Abstract
HIV-1 Nef is a major determinant in HIV-1 pathogenicity. However, its properties have mainly been associated with its biochemical activities within the producer cell. Nef is also secreted from infected and transfected cells. Our primary objective was to determine the nature of secreted Nef protein and its effect on target cells. We determined that HIV-1 Nef is secreted in the form of exosome-like vesicles. Nef protein present in these vesicles is largely protected from protease digestion, which suggests that most of the protein is present on the lumenal side of the vesicles. We observed that HEK293 cells, transfected with a Nef-GFP expression vector, can secrete vesicles containing Nef-GFP fusion protein into the extracellular medium. When the conditioned medium was used to treat Jurkat cells, we found that the cells can take up the Nef fusion protein. The pattern of distribution of the Nef-GFP fusion protein within the target cells is mainly cytoplasmic and results in a punctate staining pattern. We also observed that Nef (-) virions treated with Nef-conditioned medium have their infectivity restored to near wild-type levels. This implies that the Nef contained within vesicles has the ability to fuse with HIV-1 virions and deliver functional Nef to these virions. It also demonstrates that Nef protein delivered in trans can restore infectivity even after virion maturation has occurred. These studies suggest that secreted Nef could play a role in HIV-1 pathogenesis by inducing effects in noninfected bystander cells.
Keywords: Nef, Exosome, HEK293 Cells, Jurkat Cells, Nef-GFP Fusion Protein
Introduction
The HIV/AIDS pandemic continues to be a major public health threat throughout the world. In the United States, HIV/AIDS has increasingly become a disease of African Americans and has become a leading cause of healthcare disparities in minority populations. Most of the pathogenicity of HIV-1 can be attributed to the presence of six accessory genes coded within the viral genome, nef is one of these accessor genes that is only present within the HIV and simian immunodeficiency virus (SIV) genomes and is thought to play a key role in the progression to AIDS.1 Nef is a 27-kDa protein that is produced early during infection from multiply spliced mRNAs. It is N-terminally myristoylated at the second glycine of the highly conserved sequence MGG.2 Myristoylation of Nef is required for all of its known biological activities. Therefore, Nef's association with membranes is central to its many effects on cells. To date, most of Nef's functions have been associated with intracellular membranes or its association with virions. However, Nef is known to be secreted from infected cells3–5 and has been reported to be secreted in association with small membrane-bound vesicles.4 It is also present in the serum of HIV-infected patients,3 and regions of Nef promote the fusion of membrane vesicles.6 We have previously shown that Nef secreted into the conditioned medium from cells transfected with HIV-1, HIV-2, and SIV nef expression plasmids can induce apoptosis when applied to target Jurkat cells.7,8
Methods
Secreted Nef Protein
HEK293 cells were transfected with an expression vector PCDNAnef created by cloning the nef reading frame from the viral clone pNL4-3 as previously described.9,10 Supernatants from transfected cells were collected and either used immediately or stored at 4°C before use.
Immunoblot Analysis
The presence of Nef was determined by immunoblot analysts using a polyclonal rabbit Nef antiserum (AIDS Reagent Program, catalog #2949). Blots were detected by using a protein A horscradish-peroxidase conjugate and developed by using SuperSignal West Femto Sensitivity Substrate kit (Pierce).
Protease Protection Assays
Pellets from tissue culture supernatants were subjected to digestion by using an equal volume of subtilisin protease, 1 mg/mL final concentration (Sigma) for 16 hours at 37°C. The reaction was then terminated by the addition of a protease phenylmethylsulphonyl fluoride (5 μg/mL final concentration). The final products were then analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis stained with Coomasie blue and immunoblot as described above.
Electron Microscopy
Pellets from tissue culture supernatants were also processed for transmission electron microscopy. Pellets were embedded in 1% agar and removed from tubes and trimmed and cut into blocks. The blocks were fixed in 1% glutaraldehyde before embedding in epon plastic. Ultrathin sections were stained with uranyl acetate and placed on grids for observation and photography.
