Abstract
Objective
20-HETE promotes endothelial dysfunction by uncoupling eNOS, stimulating O2− production and reducing NO bioavailability. Moreover, 20-HETE-dependent vascular dysfunction and hypertension are associated with upregulation of the renin-angiotensin system (RAS). This study was undertaken to examine the contribution of RAS to 20-HETE actions in the vascular endothelium.
Methods and Results
In endothelial cells, 20-HETE induced ACE mRNA levels and increased ACE protein and activity by 2-3-fold; these effects were negated with addition of the 20-HETE antagonist, 20-HEDE. 20-HETE-induced ACE expression was PKC-independent and EGFR-tyrosine kinase- and IKKβ-dependent. ACE siRNA abolished 20-HETE-mediated inhibition of NO production and stimulation of O2− generation whereas AT1R siRNA attenuated these effects by 40%. 20-HETE-stimulated O2− production was negated by 20-HEDE and was attenuated by lisinopril and losartan. Importantly, 20-HETE-mediated impairment of acetylcholine-induced relaxation in rat renal interlobar arteries was also attenuated by lisinopril and losartan.
Conclusions
These results indicate that ACE and Ang II-AT1R activation contribute to 20-HETE-mediated endothelial cell and vascular dysfunction and further enforce the notion that excessive production of 20-HETE within the vasculature leads to hypertension via mechanisms that include the induction of endothelial ACE, thus perpetuating an increase in vascular Ang II which, together with 20-HETE, promotes vascular dysfunction.
INTRODUCTION
The synthesis of 20-hydroxyeicosatetraenoic acid (20-HETE), the ω-hydroxylation product of arachidonic acid, is catalyzed by enzymes of the cytochrome P450 (CYP) 4 gene family. It is a potent vasoactive eicosanoid and a key constituent of the microcirculation, most notably, the renal and cerebral microcirculations. Its synthesis within the vascular wall is primarily localized to the smooth muscle cells, increased with decreased vessel diameter, stimulated by vasoactive hormones such as angiotensin II (Ang II), endothelin and norepinephrine, and inhibited by nitric oxide (NO) 1. 20-HETE elicits vasoconstriction largely via inhibition of the smooth muscle cell large conductance Ca2+-activated K+ channel leading to depolarization and elevation in cytosolic [Ca2+] 2. It stimulates smooth muscle cell migration and proliferation 3, 4 and exerts actions on vascular endothelial function. 20-HETE is a potent angiogenic factor in vitro and in vivo 5–7 and a mitogen to endothelial cells 8 as well as a modulator of endothelial nitric oxide synthase (eNOS)-NO production and function 9–11.
Changes in 20-HETE levels have been observed in pathological conditions including ischemic cerebrovascular diseases, cardiac ischemia-reperfusion injury, kidney diseases, hypertension, diabetes and toxemia of pregnancy 1. The vascular phenotype in many of these conditions is that of injury typified by endothelial dysfunction and activation. Recent studies in our laboratory provided substantial evidence that 20-HETE contributes to both endothelial dysfunction and endothelial activation associated with hypertension. In experimental models of increased vascular synthesis of 20-HETE, the resulting endothelial dysfunction, oxidative stress and hypertension are prevented and reversed by administration of a 20-HETE synthesis inhibitor or a 20-HETE antagonist 10–12. These actions of 20-HETE within the vascular wall are believed to constitute the mechanisms by which 20-HETE contributes to the development of hypertension.
In a recent study, we identified the angiotensin-converting enzyme (ACE) as one of the few genes which are markedly upregulated upon addition of 20-HETE to cultured endothelial cells 13. Additional studies indicated that increased vascular synthesis of 20-HETE in vivo is also associated with increased vascular ACE expression and circulating Ang II levels. Moreover, in an experimental model of 20-HETE-dependent hypertension, administration of ACE inhibitors or Ang II type 1 receptor (AT1R) blockers prevented and reversed the blood pressure elevation in response to increased vascular 20-HETE levels 13. These findings provided a novel paradigm where excessive production of 20-HETE within the vasculature, such as in androgen-induced hypertension, leads to hypertension via mechanisms that include the induction of endothelial ACE, thus perpetuating an increase in vascular Ang II which, in turn and together with 20-HETE, promotes vascular dysfunction.
The current study was undertaken to further determine the cellular mechanisms by which 20-HETE induces ACE and to evaluate whether ACE is a needed component for 20-HETE actions in the vasculature and, in particular, in the endothelium. Here, we demonstrate that in endothelial cells, 20-HETE induces ACE via EGFR and IκB kinase (IKK) activation and that 20-HETE-mediated inhibition of NO and stimulation of O2− production as well as 20-HETE-mediated impairment of relaxation to acetylcholine in renal microvessels are influenced by ACE and Ang II-AT1R activation.
METHODS AND MATERIALS
Cell Culture
Human microvascular endothelial cells (HMVECs) were grown in Medium 131 containing 5% microvascular growth supplement (Invitrogen) and 5% fetal bovine serum (FBS, USA Scientific). Passages 3–5 were used for all experiments. Cells were maintained at 37°C in a humidified incubator with an atmosphere of 5% CO2:95% O2. For most experiments, cells were grown in 6-well-plates to 80–90% confluence and placed in serum-free media for 24 h prior to addition of compounds. Compounds used in this study are the following: 20-HETE (5 nmol/L), 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (20-HEDE, a 20-HETE antagonist, 5 nmol/L), 11,12-epoxyeicosatrienoic acid (11,12-EET, 5 nmol/L), SC-514 (an IKKβ inhibitor, 10 μmol/L), AG82 (an EGFR-tyrosine kinase inhibitor, 10 μmol/L), Calphostin C (a PKC inhibitor, 100 nmol/L), lisinopril (0.1–10 μmol/L), losartan (10 μmol/L) and actinomycin D (10 μmol/L).
