Abstract
Pituitary adenylate cyclase activating polypeptide (PACAP) is a potent neurotrophic and neuroprotectant that is transported across the blood-brain barrier in amounts sufficient to affect brain function. However, its short half-life in blood makes it difficult to administer peripherally. Here, we determined whether the radioactively labeled 38 amino acid form of PACAP can enter the brain after intranasal (i.n.) administration. Occipital cortex and striatum were the regions with the highest uptake, peaking at levels of about 2-4 percent of the injected dose per g of brain region. Inclusion of unlabeled PACAP greatly increased retention of I-PACAP by brain probably because of inhibition of the brain-to-blood efflux transporter for PACAP located at the blood-brain barrier. Sufficient amounts of PACAP could be delivered to the brain to affect function as shown by improvement of memory in aged SAMP8 mice, a model of Alzheimer’s disease. We found that each of three cyclodextrins when included in the i.n. injection produced a unique distribution pattern of I-PACAP among brain regions. As examples, β-cyclodextrin greatly increased uptake by the occipital cortex and hypothalamus, α-cyclodextrin increased uptake by the olfactory bulb and decreased uptake by the occipital cortex and striatum, and (2-hydropropyl)-β-cyclodextrin increased uptake by the thalamus and decreased uptake by the striatum. These results show that therapeutic amounts of PACAP can be delivered to the brain by intranasal administration and that cyclodextrins may be useful in the therapeutic targeting of peptides to specific brain regions.
Introduction
The 38 amino acid form of pituitary adenylate cyclase activating polypeptide (PACAP) has potent neurotrophic and neuroprotective effects when tested in in vitro and in vivo in neurotoxic, neurodegenerative, and ischemic models [1, 36, 44, 45]. PACAP has been proposed as a treatment of stroke, central nervous system (CNS) injuries, and neurodegenerative diseases [39]. PACAP can exert neuroprotective effects after intravenous (i.v.) injection because of its extreme potency and because it is transported across the blood-brain barrier (BBB) by a saturable system [5, 41, 44]. Transport of intravenously administered PACAP across the BBB varies among brain regions with the hypothalamus and the hippocampus having the fastest uptake [34].
However, there are problems with the use of peptides such as PACAP as therapeutics. Chief among these are rapid degradation in blood and a low bioavailability necessitating their administration by injection. PACAP also has brain-to-blood efflux transporters that limit accumulation of circulating peptide by brain [5, 9]. Some peptides have been shown to be deliverable to the brain by intranasal (i.n.) administration [8, 22, 29]. This route avoids use of needles, can bypass the blood-brain barrier (BBB), and produces lower levels in blood for a given level in brain, thus decreasing peripheral side effects. Interferon beta-1b given by the i.n route targets the CNS and lymph nodes and can produce higher levels in brain than iv administration [40]. Insulin-like growth factor-I, insulin, the glucagon-like peptide-1 antagonist exendin(9-39), vasoactive intestinal peptide, and galanin-like peptide have been shown to be taken up by brain and to exert effects on the CNS after i.n. administration [2, 7, 10, 11, 28, 35, 38, 43]. Substances given by the i.n. route reach the capillary bed which forms the BBB as shown by the ability of intranasal substances to modulate the activity of P-glycoprotein, a brain-to-blood efflux pump located in the luminal membrane of brain endothelial cells [24].
In most cases reported to date, the highest uptake occurs at the olfactory bulb with distribution to the rest of the brain being considerably lower. However, recent work with galanin-like peptide indicates that cyclodextrins may be able to alter the distribution pattern of peptides given by the intranasal route [35]. If so, this strategy could be exploited to direct peptides to or away from specific brain regions, thus improving therapeutic targeting.
Here, we investigated distribution of PACAP radioactively labeled with iodine (IPACAP) into brain regions, whole brain, and blood after i.n. administration. We showed that i.n. administration delivered amounts of PACAP sufficient to improve memory in aged SAMP8 mice, a model of Alzheimer’s disease [6, 20, 33]. We also showed that brain distribution of IPACAP can be dramatically altered by cyclodextrins with each of three cyclodextrins tested producing a unique pattern of brain distribution.
Materials and Methods
Radioactive labeling of PACAP38
The 38 amino acid form of PACAP (Peninsula Laboratories, Inc., San Carlos, CA) was radioactively labeled with 131I by the lactoperoxidase method. Briefly, 5 μg of PACAP38 was mixed with 30 μl of 0.4 M Na acetate (pH 5.6), 10 μl of lactoperoxidase (10 μg/ml), and 2 mCi of 131I. The reaction was started by adding 150 ml of H2O2 in a volume of 10 μl; 10 min later, an additional 150 ml of H2O2 in a volume of 10 μl was added. After a total of 20 min exposure to H2O2, the mixture was purified with high performance liquid chromatography (HPLC; Shimadzu USA Manufacturing Inc., Columbia, MD). Radioactively labeled PACAP38 (I-PACAP) was purified by high performance liquid chromatography with use of a C18 column (P.J. Cobert Associates, Inc., St. Louis, MO). Specific activity as previously measured was 500 Ci/g [5].
Intranasal Administration
Male ICR mice (2 month old) from our in-house colony were anesthetized with an intraperitoneal (i.p.) injection of 0.2 ml of urethane (40% solution) and the right carotid artery was exposed. Mice were given bilaterally an intranasal (i.n.) administration of 2 μl of lactated Ringer’s solution (LR) containing 1% bovine serum albumin (BSA) and 500,000 cpm/μl of IPACAP with (I-PACAP+CD) or without 5% β-cyclodextrin (CD, Sigma-Aldrich, Inc., St. Louis, MO) as used previously [2]. The 2 μl was delivered to the cribriform plate by pushing a small cannula attached to a 10 μl syringe through the right and left nares (total: 4μl/mouse) to the depth of the cribriform plate. The I-PACAP + CD solution was prepared by mixing one part of a 20% CD solution with 3 parts of I-PACAP solution to yield I-PACAP in 5% CD.
Tissue Collections
Male ICR mice (2 month old) were anesthetized with an i.p. injection of 0.2 ml of urethane (40% solution) and then given i.n. I-PACAP + CD as above. Blood was collected from the right carotid artery and the whole brain was removed at 5, 15, 30, 45, 60, and 120 min (n = 3-4 mice/time) after the i.n. administration. The brain was dissected after the method of Glowinski and Iversen [23] on ice into 11 regions of olfactory bulb (Olf), occipital cortex (OC), striatum (St), frontal cortex (FC), hypothalamus (Hyth), thalamus (Th), hippocampus (Hip), parietal cortex (PC), cerebellum (Cb), midbrain (Mb), pons-medulla (PM) and each region weighed. The whole blood was centrifuged at 5,400 g for 15 min at 4 °C and the level of radioactivity measured in 50 μl of resulting serum. The level of radioactivity in each of the11 regions and the resulting serum was determined in a gamma counter by counting for 30 min. Values for whole brain (WBr) were calculated by summing the regional weights and regional levels of radioactivity. The percentage of the injected dose present in a ml of serum (%Inj/ml) was calculated with the following formula:
where Inj is the dose administered and Cpt is the level of radioactivity in a ml of serum at time t. The percentage of injected dose taken up per gram of brain region (%Inj/g) was calculated at each time with the following formula:
where Am is the level of activity in cpm/g for a given brain region and the value 10 approximates the vascular space in units of μl/g.
