardiograms (ECG), 24-hour Holter monitoring, pacemaker log record review, and overdrive pacing at either 80 bpm or 5% faster than intrinsic rates were performed daily. ECG intervals were calculated from baseline ECGs recorded on day 5-7. To correct the QT intervals we used both Bazett's formula and Matsunaga et al's one-parameter logarithmic formula: (QTc = log600 × QT/log RR).32 For each dog, the percent of electronically induced beats was calculated daily. Biological pacemaker function on the first day after construct injection is usually minimal and is typically confounded by ectopic activity resulting from the injection trauma; therefore, we excluded data from day 1 from our analysis.
In a subset of the HCN2/AC1-injected animals (n=4) that exhibited rapid idioventricular rhythms during resting ECG recordings, we turned off the electronic pacemaker, infused ivabradine (IVB) (1 mg/kg diluted in 30 ml saline, IV.) over 5 minutes, and monitored the ECG continuously for 30 minutes to evaluate the effect of treatment. Continuous 24hr monitoring was performed thereafter. IVB was kindly provided by Servier Laboratories (Courbevoie, France).
Twenty-four hour monitoring was performed via Holter ECG (Rozinn [Scottcare], Glendale, New York, U.S.A.). We defined basal beating rate as that which was recorded daily with the animal resting quietly on the examination table. We calculated maximal beating rates daily from 30-sec strips of a stable rhythm at maximal rate. Pace-mapping was performed based on 6 lead ECG recordings obtained at the time of implantation, during pacing from the injection site. During subsequent daily 6 lead ECG testing we identified rhythms of comparable morphology and QRS-axis and recorded them simultaneously with our 3 lead Holter system. In this way we could identify and determine the characteristics of both matching and non-matching rhythms on 6-lead ECG and on Holter (Supplementary Figure 1).
We performed detailed HRV analysis on Holter recordings registered during steady-state gene expression (days 5-7; one day/animal). In this analysis we calculated the percentage of matching beats, the percentage of non-matching beats, the percentage of paced beats, and the 24hr average beating rate of the matching beats. Furthermore, we classified all pace-mapped beats as “normal” in order to calculate the standard deviation (SD) of their instantaneous RR-interval variability, SD1, and the SD of long-term continuous RR-interval variability, SD2. To analyze circadian modulation, we compared the rate of pace-mapped beats and dependence on electronic back-up pacing during sleep (2:00-4:00 AM) versus during feeding and physical activity (8:00–10:00 AM). Investigators involved in the Holter analysis were not blinded for the study group.
To evaluate β-adrenergic responsiveness at termination of the study (day 7-8), we infused epinephrine (1.0, 1.5 and 2.0 μg/kg/min, IV) for 10 min as previously reported15 and recorded the rate response of the pace-mapped rhythm.
Immunohistochemistry
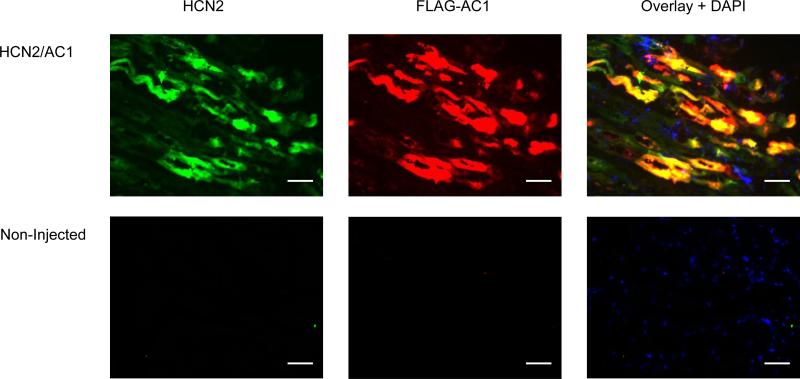
HCN2 and AC1 overexpression were validated by immunohistochemistry (Figure 1). The threshold for HCN2 detection was set above the level required to detect endogenous HCN2 in order to detect HCN2 only in animals that had received HCN2 adenovirus. Similarly, FLAG staining was positive only in animals that received AC1 adenovirus.
Figure 1.
Immunohistochemical staining for HCN2 and AC1. Positive HCN2 (green) and AC1 (red; FLAG antibody) staining were detected in the injected LBB from animals that received the corresponding adenovirus. Nuclei were stained blue using 4',6-diamidino-2-phenylindole (DAPI). Bars represent 50 μm.
Tissue blocks (LBB and surrounding endocardium) were snap-frozen in liquid nitrogen, and 5-μm serial sections were cut with a cryostat (Microm HM505E) and air dried. Sections were washed in phosphate buffered saline, pretreated for 10 minutes with 0.2% triton X, blocked for 20 minutes with 10% goat serum, and incubated overnight at 4°C with anti-FLAG antibody (1:200, Sigma-Aldrich, St Louis, MO) and anti-HCN2 antibody (1:200, Alomone, Jerusalem, Israel). Antibody bound to target antigen was detected by incubating sections for 2 hours with goat anti-mouse IgG labeled with Cy3 (red fluorescence for AC1) and goat anti-rabbit IgG labeled with Alexa 488 (green fluorescence for HCN2). Images were recorded with a Nikon E800 fluorescence microscope.
Statistical Analysis
Data are presented as means ± SEM. Statistical significance was examined by t-test or by ANOVA followed by Bonferroni's post-hoc test. In the following data sets we did not detect a normal Gaussian distribution: % matching rhythm, % non-matching rhythm, % paced (Holter), morning rate of matching, % paced (morning), and the maximal duration of electronic pacing. In these cases we examined statistical significance by Wilcoxon matched-pairs signed rank test and Kruskal-Wallis one way ANOVA followed by Dunn's multiple comparison test. P<0.05 was considered significant. The authors had full access to and take responsibility for the integrity of the data.