Measurement of NO, cGMP, O2− and H2O2
For NO measurements, cells were preincubated with 20-HETE (5 nmol/L) or its vehicle (0.1% ethanol) for 2 h and treated with or without calcium ionophore A23187 (5 μmol/L) in the presence of L-arginine (25 μmol/L) for 30 min. NO levels were measured using a NO quantitation kit (Active Motif, Carlsbad, CA) and cGMP levels were measured by immunoassay (R&D systems, Minneapolis, MN) 10, 14. For superoxide (O2−) measurements, cells were treated with or without Tiron (10 mmol/L), 20-HETE (5 nmol/L), 20-HEDE (5 nmol/L), angiotensin II (100 nmol/L), the angiotensin II receptor blocker losartan (10μmol/L) and the ACE inhibitor lisinopril (10 μmol/L) for 2 h. O2− levels were measured using dihydroethidium (DHE, 5 μmol/L) (Calbiochem, Gibbstown, NJ) as described 14. For measurements of H2O2 levels, polyethylene-glycolated superoxide dismutase (PEG-SOD, 100U/ml, Sigma Aldrich) or polyethylene-glycogated catalase (PEG-catalase, 500U/ml, Sigma Aldrich) was added 30 min prior to staining with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA, 5 μmol/L, Sigma Aldrich) for 20 min. Fluorescence intensity was measured with excitation/emission filters of 485 nm/530 nm.
ACE protein and activity assays
HMVECs were cultured on 6-well plates and placed in serum-free media for 24 h. Cells were then treated with 20-HETE (5 nmol/L) with or without 20-HEDE (5 nmol/L) for 6–24 h. ACE protein was measured in cell lysates by immunoblotting with ACE (N-20) goat polyclonal IgG (1:200, Santa Cruz Biotechnology). ACE activity in cell lysates and media was determined in the presence and absence of lisinopril (100 nmol/L) using the BÜHLMANN ACE kinetic test from ALPCO (Salem, NH).
Agonist-induced vasorelaxation
All experimental protocols were approved by the Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (n=3–5) at 7–8 wks old were used. Acetylcholine-induced relaxation was measured using renal interlobar arteries as described 11, 14. Arteries were incubated in the presence and absence of 20-HETE (1 μmol/L), lisinopril (10 μmol/L) and losartan (10 μmol/L) for 2 h, after which they were preconstricted with phenylephrine (5 μmol/L) and the relaxing responses to increasing concentrations (5×10−8−5×10−4 mol/L) of acetylcholine were measured.
Statistical analysis
Data are expressed as means±SEM. Significance of difference in mean values was determined using t-test and one-way ANOVA, followed by the Newman-Keul’s post hoc test. P<0.05 was considered to be significant.
RESULTS
20-HETE increases ACE expression and angiotensin II production in endothelial cells
Addition of 20-HETE (5 nmol/L) to HMVECs resulted in a time-dependent induction of ACE mRNA expression with a maximal 2.5-fold increase at 2 h (Figure 1A). The increase in ACE mRNA expression was abrogated by co-treatment with an equimolar concentration of 20-HEDE, a 20-HETE antagonist 15, suggesting that the increase in ACE expression is 20-HETE-dependent. The effect of 20-HETE on ACE mRNA was observed over a wide range of concentrations with a significant 2.5- and 3-fold induction at 1 and 5 nmol/L, respectively, and maximum induction of 4-fold at 10 nmol/L (Figure 1B). Additional experiments comparing the effect of 20-HETE and the known ACE inducer PMA 16 clearly indicated that while PMA significantly increased ACE mRNA 24 h after its addition, the increase in ACE mRNA in response to 20-HETE was maximal at 2 h (Figure S2). Moreover, the increase in ACE mRNA in response to 20-HETE appeared to be at the level of transcription since addition of actinomycin D abolished 20-HETE’s effect (Figure S3A).
Figure 1.
ACE mRNA levels in human microvascular endothelial cells (HMVECs) treated with (A) 20-HETE (5 nmol/L) for 30 min to 24 h (cells treated with 20-HETE and 20-HEDE (5 nmol/L) were incubated for 2 h) or with (B) increasing concentrations of 20-HETE for 2 h (n=5; *p<0.05 vs vehicle, †p<0.05 vs 2h). (C) Effect of 20-HETE (5 μmol/L) on ACE protein levels in HMVECs (n=4; *p<0.05 vs vehicle). (D) ACE protein levels in HMVECs treated with 20-HETE (5 nmol/L) in the presence and absence of 20-HEDE (5 nmol/L) for 12h (n=4; *p<0.05 vs vehicle). (E) ACE activity in HMVECs treated with and without 20-HETE (5 nmol/L) and 20-HEDE (5 nmol/L) for 24 h. Lisinopril (100 nmol/L) was added to the reaction mixture. The basal (vehicle) cellular and extracellular specific activities for ACE were 41.77±8.35 and 208.30±61.36 pmol hippuric acid/min/mg protein cell lysate, respectively (n=4–5; *p<0.05 vs vehicle, †p<0.05 vs 20-HETE).
The 20-HETE-stimulated increase in ACE mRNA was associated with an increase in ACE protein. 20-HETE increased ACE immunoreactive protein (~170 KDa) levels by 2.1±1.15 and 4.2±0.89 at 6 and 12 h; ACE protein levels remained elevated 24 h after 20-HETE addition (Figure 1C).. Moreover, the increase in ACE protein was negated by co-addition of the 20-HETE antagonist 20-HEDE (Figure 1D). Addition of 20-HETE to HMVECs stimulated cellular and extracellular ACE activity by 2-fold at 24 h. Importantly, the 20-HETE-mediated increase in ACE activity was blocked by addition of 20-HEDE (5 nmol/L) and was inhibited by lisinopril (100 nmol/L), suggesting that the increase in ACE activity is 20-HETE-dependent (Figure 1E).
The vascular wall, including the endothelium, expresses components of the RAS, including angiotensinogen, renin, ACE, ACE2, AT1R and AT2R 17. Real-time PCR analysis revealed that 20-HETE at 5 nmol/L increased angiotensinogen (AGT) mRNA by 2.2-fold at 2 h. At the same time frame, 20-HETE did not affect levels of ACE2 mRNA but did reduce AT1R and AT2R expression by 30–40% (Figure S5).
