Abstract
Background and aims
The authors’ goal was to measure pH at the gastric surface (pHo) to understand how acid secretion affects the repair of microscopic injury to the gastric epithelium.
Methods
Microscopic gastric damage was induced by laser light, during confocal/two-photon imaging of pH-sensitive dyes (Cl-NERF, BCECF) that were superfused over the mucosal surface of the exposed gastric corpus of anaesthetised mice. The progression of repair was measured in parallel with pHo. Experimental conditions included varying pH of luminal superfusates, and using omeprazole (60 mg/kg ip) or famotidine (30 mg/kg ip) to inhibit acid secretion.
Results
Similar rates of epithelial repair and resting pHo values (~pH 4) were reported in the presence of luminal pH 3 or pH 5. Epithelial repair was unreliable at luminal pH 2 and pHo was lower (2.5±0.2, P <0.05 vs pH 3). Epithelial repair was slower at luminal pH 7 and pHo was higher (6.4±0.1, P<0.001). In all conditions, pHo increased adjacent to damage. At luminal pH 3 or pH 7, omeprazole reduced maximal damage size and accelerated epithelial repair, although only at pH 3 did omeprazole further increase surface pH above the level caused by imposed damage. At luminal pH 7, famotidine also reduced maximal damage size and accelerated epithelial repair. Neither famotidine nor omeprazole raised plasma gastrin levels during the time course of the experiments.
Conclusions
Epithelial repair in vivo is affected by luminal pH variation, but the beneficial effects of acutely blocking acid secretion extend beyond simply raising luminal and/or surface pH.
INTRODUCTION
The gastric mucosa must withstand daily exposure to hydrochloric acid and digestive enzymes secreted by the stomach.1 While the physiological production of gastric acid benefits food digestion and limits colonisation by ingested microbes, the acidity in the gastric lumen can damage the gastric epithelial lining when the acid penetrates the mucosal barrier.2,3 The pre-epithelial layer is the first component of the mucosal barrier, consisting of secreted HCO3− and mucus in an unstirred pH microenvironment of the lumen that is directly found at the apical surface of the gastric epithelium.4–6 Compromising the pre-epithelial barrier renders the underlying epithelium exposed to caustic luminal contents; thus, maintenance of pre-epithelial integrity is usually considered vital to protecting the stomach from injury.1
The pH in the pre-epithelial layer is considered an important component of mucosal defense. Previous in vivo experiments using pH-sensitive electrodes or pH-sensitive dyes have shown that pH at the gastric surface (pH ~4–7) is higher than pH at the lumen when the tissue is superfused with pH ≤3 or during pharmacological inhibition of acid secretion with omeprazole.7–11 This pH gradient is due to the regulation of epithelial HCO3− secretion into the mucus gel, which, combined with the unstirred mucus layer, regulates surface pH (pHo).5 Results are more controversial when the response of the stomach to higher values of luminal pH (pH ≥5), such as those that occur during a meal, is evaluated. Although it is clear that increased luminal pH is a stimulus for acid secretion,1 the pH at the gastric surface is variably reported to be higher or lower than the luminal pH for reasons that remain unresolved.4,9–11 We have previously used in vivo confocal microscopy of pH indicator dyes to measure pHo without physical disruption of the mucus layer and have reported that when luminal pH was raised to pH 5, gastric pHo was more acidic than this value due to the action of gastric H+, K+-ATPase.5,7,12 These results are derived from healthy tissues, and it remains unknown how this response to a higher luminal pH affects the ability of the stomach to respond to injury or insult.
In various models of gastric damage, it has been shown that an environment of raised pH exists over the damaged area.13–15 These environments contain mucus, HCO3− and fibrin, and can be disrupted with agents that reduce protective HCO3− secretion.14 Furthermore, we have previously shown that a transient rise in pHo over damage occurs in response to two-photon photodamage,13,16 and that mice with a more acidic pHo have impaired epithelial repair.13 It is also widely recognised that, in a clinical setting, acid secretion hinders long-term ulcer repair, whereas pharmacological inhibition of acid secretion with proton-pump inhibitors such as omeprazole can enhance gastric healing.17 Omeprazole, in addition to blocking the acid pump, has been reported to stimulate ulcer healing days or weeks after damage due to elevation of circulating gastrin, which occurs under H+,K+-ATPase inhibition.18,19 However, it has not been possible to observe the in vivo effects of such treatment on pHo immediately after damage occurs.
In this study, we measured the repair of microscopic injury to the gastric surface epithelium in vivo while manipulating gastric acid secretion and luminal pH. By simultaneous measurements of the pHo values of a repairing epithelium, results help distinguish which parameters affect the dynamics of epithelial repair.