Results
Basal function
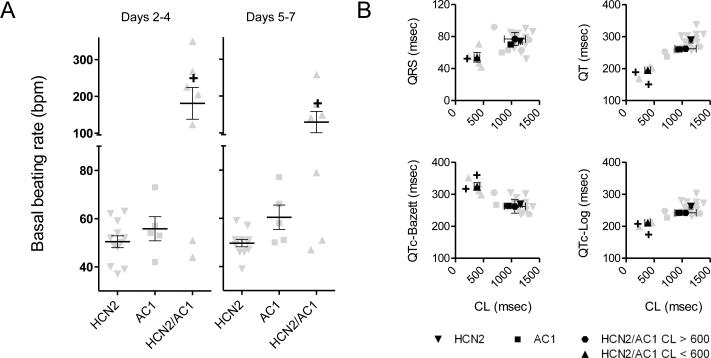
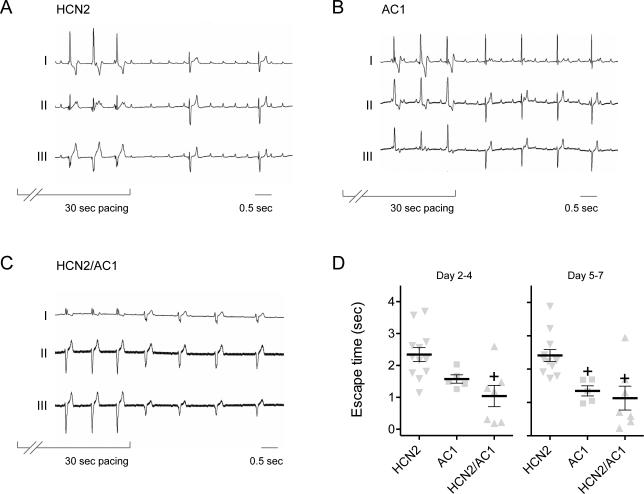
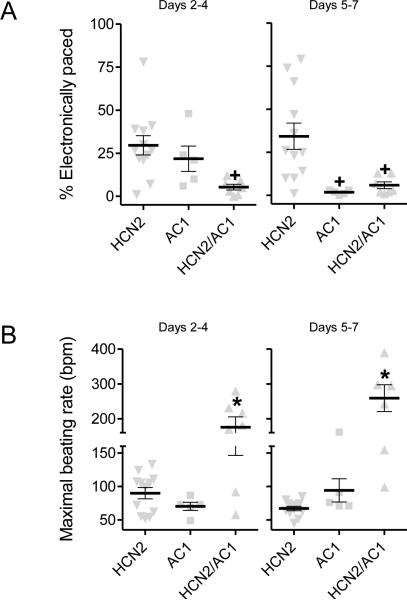
Basal beating rates did not significantly differ in the AC1 and HCN2 groups, but that for the HCN2/AC1 group was more rapid than desirable and significantly faster than the HCN2 group (Figure 2A and Supplementary Figure 2). The analysis of ECG intervals indicated significantly shorter cycle lengths in HCN2/AC1 as compared to HCN2 alone. Furthermore, in HCN2/AC1-injected animals with cycle lengths (CL) < 600ms, QT and QTc-log were significantly shortened (P<0.05 vs. HCN2) using the Matsunaga et al formula32 whereas QTc-Bazett was significantly prolonged (P<0.05 vs. HCN2; Figure 2B). The reemergence of pacemaker activity following overdrive pacing was significantly more rapid in the AC1 and HCN2/AC1 groups than HCN2 alone (Figure 3). The differences in basal rate and escape time had a sizeable impact on the dependence on electronic back-up pacing, which was significantly reduced in the AC1 and HCN2/AC1 groups as compared to HCN2 alone (Figure 4A).
Figure 2.
Basal beating rates in HCN2/AC1-injected animals are faster than the other groups. A, Summary data pooled for days 2-4 and 5-7. B, Summary data on ECG intervals pooled for days 5-7. HCN2 n=12, AC1 n=5, HCN2/AC1 n=7. + = P<0.05 vs. HCN2 alone.
Figure 3.
Reemergence of pacemaker activity after overdrive suppression in AC1 and HCN2/AC1-injected animals is faster than HCN2. A-C, Representative examples of overdrive suppression experiments in HCN2, AC1 and HCN2/AC1-injected animals respectively. D, Summary data pooled for days 2-4 and 5-7. HCN2 n=12, AC1 n=5, HCN2/AC1 n=7. + = P<0.05 vs. HCN2 alone.
Figure 4.
Dependence on electronic back-up pacing and maximal beating rates achieved. A, Reduced dependence on electronic back-up pacing in both AC1 alone and HCN2/AC1-injected animals. B, Excessively rapid maximal beating rates in HCN2/AC1-injected animals. HCN2 n=12, AC1 n=5, HCN2/AC1 n=7. + = P<0.05 vs. HCN2 alone, * = P<0.05 vs. HCN2 or AC1 alone.
Maximal beating rates and response to Ivabradine
Using 24hr Holter recordings, we investigated the maximal rates achieved in the three groups. Rates were significantly more rapid in HCN2/AC1-injected animals than in the other groups throughout the study (Figure 4B). Given the maximal rates attained in the HCN2/AC1 group, sometimes exceeding 250 bpm, we treated 4 of these animals with the If blocker IVB. This significantly slowed but did not silence pacemaker activity, as the pace-mapped rhythms continued at slower rates (Figure 5).