Acid Precipitation
Male ICR mice (2 month old) were anesthetized, prepared, and administered i.n. IPACAP as above. Blood was collected from the right carotid artery and the WBr was removed at 5, 30, 60, and 120 min (n = 3 mice/time) after the i.n.administration of I-PACAP . Five regions (Olf, OC, Hyth, Hip, PM) were dissected from brain on ice and 150 μl of 0.25 M phosphate buffer solution (PBS) and 150 μl of 10 mM enzymatic cocktail (19.82 mg/ml 100mM 1,10-Phenanthroline, 12.51 mg/ml 100mM N-Ethylmaleimide, 37.22 mg/ml 100mM EDTA, 88.89 mg/ml 100mM L-Thyroxine in distilled water) added to the brain region. The brain region was homogenized on ice with a Tissue tearor (Biospec Products, Inc., Dremel Racine, WI) for 20 sec at a setting of 20. The homogenate was centrifuged at 5,400 g for 15 min. A portion of the supernatant (150 μl) was transferred to a second tube and 150 μl of 30% trichloroacetic acid (TCA) added to precipitate I-PACAP. The whole blood was centrifuged at 5,400 g for 15 min at 4 C and the level of radioactivity measured in 50 μl of the resulting serum. 10 μl of serum was added to a tube containing 500 μl of 1% BSA in LR, mixed, and 500 μl of 30% TCA added. This combination was then mixed and centrifuged. To determine the amount of degradation that occurred with processing, 500,000 cpm was added in vitro to a serum or a brain region obtained from a mouse that had not received an i.n. administration (n = 2). Samples were then processed as above. This was then homogenized and centrifuged as above. After addition of TCA, the serum, brain, and processing controls were centrifuged and the supernatant was separated from the pellet. The percent of radioactivity precipitated was calculated by the following formula:
The percent precipitated from samples was divided by that which precipitated in the processing controls and multiplied by 100 to give an index of intact I-PACAP.
I-PACAP and I-PACAP + unlabeled PACAP
Anesthetized male ICR mice (2 month old) were given i.n. I-PACAP with or without 1 μg (0.25 μg/μl) of unlabeled PACAP38 (n = 7/group). Blood was collected from the right carotid artery and WBr was removed at 30 min after the i.n. administration of I-PACAP. Six brain regions (Olf, OC, Hyth, Hip, Cb, PM) were dissected on ice from brain, weighed, and the level of radioactivity determined. The whole blood was centrifuged at 5,400 g for 15 min at 4 C and the level of radioactivity measured in 50 μl of the resulting serum.
I-PACAP without CD, with (2-Hydroxypropyl)-β-cyclodextrin, or with α-Cyclodextrin
Male ICR mice (2 month old) were anesthetized and prepared as above and given i.n. administration. I-PACAP with or without one of the CD (Sigma-Aldrich, Inc., St. Louis, MO): (2-hydroxypropyl)-β-cyclodextrin (hydro-ß-CD) or α-cyclodextrin (alpha-CD). After 60 min, the brain was dissected on ice into 11 regions (Olf, OC, St, FC, Hyth, Th, Hip, PC, Cb, Mb, PM) and each region weighed and the level of radioactivity measured. Values for WBr were calculated by adding the regional weights and levels of radioactivity for each mouse. The whole blood was centrifuged at 5,400 g for 15 min at 4 C and the level of radioactivity measured in 50 μl of the resulting serum. Results were expressed as the %Inj/g for each brain region and WBr.
T-Maze Assessment of Memory
The T-maze is a reference-memory task. We have demonstrated that it tests hippocampal dependence of memory and has been widely applied by us to investigate aspects of memory [14, 15, 18]. The T-maze consisted of a black plastic alley with a start box at one end and two goal boxes at the other. The start box was separated from the alley by a plastic guillotine door that prevented movement down the alley until raised at the onset of training. An electrifiable floor of stainless steel rods ran throughout the maze to deliver a mild scrambled foot-shock.
Mice were not permitted to explore the maze prior to training. A block of training trials began when a mouse was placed into the start box. The guillotine door was raised and a cue buzzer sounded simultaneously; 5 sec later foot-shock was applied. The arm of the maze entered on the first trial was designated “incorrect” and the mild foot-shock was continued until the mouse entered the other goal box, which in all subsequent trials was designated as “correct” for the particular mouse. At the end of each trial, the mouse was returned to its home cage until the next trial.
Mice were trained until they made 1 avoidance. Training used an inter-trial interval of 35 sec, the buzzer was of the door-bell type sounded at 55 dB, and shock was set at 0.35 mA (Coulbourn Instruments scrambled grid floor shocker model E13-08). Retention was tested one week later by continuing training until mice reached the criterion of 5 avoidances in 6 consecutive trials. The results were reported as the number of trials to criterion for the retention test.
Immediately after training, the mouse was anesthetized to effect in an induction chamber using 4% isoflurane delivered via a precision vaporizer with appropriate waste gas scavenging. A 0.5 to 2.5μl pipette with an electrophoresis or gel-loading tip was used to administer the test agent. One μl of test agent was drawn up into the pipette tip, the outside surface of the tip coated with lubricating jelly then, while holding the anesthetized mouse in a supine position, the pipette tip was gently placed into the right nares a distance of 4-5mm and the agent evacuated from the tip. This process applied the PACAP to the cribriform plate, allowing absorption directly into the brain via the olfactory nerves. The mouse returned to its home cage.
Statistical analysis
Means are reported with their standard errors and n. More than two means were compared by analysis of variance (ANOVA) followed by Newman-Keuls post-test. Statistical significance was taken as p<0.05. The Prism 5.0 statistical software program (GraphPad Software Inc., San Diego, CA) was used in statistical analysis.