MATERIALS AND METHODS
Animals
Male and female B6-129SF1/J mice (mixed B6 background) and C57BL/6J mice were purchased from Jackson (Bar Harbor, Maine, USA). All animals had free access to standard rodent chow diet and water, and were used for experiments at 2–5 months of age. The surgical method for these experiments has been previously described.12,13,16,20 Briefly, mice were anaesthetised with Ketaject (50 mg/kg intraperitoneally; Phoenix Scientific, St. Joseph, Missouri, USA) and Inactin (100 mg/kg intraperitoneally; Sigma, St. Louis, Missouri, USA), and tracheotomy was performed to facilitate breathing. The stomachs were exteriorised through a midline abdominal incision, and the gastric mucosa was surgically exposed for imaging on an inverted confocal/two-photon microscope (Zeiss LSM 510 NLO). Animals were positioned such that a portion of the corpus mucosa protruded into a temperature-controlled perfusion chamber, facing a Zeiss C-Apo X40 water-immersion objective lens.16 All experimental procedures have been approved by the institutional animal care and use committee of the University of Cincinnati.
Drugs and tissue perfusion
The exposed gastric mucosa was superfused at a rate of 0.2 ml/ min (push/pull syringe pump; KD Scientific) with lightly buffered pH 2, pH 3, pH 5 (150 mM NaCl+4 mM HOMOPIPES) or pH 7 (150 mM NaCl+4 mM HEPES) saline solution, each containing fluorometric pH probes for the reporting of extracellular (surface) pH. In some experiments, omeprazole (60 mg/kg; Sigma) or famotidine (30 mg/kg; Sigma) was injected intraperitoneally prior to surgery and at least 30 min after the drug has elapsed prior to imaging to allow inhibition of gastric acid secretion. Omeprazole was suspended via sonication in 0.5% carboxymethylcellulose (CMC; Sigma), while famotidine was suspended in 0.5% CMC without sonication. Control experiments confirmed that 0.5% CMC alone had no effect on pHo or on the response to microlesions at either pH 3 or pH 7 (data not shown); thus, controls with and without vehicle were combined in experiments evaluating the effects of omeprazole.
Gastrin radioimmunoassay
Mice were injected with vehicle (CMC), omeprazole (60 mg/kg) or famotidine (30 mg/kg) intraperitoneally 1 h prior to the perfusion of the exposed gastric mucosa. After 3 h of tissue perfusion with pH 3 or pH 7 lightly buffered saline, the animals were killed, and blood was collected in a heparinised tube (Sarstedt, Nümbrecht, Germany). Blood samples were centrifuged (15 min×1600 rpm) and plasma fractions were assayed for total amidated gastrin using antibody L2 (reactive to G-17 and G-34), as previously described.21
Creation of two-photon microlesions
The method for creating microlesions has been previously reported.13,16 Briefly, a small region (3–5 cells, ~200 μm2) of the gastric epithelium was selectively photodamaged by repetitive scanning of two-photon laser light (710–730 nm) over the targeted region for 5–8 s. Subsequently, time-course experiments collected images of cellular NAD(P)H autofluorescence (excitation 710–730 nm, emission 435–485 nm) and surface epithelial cell structure (confocal reflectance 710–730 nm). Multiple cycles of photodamage and repair (cycles 1–5) were induced in each animal, and we have previously reported that the tissue responses to each microlesion in initial versus later lesions are similar.13,16
In vivo confocal imaging of pH
For experiments performed using pH 2, pH 3 and pH 5 lightly buffered saline solutions, the ratiometric pH indicator Cl-NERF (10 μM, excitation 514 nm and 458 nm, emission >530 nm; Invitrogen) was added to the perfusate. For experiments performed during superfusion with pH 7 solution, 10 μM BCECF (2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein) (excitation 488 nm and 458 nm, emission >505 nm; Invitrogen) was added to the perfusate.
Image collection and analysis
Time-course experiments were performed by collecting NAD(P)H autofluorescence, confocal reflectance and images for pH measurement at each time point. The area of epithelial cells that lost NAD(P)H over time was used to quantify the damage area while simultaneously examining confocal reflectance images to ensure that only areas of dead/dying cells were included in the analysis13,16 (MetaMorph software; Molecular Devices, Downington, Pennsylvania, USA). These measures defined the initial and maximal size of damage (the latter being 4-fold to 6-fold greater than the initial size).13,16 With the time of photodamage defined as time zero, we quantified the time of epithelial repair as the time point at which the damage area shrank to the size of the initial damage after cell exfoliation.
To quantify pHo, we calculated the background-corrected ratio images of Cl-NERF or BCECF. A 100–200 μm2 region directly adjacent to the tissue surface was selected, and the average ratio was measured within that region during the time course of damage and repair.16 The fluorescence ratios were then converted into pH based on the pH calibration curves created for each dye, as described previously.7
Statistics
Data were analysed (GraphPad Prism 4, La Jolla, California, USA) using unpaired t test, one-way ANOVA or two-way ANOVA (Bonferroni’s post hoc test), and the results are presented as mean±SE. p<0.05 was considered significant.
RESULTS
We first determined if varying the luminal pH affected the repair of microscopic injury caused by two-photon photodamage.