Figure 5.
Significant slowing of ventricular tachycardia in HCN2/AC1 by ivabradine (IVB). A, Representative ECG tracings of pace-mapped rhythm before and after IVB infusion. B, Summary data of beating rates before, during and after IVB administration. n=4. † = P<0.05 vs. baseline
24 hour analysis of pace-mapped rhythms and of autonomic modulation
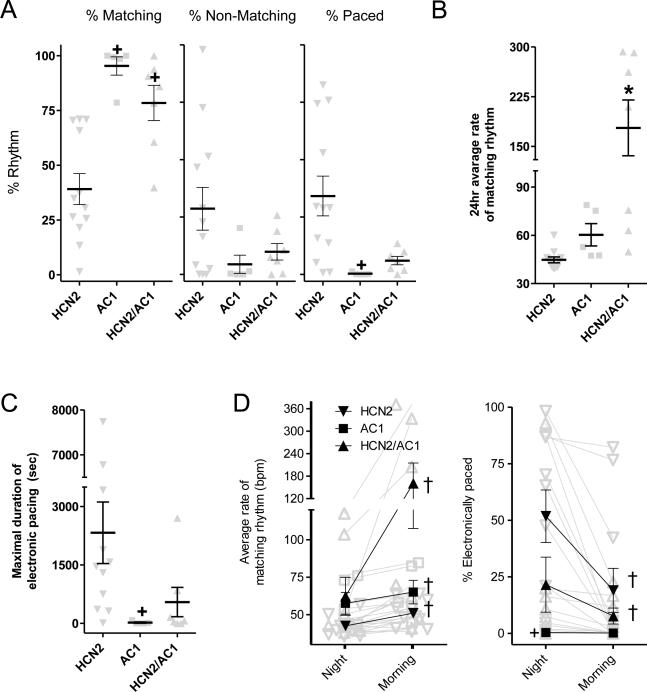
The percentage of matching pace-mapped beats (Supplementary Figure 1 and 3) was significantly higher in the AC1 and HCN2/AC1 groups (>95% and >75%, respectively) compared to HCN2 alone (~40%, P<0.05 vs. the other groups) (Figure 6A, left panel). This increase was accompanied by a significant reduction in the percentage of electronically paced beats in AC1 vs. HCN2-injected animals (P<0.05; Figure 6A, right panel). The 24h average rate of pace-mapped rhythms is summarized in Figure 6B, showing a significant increase in rate in HCN2/AC1 vs. the other groups (P<0.05), which correlated with the day 5-7 averages of basal and maximal beating rates reported above. The greater dependence on electronic back-up pacing in the HCN2/AC1 vs. AC1 groups derived from the finding that HCN2/AC1-induced rhythms sometimes overdrive-suppressed themselves, a process that did not occur in animals injected with AC1 (sample tracings of overdrive suppression are shown in Supplementary Figure 4). The maximal durations of episodes of electronic pacing are summarized in Figure 6C. In AC1-injected animals the maximal duration of electronic pacing was significantly shorter than HCN2.
Figure 6.
A, Summary data of percentage of matching beats (corresponding to pace-mapping), non-matching, and electronically paced beats. HCN2 n=12, AC1 n=5, HCN2/AC1 n=7. B, 24hr average rate of matching beats. C, Summary data of day/night modulation of pace-mapped beating rates (left panel) and dependence on electronic back-up pacing (right panel). D, Summary data on the maximal duration of electronic pacing. Panel B-D: HCN2 n=11, AC1 n=5, HCN2/AC1 n=7. + = P<0.05 vs. HCN2 alone; * = P<0.05 vs. HCN2 or AC1 alone; † = P<0.05 for morning vs. night.
To test whether the changes in beating rate and dependence on back-up electronic pacing occurred in accordance with what would be expected based on a normal circadian rhythm, we compared these parameters during 2h of sleep (2:00-4:00 AM) with 2h of feeding and activity (8:00-10:00 AM). Slower matching rhythms and higher percentages of electronically paced beats occurred in the HCN2-injected animals, primarily during the night (P<0.05 vs. AC1 and HCN2/AC1; Figure 6D). In addition, beating rates increased in all groups from the resting state during the night to the active state in the morning (P<0.05). In the HCN2 and HCN2/AC1 groups, the percentage of electronically paced beats in the morning was also lower than in the night (P<0.05).
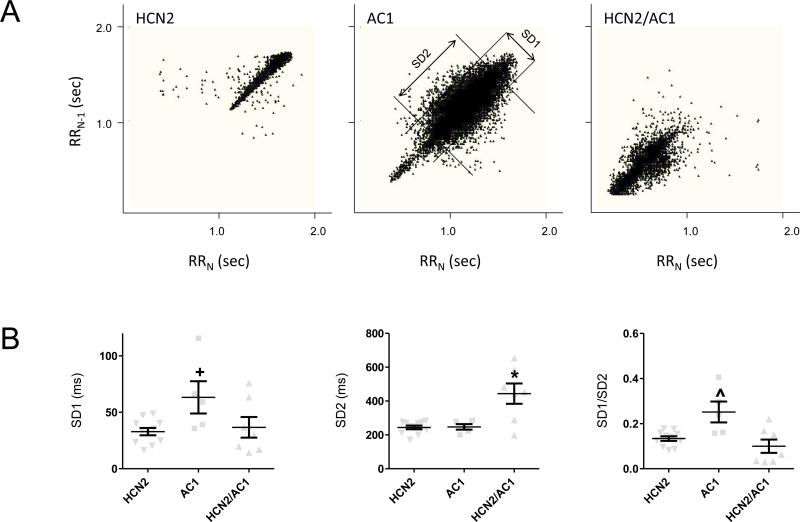
Poincaré plots of pace-mapped rhythms showed differences in HRV among the three groups (Figure 7A). Significant modulation of heart rates occurred in HCN2-injected animals, (Figure 7A, left panel). AC1-injected animals showed more pronounced modulation that occurred over a wider range of heart rates than HCN2 (Figure 7A, center panel). HCN2/AC1-injected animals showed pronounced modulation that primarily occurred during intermediate to very rapid heart rates (Figure 7A, right panel).