Results
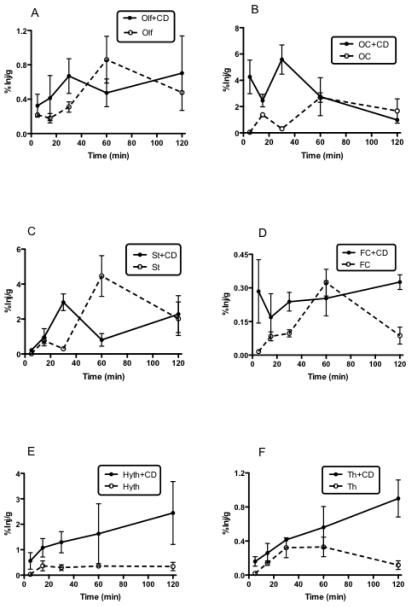
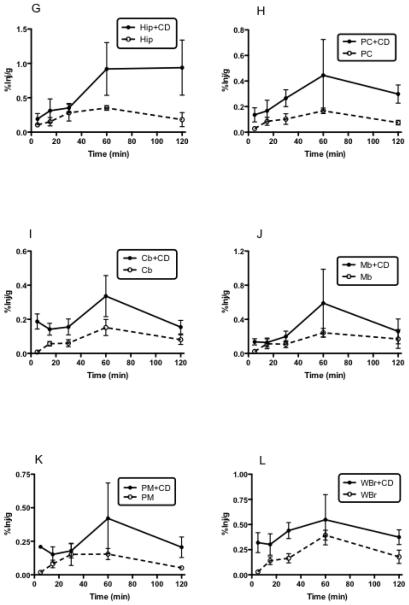
Fig. 1A-L shows the percent of i.n. I-PACAP administered with or without CD taken up per gram of brain region (%Inj/g) at various times. All regions of brain showed uptake of IPACAP regardless of whether administered with or without CD. In the absence of CD, most regions peaked or reached a plateau 60 min after i.n. administration, with the regions of highest uptake being striatum, occipital cortex, and olfactory bulb. Figure 2 shows a similar plateau in blood uptake after about 30 min. CD greatly increased the appearance of I-PACAP in blood as shown by 2-way ANOVA (p<0.005), where CD treatment and time were the two independent variables.
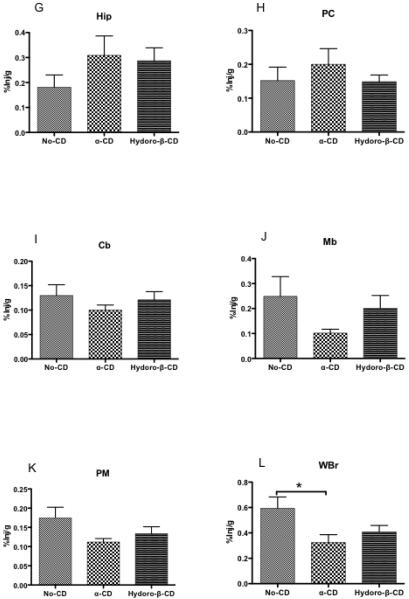
Fig. 1.
A-L. Appearance of I-PACAP in olfactory bulb (A: Olf), occipital cortex (B: OC), striatum (C: St), frontal cortex (D: FC), hypothalamus (E: Hyth), thalamus (F: Th), hippocampus (G: Hip), parietal cortex (H: PC), cerebellum (I: Cb), midbrain (J: Mb), pons-medulla (K: PM) and whole brain (L: WBr) at 5, 15, 30, 60, and 120 min after i.n. administration of I-PACAP with or without CD. The peak value without CD was at 60 min and with CD at 30 min in Olf (A), OC (B), and St (C). The peak value without CD was at 60 min and without CD at 120 min in FC (D) and Th (F). The peak value without CD was at 15 min and with CD at 120 min in Hyth (E). The peak value without CD was at 60 min and with CD at 60 min in Hip (G), PC (H), Cb (I), Mb (J), PM (K), and WBr (L).
Fig. 2.
Appearance of I-PACAP with or without CD in serum after i.n. administration. The peak value with or without CD was at 60 min. I-PACAP without CD produced much lower levels in serum as shown by w-way ANOVA.
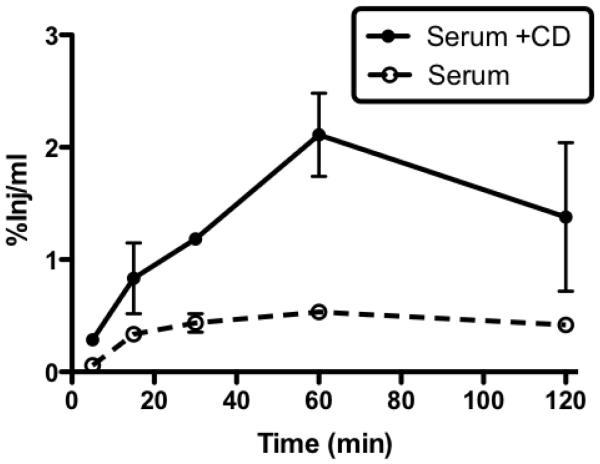
Fig. 3 shows results from figure 1 averaged over time. This more clearly shows that in the absence of CD, the regions of striatum and occipital cortex took up more I-PACAP than other regions, including the olfactory bulb. CD produced the greatest increase in PACAP uptake in the hypothalamus, but statistically significant increases also occurred in the occipital cortex, frontal cortex, parietal cortex, thalamus, hippocampus, cerebellum, midbrain, pons-medulla, and whole brain. CD did not increase PACAP uptake by the olfactory bulb or the striatum.
Fig. 3.
Data in Figure 1 combined over time period of study and presented as single value per animal. Effect of CD on I-PACAP uptake by individual regions was compared by t-tests. CD produced a statistically significant (p<0.05) increase in values for occipital cortex (OC), frontal cortex (FC), hypothalamus (Hyth), thalamus (Th), hippocampus (Hip), parietal cortex (PC), cerebellum (Cb), midbrain (Mb), pons-medulla (PM), and whole brain (WBr).
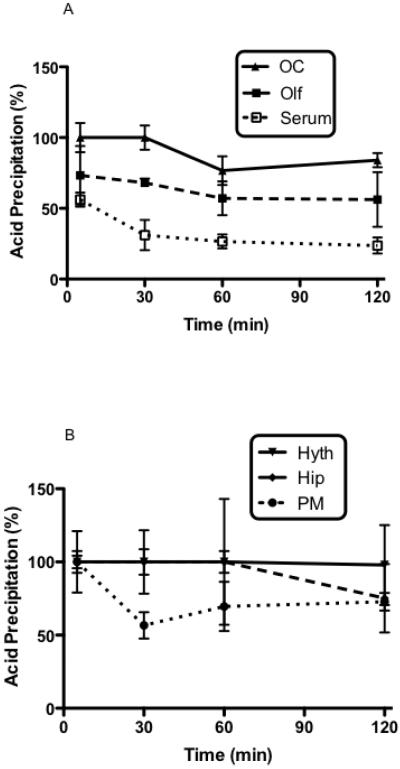
Figure 4 shows the percent of radioactivity from serum and 5 brain regions that was precipitated by acid, an indicator of the amount of radioactivity was still attached to PACAP. From 30 min onward, the majority of the radioactivity in serum did not precipitate with acid consistent with the rapid enzymatic degradation of PACAP and I-PACAP in blood. However, IPACAP was more stable in brain. I-PACAP degradation showed regional variation, with it being particularly stable in occipital cortex and hypothalamus and less stable in pons-medulla and olfactory bulb.