The 20-HETE-mediated increase in ACE expression is tyrosine kinase- and IKK-dependent
Previous studies in our lab have demonstrated that 20-HETE-mediated endothelial cell dysfunction is tyrosine kinase (EGFR)- and IkappaB kinase (IKKβ)-dependent 18, but PKC-independent (Figure S6). Moreover, inhibition of tyrosine kinase-EGFR activation abrogated 20-HETE-stimulated IKKβ as well as IκB phosphorylation (Figure S7), placing EGFR activation as the upstream step in the 20-HETE signaling pathway18. Therefore, we examined whether 20-HETE-mediated ACE induction is also tyrosine kinase- and IKKβ-dependent. Pretreatment with either AG82, a tyrosine kinase inhibitor specific for EGFR, or SC-514, a specific IKKβ inhibitor, prevented 20-HETE from increasing ACE expression. The PKC inhibitor calphostin C had no effect on 20-HETE-induced ACE mRNA (Figure 2A) or ACE activity (Figure S6B). 11,12-EET, an endothelial-derived CYP- eicosanoid which has been shown to activate EGFR in endothelial cells 19, had no effect on ACE mRNA (Figure S3B).
Figure 2.
20-HETE-mediated ACE induction is PKC-independent and EGFR- and IKKβ-dependent. (A) HMVECs were pretreated with the tyrosine kinase/EGFR inhibitor, AG82 (10 μmol/L), IKKβ inhibitor, SC514 (10 μmol/L) or the PKC inhibitor calphostin C (CC, 100 nmol/L) for 30 min prior to addition of 20-HETE (5 nmol/L) for 2 h (n=4, *p<0.05 vs vehicle, †p<0.05 vs 20-HETE). (B) Representative Western blot and densitometry analysis of IKKβ and ACE protein levels in cells treated with IKKβ-specific siRNA (n=4, *p<0.01 vs control siRNA). (C) Effect of 20-HETE on ACE mRNA in cells transfected with IKKβ siRNA and treated with 20-HETE (5 nmol/L) or its vehicle, 0.1% ethanol, for 2 h (n= 4; *p<0.05 vs vehicle, ‡p<0.05 vs vehicle-treated control siRNA, #p<0.05 vs 20-HETE-treated control siRNA).
To ascertain that 20-HETE-mediated ACE induction is dependent on IKKβ activation, a specific IKKβ siRNA was employed. IKKβ siRNA suppressed the expression of IKKβ by 70±6% and ACE by 49±7% when compared to control siRNA (Figure 2B). Furthermore, incubation with IKKβ siRNA prevented 20-HETE-mediated induction of ACE, whereas, incubation with the control siRNA had no effect on 20-HETE-induced ACE expression; in the latter, ACE expression in response to 20-HETE increased by 2.5-fold (Figure 2C).
Effect of ACE and AT1R knockdown on 20-HETE-mediated inhibition of NO and stimulation of O2− production
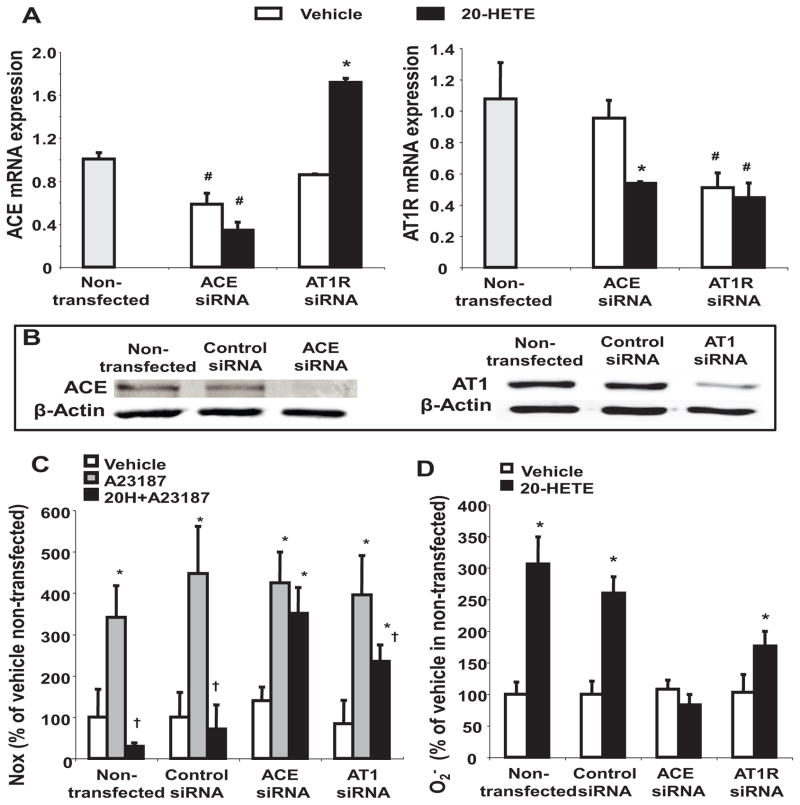
We have shown that in short-term experiments, 20-HETE inhibits NO production (and cGMP production, Figure S8) and stimulates O2− formation primarily by uncoupling eNOS 14 (Figure S9A). In long-term conditions, it also stimulates NADPH oxidase activity and increases O2−, which is rapidly converted to H2O2 in some cells 8, 11, 20. The role of ACE induction in 20-HETE-mediated NO inhibition and O2− production was assessed using ACE- and AT1R-specific siRNAs. In cells transfected with the ACE siRNA, ACE mRNA was reduced by 42% while levels of AT1R mRNA were unaffected. Transfection of cells with AT1R siRNA reduced AT1R mRNA by 47% but had no significant effect on ACE mRNA (Figure 3A). As expected, in cells transfected with the ACE siRNA, 20-HETE failed to increase ACE mRNA but did decrease AT1R mRNA by 45% (p<0.05). On the other hand, in cells transfected with the AT1R siRNA, 20-HETE had no further effect on AT1R mRNA, while its inducing effect on ACE was unaffected; ACE mRNA increased by 2-fold in response to 20-HETE in AT1R siRNA-transfected cells (Figure 3A). The efficacy of the siRNAs was further confirmed by Western blot (Figure 3B).
Figure 3.
Effect of ACE and AT1R siRNAs on 20-HETE-mediated endothelial cell dysfunction. HMVECs transfected with siRNA were treated with 20-HETE (5 nmol/L) or its vehicle (0.1% ethanol) for 2 h and processed for (A) mRNA expression of ACE and AT1R, (B) protein levels of ACE and AT1R (representatives blots of four separate experiments), (C) nitric oxide (NOx) levels, and (D) superoxide (O2−) production (n=4–6; #p<0.05 vs non-transfected. *p<0.05 vs vehicle, †p<0.05 vs A23187).