Take Up of Nef-GFP by Target Cells
In some cases, HEK293 cells were transfected with a Nef-GFP construct (Q.BIOGENE). Tissue culture supernatants were collected and used to treat untransfected Jurkat T cells. Cells were then directly observed or counterstained with DRAQ5 before analysis by confocal microscopy.
Complementation of Nef (-) Virions
Virions lacking Nef protein were produced as previously described.9,10 Intact virions were treated with tissue culture supernatants from nef transfected cells. Mock-transfected supernatants were used as a control. Samples were normalized by p24 enzyme-linked immunosorbent assay before determining infectivity by MAGI infectivity assay.
Results
We have previously established that Nef is expressed into the extracellular medium of cells transfected with HIV-1, HIV-2, or SIV nef expression plasmids. To determine the nature of the secreted protein, we subjected conditioned tissue culture supernatants to ultracentrifugation at 400,000 × g for 1 hour. The results are summarized in Figure 1. Nef was present in the conditioned medium (lane 3). Upon centrifugal ion, most Nef was present in the pellet (lane 5) and absent in the supernatant (lane 1). Since Nef is a myristoylated protein, we included a control that lacked the site of mytistoylation (G2A). However, the mutant behaved essentially the same as wild-type protein (lanes 2, 4, and 6).
Fig 1.
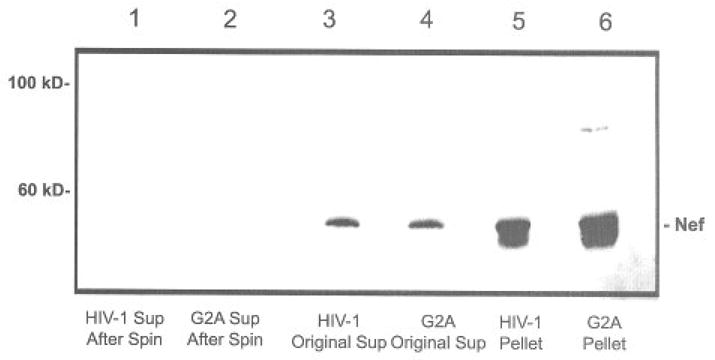
Conditioned tissue culture supernatant was collected from HEK293 cells that were transfected with pNef expression plasmid or a G2A mutation in the Nef sequence. The supernatants were spun in a TLA100 rotor at 400,000 × g for 1 h to create the pellets. Medium, supernatants, and pellets were each separated on an 12.5% SDS PAGE and Nef was detected by immunoblot using anti-Nef antiserum. Note that the majority of Nef present in these blots is actually in the form of a dimmer.
Nef Protein in High-Speed Pellets Protected from Protease Digestion
Since Nef is associated with endosomes and the secreted Nef from conditioned medium was in a higher molecular weight form, we considered whether Nef may be associated with membranous structures. To test this hypothesis, we treated the high-speed pellets from conditioned media with subtilisin. This treatment results in the degradation of proteins that are not protected by structures such as a membrane. The results are summarized in Figure 2. A Coomasic stain of the gel revealed that the original pellet contained a large number of proteins of all various molecular masses (lane 1). In contrast, the lane containing treated pellet had only a few prominent bands (lane 2). This suggests that most of the protein present was unprotected and could represent proteins on the surface of vesicles. Further studies using serum-free medium and other means of vesicle purification are currently underway. An immunoblot of the same material showed that Nef was present in the untreated sample as 4–6 bands of various molecular weights (lane 1). After subtilisin treatment, Nef was present in the pellet but appeared to have coalesced into a single band that corresponded to the molecular weight of a Nef dimer (lane 2). We believe that the different forms of Nef in the untreated sample coalesced into a single band in the created simple. It is unclear why removal of the unprotected proteins would cause a change in the mobility of Nef, but in some cases they could promote the formation of higher molecular weight aggregates. In other cases, the treatment appeared to interfere with the formation of dimers that result in the lower molecular weight material. As a control, the pellet was also treated with 1% Triton prior to subtilisin treatment, which resulted in no detectable bands by Coomasic or immunoblot (data not shown).
Fig 2.