Luminal pH and gastric pHo
Prior to imposition of damage, we superfused the gastric epithelium with pH 2, pH 3, pH 5 or pH 7 lightly buffered saline and used confocal imaging of pH-sensitive fluorescent dyes in the luminal perfusate to measure steady-state pH directly at the gastric surface (pHo). Confirming previous findings,5,7 figure 1A shows that the resting gastric pHo values at luminal pH 3 (3.9±0.3, n = 9) and pH 5 (4.5±0.5, n = 5) are similar, but that exposure of the stomach epithelium to luminal pH 2 caused a significant decrease in pHo (2.5±0.2, p<0.05 vs pH 3 condition, n = 4). Conversely, exposure to luminal pH 7 produced a significant rise in pHo (6.4±0.1, p<0.001 vs pH 3 condition, n = 8). The difference between perfusate solution pH and pHo (ΔpHo) is also informative. The ΔpHo during luminal pH 3 perfusion was similar to the value observed at pH 2, but was significantly higher than the ΔpHo in response to a pH 5 or pH 7 luminal perfusate (figure 1B). The switch from positive to negative ΔpHo values suggests that net acid secretion dominates pHo control when the perfusate pH is >3.
Figure 1.
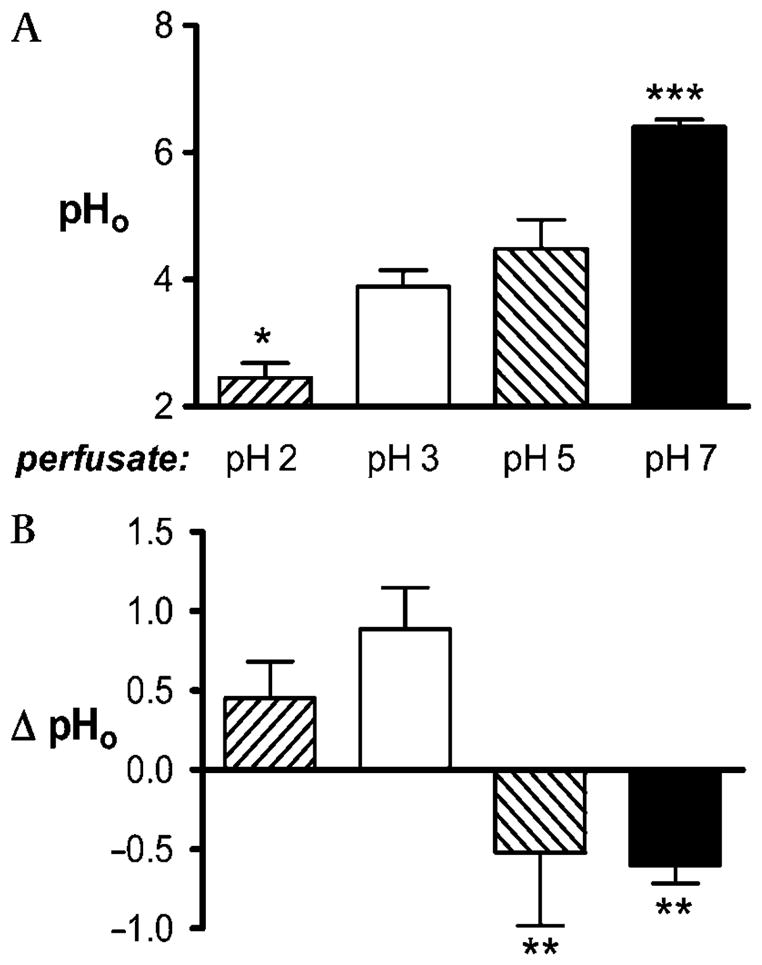
Measurements of gastric surface pH (pHo) at varying luminal pH values. The gastric mucosa of anaesthetised mice was superfused with either pH 2 (n = 4), pH 3 (n = 9), pH 5 (n = 5) or pH 7 (n = 8) lightly buffered saline and equilibrated for >20 min. (A) Resting gastric pHo values at each luminal pH. (B) Change in pH between perfusate and pHo values under each condition. Data are presented as mean±SE and analysed using ANOVA, with Bonferroni’s post hoc test comparing values to those at pH 3. *p<0.05, **p<0.01, ***p<0.001.
Luminal pH and response to damage
We have previously reported that during luminal perfusion with pH 3, a microscopic damage of two to five epithelial cells leads to damage expansion to surrounding cells and to a transient increase in pHo due to bicarbonate secretion.13,16 Both features return to normal upon shedding of the damaged cells and epithelial restitution at ~10–15 min. Here we report the response to damage when gastric surface cells are superfused with a wider range of luminal pH values.