The effect of downregulating ACE and AT1R expression on 20-HETE-mediated NO production is shown in Figure 3C. Treatment of cells with 20-HETE inhibited ionophore-stimulated NO production by 11.7- and 6.3-fold in non-transfected and control siRNA-transfected cells, respectively. ACE siRNA transfection negated the ability of 20-HETE to inhibit NO production. On the other hand, 20-HETE-mediated inhibition of NO production was attenuated by 40% in AT1R siRNA-transfected cells (Figure 3C), suggesting that Ang II contributes to 20-HETE-mediated inhibition of NO production via AT1R-dependent mechanisms.
The effect of downregulation of ACE and AT1R expression on the 20-HETE-mediated increase in O2− formation is depicted in Figure 3D. 20-HETE increased Tiron-sensitive O2− levels by 306±43% and 260±25% in non-transfected and control siRNA-transfected cells, respectively. Downregulation of ACE expression abolished the increase in O2− levels by 20-HETE but had no effect on basal levels of O2−. On the other hand, knockdown of AT1R expression had no effect on basal O2− levels but did reduce 20-HETE-stimulated O2− production by 40% (Figure 3D). Under the same conditions, 20-HETE had no effect on H2O2 levels in HMVECs (Figure S9B).
Effects of ACE inhibition and AT1R blockade on 20-HETE-stimulated O2− production
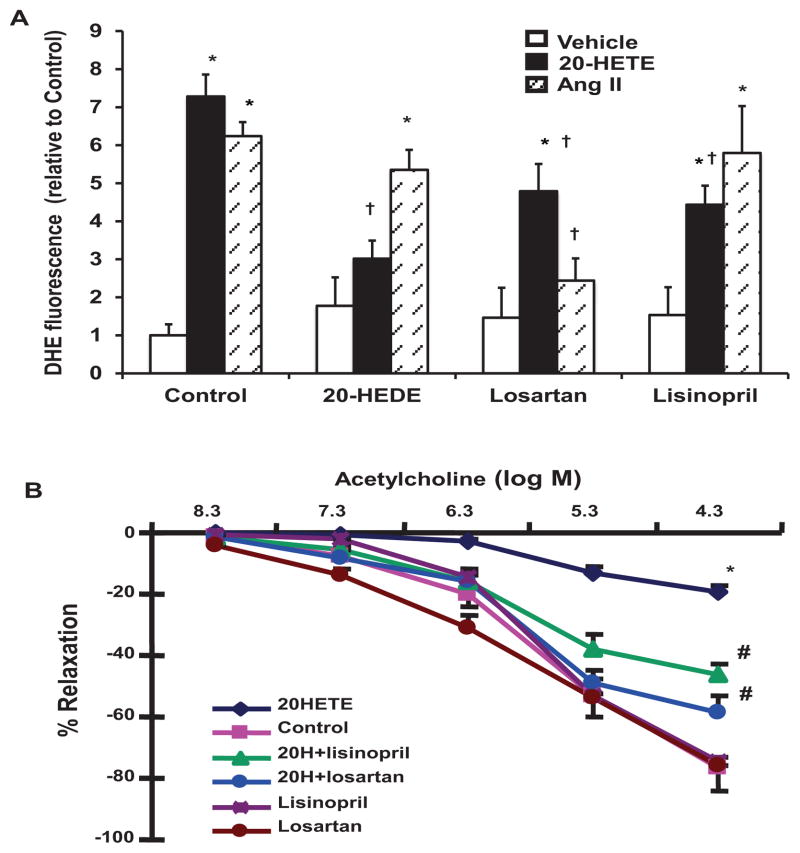
Ang II has been shown to stimulate O2− production in vascular cells, including endothelial cells. Therefore, its contribution to 20-HETE-mediated O2− production was further evaluated using lisinopril and losartan. As seen in Figure 4A, incubation of cells with Ang II increased O2− production to levels similar to that achieved with 20-HETE. While addition of 20-HEDE inhibited 20-HETE-mediated O2− generation, it did not affect Ang II-mediated O2− generation. On the other hand, losartan negated Ang II-stimulated O2− generation and reduced 20-HETE-stimulated O2− levels by 30%. Lisinopril also reduced 20-HETE-stimulated O2− levels by 40% but had no effect on O2− production in response to Ang II. Lisinopril and losartan had no significant effect on O2− production in the absence of 20-HETE or Ang II (Figure 4A). These results further support the notion that Ang II contributes to the 20-HETE-mediated stimulation of O2− production.
Figure 4.
Effect of ACE inhibition and AT1R blockade on (A) 20-HETE-induced O2− production and (B) 20-HETE-induced impairment of relaxation to acetylcholine in renal microvessels. (A) HMVECs were pretreated with 20-HEDE (5 nmol/L), losartan (10 μmol/L) or lisinopril (10 μmol/L) for 30 min. 20-HETE (5 nmol/L) and angiotensin II (Ang II; 100 nmol/L) were then added to the cells for 2 h (n=4; *p<0.05 vs vehicle, †p<0.05 vs corresponding controls). (B) The relaxing responses to increasing concentrations of acetylcholine (5×10−8 – 5×10−4 M) were measured in renal interlobar arteries pre-constricted with phenylephrine (5 μmol/L) and treated with 20-HETE (1 μmol/L), lisinopril (10 μmol/L) and losartan (10 μmol/L). Results are mean ± SEM (n=3–5; *p<0.05 vs control, #p<0.05 vs 20-HETE).
20-HETE-mediated impairment in acetylcholine-induced vasorelaxation is partially restored with inhibitors of ACE and AT1R
The results in endothelial cells suggested that ACE and AT1R may play a role in the 20-HETE-mediated inhibition of acetylcholine-induced relaxation. As seen in Figure 4B, incubation of rat renal interlobar arteries with 20-HETE markedly impaired the relaxing response to acetylcholine, with maximal relaxation reduced from 76.8±3.8 to 19.6±2.6%. Treatment of arteries with lisinopril or losartan significantly improved 20-HETE-impaired relaxation responses to 46.4±3.5 and 58.5±5.2%, respectively. Addition of lisinopril to the bath in the absence of 20-HETE did not alter acetylcholine-induced relaxation response (74.3±10.0%). A similar response was achieved in arteries treated with losartan alone (75.7±0.1%) (Figure 4B).