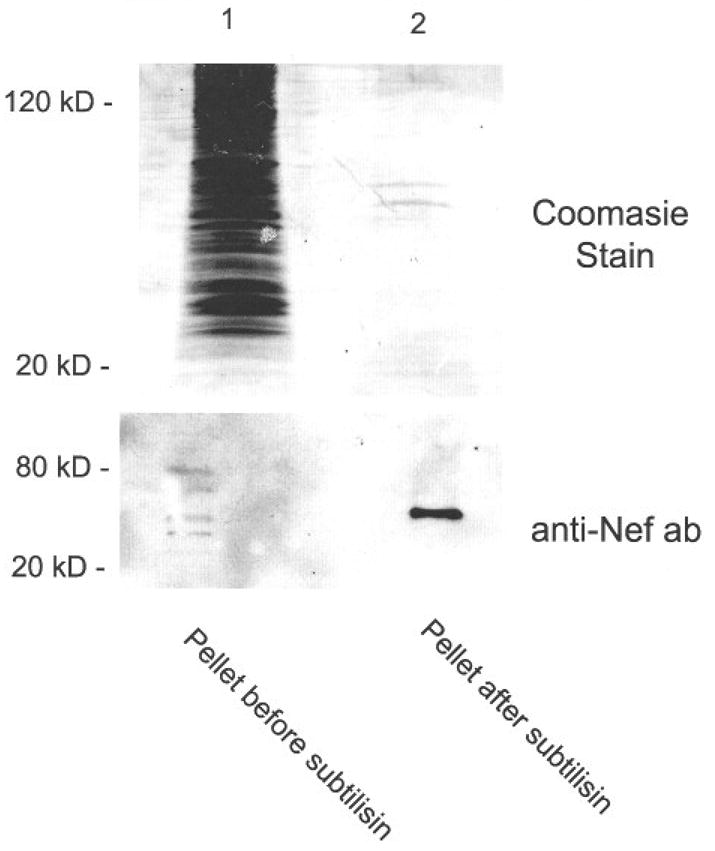
Pellets from high-speed spins were treated with equal volumes of subtilisin (1mg/mL final concentration) for 16 h at 37°C. The reaction was then terminated by adding PMSF (5μg/mL final concentration). The products were then separated on an 12.5% gel and either stained with Coomasie blue or immunoblot using anti-Nef antiserum.
High-Speed Pellets from Conditioned Media Contain Exosome-Like Vesicles by Electron Microscopy
To determine the nature of the higher molecular weight form of Nef present in pellets, we subjected the material to analysis by election microscopy (Figure 3A). The results show that the material in pellets from Nef containing supernatants contained numerous vesicles (Figure 3A). In contrast, pellets from mock-transfected cells showed only debris (Figure 3B). The vesicles present in Nef supernatants averaged approximately 50–200 nm in diameter and varied greatly in size and shape (sec Figure 3A). The shape and size of these vesicular components were consistent with those of exosomes.11 Exosomes are vesicular bodies that can be shed from a variety of cells and function as a mechanism for transporting material from cell to cell.12 HEK293 cells do not normally produce large numbers of exosomes. However, when the HIV-1 nef gene is expressed in these cells, they produce large numbers of exosomes. Since it appears that the Nef protein is inducing this effect, it seems likely that most, if not all, of the exosomes present will contain at least some Nef protein.
Fig 3.