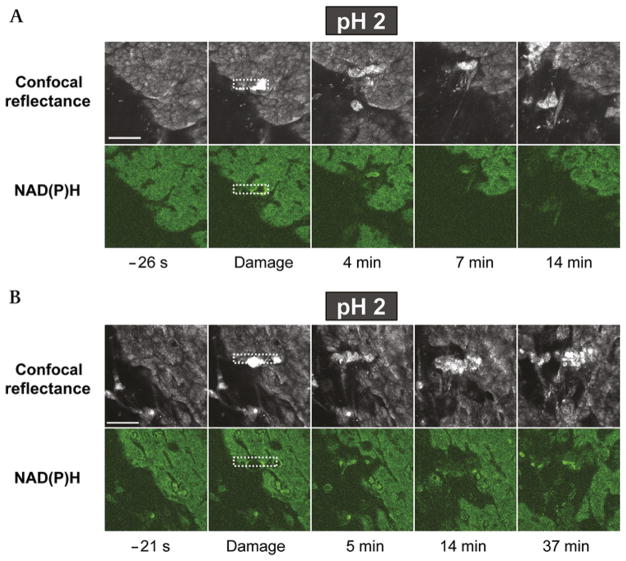
At luminal pH 2, pHo significantly increased after damage (3.1±0.1 vs 2.5±0.2 prior to damage, p<0.01, n = 4), but the ability to repair the damaged epithelium was heterogeneous (figure 2). In all experiments, the damage area expanded, but some experiments demonstrated prompt epithelial repair (figure 2A), whereas in others, the damaged area did not repair (and may have even expanded) for >35 min after damage imposition (figure 2B). In these and all subsequent experiments, the region of damage was defined as the area in which cells were present (defined by confocal reflectance) but had compromised metabolism (absent NAD[P]H autofluorescence).13,16 These results indicate that the low luminal pH value is too stressful for tissues to undergo a reliable repair of microscopic damage, despite the ability of bicarbonate secretion to raise pHo higher than the perfusate value.16
Figure 2.
Heterogeneous response to microscopic damage at luminal pH 2. The exposed gastric tissue of anaesthetised mice was superfused with a pH 2 lightly buffered saline solution during time-course imaging. Shown are results from two separate experiments in which (A) the tissue repaired in response to damage and (B) the tissue did not repair and damage continually expanded. For each experiment, representative images of confocal reflectance (upper image series) and NAD(P)H autofluorescence (lower image series) are shown. In both experiments, images were collected at the indicated times, where time zero is the time of the imposition of photodamage (in the rectangular region demarcated by the white dotted line). Images are representative of four experiments. Bar = 50 μm.
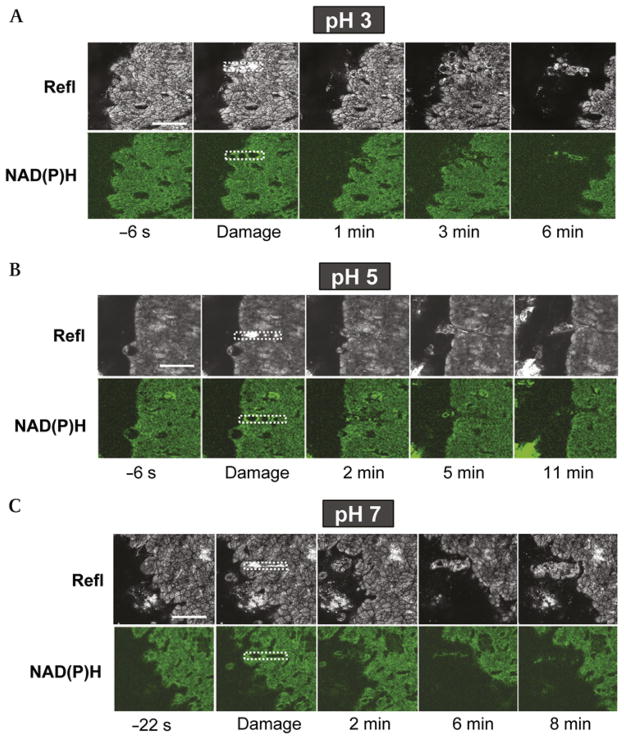
Overall, the response to microscopic damage was more predictable in response to luminal pH 3–7 values. Under all these pH conditions, pHo significantly increased in response to damage. At luminal pH 5, pHo increased to 5.4±0.5 vs 4.5±0.5 prior to damage (n = 5, p<0.001). The damage-induced pHo increases at luminal pH 3 and pH 7 are documented as part of the control experiments in figure 5. Under all these pH conditions, initial damage spread to neighbouring cells, resulting in a larger damaged area that rapidly stabilised and started to repair. This is shown qualitatively in figure 3, which presents representative images of confocal reflectance and NAD(P)H autofluorescence images from time-course experiments evaluating damage response during superfusion of the gastric surface at luminal pH 3 (figure 3A), pH 5 (figure 3B) or pH 7 (figure 3C). These findings are quantified from multiple experiments in figure 4. This analysis shows that, at all pH values, initial damage sizes were similar and that damage significantly expanded to a maximal area that was 2.4-fold to 6-fold greater than the initial damage size. The maximal damage area at luminal pH 5 was significantly smaller (p<0.05) than that at either pH 3 or pH 7, but this observation was not pursued further. The times taken to repair the damaged epithelium were similar at luminal pH 3 (18±5 min, n = 5) and pH 5 (12±3 min, n = 5). However, at luminal pH 7, the majority of experiments in this series showed incomplete epithelial repair that could be conducted for the duration of the experiment (still incomplete by 32±2 min, n = 6), while only two out of eight experiments exhibited complete repair. Animals in this experimental series were of a different background (mixed B6; see Materials and Methods) compared to pure B6 animals used in subsequent luminal pH 7 studies, which may explain why repair was modestly more rapid in later experimental series at pH 7.