Fig. 4.
Acid precipitation (%) of I-PACAP without CD at 5, 30, 60, and 120 min after i.n. administration. Panel A shows the occipital cortex (OC), olfactory bulb (Olf), and serum and panel B shows the hypothalamus (Hyth), hippocampus (Hip), and pons-medulla (PM).
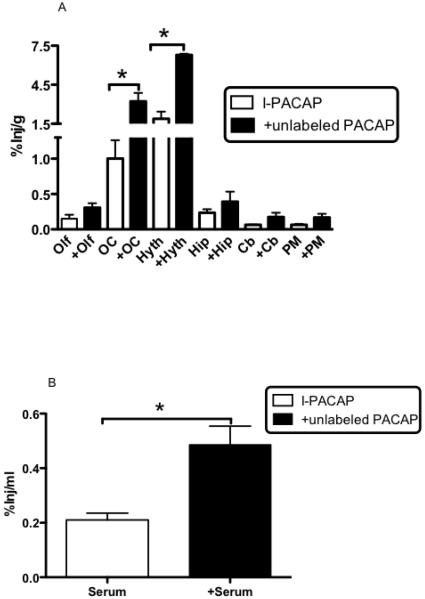
Inclusion of unlabeled PACAP in the i.n. administration increased I-PACAP uptake by the occipital cortex [t(8) = 3.7, p<0.01] and hypothalamus [t(6) = 6.7, p<0.001] , with trends (0.1>p>0.05) for olfactory bulb and pons-medulla and arithmetic increases for all brain regions examined (Fig. 5A). Based on specific activity, the dose for I-PACAP only was about 1 ng/mouse or about 1/1000th that of the dose given when unlabeled PACAP was included. Uptake by serum was also increased [t(11) = 4.1, p<0.005] (Fig. 5B).
Fig. 5.
%Inj/g of I-PACAP ± unlabeled PACAP in olfactory bulb (Olf), occipital cortex (OC), hypothalamus (Hyth), hippocampus (Hip), cerebellum (Cb), and pons-medulla (PM) at 30 min after i.n. administration. Unlabeled PACAP produced significant increases (* p < 0.05) in OC and Hyth and arithmetic increases in other brain regions (Panel A) and a significant increase in %Inj/ml in serum (Panel B) as assessed by t-test.
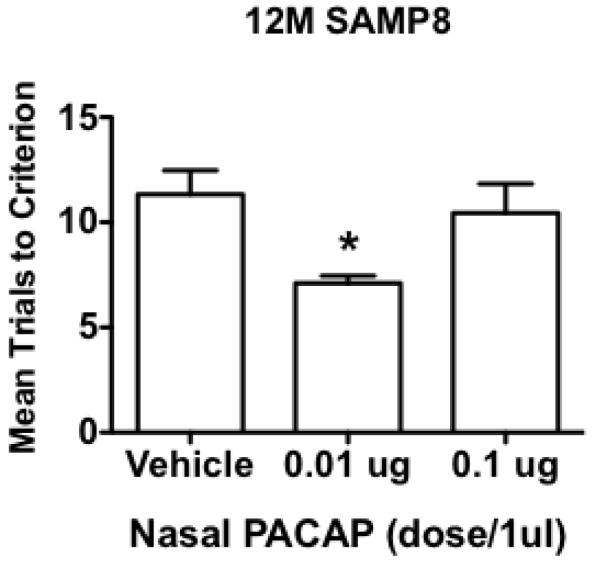
Intranasal PACAP was effective in improving memory in the aged SAMP8 mouse, a model of Alzheimer’s disease (Fig 6): F(2.26) = 4.48, p<0.05. Neuman-Keuls post-test showed that the 0.01 microg dose was statistically different from vehicle and from the 0.1 microg dose (p<0.05).
Figure 6.
Intranasal PACAP dose of 0.01 microg improves memory in the SAMP8 mouse model of Alzheimer’s disease. *p<0.05 compared to vehicle or 0.1 microg dose, n = 9/group.
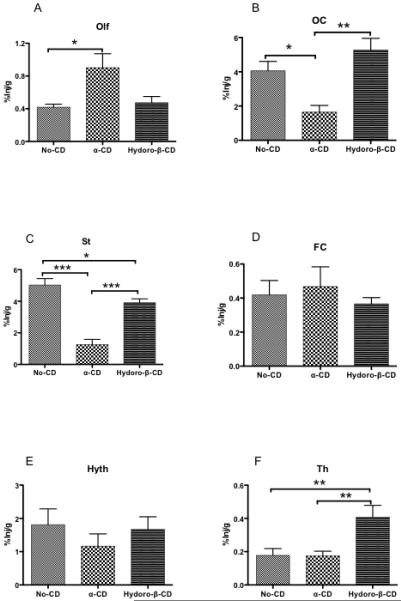
Figures 1 and 3 show that β-CD increased brain uptake for most regions. We compared the abilities of two other cyclodextrans to affect uptake of I-PACAP after i.n. adminstration (Fig. 7). Neither alpha-CD nor hydro-β-CD affected uptake by frontal cortex (Fig. 6D), hypothalamus (Fig. 7E), hippocampus (Fig. 7G), parietal cortex (Fig. 6H), cerebellum (Fig. 6I), midbrain (Fig. 6J) and pons-medulla (Fig. 7K). For olfactory bulb [F(2,22)=5.49, p<0.05], uptake was increased by alpha-CD (p<0.05), but not by hydro-β-CD (Fig. 7A). For occipital cortex [F(2,18)=9.14, p<0.05], uptake was decreased by alpha-CD (p<0.05), but not by hydro-β-CD (Fig. 7B). For striatum [F(2,17)=36.13, p<0.0001], uptake was decreased by alpha-CD (p<0.001) and uptake was decreased by hydro-β-CD (p<0.05) (Fig. 7C). For thalamus [F(2,26)=7.16, p<0.05], uptake was not change by alpha-CD, but uptake was increased by hydro-β-CD (p<0.01) (Fig. 7F). For whole brain [F(2,25)=3.96, p<0.05], uptake was decreased by alpha-CD (p<0.05), but not by hydro-β-CD (Fig. 7L). Neither alpha-CD nor hydro-ß-CD affected blood levels of I-PACAP (Fig. 8). Table I compares the effects of the 3 cyclodextrins on I-PACAP distribution.