Figure 7.
Analysis of heart rate variability. A, Representative Poincaré plots of pace-mapped beats in 24hr Holter recordings of HCN2, AC1, and HCN2/AC1-injected animals. The middle panel (AC1-injected animal) also shows standard deviation (SD) of instantaneous RR-interval variability (SD1) and SD of long term continuous RR-interval variability (SD2). B, Summary data of SD1, SD2 and SD1/SD2. HCN2 n=11, AC1 n=5, HCN2/AC1 n=7. + P<0.05 vs. HCN2 * = P<0.05 vs. HCN2 or AC1 alone; ^ = P<0.05 vs. HCN2 alone or HCN2/AC1.
Quantitative analysis of SD1, SD2, and SD1/SD2 revealed that the level of sympathetic modulation expressed by long-term variation of heart rates (SD2) was comparable between HCN2 and AC1 and was maximal in animals injected with HCN2/AC1 (P<0.05 vs. HCN2 and AC1; Figure 7B, middle panel). Parasympathetic modulation, as expressed by short-term variation of heart rates (SD1) was comparable between HCN2 and HCN2/AC1 and was maximal in animals injected with AC1 (P<0.05 vs. HCN2; Figure 7B, left panel). Furthermore, the significantly increased ratio of SD1/SD2 in the AC1-injected animals supports the notion of greater sensitivity to parasympathetic modulation in this group than in the other groups (P<0.05 vs. HCN2 and HCN2/AC1; Figure 7B, right panel).
On the final day of the study, we tested the response to epinephrine. Figure 8A illustrates the individual rate responses during 10 minutes of infusion with 1.0 μg/kg/min epinephrine. After the 10 minute exposure to epinephrine, all groups exhibited a significant increase in rate compared to baseline (P<0.05) and beating rates in the HCN2/AC1 group were significantly faster than in the HCN2 group (P<0.05; Figure 8B). Animals that responded with an increase in rate <50% received higher doses of epinephrine up to 2.0 μg/kg/min. In the HCN2 group this did not result in a more extensive effect, in the other groups numbers were to low top draw further conclusions in relation to the higher doses.
Figure 8.
Catecholamine-stimulated rates in HCN2/AC1 group are greater than in HCN2. A, Time course of effect during first 10 minutes of epinephrine (1.0μg/kg/min) infusion. B, Summary data for the complete epinephrine protocol; animals that did not show an increase in beating rates of >50% during the first 10 minutes received additional doses of 1.5 μg/kg/min and 2.0 μg/kg/min epinephrine respectively. For all three groups, epinephrine increased rate significantly over baseline. One of the HCN2/AC1 injected animals exhibited a rapid rhythm of > 200 bpm at the time of study termination and was therefore not further challenged with epinephrine infusion. HCN2 n=12, AC1 n=5, HCN2/AC1 n=5. † = P<0.05 vs. baseline; + = P<0.05 vs. HCN2 alone.
Discussion
This study demonstrates significant changes in biological pacemaker function achieved with AC1 or HCN2/AC1 gene transfer. It is noteworthy that biologically induced rhythms at physiological beating rates based on overexpression of AC1 alone were generated for more than 95% of beats. Additionally, AC1 gene transfer resulted in robust sensitivity to sympathetic and parasympathetic modulation to a greater degree than has been reported for any single gene-based pacemaker strategies. Sensitivity to sympathetic modulation was further enhanced when HCN2 and AC1 were injected together, but this combination also resulted in an excessive increase in basal beating rate.
Biological pacemaker function in relation to other approaches
In comparing the approaches tested we used the following criteria as optimal biological pacing outcomes: basal beating rates of 60-90 bpm, escape times closely matching the basal cycle length (i.e. if the basal rate is 60 bpm – escape times of ~1sec would be optimal and represent a situation in which no beat is missed), an autonomic response resulting in rate increases to 130-160 bpm and low-to-absent dependence on electronic back-up pacing. Among the three groups studied, overall outcomes in the AC1 group were superior to those with the HCN2 and HCN2/AC1 groups. For AC1 alone on days 5-7, beating rates were 60-70 bpm (Figure 2), escape times were <1.5 sec (Figure 3), and the dependence on electronic back-up pacing was reduced to <2% (Figure 4A).
These outcomes compare favorably to those reported previously for gene and cell based pacemakers. Biological pacemakers based on HCN2 or the mutant HCN2-E324A have consistently exhibited episodes of excess bradycardia requiring significant (~35%) dependence on electronic back-up pacing.15, 33 A truncated HCN1 construct administered to the left atrium shifted the kinetics of activation positively and reduced dependence on electronic back-up pacing to only ~15%.18 This construct was used to provide atrial pacing in a porcine model of SA node dysfunction, which is a different setting than atrioventricular block and demand ventricular pacing. Finally, preliminary experiments have shown highly efficient biological pacemaker function based on combined gene transfer of HCN2 and the skeletal muscle sodium channel (SkM1).34 Potential advantages of the AC1-based approach may include the smaller gene size of AC1 than SkM1, facilitating packaging into smaller delivery vehicles such as adeno-associated virus, and the lesser complexity of a single gene-based approach.