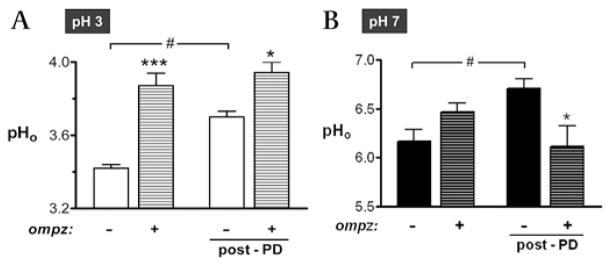
Figure 5.
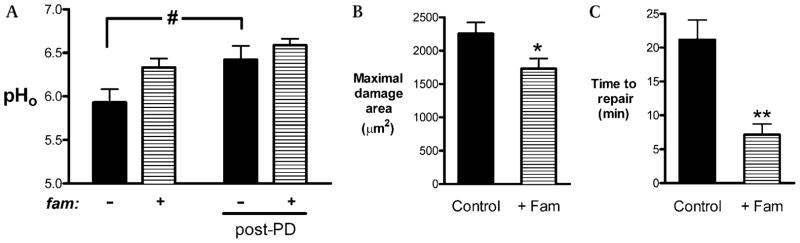
Effects of omeprazole on gastric surface pH (pHo) at luminal pH 3 and pH 7. Prior to surgery for imaging, some mice were injected with omeprazole (omepz; 60 mg/kg intraperitoneally). After surgery, the exposed gastric tissue was superfused with (A) pH 3 or (B) pH 7 saline solution. Resting gastric pHo was measured before photodamage, and the maximal change in pHo was recorded after photodamage (post-PD). Results compile n = 7 experiments for each condition in (A), and n = 6–16 for conditions in (B). Data are presented as mean±SE and analysed using ANOVA, with Bonferroni’s post hoc test. *p<0.05 and ***p<0.001 versus lack of omeprazole. #p<0.05 versus the indicated conditions.
Figure 3.
Epithelial repair of microscopic damage at luminal pH 3, pH 5 and pH 7. The exposed gastric tissue of anaesthetised mice was superfused with (A) pH 3, (B) pH 5 or (C) pH 7 saline solution during time-course imaging. Otherwise, experiments were performed and results are presented as described in figure 2. Results are representative of multiple experiments performed at luminal pH 3 (n = 9), pH 5 (n = 5) and pH 7 (n = 8). Bar = 50 μm. Refl, confocal reflectance.
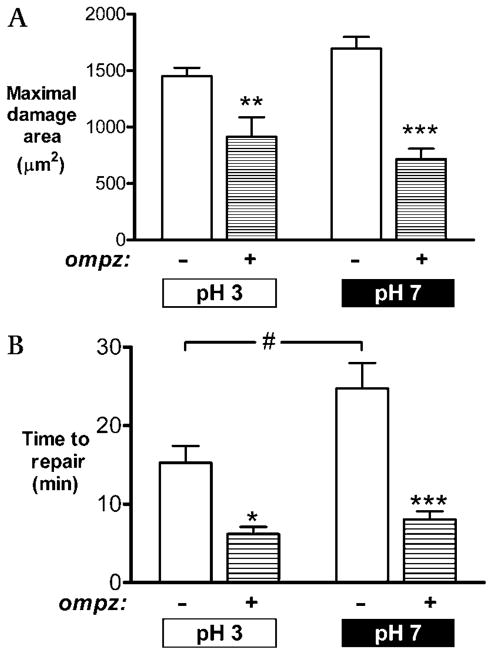
Figure 4.
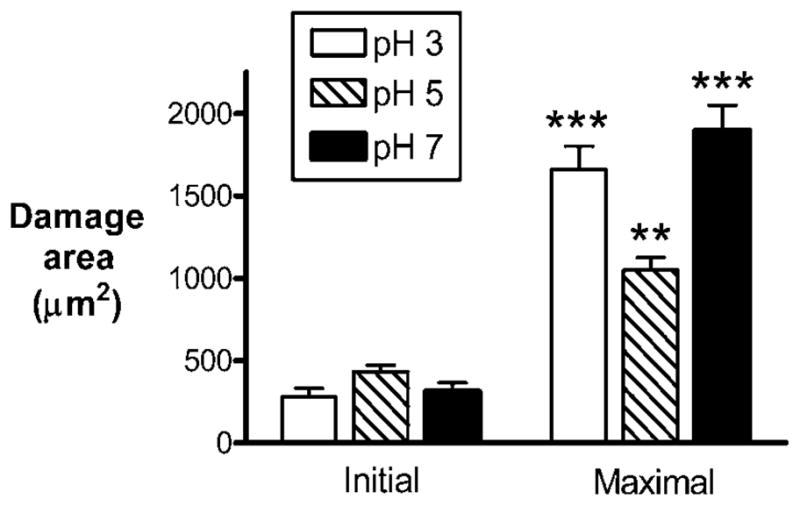
Quantification of damage size at luminal pH 3, pH 5 and pH 7. The gastric mucosa of anaesthetised mice was exposed for imaging and superfused with either pH 3 (n = 9), pH 5 (n = 5) or pH 7 (n = 8) solution. Damage size was measured from images as described in Materials and Methods. The initial damage size was measured <10 s after the targeted region was exposed to repetitive scanning by high-power two-photon laser light. The maximal damage size indicates the magnitude of damage expansion (usually 3–5 min after the initial damage). Data are presented as mean±SE and analysed using ANOVA, with Bonferroni’s post hoc test. **p<0.01 and ***p<0.001 versus the respective initial damage sizes.