Fig. 7.
A-L. %Inj/g of I-PACAP without CD, I-PACAP with α-CD, and I-PACAP with Hydro-β-CD in olfactory bulb (A: Olf), occipital cortex (B: OC), striatum (C: St), frontal cortex (D: FC), hypothalamus (E: Hyth), thalamus (F: Th), hippocampus (G: Hip), parietal cortex (H: PC), cerebellum (I: Cb), midbrain (J: Mb), pons-medulla (K: PM) and whole brain (L: WBr) at 60 min after i.n. administration. ANOVAs showed difference for Olf, OC, St, FC, Hyth, Th, Hip, PC, Cb, Mb, PM and WBr. Newman-Keuls Multiple Comparison Test showed Olf, OC, St, FC, Hyth, Th, Hip, PC, Cb, Mb, PM, and WBr in I-PACAP without CD, I-PACAP with α-CD, and IPACAP with Hydro-β-CD (* p < 0.05, ** p < 0.01, *** p < 0.001). n = 6 - 11 mice/groups.
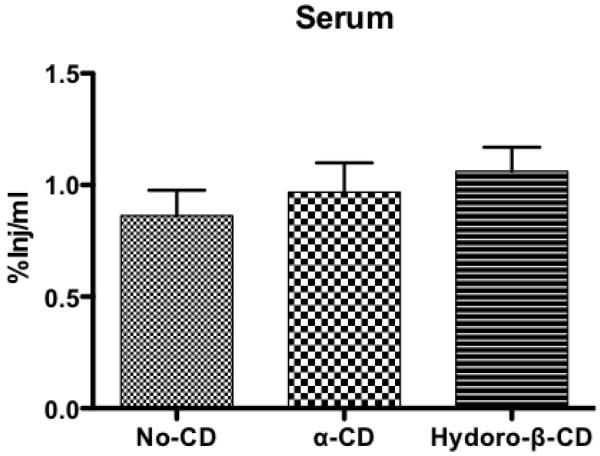
Fig. 8.
%Inj/ml of I-PACAP without CD, I-PACAP with α-CD, and I-PACAP with Hydro-β-CD in serum at 60 min after i.n. administration. No change occurred in serum levels as assessed by ANOVA.
Table I.
Comparative Effects of Three Cyclodextrins on the Uptake of I-PACAP After Intranasal Administration
| Olf | OC | St | FC | Hyth | Th | Hip | PC | Cb | Mb | PM | WBr | Serum | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-CD | NS | ↑ | NS | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| α-CD | ↑ | ↓ | ↓ | NS | NS | NS | NS | NS | NS | NS | NS | ↓ | NS |
| H-β-CD | NS | NS | ↓ | NS | NS | ↑ | NS | NS | NS | NS | NS | NS | NS |
Olfactory bulb (Olf), occipital cortex (OC), striatum (St), frontal cortex (FC), hypothalamus (Hyth), parietal cortex (PC), cerebellum (Cb), midbrain (Mb), pons-medulla (PM), whole brain (WBr); beta-cyclodextrin (β-CD ), alpha-cyclodetrin (α-CD), (2-hydroxypropyl)-betacyclodextrin (H-β-CD); no statistically significant change (NS), statistically significant increase (↑), statistically significant decrease (↓).
Discussion
These studies evaluated the effectiveness of i.n. administration in the delivery of I-PACAP to the CNS. Our previous work had shown that brain uptake of the glucagon-like peptide-1 antagonist exendin(9-39) after i.n.administration [2] was especially high at the olfactory bulb with distribution to other regions of the brain being much lower. However, delivery of both a glucagon-like peptide-1 agonist and antagonist reached the hippocampus in sufficient amounts to affect cognition [11]. This is consistent with work by Frey, Thorne, and others whose work has shown that many peptides can be delivered to the brain by i.n. administration [8, 11, 22, 29, 38].
Here, we investigated distribution into brain and serum of I-PACAP after intranasal administration into the brain. We detected I-PACAP uptake into all regions of the brain; however, some regions took up much more I-PACAP than others. Specifically, I-PACAP uptake by occipital cortex and striatum was much higher than to other regions of the brain. This is in marked contrast to many other studies where olfactory bulb uptake of the intranasally administered substance is the highest. This pattern of distribution is also different from that found for I-PACAP given by intravenous injection in that the highest uptakes were by hippocampus and hypothalamus [34]. After i.n. administration, I-PACAP was also found in serum, indicating absorption into blood either directly from the nose or by way of cerebrospinal fluid absorption.
Acid precipitation was used to determine the percentage of radioactivity still bound to IPACAP. We found that most of the radioactivity from various brain regions was at all time points precipitated by acid, indicating it was bound to PACAP. Radioactivity in serum, however, showed significant degradation. This is consistent with the rapid degradation of I-PACAP in the circulation [5]. I-PACAP was more stable in some brain regions, such as hypothalamus and hippocampus, than in other brain regions, such as olfactory bulb. Overall, acid precipitation shows that I-PACAP is stable in brain regions for a prolonged period after its intranasal administration.
PACAP38 is transported across the BBB in both the blood-to-brain and the brain-to-blood directions [5, 9]. That work indicates that the blood-to-brain transporter is a different protein than the brain-to-blood transporter. We hypothesized that inclusion of unlabeled PACAP would enhance the retention of I-PACAP by competitively inhibiting the brain-to-blood transporter. The dose of 1 μg/mouse of unlabeled PACAP in the i.n. administration increased the amount of PACAP by about 1000 fold in comparison to that given as I-PACAP only. The unlabeled PACAP increased uptake by the occipital cortex and hypothalamus in a statistically significant manner. Arithmetic increases in all the other regions did not reach statistical significance. A statistically significant increase in blood levels of I-PACAP with the inclusion of unlabeled PACAP in the intranasal administration is likely caused by the unlabeled PACAP inhibiting clearance and uptake of I-PACAP by peripheral tissues.
Four factors may explain the unique distribution in brain for I-PACAP after its nasal administration. First, the concentration of PACAP in brain regions exceeding that of the olfactory bulb suggests a unique mechanism of brain distribution. Second, brain region differences in enzymatic degradation affect I-PACAP levels. Third, the brain-to-blood transport of I-PACAP may be non-homogeneously distributed given the different effects of unlabeled PACAP on I-PACAP retention by different brain regions as shown in Figure 5A. Fourth, regional differences in the distribution of the PACAP receptors may also affect retention of IPACAP. In particular, regional differences in the competition for I-PACAP between the brain-to-blood transporter and the receptors could contribute to this unique distribution of I-PACAP.