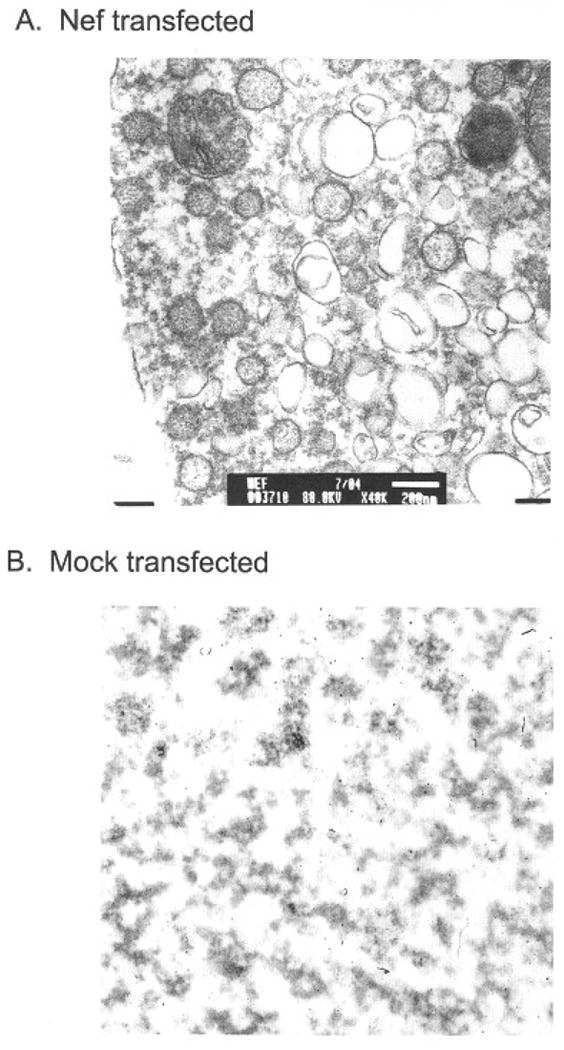
Pellets from high-speed spins were immobilized in 1% agar and then fixed, embedded and stained with 2% uranyl acetate. They were then visualized using a JEOL 1200EX transmission microscope. A control using cells that were mock transfected is also included. The size bar in the transfected sample is 200nm in size.
Conditioned Medium from Cells Transfected with a Nef-GFP Expression Vector Can Accumulate in Target Cells
To determine if Nef had an effect on nontransfected target cells, we transfected HEK293 cells with a Nef-GFP expression vector and collected conditioned medium. Transfected cells began to show fluorescence within an hour of transfection. Fluorescence became detectable in the conditioned tissue culture medium within 24 hours of transfection as determined on a fluorescence plate reader (data not shown). The conditioned medium contained Nef-GFP fusion protein as determined by immunoblot with anti-Nef antisera. When conditioned medium was placed onto an equal number of fresh, non-transfected Jurkat T cells, some cells begin to accumulate fluorescence within 30 minutes after exposure at 37°C, as determined by confocal microscopy of the treated cells (Figure 4A). Observation at higher magnifications (Figure 4) revealed small points of fluorescence, which could represent individual Nef vesicles before fusion with target cells.
Fig 4.
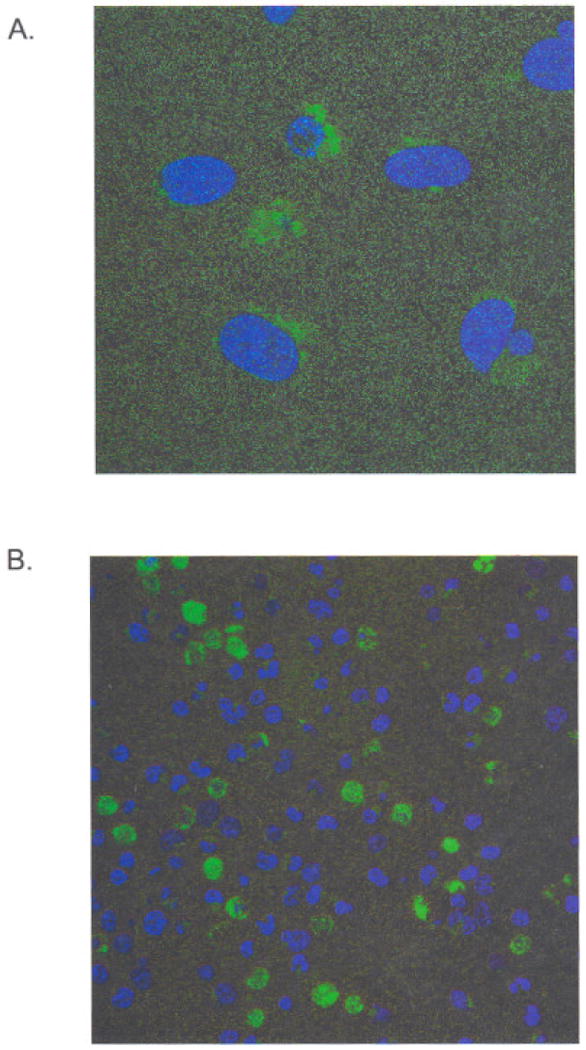
Conditioned medium was collected from Nef-GFP transfected cells after 48h. Fresh Jurkat cells were then added and allowed to come in contact with the medium for 30min. The cells were then observed by confocal microscopy for the localization of Nef-GFP. Cells were counterstained with DRAQ5. (A) Note that the small dots in this high magnification field are thought to be individual Nef vesicles. (B) A lower magnification image shows that some cells have taken up significant quantities of the fusion protein.