Acid secretion and response to damage
Results suggest that exposing gastric tissue to luminal pH 7 does not measurably protect against microscopic damage compared to a luminal pH 3 exposure, although the pH at the gastric surface is two pH units more alkaline under the former condition. In fact, by some measures, repair was compromised at pH 7. It has been established that acid secretion and alkali secretion have different contributions to pHo at luminal pH 7 versus pH 3 (figure 1 and Baumgartner et al12), and that proton-pump inhibitors are well known for their ability to enhance ulcer repair. Therefore, we asked if blocking gastric acid secretion with omeprazole differentially affected the response to damage at luminal pH 3 and pH 7. In these experiments, mice were injected with omeprazole prior to surgery.
The results compiled in figure 5 evaluate the pHo response to omeprazole and the damage incurred while superfusing luminal pH 3 (figure 5A) or pH 7 (figure 5B) solutions over the gastric surface. In the absence of omeprazole, imposition of damage caused a significant rise in pHo in both luminal pH perfusates. At luminal pH 3, addition of omeprazole caused alkalinisation of pHo when evaluated either in the absence or in the presence of damage (figure 5A). It was also noted that in omeprazole-treated mice, damage did not cause any further increase in pHo beyond that caused by omeprazole alone. At luminal pH 7, addition of omeprazole had no effect on pHo prior to damage; in the presence of the drug, pHo did not change in response to photodamage (figure 5B). This was qualitatively similar to the pH 3 condition, where damage did not cause a pHo increase in omeprazole-treated mice. In statistical analysis comparing the absence versus the presence of omeprazole after damage, a modestly more acidic pHo was noted for the presence of omeprazole at pH 7. Since prior results show that pHo rises after damage due to bicarbonate secretion via SLC26A9,16 this result could be explained by an omeprazole-induced reduction of bicarbonate secretion. Thus, only under the pH 3 condition is there a possibility that the beneficial effects of omeprazole on the repair of the gastric surface epithelium could be explained by an action that raises pHo at the site of repair.
The results compiled in figure 6 ask if omeprazole affects the repair of damaged gastric surface epithelium superfused with luminal pH 3 or pH 7. On a comparison between luminal pH 3 and pH 7, initial damage sizes were found to be similar in control animals (314±49 μm2 [pH 3] vs 336±28 μm2 [pH 7]) and omeprazole-treated animals (245±37 μm2 [pH 3] vs 194±36 μm2 [pH 7]). Initial damage expanded under both treatment conditions. As shown in figure 6A, the maximal damage sizes were similar at pH 3 and pH 7, and treatment with omeprazole reduced the maximal damage size at either luminal pH 3 (p<0.01) or pH 7 (p<0.001). As shown in figure 6B, omeprazole was strikingly effective in speeding the repair of microscopic damage at luminal pH 3 (p<0.05) or pH 7 (p<0.001). It was also noted that in the absence of omeprazole, epithelial repair at luminal pH 7 was significantly slower than at luminal pH 3 (25±3 min vs 15±2 min, p = 0.04). These results suggest that the severity of damage and the efficiency of epithelial repair can be improved with omeprazole whether or not the inhibition of gastric H+,K+-ATPase is allowed to raise pH in the luminal environment impinging on the damaged cells.
Figure 6.
Effects of omeprazole on damage and repair at luminal pH 3 and pH 7. Prior to surgery, some mice were injected with omeprazole (omepz). After surgery, the exposed gastric tissue was superfused with pH 3 or pH 7 solution as indicated in the figure panels. Results compiled from n = 6 experiments in each condition evaluating (A) the maximal damage size and (B) the time to complete epithelial repair. Data are presented as mean±SE and analysed using ANOVA, with Bonferroni’s post hoc test. *p<0.05, **p<0.01 and ***p<0.001 versus lack of omeprazole. #p<0.05 versus the indicated conditions.
We tested if blocking acid secretion by another mechanism (H2 receptor antagonism) caused a similar improvement in epithelial repair. Tissue was treated with famotidine, and epithelial repair was evaluated at luminal pH 7. Results compiled in figure 7 show the effects of famotidine on pHo (figure 7A), maximal damage size (figure 7B) and epithelial repair (figure 7C). Similar to outcomes with omeprazole, addition of famotidine did not increase the resting pHo before damage, nor did it affect pHo after damage. However, famotidine significantly reduced the maximal damage size (p<0.05; figure 7B) and accelerated epithelial repair (p<0.01; figure 7C). Similar results after omeprazole or famotidine exposure suggest that outcomes were due to inhibition of acid secretion and were not dependent on the specific mode (or drug) of inhibition.