DISCUSSION
The relationship between the renin-angiotensin system (RAS) and 20-HETE has been suggested in several studies but has not been fully elucidated. Ang II has been shown to stimulate the synthesis of 20-HETE in isolated human neutrophils and platelets 21 and in rat kidney and preglomerular vessels 22, 23. In hypertensive humans, increased plasma levels of 20-HETE are correlated with increased plasma renin activity 24, whereas in high salt diet-fed rats in which the RAS is suppressed, CYP4A expression is reduced 25. Other studies demonstrated that increased 20-HETE in the peripheral vasculature contributes to the acute vasoconstrictor response to Ang II 26, acute and chronic inhibition of 20-HETE synthesis, respectively, attenuates the renal pressor response to Ang II 22 and the development of Ang II-dependent hypertension 27. 20-HETE is known to mediate Ang II-induced mitogenic effects in cultured aortic vascular smooth muscle cells and contribute to the vascular injury, hypertrophy and hypertension caused by Ang II in rats 28–30. Interestingly, experimental models of hypertension that show increased vascular 20-HETE production such as the SHR 31, 32 and the androgen-induced hypertensive rats 11, 33–35 are also RAS-mediated. The nature of these interactions is yet to be fully identified. In a recent study, we showed that endothelial-specific CYP4A2 expression produced 20-HETE-dependent hypertension 36, which was abrogated by either AT1R blockade or ACE inhibition 13. Based on these findings and data from microarray analysis indicating that 20-HETE is a potent inducer of endothelial ACE, we postulated that excessive production of 20-HETE within the vasculature leads to hypertension via mechanisms that include the induction of endothelial ACE, thus perpetuating an increase in vascular Ang II which, in turn and together with 20-HETE, promotes vascular dysfunction. The mechanisms and the extent to which ACE contributes to 20-HETE-mediated vascular endothelial dysfunction are explored in the current study.
Endothelial cells are known to express most of the components of the RAS, including angiotensinogen, ACE, ACE2, AT1R and AT2R. Moreover, the endothelium has been shown to actively participate in the vascular production of Ang II 17, 37, 38, which, in turn, alters endothelial function by increasing the production of ROS, most prominently O2−, and decreasing NO bioavailability. The vascular endothelium is also a target for 20-HETE’s actions where it interferes with NO synthesis and stimulates O2− production 14, 20, 39. We have recently demonstrated that, in endothelial cells, 20-HETE uncouples eNOS via EGFR-MAPK-IKKβ signaling pathway leading to decreased NO levels and increased O2− production 14, 18. Furthermore, long-term, 20-HETE also increases O2− production by activating the NADPH oxidase 11. Here, we demonstrated that 20-HETE induces ACE expression and increases its activity; an effect abrogated by co-treatment with the 20-HETE antagonist 20-HEDE. The ability of 20-HETE to increase ACE mRNA within 2 h and the demonstration that inhibition of EGFR or IKKβ activation, both of which mediate 20-HETE actions in endothelial cells18, abolished the ability of 20-HETE to increase ACE mRNA levels further points to the specificity of 20-HETE effect and suggests a novel mechanism not shared with known ACE inducers such as PMA and VEGF 16, 40. PMA-activated PKC has been shown to increase ACE mRNA level and ACE gene transcription as well as ACE secretion in HUVEC 41. The demonstration that a PKC inhibitor had no effect on 20-HETE-mediated increases in ACE mRNA and activity further substantiate the notion of a distinct mechanism for 20-HETE actions on ACE expression and activity. Moreover, the fact that 11,12-EET, which has been shown to activate EGFR in endothelial cells 19, does not induce ACE mRNA suggests that activation of IKK downstream of EGFR is a key feature of 20-HETE action. A recent study by Wu et al demonstrated that inhibition of IKK activation in vivo abrogate 20-HETE-dependent vascular dysfunction and hypertension 42. The mechanism that links activation of EGFR-IKK and possibly NFκB, the downstream effector molecule of IKKβ activation, to ACE expression is unclear. There are no reports regarding the presence of NF-κB response elements on the ACE promoter and studies to examine the effect of NF-κB inhibition on intrarenal RAS expression in a model of proteinuric renal injury suggested that ACE expression is not subjected to regulation by NF-κB 43. On the other end, IKKβ and/or NF-κB may activate a distinct ACE-responsive transcriptional pathway such as AP-1. A connection between NF-κB and AP-1 has been documented 44 and AP-1 has been shown to activate the transcription of endothelial ACE 16. Clearly, further studies are needed to fully understand the mechanism by which 20-HETE induces endothelial ACE.
This study demonstrated that induction of ACE expression and activity is an important mechanism by which 20-HETE affects endothelial and vascular function. In cells transfected with ACE siRNA, 20-HETE-mediated inhibition of NO and stimulation of O2− production were completely prevented. However, suppression of AT1R expression by siRNA attenuated but did not prevent the 20-HETE-mediated effects on NO and O2− production, suggesting that Ang II actions through the AT1R, e.g., stimulation of NADPH oxidase 45, are required but not necessary for achieving the maximum effect of 20-HETE. As for ACE, it is possible that its induction by 20-HETE elicits actions independent of Ang II and its actions through the AT1R such as degradation of bradykinin (BK), a key mediator of NO production in the endothelium and a substrate for ACE 46. On the other hand, we cannot exclude the possibility that ACE contribution could be independent of its catalytic activity 47. Certainly, additional studies are needed to fully determine the mechanism by which ACE affects 20-HETE. The use of lisinopril and losartan to inhibit ACE activity and block the AT1R, respectively, provided additional evidence that ACE and AT1R contribute to the stimulatory effect of 20-HETE on O2− levels. In addition, treatment of renal interlobar arteries with either lisinopril or losartan improved the 20-HETE-induced impairment in relaxation response to acetylcholine, an effect that has been attributed to 20-HETE-induced reduction in NO production through eNOS uncoupling 10, 14.