Rates generated in the HCN2/AC1 group were excessive, but the outcomes provide insight at several levels. First, they show that when co-expressed, AC1 and HCN2 can function synergistically to increase basal beating rates (Figure 2) and sensitivity to sympathetic modulation (Figure 7A-B and 8). Second, although rhythms with the AC1/HCN2 combination were excessively rapid (Figure 4B), pacemaker activity remained stable, as indicated by the infrequent need for electronic back-up pacing (Figure 4A), high number of beats that pace-mapped to the injection site (Figure 6A), and intact autonomic modulation (Figures 6-8). Third, the results support the earlier finding that if excessive rate accelerations occur as a result of HCN-associated pacing, then effective treatment is provided by If blockade (Figure 5).24 Finally, the outcomes in the HCN2, AC1 and HCN2/AC1 groups suggest that a biological pacemaker profile superior to the approaches using either HCN2 or AC1 may be generated if gene expression is sufficiently down-titrated in the combination strategy.
Mechanisms underlying AC1-based biological pacemaker function
In vitro studies have confirmed some of the mechanisms contributing to AC1-induced pacemaker function.30 They demonstrated that in AC1-overexpressing neonatal myocytes, cAMP was increased in basal conditions and that the increase in cAMP impacted on downstream targets of cAMP. The outcome was the positive activation shift of co-expressed HCN2 channels, rendering more channels available for membrane depolarization. Additionally, in the setting of overexpressed HCN2-R/E, a mutant channel that is insensitive to modulation by cAMP, kinetics of If were, as expected, unmodified by overexpression of AC1. Yet, neonatal myocytes that co-expressed HCN2-R/E and AC1 had significantly faster beating rates than cells that overexpressed HCN2-R/E alone, further supporting the notion that AC1 can enhance pacemaker mechanisms other than If.30 Therefore, it is likely that the pacemaker function generated by overexpressing AC1 results from direct effects of cAMP elevation on targets that are sensitive to cyclic nucleotides such as HCN channels and from indirect effects on Ca-handling proteins that may be enhanced via PKA-mediated phosphorylation. Examples of phosphorylation targets that may impact importantly on pacemaker function include the L-type Ca-channels, phospholamban, ryanodine receptors, and K-channels.29 Consistent with the above-mentioned preliminary data suggesting that AC1 stimulates pacemaker mechanisms other than If was our finding that pacemaker activity in HCN2/AC1 in vivo was not silenced completely upon administration of IVB (Figure 5). This observation was in contrast to our previous investigation with the chimera HCN212, in which a similar IVB protocol completely suppressed HCN212-based and endogenous idioventricular pacemaker activity24 while SAN pacemaker activity remained unaffected. In sum, in vitro and in vivo data are consistent with enhancement of If and other likely Ca-based pacemaker mechanisms by AC1. With regard to the debate over Ca-clock versus HCN roles in pacemaker function29, our work supports the likelihood that a combination of Ca-based and If-based mechanisms are responsible.
Autonomic modulation of HCN2, AC1 and HCN2/AC1-based pacemakers
Direct sensitivity to autonomic modulation is a potential key advantage of biological over electronic pacemakers.22 With this in mind, we concluded that HCN2 and AC1-based biological pacemakers incorporate a similar degree of sensitivity to sympathetic modulation based on SD2 (Figure 7B, middle panel), night vs. morning rhythms (Figure 6B, left panel, and response to catecholamine infusion (Figure 8). Furthermore, the higher SD2 in HCN2/AC1 vs. HCN2 or AC1 alone (Figure 7B, middle panel), and increased beating rates in the morning (Figure 6B, left panel) or following catecholamine administration (Figure 8), indicate that the response to sympathetic modulation may be further enhanced when HCN2 and AC1 are combined.
Also of interest is the high degree of HRV in the AC1 group observed in the fan-like pattern in the Poincaré plot (Figure 7A, middle panel), which closely resembles the pattern of sinus rhythm and is indicative of vagosympathetic modulation. Heightened sensitivity to parasympathetic stimulation was confirmed by the significant increase in SD1 and SD1/SD2 (Figure 7B). Given the strong correlation between higher HRV values and improved cardiovascular health38, 39 and the association of severe phenotypes of diabetic neuropathy, myocardial infarction, and heart failure, with reduced HRV and poor prognosis, the increased HRV in the AC1 group might suggest potential benefit in the modulation of cardiovascular chronotropy. This would be consistent with the association of increased HRV with interventions that improve prognosis such as disease-altering pharmacotherapy and physical exercise.40, 41
QT interval measurements
The QT and QTc intervals (Figure 2B) were not affected by AC1 alone, while for the HCN2/AC1 intervention – at cycle lengths bellow 600 msec – a significant shortening of QT and QTc-log was seen with the Matsunaga et al formula32 while QTc with the Bazett formula was significantly prolonged. It should be noted that Bazett's formula overestimates the actual values at short RR intervals, while underestimating them at long RR intervals in humans (see 30 for review) and dogs.45, 46 Without entering into the remaining controversies regarding which QTc correction to use in which experimental setting, the following should be noted: (1) the Matsunaga formula32 has been found preferable to Bazett for testing QTc prolongation in beagle dogs and likely other breeds; (2) despite the first point, the QTc prolongation with the Bazett formula in the combined AC1/HCN2 biological pacemaker setting (at cycle lengths < 600 ms) should call for vigilance in evaluating the occurrence of proarrhythmia either spontaneously or induced via electrophysiological testing; (3) the occurrence of QTc prolongation with overexpressed HCN2/AC1 at low cycle lengths, but not with HCN2/AC1 at higher cycle lengths nor with sole overexpression of AC1 or HCN2 points out the complexity of events that may occur when various gene therapies are administered singly versus together in settings where their impact on a physiologic process is being assessed.