Figure 7.
Effects of famotidine on damage and repair at luminal pH 7. Prior to surgery, mice were injected with 0.5% carboxymethylcellulose (CMC) or famotidine (fam; 30 mg/kg intraperitoneally). After surgery, the exposed gastric tissue was superfused with pH 7 solution, and then (A) gastric surface pH (pHo), (B) maximal damage size and (C) epithelial repair were quantified. Data are presented as mean±SE and analysed using (A) two-way ANOVA or (B and C) unpaired t test. *p<0.05 and **p<0.01 versus lack of famotidine. #p<0.05 versus the condition specified.
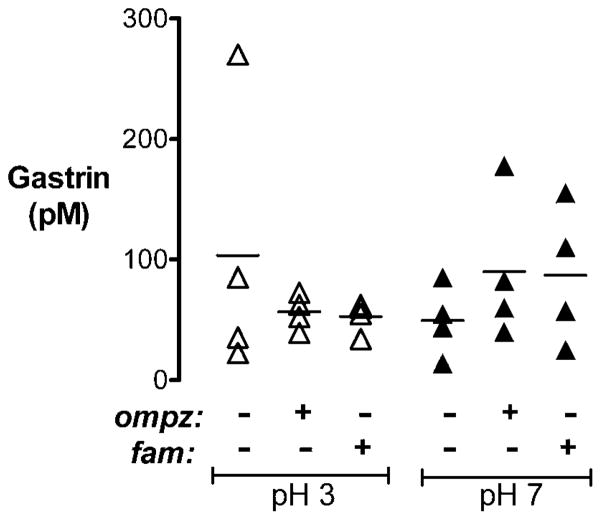
One consequence of acid blockade can be a rise in plasma gastrin, a hormone shown to promote wound healing both in vitro and in vivo.18,19,22 We analysed serum gastrin concentrations in anaesthetised, surgically prepared animals treated with CMC (vehicle), omeprazole or famotidine and tissue-superfused at luminal pH 3 and pH 7 as in imaging experiments. As shown in figure 8, serum gastrin levels were similar in all treatment groups. Therefore, under our experimental conditions, the observed healing benefit of blocking acid secretion is not due to changes in gastrin levels.
Figure 8.
Serum gastrin concentrations in surgically prepared animals superfused at different luminal pH values. Serum gastrin levels (pM) were measured via radioimmunoassay in anaesthetised animals treated for 1 h with carboxymethylcellulose (CMC; vehicle), omeprazole or famotidine, as indicated in the figure, then surgically prepared and superfused with pH 3 (△) or pH 7 (▲) luminal perfusates as in imaging experiments. Results from individual animals are shown as data points (n = 4 animals per group), with a horizontal bar indicating the mean value of each group. Significance was tested by two-way ANOVA.
DISCUSSION
Gastric acid, along with secreted digestive enzymes, poses a threat to the gastric epithelial lining if the myriad mucosal defense mechanisms become impaired.1 In this report, we evaluate the dynamic and local pH changes that occur at the gastric surface in response to microscopic damage, and we examine the role of luminal pH and gastric acid secretion in the progression of gastric repair from these microscopic lesions. Our model provides a unique insight into the repair of superficial damage, probably most analogous to the process of repair after biopsy, surface abrasion or non-steroidal anti-inflammatory drug lesions.12,13,20 Since the repair of individual punctuate lesions has been difficult to examine in any in vivo model, our model provides information not available elsewhere.
Limits to the effectiveness of pre-epithelial defense and pHo control
One of the first lines of gastric defense is a pH microenvironment at the gastric surface (termed pHo) that helps keep surface epithelial cells from being exposed to the dramatic pH changes that commonly occur in the stomach lumen during the digestive cycle. We have shown previously that in response to the fasted–fed pH transition in rodents (from luminal pH 3 to pH 5), pHo has similar steady-state values near pH 4.5,7 Our results now show that efficient epithelial repair of microscopic lesions also occurs in this physiological range of luminal pH values. When experiments evaluated the efficiency of repair at more extreme luminal pH values that compromised pHo constancy (pH 2 and pH 7), the repair of microscopic lesions was less robust (slowed at pH 7 and unreliable at pH 2). It is well recognised that acid secretion is activated when luminal pH increases.5,7 Interestingly, a similar time course of repair occurred at pH 3 versus pH 5, despite the demonstrated predominance of net alkali secretion versus net acid secretion between these two conditions, respectively (figure 1).5,7 Combined, these results suggest that repair may be more sensitive to the absolute value of pHo, rather than to the predominant net secretion (acid vs bicarbonate secretion).