We also found that 20-HETE induces angiotensinogen expression in endothelial cells. Angiotensinogen is transcriptionally activated by NF-κB and the IKK-NF-κB pathway may be the mechanism by which 20-HETE induces its expression. All together, 20-HETE’s ability to induce angiotensinogen in the same time frame as ACE suggests that 20-HETE contributes to activation of the RAS at multiple points. It also raises the possibility that 20-HETE perpetuates at least two positive feedback loops. As seen in Figure 5, there are several ways by which the initial response to 20-HETE can be amplified. We argue that ACE induction is an important component of 20-HETE-induced endothelial and vascular dysfunction. We further postulate that induction of endothelial ACE sets in motion a functional amplification circuit that combines the actions of 20-HETE, ACE (generation of Ang II and degradation of BK) and Ang II (ROS-derived endothelial dysfunction, vasoconstriction) and further increases vascular dysfunction. The ability of 20-HETE to increase angiotensinogen expression provides an additional amplification of the aforementioned processes. The relevance of this study to human health is supported by numerous reports indicating a correlation between urinary 20-HETE, oxidative stress and endothelial dysfunction in hypertensive subjects 48, 49 as well as associations between CYP4F2 (a major 20-HETE-synthesizing enzyme in humans) polymorphism and hypertension 50, 51 and ischemic stroke 52.
Figure 5.
Postulated interactions between 20-HETE and RAS in the vascular endothelium. 20-HETE produced within the vascular wall acts on endothelial cells to uncouple eNOS via an EGFR-MAPK-IKKβ signaling pathway, decreasing and increasing levels of NO and O2−, respectively 14, 18. The activation of IKKβ through EGFR- and MAPK- dependent pathways also constitute the mechanism by which 20-HETE induces ACE expression, leading to increased ACE activity. The increase in O2− levels in response to 20-HETE is initially the consequence of eNOS uncoupling 8, 14 and later the result of activation of NADPH oxidase 8, 11. 20-HETE may also stimulate, either directly or indirectly through the NF-κB-activated pathway, the expression of angiotensinogen (AGT). The induction of ACE alone and together with AGT can lead to increases in the vascular production of Ang II, which, through its interaction with AT1R, further amplifies 20-HETE actions on the endothelium, namely, decreases in NO bioavailability and increases in O2− levels, thus exacerbating endothelial dysfunction and impeding on vascular function.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported by NIH grants HL34300 (MLS), DK38226 (JRF), F30 HL097402 (CCW) and NHLBI Diversity Supplement to HL034300 (VG), and by the Robert A. Welch Foundation (GL625910) (JRF) and American Heart Association pre-doctoral fellowship (0715781T) to Jennifer Cheng.
Footnotes
Disclosures
None
References
- 1.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-hete) in vascular system. J Smooth Muscle Res. 2005;41:175–193. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 2.Harder DR, Gebremedhin D, Narayanan J, Jefcote C, Falck JR, Campbell WB, Roman R. Formation and action of a p450 4a metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol. 1994;266:H2098–H2107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- 3.Muthalif MM, Benter IF, Karzoun N, Fatima S, Harper J, Uddin MR, Malik KU. 20-hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase ii-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci US A. 1998;95:12701–12706. doi: 10.1073/pnas.95.21.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stec D, Gannon KP, Beaird JS, Drummond HA. 20-hydroxyeicosatetraenoic acid (20-hete) stimulates migration of vascular smooth muscle cells. Cell Physiol Biochem. 2007;19:121–128. doi: 10.1159/000099200. [DOI] [PubMed] [Google Scholar]
- 5.Amaral SL, Maier KG, Schippers DN, Roman RJ, Greene AS. Cyp4a metabolites of arachidonic acid and vegf are mediators of skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol. 2003;284:H1528–H1535. doi: 10.1152/ajpheart.00406.2002. [DOI] [PubMed] [Google Scholar]
- 6.Jiang M, Mezentsev A, Kemp R, Byun K, Falck JR, Miano JM, Nasjletti A, Abraham NG, Laniado-Schwartzman M. Smooth muscle--specific expression of cyp4a1 induces endothelial sprouting in renal arterial microvessels. Circ Res. 2004;94:167–174. doi: 10.1161/01.RES.0000111523.12842.FC. [DOI] [PubMed] [Google Scholar]
- 7.Chen P, Guo M, Wygle D, Edwards PA, Falck JR, Roman RJ, Scicli AG. Inhibitors of cytochrome p450 4a suppress angiogenic responses. Am J Pathol. 2005;166:615–624. doi: 10.1016/S0002-9440(10)62282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo AM, Arbab AS, Falck JR, Chen P, Edwards PA, Roman RJ, Scicli AG. Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. J Pharmacol Exp Ther. 2007;321:18–27. doi: 10.1124/jpet.106.115360. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Medhora MM, Falck JR, Pritchard KA, Jacobs ER. Mechanisms of activation of enos by 20-hydroxyeicosatetraenoic acid and vegf in bovine pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L369–L377. doi: 10.1152/ajplung.00424.2005. [DOI] [PubMed] [Google Scholar]
- 10.Wang JS, Singh H, Zhang F, Ishizuka T, Deng H, Kemp R, Wolin MS, Hintze TH, Abraham NG, Nasjletti A, Laniado-Schwartzman M. Endothelial dysfunction and hypertension in rats transduced with cyp4a2 adenovirus. Circ Res. 2006;98:962–969. doi: 10.1161/01.RES.0000217283.98806.a6. [DOI] [PubMed] [Google Scholar]
- 11.Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML. Vascular cytochrome p450 4a expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension. 2007;50:123–129. doi: 10.1161/HYPERTENSIONAHA.107.089599. [DOI] [PubMed] [Google Scholar]