Clinical applicability
The clinical applicability of biological pacing is still uncertain, as much remains to be discovered and there are contemporaneous improvements in electronic pacing.3 Within the framework of a biological approach, we believe that gene transfer in the HCN2, AC1, and HCN2/AC1 spectrum has significant potential. However, the demonstration of safe and long-term function will be pivotal for translation to clinical application. The next steps in proof-of-concept of viral HCN2, AC1, and HCN2/AC1 approaches will be to use vectors that induce long-term gene expression, e.g., lentiviral or adeno-associated viral vectors.42-44 Stability of pacemaker function based on HCN2, AC1, or HCN2/AC1 overexpression is not known in these settings and its demonstration will be crucial. There is also need for caution related to the overexpression of AC1, either alone or in combination with HCN2. In settings of myocardial infarction or heart failure, elevating cAMP is known to have arrhythmogenic consequences. Safety testing for proarrhythmia during ischemia or cardiac hypertrophy will be useful in the further exploration of these biological pacemaker strategies.
Conclusions
AC1 generates efficient biological pacemaker function either alone or when co-expressed with HCN2. In addition, the baseline function and autonomic responsiveness generated by AC1 are superior to those of HCN2 alone. Finally, our in vivo as well as previous in vitro30 studies suggest that AC1 enhances If-dependent and If-independent pacemaker mechanisms, which may explain why pacemaker activity is more robust than in the setting of HCN2 overexpression alone.
Supplementary Material
Clinical Perspective.
In the United States, approximately 300.000 pacemakers are implanted annually, 5% of which result in serious complications requiring surgical revision or other invasive procedures. In addition, electronic pacemakers have limitations such as an inadequate autonomic response, limited battery life and restrictions with regard to stable lead positioning. These issues may in part be dealt with by the development of biological pacemakers. Within the framework of biological pacing the AC1-based approach shows potential because it generates highly stable pacemaker function at beating rates of approximately 60 bpm and incorporates sensitivity to sympathetic and parasympathetic input. Two important hurdles on the road toward clinical application include 1) demonstration of stable long term-function, and 2) demonstration of safety with regard to both potential proarrhythmia and toxicity. The continued development of electronic pacemakers may obviate the need for biological alternatives, but regardless of whether biological pacemakers find clinical application, their development will continue to increase our understanding of pacemaker function and of cardiac gene therapy.
Acknowledgments
Sources of Funding
This work was supported by United States Public Health Service, National Heart, Lung, and Blood Institute grant HL-094410. GJJB received grant support from the Netherlands Heart Foundation, the Netherlands Foundation for Cardiovascular Excellence, the Dr. Saal van Zwanenberg Foundation and the Interuniversity Cardiology Institute of the Netherlands (ICIN). HLT received grant support from the Netherlands Heart Foundation (2005B180) and the Netherlands Organization for Scientific Research (NWO, grant ZonMW Vici 918.86.616).
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eberhardt F, Bode F, Bonnemeier H, Boguschewski F, Schlei M, Peters W, Wiegand UK. Long term complications in single and dual chamber pacing are influenced by surgical experience and patient morbidity. Heart. 2005;91:500–506. doi: 10.1136/hrt.2003.025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siu CW, Lieu DK, Li RA. Hcn-encoded pacemaker channels: From physiology and biophysics to bioengineering. J Membr Biol. 2006;214:115–122. doi: 10.1007/s00232-006-0881-9. [DOI] [PubMed] [Google Scholar]
- 3.Rosen MR, Robinson RB, Brink PR, Cohen IS. The road to biological pacing. Nat Rev Cardiol. 2011;8:656–666. doi: 10.1038/nrcardio.2011.120. [DOI] [PubMed] [Google Scholar]
- 4.Marban E, Cho HC. Biological pacemakers as a therapy for cardiac arrhythmias. Curr Opin Cardiol. 2008;23:46–54. doi: 10.1097/HCO.0b013e3282f30416. [DOI] [PubMed] [Google Scholar]
- 5.Boink GJ, Seppen J, de Bakker JM, Tan HL. Gene therapy to create biological pacemakers. Med Biol Eng Comput. 2007;45:167–176. doi: 10.1007/s11517-006-0112-7. [DOI] [PubMed] [Google Scholar]