Treatment of a nef-Deleted Strain of HIV-1 with Conditioned Medium Restored Infectivity to Near Wild-Type Levels
As a further means of showing that Nef from conditioned medium had biological effects, we treated virions (NL4-3KFSΔNef) that lacked Nef with conditioned supernatant. We found that the nef-deleted strain was approximately fivefold reduced in infection. When this virus was incubated with conditioned medium for 4 hours before determining infectivity by MAGI assay, there was an increase to almost wild-type levels (data not shown). The increase in infectivity was proportional to the amount of conditioned medium used to treat the nef-deleted virus.
Discussion
To date, most of the activities of the Nef protein have been attributed to the intracellular expression of the protein or its association with virions. Inside cells, Nef is known to downregulate CD4 and major histocompatibility receptors.13,14 To accomplish these functions, Nef acts as a sort of connector between the receptor and components of the cell's trafficking machinery.15 Nef binds to CD4 by a dileucine-based signal in the CD4 cytoplasmic tail. Nef also binds to cellular partners such as adaptor proteins and COP I that are involved in protein sorting16 and internalizes CD4 to specific clathrin-coated pits. Nef's N-terminal myristoylation signal directs it to membranes and its site of action, though recent reports implicate basic residues near the N-terminus as also important.17 Since Nef associates with membranes, it is perhaps not surprising that it could ultimately be secreted into vesicles. Our studies show that Nef can be secreted from HEK293 cells (Figure 1, lane 3). In our experiments, HIV-1 Nef lacking an N-terminal myristoylation site (G2A) was as efficiency as wild-type virus in its secretion (Figure 1, lane 4). Therefore, secretion also appears to involve residues independent of N-terminal myristoylation. The Nef that is secreted from these cells is also in a form that can be sedimented at high centrifugal forces (Figure 1). When these high-speed pellets were characterized, we found that the Nef contained within them was protected from protease digestion (Figure 2) and associated with vesicles (Figure 3). This suggests that most of the Nef present was on the lumenal side of these vesicles. Using a Nef-GFP expression vector, we also show that Nef vesicles can be visualized and that the vesicles can fuse with target Jurkat cells (Figure 4). This demonstrates that secreted Nef can do at least two important things: 1) induce apoptosis in bystander cells and 2) fuse with bystander cells. In other experiments we also demonstrate that Nef vesicles can trans complement a nef-deleted virus and restore its infectivity to near wild-type levels. All of these data point toward a potentially significant role for secreted Nef in viral pathogenesis. Our future studies should help determine the relative importance of these effects in the context of T-cell depletion during the course of AIDS.
Implications for Improving Health Disparities
Perhaps one of the best ways to prevent the health disparities that result as a consequence of HIV/AIDS is to devise an adequate treatment. Our studies suggest that the pathogenesis of HIV-1 infection could be reduced or eliminated by therapeutic vaccines or drug therapies directed against Nef-induced effects on bystander cells.
Acknowledgments
We acknowledge the support of grants S06-GM08428, R21 AI060370, and the RCMI core facilities supported by RR003034. This work was also supported, in part, through the Emory Center for AIDS Research (p30 AI050409). We also acknowledge the support of the AIDS Reagent Program.