To determine whether enough PACAP can enter brain after intranasal administration sufficient to exert effects on CNS function, we used the SAMP8 mouse, an animal model of Alzheimer’s disease. We have previously established that PACAP has effects on cognition in the SAMP8 [9]. The SAMP8 strain of mouse has a natural mutation so that, although as a young mouse it has no distinguishing physical phenotype, as it ages it develops defects in both learning and memory, cholinergic deficits, oxidative stress in brain, decreased reabsorption of cerebrospinal fluid, impaired brain-to-blood efflux of amyloid beta proein, and elevated levels of amyloid beta protein [4, 13, 16, 19, 27, 31, 33, 37, 42]. These features, also found in Alzheimer’s disease, can be reversed by treating the mice with antisense directed against amyloid precursor protein or by administering antibodies directed against amyloid beta protein, thus demonstrating that the memory deficits in the SAMP8 are dependent on APP/amyloid beta protein [3, 13, 16, 21, 26, 32]. PACAP27 can improve memory function in aged SAMP8 mice when it is administered peripherally and its efflux system blocked and PACAP protects against CA1 hippocampal neuron loss when given peripherally after stroke [9, 44]. Here, we showed that intranasal PACAP was also effective in improving memory at the very low dose of 0.01 microg. Testing memory has advantages over testing learning in that drug is administered after training. This means that any side effects a drug that could interfere with training are negated as the drug is given after the training episode. Likewise, any acute effects that might affect performance are negated as retesting occurs a week after drug administration. Thus, the drug can only affect memory by affecting post-training memory consolidation. The higher dose of 0.1 microg had no effect on memory. A common feature of peptides, especially those that are mnemonics, is their inverted-U shaped curve [12, 25]; indeed, at extreme doses, mnemonics can even impair cognition [15, 17].
We also examined the effects of cyclodextrin on regional brain distribution of I-PACAP. Cyclodextrin has several forms, including alpha (six glucose residue), beta (seven glucose residue), and gamma (eight glucose residue) forms. Cyclodextrin has been used to increase absorption of peptides across biological membranes [46]. Overall ß-cyclodextrin increased uptake of i.n. I-PACAP by most, but not all brain regions. Most notably were increases in uptake by the occipital cortex and hypothalamus, the same regions where uptake was most increased by unlabeled PACAP. This suggests that CD acted primarily not by increasing transport from the cribriform plate to brain but by preventing I-PACAP from binding to its brain-to-blood transporter. Serum levels of I-PACAP were also increased with CD, suggesting that it also prevented I-PACAP from binding to the uptake and clearance sites in peripheral tissues.
We compared the abilities of two other cyclodextrins, alpha-CD and (2-hydropropyl)-β-CD (hydro-ß-CD) to affect uptake of I-PACAP after i.n. administration. We found that each CD produced unique variations in the regional distribution of I-PACAP. For example, olfactory bulb uptake was increased by alpha-CD and uptake by the thalamus was increased by hydro-β-CD. Occipital cortex was decreased by alpha-CD, striatum uptake was decreased by both of alpha-CD and hydro-β-CD and whole brain uptake was decreased by alpha-CD. Neither alpha-CD nor hydro-β-CD affected blood levels of I-PACAP. These results show that different cyclodextrins have different effects on the distribution in brain of i.n. I-PACAP. As such, individual cyclodextrins could be used to direct PACAP or other peptides to or away from specific brain regions. These targeting effects of CD could result in lower doses of PACAP being needed to affect brain function, but the main advantage would be in reducing off target effects mediated through other CNS and peripheral sites.
Some of the mechanisms by which cyclodextrins could affect regional distribution of PACAP are understood [30, 46]. Cyclodextrins interact with peptides and proteins, primarily by complexing with their aromatic or other hydrophobic side chains. These reversible supramolecular cyclodextrin-protein complexes can have different tertiary structures, solubilities, molecular interactions, and susceptibilities to enzymatic degradation than the native peptide or protein. The cyclodextrins can also directly interact with the target cell by extracting cholesterol from the cell membrane. These effects could alter both ligand and cell in ways that would result in unique distribution patterns for each peptide-cyclodextrin combination.
In conclusion, we found that intranasal administration of I-PACAP was absorbed into brain and distributed to all brain regions. Distribution was unique from traditional distribution after intranasal administration in that occipital cortex and striatum has higher uptakes than the olfactory bulb. PACAP given by intranasal administration was therapeutic in that it improved memory in aged SAMP8 mice, an animal model of Alzheimer’s disease. Inclusion of unlabeled PACAP in the i.n. administration significantly increased uptake of I-PACAP by occipital cortex and hypothalamus, probably by competitively inhibiting the brain-to-blood efflux transporter for I-PACAP. Cyclodextrins altered uptake patterns of intranasal I-PACAP with each of three cyclodextrins tested producing its own unique effect on I-PACAP distribution. We conclude that intranasal delivery is a viable route for delivering therapeutic amounts of PACAP to the brain and that cyclodextrins can be used to target PACAP to or away from specific brain regions.
Highlights.
-
-
PACAP is taken up by hippocampus and hypothalamus after intranasal administration
-
-
Cyclodextrins can be used to target intranasal PACAP to specific brain regions
-
-
Intranasal PACAP improves memory in a mouse model of Alzheimer’s disease
Acknowledgements
Supported by VA Merit Review, R0-1 AG029839 (WAB), R0-1 NS051134, and Grant-in-Aid for Scientific Research (20592148, 21592342) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (NN)
Nonstandard abbreviations
- BBB
Blood-brain barrier
- CD
Cyclodextrin
- I.N.
Intranasal
- %Inj/g
Percent of injected dose/g of brain
- %Inj/ml
Percent of injected dose/ml of serum
- LR
Lactated Ringer’s solution
- PACAP
Pituitary adenylate cyclase activating polypeptide
- Brain Regions:Cb
Cerebellum
- FC
frontal cortex
- Hip
hippocampus
- Hyth
hypothalamus
- OB
olfactory bulb
- OC
occipital cortex
- MB
midbrain
- PC
parietal cortex
- PM
pons medulla
- St
striatum
- Th
thalamus
- WBr
whole brain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Naoko Nonaka, Department of Oral Anatomy and Developmental Biology, Showa University School of Dentistry, Tokyo, Japan.
Susan A. Farr, Geriatric Research, Education, and Clinical Center, Veterans Affairs Medical Center-St. Louis, St. Louis, MO, USA 63108; Department of Internal Medicine, Division of Geriatrics, Saint Louis University School of Medicine, St. Louis, MO, USA 63104
Tomoya Nakamachi, Department of Anatomy 1, Showa University School of Medicine, Tokyo, Japan.