- 6.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 7.Xue T, Cho HC, Akar FG, Tsang SY, Jones SP, Marban E, Tomaselli GF, Li RA. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: Insights into the development of cell-based pacemakers. Circulation. 2005;111:11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- 8.Novak A, Shtrichman R, Germanguz I, Segev H, Zeevi-Levin N, Fishman B, Mandel YE, Barad L, Domev H, Kotton D, Mostoslavsky G, Binah O, Itskovitz-Eldor J. Enhanced reprogramming and cardiac differentiation of human keratinocytes derived from plucked hair follicles, using a single excisable lentivirus. Cell Reprogram. 2010;12:665–678. doi: 10.1089/cell.2010.0027. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelberg JM, Huang DT, Josephson ME, Rosenberg RD. Molecular enhancement of porcine cardiac chronotropy. Heart. 2001;86:559–562. doi: 10.1136/heart.86.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelberg JM, Aird WC, Rosenberg RD. Enhancement of murine cardiac chronotropy by the molecular transfer of the human beta2 adrenergic receptor cdna. J Clin Invest. 1998;101:337–343. doi: 10.1172/JCI1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruhparwar A, Kallenbach K, Klein G, Bara C, Ghodsizad A, Sigg DC, Karck M, Haverich A, Niehaus M. Adenylate-cyclase vi transforms ventricular cardiomyocytes into biological pacemaker cells. Tissue Eng Part A. 2010;16:1867–1872. doi: 10.1089/ten.TEA.2009.0537. [DOI] [PubMed] [Google Scholar]
- 13.Miake J, Marban E, Nuss HB. Biological pacemaker created by gene transfer. Nature. 2002;419:132–133. doi: 10.1038/419132b. [DOI] [PubMed] [Google Scholar]
- 14.Boink GJ, Verkerk AO, van Amersfoorth SC, Tasseron SJ, van der Rijt R, Bakker D, Linnenbank AC, van der Meulen J, de Bakker JM, Seppen J, Tan HL. Engineering physiologically controlled pacemaker cells with lentiviral hcn4 gene transfer. J Gene Med. 2008;10:487–497. doi: 10.1002/jgm.1172. [DOI] [PubMed] [Google Scholar]
- 15.Bucchi A, Plotnikov AN, Shlapakova I, Danilo P, Jr., Kryukova Y, Qu J, Lu Z, Liu H, Pan Z, Potapova I, KenKnight B, Girouard S, Cohen IS, Brink PR, Robinson RB, Rosen MR. Wild-type and mutant hcn channels in a tandem biological-electronic cardiac pacemaker. Circulation. 2006;114:992–999. doi: 10.1161/CIRCULATIONAHA.106.617613. [DOI] [PubMed] [Google Scholar]
- 16.Plotnikov AN, Sosunov EA, Qu J, Shlapakova IN, Anyukhovsky EP, Liu L, Janse MJ, Brink PR, Cohen IS, Robinson RB, Danilo P, Jr., Rosen MR. Biological pacemaker implanted in canine left bundle branch provides ventricular escape rhythms that have physiologically acceptable rates. Circulation. 2004;109:506–512. doi: 10.1161/01.CIR.0000114527.10764.CC. [DOI] [PubMed] [Google Scholar]
- 17.Qu J, Plotnikov AN, Danilo P, Jr., Shlapakova I, Cohen IS, Robinson RB, Rosen MR. Expression and function of a biological pacemaker in canine heart. Circulation. 2003;107:1106–1109. doi: 10.1161/01.cir.0000059939.97249.2c. [DOI] [PubMed] [Google Scholar]
- 18.Tse HF, Xue T, Lau CP, Siu CW, Wang K, Zhang QY, Tomaselli GF, Akar FG, Li RA. Bioartificial sinus node constructed via in vivo gene transfer of an engineered pacemaker hcn channel reduces the dependence on electronic pacemaker in a sick-sinus syndrome model. Circulation. 2006;114:1000–1011. doi: 10.1161/CIRCULATIONAHA.106.615385. [DOI] [PubMed] [Google Scholar]
- 19.Kashiwakura Y, Cho HC, Barth AS, Azene E, Marban E. Gene transfer of a synthetic pacemaker channel into the heart: A novel strategy for biological pacing. Circulation. 2006;114:1682–1686. doi: 10.1161/CIRCULATIONAHA.106.634865. [DOI] [PubMed] [Google Scholar]
- 20.Miake J, Marban E, Nuss HB. Functional role of inward rectifier current in heart probed by kir2.1 overexpression and dominant-negative suppression. J Clin Invest. 2003;111:1529–1536. doi: 10.1172/JCI17959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic amp. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- 22.Shlapakova IN, Nearing BD, Lau DH, Boink GJ, Danilo P, Jr., Kryukova Y, Robinson RB, Cohen IS, Rosen MR, Verrier RL. Biological pacemakers in canines exhibit positive chronotropic response to emotional arousal. Heart Rhythm. 2010;7:1835–1840. doi: 10.1016/j.hrthm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Xue T, Siu CW, Lieu DK, Lau CP, Tse HF, Li RA. Mechanistic role of i(f) revealed by induction of ventricular automaticity by somatic gene transfer of gating-engineered pacemaker (hcn) channels. Circulation. 2007;115:1839–1850. doi: 10.1161/CIRCULATIONAHA.106.659391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plotnikov AN, Bucchi A, Shlapakova I, Danilo P, Jr., Brink PR, Robinson RB, Cohen IS, Rosen MR. Hcn212-channel biological pacemakers manifesting ventricular tachyarrhythmias are responsive to treatment with i(f) blockade. Heart Rhythm. 2008;5:282–288. doi: 10.1016/j.hrthm.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattick P, Parrington J, Odia E, Simpson A, Collins T, Terrar D. Ca2+-stimulated adenylyl cyclase isoform ac1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the i(f) pacemaker current. J Physiol. 2007;582:1195–1203. doi: 10.1113/jphysiol.2007.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Younes A, Lyashkov AE, Graham D, Sheydina A, Volkova MV, Mitsak M, Vinogradova TM, Lukyanenko YO, Li Y, Ruknudin AM, Boheler KR, van Eyk J, Lakatta EG. Ca(2+) - stimulated basal adenylyl cyclase activity localization in membrane lipid microdomains of cardiac sinoatrial nodal pacemaker cells. J Biol Chem. 2008;283:14461–14468. doi: 10.1074/jbc.M707540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fagan KA, Mahey R, Cooper DM. Functional co-localization of transfected ca(2+)-stimulable adenylyl cyclases with capacitative ca2+ entry sites. J Biol Chem. 1996;271:12438–12444. doi: 10.1074/jbc.271.21.12438. [DOI] [PubMed] [Google Scholar]