References
- 1.Trono D. HIV accessory proteins: leading roles for the supporting cast. Cell. 1995;82:189–192. doi: 10.1016/0092-8674(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 2.Niederman TM, Hastings WR, Ratner L. Myristoylation-enhanced binding of the HIV-1 Nef protein to T cell skeletal matrix. Virology. 1993;197:420–425. doi: 10.1006/viro.1993.1605. [DOI] [PubMed] [Google Scholar]
- 3.Fujii Y, Otake K, Tashiro M, Adachi A. Soluble Nef antigen of HIV-1 is cytotoxic for human CD4+ T cells. FEBS Lett. 1996;313:93–96. doi: 10.1016/0014-5793(96)00859-9. [DOI] [PubMed] [Google Scholar]
- 4.Guy B, Riviere Y, Dott D, Regnault A, Kieny MP. Mutational analysis of the HIV Nef protein. Virology. 1990;176:413–425. doi: 10.1016/0042-6822(90)90011-f. [DOI] [PubMed] [Google Scholar]
- 5.Macreadie IG, Fernley R, Castelli LA, Lucantoni A, White J, Azad A. Expression of HIV-1 nef in yeast causes membrane perturbation and release of the myristylated Nef protein. J Biomed Sci. 1998;5:203–210. doi: 10.1007/BF02253470. [DOI] [PubMed] [Google Scholar]
- 6.Curtain CC, Separovic F, Rivett D, et al. Fusogenic activity of amino-terminal region of HIV type 1 Nef protein. AIDS Res Hum Retroviruses. 1944;10:1231–1240. doi: 10.1089/aid.1994.10.1231. [DOI] [PubMed] [Google Scholar]
- 7.Huang MB, Jin LL, James CO, Khan M, Powell MD, Bond VC. Characterization of Nef-CXCR4 interactions important for apoptosis induction. J Virol. 2004;78:11084–11096. doi: 10.1128/JVI.78.20.11084-11096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James COM, Huang MB, Khan M, Garcia-Barrio M, Powell MD, Bond VC. Extracellular Nef protein targets CD4+ T cells for apoptosis by interacting with CXCR4 surface receptors. J Virol. 2004;78:3099–3109. doi: 10.1128/JVI.78.6.3099-3109.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan M, Jin L, Huang MB, Miles L, Bond VC, Powell MD. Chimeric HIV-1 virions containing H1V-2 or SIV Nef are resistant to cyclosporine A treatment. J Virol. 2004;78:1843–1850. doi: 10.1128/JVI.78.4.1843-1850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan M, Jin L, Miles L, Bond VC, Powell MD. Chimeric human immunodeficiency virus type 1 virions that contain the simian immunodeficiency virus nef gene are cyclosporin A resistant. J Virol. 2005;79:3211–3216. doi: 10.1128/JVI.79.5.3211-3216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvegel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(Pt 19):3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 12.Gould SJ, Booth AM, Hildreth JE. The Trojan exosome hypothesis. Proc Natl Acad Sci U S A. 2003;100:10592–10597. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson SD, Shugars C, Swanstrom R, Gareia JV. Nef from primary isolates of human immunodeficiency virus type 1 suppresses surface CD4 expression in human and mouse T cells. J Virol. 1993;67:4923–4931. doi: 10.1128/jvi.67.8.4923-4931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg ME, Bronson S, Lock M, Neumann M, Pavlakis GN, Skowronski J. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 1997;16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piguet V, Trono D. A structure-function analysis of the Nef protein of primate lentiviruses. doi: 10.1002/(sici)1099-1654(199904/06)9:2<111::aid-rmv245>3.0.co;2-p. Available at: http://www.hiv.lanl.gov/content/hiv-db/REVIEWS/reviews.html. [DOI] [PubMed]
- 17.Giese SI, Woerz I, Homann S, Tibroni N, Geyer M, Fackler OT. Specific and distinct determinants mediate membrane binding and lipid raft incorporation of HIV-1(SF2) Nef. Virology. 2006;355:175–191. doi: 10.1016/j.virol.2006.07.003. [DOI] [PubMed] [Google Scholar]