John E. Morley, Geriatric Research, Education, and Clinical Center, Veterans Affairs Medical Center-St. Louis, St. Louis, MO, USA 63108; Department of Internal Medicine, Division of Geriatrics, Saint Louis University School of Medicine, St. Louis, MO, USA 63104
Masanori Nakamura, Department of Oral Anatomy and Developmental Biology, Showa University School of Dentistry, Tokyo, Japan.
Seiji Shioda, Department of Anatomy 1, Showa University School of Medicine, Tokyo, Japan.
William A. Banks, Geriatric Research, Education, and Clinical Center, Veterans Affairs Puget Sound Health Care System, Seattle, WA 98108; Department of Medicine, Division of Gerontology and Geriatric Medicine, University of Washington School of Medicine, Seattle, WA 98104
References
- [1].Arimura A, Somogyvari-Vigh A, Weill C, Fiore RC, Tatsuno I, Bay V, et al. PACAP functions as a neurotrophic factor. Annals of the New York Academy of Sciences. 1994;739:228–43. doi: 10.1111/j.1749-6632.1994.tb19825.x. [DOI] [PubMed] [Google Scholar]
- [2].Banks WA, During MJ, Niehoff ML. Brain uptake of glucagon-like peptide-1 antagonist exendin(9-39) after intranasal administration. Journal of Pharmacology and Experimental Therapeutics. 2004;309:469–75. doi: 10.1124/jpet.103.063222. [DOI] [PubMed] [Google Scholar]
- [3].Banks WA, Farr SA, Butt W, Kumar VB, Franko MW, Morley JE. Delivery across the blood-brain barrier of antisense directed againt amyloid ·: reversal of learning and memory deficits in mice overexpressing amyloid precursor protein. Journal of Pharmacology and Experimental Therapeutics. 2001;297:1113–21. [PubMed] [Google Scholar]
- [4].Banks WA, Farr SA, Morley JE, Wolf KM, Geylis V, Steinitz M. Anti-amyloid beta protein antibody passage across the blood-brain barrier in the SAMP8 mouse model of Alzheimer’s disease: An age related selective uptake with reversal of learning impairment. Experimental Neurology. 2007;206:248–56. doi: 10.1016/j.expneurol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Banks WA, Kastin AJ, Komaki G, Arimura A. Passage of pituitary adenylate cyclase activating polypeptide 1-27 and pituitary adenylate cyclase activating polypeptide 1-38 across the blood-brain barrier. Journal of Pharmacology and Experimental Therapeutics. 1993;267:690–6. [PubMed] [Google Scholar]
- [6].Banks WA, Morley JE. Memories are made of this: recent advances in understanding cognitive impairments and dementia. JGerontology. 2003;58A:M314–M21. doi: 10.1093/gerona/58.4.m314. [DOI] [PubMed] [Google Scholar]
- [7].Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–34. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- [8].Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neuroscience. 2002;5:514–6. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- [9].Dogrukol-Ak D, Kumar VB, Ryerse JS, Farr SA, Verma S, Nonaka K, et al. Isolation of peptide transport system-6 from brain endothelial cells: therapeutic effects with antisense inhibition in Alzheimer’s and stroke models. J Cereb Blood Flow Metab. 2009;29:411–22. doi: 10.1038/jcbfm.2008.131. [DOI] [PubMed] [Google Scholar]
- [10].Dufes C, Olivier JC, Gaillard F, Gaillard A, Couet W, Muller JM. Brain delivery of vasoactive intestinal peptide (VIP) following nasal administration to rats. International Journal of Pharmaceutics. 2003;255:87–97. doi: 10.1016/s0378-5173(03)00039-5. [DOI] [PubMed] [Google Scholar]
- [11].During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nature Medicine. 2003;9:1173–9. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- [12].Ehrensing RH, Kastin AJ. Dose-related biphasic effect of prolyl-leucyl-glycinamide (MIF-1) in depression. American Journal of Psychiatry. 1978;135:562–6. doi: 10.1176/ajp.135.5.562. [DOI] [PubMed] [Google Scholar]
- [13].Erickson MA, Niehoff ML, Farr SA, Morley JE, Dillman LA, Lynch KM, et al. Peripheral administration of antisense targeting the amyloid beta protein precursor reverses ABPP and LRP-1 overexpression in aged SAMP8 mouse brain. J Alzheimer’s Dis. 2011 doi: 10.3233/JAD-2011-111517. in press. [DOI] [PubMed] [Google Scholar]
- [14].Farr SA, Banks WA, La Scola ME, Flood JF, Morley JE. Permanent and tempory inactivation of the hippocampus impairs T-maze footshock avoidance acquisition and retention. Brain Research. 2000;872:242–9. doi: 10.1016/s0006-8993(00)02495-1. [DOI] [PubMed] [Google Scholar]
- [15].Farr SA, Banks WA, Uezu K, Freed EO, Kumar VB, Morley JE. Mechanisms of HIV-1 induced cognitive impairment: evidence for hippocampal cholinergic involvement with overstimulation of the VIPergic system by the viral coat protein core. AIDS Research and Human Retroviruses. 2002;18:1189–95. doi: 10.1089/08892220260387931. [DOI] [PubMed] [Google Scholar]
- [16].Farr SA, Banks WA, Uezu K, Sano A, Gaskin FS, Morley JE. Antibody to beta-amyloid protein increases acetylcholine in the hippocampus of 12 month SAMP8 male mice. Life Sciences. 2003;73:555–62. doi: 10.1016/s0024-3205(03)00322-9. [DOI] [PubMed] [Google Scholar]
- [17].Farr SA, Flood JF, Morley JE. The effect of cholinergic, GABAergic, serotonergic and glutamatergic receptor modulation on post-trial memory processing in the hippocampus. Neurobiology of Learning and Memory. 2000;73:150–67. doi: 10.1006/nlme.1999.3927. [DOI] [PubMed] [Google Scholar]
- [18].Farr SA, Uezu K, Flood JF, Morley JE. Septo-hippocampal drug interactions in post-trial memory processing. Brain Research. 1999;847:221–30. doi: 10.1016/s0006-8993(99)02049-1. [DOI] [PubMed] [Google Scholar]
- [19].Flood JF, Morley JE. Age-related changes in the pharmacological improvement of retention in SAMP8 mice. In: Takeda T, editor. The SAM Model of Senescence. Excerpta Medica; Kyoto: 1994. pp. 89–94. [Google Scholar]
- [20].Flood JF, Morley JE. Learning and memory in the SAMP8 mouse. Neuroscience and Biobehavioral Reviews. 1998;22:1–20. doi: 10.1016/s0149-7634(96)00063-2. [DOI] [PubMed] [Google Scholar]
- [21].Flood JF, Morley PM, Morley JE. Age-related changes in learning, memory, and lipofuscin as a function of the percentage of SAMP8 genes. Physiology and Behavior. 1995;58:819–22. doi: 10.1016/0031-9384(95)00125-3. [DOI] [PubMed] [Google Scholar]