- 12.Singh H, Schwartzman ML. Renal vascular cytochrome p450-derived eicosanoids in androgen-induced hypertension. Pharmacol Rep. 2008;60:29–37. [PubMed] [Google Scholar]
- 13.Sodhi K, Wu CC, Cheng J, Gotlinger K, Inoue K, Goli M, Falck JR, Abraham NG, Schwartzman ML. Cyp4a2-induced hypertension is 20-hydroxyeicosatetraenoic acid- and angiotensin ii-dependent. Hypertension. 2010;56:871–878. doi: 10.1161/HYPERTENSIONAHA.110.154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA, Jr, Schwartzman ML. 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via enos uncoupling. Am J Physiol Heart Circ Physiol. 2008;294:H1018–1026. doi: 10.1152/ajpheart.01172.2007. [DOI] [PubMed] [Google Scholar]
- 15.Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ. 20-hete agonists and antagonists in the renal circulation. Am J Physiol. 1999;277:F790–796. doi: 10.1152/ajprenal.1999.277.5.F790. [DOI] [PubMed] [Google Scholar]
- 16.Eyries M, Agrapart M, Alonso A, Soubrier F. Phorbol ester induction of angiotensin-converting enzyme transcription is mediated by egr-1 and ap-1 in human endothelial cells via erk1/2 pathway. Circ Res. 2002;91:899–906. doi: 10.1161/01.res.0000042703.39845.b4. [DOI] [PubMed] [Google Scholar]
- 17.Muller DN, Luft FC. The renin-angiotensin system in the vessel wall. Basic research in cardiology. 1998;93 (Suppl 2):7–14. doi: 10.1007/s003950050194. [DOI] [PubMed] [Google Scholar]
- 18.Cheng J, Wu CC, Gotlinger KH, Zhang F, Narsimhaswamy D, Falck JR, Schwartzman ML. 20-hete mediates endothelial dysfunction via ikk-dependent enos uncoupling. J Pharmacol Exp Ther. 2009;332:57–65. doi: 10.1124/jpet.109.159863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaelis UR, Fisslthaler B, Medhora M, Harder D, Fleming I, Busse R. Cytochrome p450 2c9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (egfr) FASEB J. 2003;17:770–772. doi: 10.1096/fj.02-0640fje. [DOI] [PubMed] [Google Scholar]
- 20.Medhora M, Chen Y, Gruenloh S, Harland D, Bodiga S, Zielonka J, Gebremedhin D, Gao Y, Falck JR, Anjaiah S, Jacobs ER. 20-hete increases superoxide production and activates napdh oxidase in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L902–911. doi: 10.1152/ajplung.00278.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai IJ, Croft K, Puddey IB, Beilin LJ, Barden AE. 20-hydroxyeicosatetraenoic acid synthesis is increased in human neutrophils and platelets by angiotensin ii and endothelin-1. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00733.2010. [DOI] [PubMed] [Google Scholar]
- 22.Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Jr, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin ii. Am J Physiol Regul Integr Comp Physiol. 2002;283:R60–R68. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- 23.Croft KD, McGiff JC, Sanchez-Mendoza A, Carroll MA. Angiotensin ii releases 20-hete from rat renal microvessels. Am J Physiol Renal Physiol. 2000;279:F544–F551. doi: 10.1152/ajprenal.2000.279.3.F544. [DOI] [PubMed] [Google Scholar]
- 24.Minuz P, Jiang H, Fava C, Turolo L, Tacconelli S, Ricci M, Patrignani P, Morganti A, Lechi A, McGiff JC. Altered release of cytochrome p450 metabolites of arachidonic acid in renovascular disease. Hypertension. 2008;51:1379–1385. doi: 10.1161/HYPERTENSIONAHA.107.105395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito O, Roman RJ. Regulation of p-450 4a activity in the glomerulus of the rat. Am J Physiol. 1999;276:R1749–R1757. doi: 10.1152/ajpregu.1999.276.6.R1749. [DOI] [PubMed] [Google Scholar]
- 26.Joly E, Seqqat R, Flamion B, Caron N, Michel A, Imig JD, Kramp R. Increased renal vascular reactivity to ang ii after unilateral nephrectomy in the rat involves 20-hete. Am J Physiol Regul Integr Comp Physiol. 2006;291:R977–986. doi: 10.1152/ajpregu.00401.2005. [DOI] [PubMed] [Google Scholar]
- 27.Chabova VC, Kramer HJ, Vaneckova I, Vernerova Z, Eis V, Tesar V, Skaroupkova P, Thumova M, Schejbalova S, Huskova Z, Vanourkova Z, Kolsky A, Imig JD, Cervenka L. Effects of chronic cytochrome p-450 inhibition on the course of hypertension and end-organ damage in ren-2 transgenic rats. Vascular pharmacology. 2007;47:145–159. doi: 10.1016/j.vph.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Muthalif MM, Karzoun NA, Gaber L, Khandekar Z, Benter IF, Saeed AE, Parmentier JH, Estes A, Malik KU. Angiotensin ii-induced hypertension: Contribution of ras gtpase/mitogen-activated protein kinase and cytochrome p450 metabolites. Hypertension. 2000;36:604–609. doi: 10.1161/01.hyp.36.4.604. [DOI] [PubMed] [Google Scholar]
- 29.Parmentier JH, Muthalif MM, Nishimoto AT, Malik KU. 20-hydroxyeicosatetraenoic acid mediates angiotensin ii-induced phospholipase d activation in vascular smooth muscle cells. Hypertension. 2001;37:623–629. doi: 10.1161/01.hyp.37.2.623. [DOI] [PubMed] [Google Scholar]
- 30.Yaghini FA, Zhang C, Parmentier JH, Estes AM, Jafari N, Schaefer SA, Malik KU. Contribution of arachidonic acid metabolites derived via cytochrome p4504a to angiotensin ii-induced neointimal growth. Hypertension. 2005;45:1182–1187. doi: 10.1161/01.HYP.0000168051.04275.ea. [DOI] [PubMed] [Google Scholar]
- 31.Dunn KM, Renic M, Flasch AK, Harder DR, Falck J, Roman RJ. Elevated production of 20-hete in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:H2455–2465. doi: 10.1152/ajpheart.00512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang F, Wang MH, Krishna UM, Falck JR, Laniado-Schwartzman M, Nasjletti A. Modulation by 20-hete of phenylephrine-induced mesenteric artery contraction in spontaneously hypertensive and wistarkyoto rats. Hypertension. 2001;38:1311–1315. doi: 10.1161/hy1201.096116. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa K, Marji JS, Schwartzman ML, Waterman MR, Capdevila JH. Androgen-mediated induction of the kidney arachidonate hydroxylases is associated with the development of hypertension. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1055–R1062. doi: 10.1152/ajpregu.00459.2002. [DOI] [PubMed] [Google Scholar]