- 28.Wu ZL, Thomas SA, Villacres EC, Xia Z, Simmons ML, Chavkin C, Palmiter RD, Storm DR. Altered behavior and long-term potentiation in type i adenylyl cyclase mutant mice. Proc Natl Acad Sci U S A. 1995;92:220–224. doi: 10.1073/pnas.92.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakatta EG, DiFrancesco D. What keeps us ticking: A funny current, a calcium clock, or both? J Mol Cell Cardiol. 2009;47:157–170. doi: 10.1016/j.yjmcc.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kryukova YN, Protas L, Robinson RB. Ca(2+)-activated adenylyl cyclase 1 introduces ca(2+)-dependence to beta-adrenergic stimulation of hcn2 current. J Mol Cell Cardiol. 2012 Mar 29; doi: 10.1016/j.yjmcc.2012.03.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu J, Barbuti A, Protas L, Santoro B, Cohen IS, Robinson RB. Hcn2 overexpression in newborn and adult ventricular myocytes: Distinct effects on gating and excitability. Circ Res. 2001;89:E8–14. doi: 10.1161/hh1301.094395. [DOI] [PubMed] [Google Scholar]
- 32.Matsunaga T, Mitsui T, Harada T, Inokuma M, Murano H, Shibutani Y. Qt corrected for heart rate and relation between qt and rr intervals in beagle dogs. J Pharmacol Toxicol Methods. 1997;38:201–209. doi: 10.1016/s1056-8719(97)00098-1. [DOI] [PubMed] [Google Scholar]
- 33.Plotnikov AN, Shlapakova I, Szabolcs MJ, Danilo P, Jr., Lorell BH, Potapova IA, Lu Z, Rosen AB, Mathias RT, Brink PR, Robinson RB, Cohen IS, Rosen MR. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation. 2007;116:706–713. doi: 10.1161/CIRCULATIONAHA.107.703231. [DOI] [PubMed] [Google Scholar]
- 34.Boink GJJ, Duan L, Shlapakova I, Sosunov EA, Anyukhovsky EP, Kryukova Y, Lau DH, Ozgen N, Danilo P, Jr., Cohen IS, Robinson RB, Rosen MR. Hcn2/skm1 gene transfer into the canine left bundle branch induces highly stable biological pacing at physiological beating rates [abstract ab24–2] 2011. p. S54. [DOI] [PMC free article] [PubMed]
- 35.Lu HH, Lange G, Brooks MC. Factors controlling pacemaker action in cells of the sinoatrial node. Circ Res. 1965;17:460–471. doi: 10.1161/01.res.17.5.460. [DOI] [PubMed] [Google Scholar]
- 36.Graziani AT, Vassalle M. Mechanisms underlying overdrive suppression and overdrive excitation in guinea pig sino-atrial node. J Biomed Sci. 2006;13:703–720. doi: 10.1007/s11373-006-9089-3. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg YJ, Vassalle M. On the mechanism of overdrive suppression in the guinea pig sinoatrial node. J Electrocardiol. 1990;23:53–67. doi: 10.1016/0022-0736(90)90151-q. [DOI] [PubMed] [Google Scholar]
- 38.Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease: Physiological basis and prognostic implications. J Am Coll Cardiol. 2008;51:1725–1733. doi: 10.1016/j.jacc.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 39.Verrier RL, Tan A. Heart rate, autonomic markers, and cardiac mortality. Heart Rhythm. 2009;6:S68–75. doi: 10.1016/j.hrthm.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernardi L, Valle F, Coco M, Calciati A, Sleight P. Physical activity influences heart rate variability and very-low-frequency components in holter electrocardiograms. Cardiovasc Res. 1996;32:234–237. doi: 10.1016/0008-6363(96)00081-8. [DOI] [PubMed] [Google Scholar]
- 41.Sandrone G, Mortara A, Torzillo D, La Rovere MT, Malliani A, Lombardi F. Effects of beta blockers (atenolol or metoprolol) on heart rate variability after acute myocardial infarction. Am J Cardiol. 1994;74:340–345. doi: 10.1016/0002-9149(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 42.Gwathmey JK, Yerevanian AI, Hajjar RJ. Cardiac gene therapy with serca2a: From bench to bedside. J Mol Cell Cardiol. 2011;50:803–812. doi: 10.1016/j.yjmcc.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Wang D, Qian S, Chen Z, Zhu T, Xiao X. Efficient and long-term intracardiac gene transfer in delta-sarcoglycan-deficiency hamster by adeno-associated virus-2 vectors. Gene Ther. 2003;10:1807–1813. doi: 10.1038/sj.gt.3302078. [DOI] [PubMed] [Google Scholar]
- 44.Niwano K, Arai M, Koitabashi N, Watanabe A, Ikeda Y, Miyoshi H, Kurabayashi M. Lentiviral vector-mediated serca2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol Ther. 2008;16:1026–1032. doi: 10.1038/mt.2008.61. [DOI] [PubMed] [Google Scholar]
- 45.Todt H, Krumpl G, Krejcy K, Raberger G. Mode of qt correction for heart rate: Implications for the detection of inhomogeneous repolarization after myocardial infarction. Am Heart J. 1992;124:602–609. doi: 10.1016/0002-8703(92)90266-x. [DOI] [PubMed] [Google Scholar]
- 46.Van de Water A, Verheyen J, Xhonneux R, Reneman RS. An improved method to correct the qt interval of the electrocardiogram for changes in heart rate. J Pharmacol Methods. 1989;22:207–217. doi: 10.1016/0160-5402(89)90015-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.