- [22].Frey WH., II Bypassing the blood-brain barrier to deliver therapeutic agents to the brain and spinal cord. Drug Delivery Technology. 2002;2:46–9. [Google Scholar]
- [23].Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. Journal of Neurochemistry. 1966;13:655–69. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- [24].Graff CL, Pollack GM. P-glycoprotein attenuates brain uptake of substrates after nasal installation. Pharm Res. 2003;20:1225–30. doi: 10.1023/a:1025053115583. [DOI] [PubMed] [Google Scholar]
- [25].Kastin AJ, Banks WA, Zadina JE, Graf MV. Minireview: Brain peptides: the dangers of constricted nomenclatures. Life Sciences. 1983;32:295–301. doi: 10.1016/0024-3205(83)90073-5. [DOI] [PubMed] [Google Scholar]
- [26].Kumar VB, Farr SA, Flood JF, Kamlesh V, Franko M, Banks WA, et al. Site-directed antisense oligonucleotide decreases the expression of amyloid precursor protein and reverses deficits in learning and memory in aged SAMP8 mice. Peptides. 2000;21:1769–75. doi: 10.1016/s0196-9781(00)00339-9. [DOI] [PubMed] [Google Scholar]
- [27].Kumar VB, Vyas K, Franko M, Choudhary V, Buddhiraju C, Alvarez J, et al. Molecular cloning, expression and regulation of hippocampal amyloid precursor protein of senescence accelerated mouse (SAMP8) Biochemistry and Cell Biology. 2000;79:57–67. [PubMed] [Google Scholar]
- [28].Liu XF, Fawcett JR, Thorne RG, Frey WH. Non-invasive intranasal insulin-like growth factor-1 reduces infarct volume and improves neurologic function in rats following middle cerebral artery occlusion. Neurosci Lett. 2001;308:91–4. doi: 10.1016/s0304-3940(01)01982-6. [DOI] [PubMed] [Google Scholar]
- [29].Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2011 doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- [30].Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: basic science and product development. J of Pharmacy and Pharmacology. 2010;62:1607–21. doi: 10.1111/j.2042-7158.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- [31].Morley JE. The SAMP8 mouse: a model of Alzheimer’s disease? Biogerontology. 2002;31:57–60. doi: 10.1023/a:1015207429786. [DOI] [PubMed] [Google Scholar]
- [32].Morley JE, Farr SA, Flood JF. Antibody to amyloid beta protein alleviates impaired acquisition, retention, and memory processing in SAMP8 mice. Neurobiology of Learning and Memory. 2002;78:125–38. doi: 10.1006/nlme.2001.4047. [DOI] [PubMed] [Google Scholar]
- [33].Morley JE, Farr SA, Kumar VB, Banks WA. Alzheimer’s disease through the eye of a mouse: Acceptance lecture for the 2001 Gayle A. Olson and Richard D. Olson prize. Peptides. 2002;23:589–99. doi: 10.1016/s0196-9781(01)00630-1. [DOI] [PubMed] [Google Scholar]
- [34].Nonaka N, Banks WA, Mizushima H, Shioda S, Morley JE. Regional differences in PACAP transport across the blood-brain barrier in mice: A possible influence of strain, amyloid · protein, and age. Peptides. 2002;23:2197–202. doi: 10.1016/s0196-9781(02)00248-6. [DOI] [PubMed] [Google Scholar]
- [35].Nonaka N, Farr SA, Kageyama H, Shioda S, Banks WA. Delivery of galanin-like peptide to the brain: Targeting with intranasal delivery and cyclodextrins. Journal of Pharmacology and Experimental Therapeutics. 2008;325:513–9. doi: 10.1124/jpet.107.132381. [DOI] [PubMed] [Google Scholar]
- [36].Ohtaki H, Nakamachi T, Dohi K, Aizawa Y, Takaki A, Hodoyama K, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with interleukin-6 (IL-6) signaling. Proceedings of the National Academy of Sciences USA. 2006;103:7488–93. doi: 10.1073/pnas.0600375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Poon HF, Castegna A, Farr SA, Thongboonkerd V, Lynn BC, Banks WA, et al. Quantitative proteomics analysis of specific protein expression and oxidative modification in aged senescence-accelerated-prone 8 mice brain. Neuroscience. 2004;126:915–26. doi: 10.1016/j.neuroscience.2004.04.046. [DOI] [PubMed] [Google Scholar]
- [38].Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired adults. Journal of Alzheimer’s Disease. 2008;13:323–31. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Reglodi D, Kiss P, Lubics A, Tamas A. Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Current Pharmaceutical Design. 2011;17:962–72. doi: 10.2174/138161211795589355. [DOI] [PubMed] [Google Scholar]
- [40].Ross TM, Martinez PM, Renne JC, Thorne RG, Hanson LR, Frey WH. Intranasal administration of interferon beta bypasses the blood-brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J Neuroimmunol. 2004;151:66–77. doi: 10.1016/j.jneuroim.2004.02.011. [DOI] [PubMed] [Google Scholar]
- [41].Somogyvari-Vigh A, Pan W, Reglodi D, Kastin AJ, Arimura A. Effect of middle cerebral artery occulsion on the passage of pituitary adenylate cyclase activating polypeptide across the blood-brain barrier in the rat. Regulatory Peptides. 2000;91:89–95. doi: 10.1016/s0167-0115(00)00123-3. [DOI] [PubMed] [Google Scholar]
- [42].Strong R, Reddy V, Morley JE. Cholinergic deficits in the septal-hippocampal pathway of the SAM-P8 senescence accelerated mouse. Brain Research. 2003;966:150–6. doi: 10.1016/s0006-8993(02)04192-6. [DOI] [PubMed] [Google Scholar]
- [43].Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., II Delivery of insulin-like growth factor-1 to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–96. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- [44].Uchida D, Arimura A, Somogyvari-Vigh A, Shioda S, Banks WA. Prevention of ischemia-induced death of hippocampal neurons by pituitary adenylate cyclase activating polypeptide. Brain Research. 1996;736:280–6. doi: 10.1016/0006-8993(96)00716-0. [DOI] [PubMed] [Google Scholar]
- [45].Uchida D, Shloda S, Takaki A, Arimura A. Cytoprotective action of pituitary adenylate cyclase activating polypeptide (PACAP) in ischemia-induced neuronal cell death in rat hippocampus. Society for Neuroscience Abstracts. 1994;193.10 [Google Scholar]
- [46].Varca GHC, Andreo-Fiho N, Lopes PS, Ferraz HG. Cycldextrins: An overview of the complexation of pharmaceutical proteins. Current Protein and Peptide Science. 2010;11:255–63. doi: 10.2174/138920310791233387. [DOI] [PubMed] [Google Scholar]