- 34.Holla VR, Adas F, Imig JD, Zhao X, Price E, Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen-sensitive cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci U S A. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller DN, Schmidt C, Barbosa-Sicard E, Wellner M, Gross V, Hercule H, Markovic M, Honeck H, Luft FC, Schunck WH. Mouse cyp4a isoforms: Enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. The Biochemical journal. 2007;403:109–118. doi: 10.1042/BJ20061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue K, Sodhi K, Puri N, Gotlinger KH, Cao J, Rizzani R, Falck JR, Abraham NG, Laniado-Schwartzman M. Endothelial-specific cyp4a2 overexpression leads to renal injury and hypertension via increased production of 20-hete. Am J Physiol Renal Physiol. 2009 doi: 10.1152/ajprenal.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lilly LS, Pratt RE, Alexander RW, Larson DM, Ellison KE, Gimbrone MA, Jr, Dzau VJ. Renin expression by vascular endothelial cells in culture. Circ Res. 1985;57:312–318. doi: 10.1161/01.res.57.2.312. [DOI] [PubMed] [Google Scholar]
- 38.Hilgers KF, Veelken R, Muller DN, Kohler H, Hartner A, Botkin SR, Stumpf C, Schmieder RE, Gomez RA. Renin uptake by the endothelium mediates vascular angiotensin formation. Hypertension. 2001;38:243–248. doi: 10.1161/01.hyp.38.2.243. [DOI] [PubMed] [Google Scholar]
- 39.Guo AM, Scicli G, Sheng J, Falck JC, Edwards PA, Scicli AG. 20-hete can act as a nonhypoxic regulator of hif-1alpha in human microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H602–613. doi: 10.1152/ajpheart.00874.2008. [DOI] [PubMed] [Google Scholar]
- 40.Saijonmaa O, Nyman T, Kosonen R, Fyhrquist F. Upregulation of angiotensin-converting enzyme by vascular endothelial growth factor. Am J Physiol Heart Circ Physiol. 2001;280:H885–891. doi: 10.1152/ajpheart.2001.280.2.H885. [DOI] [PubMed] [Google Scholar]
- 41.Villard E, Alonso A, Agrapart M, Challah M, Soubrier F. Induction of angiotensin i-converting enzyme transcription by a protein kinase c-dependent mechanism in human endothelial cells. J Biol Chem. 1998;273:25191–25197. doi: 10.1074/jbc.273.39.25191. [DOI] [PubMed] [Google Scholar]
- 42.Wu CC, Cheng J, Zhang FF, Gotlinger KH, Kelkar M, Zhang Y, Jat JL, Falck JR, Schwartzman ML. Androgen-dependent hypertension is mediated by 20-hydroxy-5,8,11,14-eicosatetraenoic acid-induced vascular dysfunction: Role of inhibitor of {kappa}b kinase. Hypertension. 2011;57:788–794. doi: 10.1161/HYPERTENSIONAHA.110.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takase O, Marumo T, Imai N, Hirahashi J, Takayanagi A, Hishikawa K, Hayashi M, Shimizu N, Fujita T, Saruta T. Nf-kappab-dependent increase in intrarenal angiotensin ii induced by proteinuria. Kidney international. 2005;68:464–473. doi: 10.1111/j.1523-1755.2005.00424.x. [DOI] [PubMed] [Google Scholar]
- 44.Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL, Chiao PJ. Nf-kappab and ap-1 connection: Mechanism of nf-kappab-dependent regulation of ap-1 activity. Molecular and cellular biology. 2004;24:7806–7819. doi: 10.1128/MCB.24.17.7806-7819.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toda N, Ayajiki K, Okamura T. Interaction of endothelial nitric oxide and angiotensin in the circulation. Pharmacological reviews. 2007;59:54–87. doi: 10.1124/pr.59.1.2. [DOI] [PubMed] [Google Scholar]
- 46.Ignjatovic T, Stanisavljevic S, Brovkovych V, Skidgel RA, Erdos EG. Kinin b1 receptors stimulate nitric oxide production in endothelial cells: Signaling pathways activated by angiotensin i-converting enzyme inhibitors and peptide ligands. Mol Pharmacol. 2004;66:1310–1316. doi: 10.1124/mol.104.001990. [DOI] [PubMed] [Google Scholar]
- 47.Fleming I. Signaling by the angiotensin-converting enzyme. Circ Res. 2006;98:887–896. doi: 10.1161/01.RES.0000217340.40936.53. [DOI] [PubMed] [Google Scholar]
- 48.Ward NC, Puddey IB, Hodgson JM, Beilin LJ, Croft KD. Urinary 20-hydroxyeicosatetraenoic acid excretion is associated with oxidative stress in hypertensive subjects. Free Radic Biol Med. 2005;38:1032–1036. doi: 10.1016/j.freeradbiomed.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 49.Ward NC, Rivera J, Hodgson J, Puddey IB, Beilin LJ, Falck JR, Croft KD. Urinary 20-hydroxyeicosatetraenoic acid is associated with endothelial dysfunction in humans. Circulation. 2004;110:438–443. doi: 10.1161/01.CIR.0000136808.72912.D9. [DOI] [PubMed] [Google Scholar]
- 50.Liu H, Zhao Y, Nie D, Shi J, Fu L, Li Y, Yu D, Lu J. Association of a functional cytochrome p450 4f2 haplotype with urinary 20-hete and hypertension. J Am Soc Nephrol. 2008;19:714–721. doi: 10.1681/ASN.2007060713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward NC, Tsai IJ, Barden A, van Bockxmeer FM, Puddey IB, Hodgson JM, Croft KD. A single nucleotide polymorphism in the cyp4f2 but not cyp4a11 gene is associated with increased 20-hete excretion and blood pressure. Hypertension. 2008;51:1393–1398. doi: 10.1161/HYPERTENSIONAHA.107.104463. [DOI] [PubMed] [Google Scholar]
- 52.Fava C, Montagnana M, Almgren P, Rosberg L, Lippi G, Hedblad B, Engstrom G, Berglund G, Minuz P, Melander O. The v433m variant of the cyp4f2 is associated with ischemic stroke in male swedes beyond its effect on blood pressure. Hypertension. 2008;52:373–380. doi: 10.1161/HYPERTENSIONAHA.108.114199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.