The compromise of epithelial repair at luminal pH 2 is expected. Such low luminal pH is observed in rodents after pyloric ligation, and its impact to damage promotion has been consistently noted, although the human stomach can routinely tolerate such low pH values.23,24 Previous studies that examined isolated rabbit gastric glands found that cell viability was markedly lower when glands were exposed to an acidic (pH 2 or pH 3) environment.23 Additionally, studies using methods that create pervasive superficial gastric damage in intact frog fundic gastric mucosa (ie, absolute ethanol or hypertonic saline) have shown that poor surface epithelial restitution (repair) at low luminal pH values (ie, pH 3.0) could be improved by increasing plasma HCO3−.24,25 A consistent observation of impaired tissue defenses at low luminal pH forms part of the basis for the belief that luminal acid and/or control of gastric pHo is a central determinant of gastric damage.
This belief is challenged by the finding that repair was significantly slowed at luminal pH 7 (figure 6B) despite a direct verification that pHo values over the site of damage were higher than those observed under other luminal pH conditions (figure 5). Previous studies performed in isolated rabbit gastric glands have shown that cell viability decreases when tissue is exposed to an alkaline (pH 8) environment.23 In contrast, studies in isolated frog fundic gastric mucosa found that restitution in response to absolute ethanol or hypertonic saline was more favourable at higher luminal pH values (pH 7.4).24,25 It is difficult to resolve differences among studies when, in most cases, pHo has not been measured, the extent of damage varies dramatically (eg, a handful of cells vs the entire surface epithelium) and it is not always possible to expose just the apical surface of the cells to variable pH stress (eg, in isolated glands). It should also be noted that our experimental setting of tissue superfusion acts to hold luminal pH constant, instead of allowing the stomach secretions to cause changes in the bulk luminal pH, as would occur under normal conditions.
In aggregate, the results show that a high pH at the gastric surface (pH ~6) is not sufficient to promote efficient damage repair and that the speed of repair can be similar whether the stomach undergoes repair during net acid secretion or the stomach undergoes repair during net alkali secretion (eg, comparing pH 3 vs pH 5 luminal perfusates).
Acid secretion, pHo and epithelial repair
The striking efficacy of proton-pump inhibitors as a treatment for acid diseases is widely believed to be due to their ability to raise gastric luminal pH and thereby reduce the acid stress impinging on exposed gastric or esophageal cells. This has lent strong support to the hypothesis that an increased pHo over the damaged epithelium provides a protective environment to ensure gastric restitution.4,6–8,14 Consequently, we were surprised to find that microscopic lesions repaired slowly at the high pHo observed under luminal pH 7 conditions. Therefore, we evaluated the dynamic changes occurring in pHo—while manipulating acid secretion—physiologically by changing the luminal pH or pharmacologically with the proton-pump inhibitor omeprazole or the H2 receptor antagonist famotidine.
Our results showed that blocking acid secretion with omeprazole or famotidine can strongly enhance the repair of microscopic damage to the gastric epithelium. These data are consistent with a vast number of clinical reports of acid inhibitors benefiting ulcer healing, but also provide new insights from a real-time analysis of acute healing immediately after microscopic damage has been induced. We find that both omeprazole and famotidine blunt the expansion of damage among adjacent cells, suggesting that acid secretion is one factor (directly or indirectly) influencing the rapid spread of surface damage. At luminal pH 7, either a proton-pump inhibitor or an H2 receptor antagonist accelerated repair, providing two independent observations that confirm that repair is stimulated by blocking acid secretion despite a consistently high luminal pH at the site of damage.
Results at luminal pH 7 were surprising because they suggested that the beneficial effects of inhibiting acid secretion were not due to simply increasing either pHo or luminal pH. It is expected that inhibitors of acid secretion will change pH within the gastric gland lumen, but we focused on luminal pH at the site of induced damage so that we could test the hypothesis that local changes in juxtamucosal pH were required to promote surface cell recovery. Unlike results at luminal pH 3, blocking acid secretion with omeprazole or famotidine at pH 7 did not lead to a measurably higher pHo either before or after damage. This is probably because the competing process of bicarbonate secretion is less active at higher luminal pH.7,12,26 Importantly, whether or not acid blockers were present, all values of pHo under the pH 7 conditions were higher than in experiments using luminal pH 3. Furthermore, although neither omeprazole nor famotidine was effective at changing resting pHo under the luminal pH 7 conditions (figures 5B and 7A), both drugs were clearly effective in accelerating the time of gastric repair 3-fold under the same experimental conditions (figures 6B and 7C). This suggests that the ability of acid blockers to improve epithelial healing is not primarily due to a locally increased pHo but is more likely due to another consequence of blocking gastric acid secretion. Since serum gastrin levels did not increase under these experimental conditions (figure 8), the results eliminated the possibility that elevated gastrin mediated the beneficial effect of acid blockers. Our results do not contest the well-established beneficial effects of acid blockers that are mediated by changing acidic stress in the lumen19,27,28 but do show a dramatic improvement in repair that must occur independent of those actions. We speculate that acid blockers may also affect pH in other extracellular compartments (eg, interstitial pH adjacent to surface cells), which could lead to enhanced repair. However, such measurements are not currently feasible, so this and other possible mechanisms may need to be tested in the future.
In summary, we find that the repair of microscopic injury to the gastric epithelium is sensitive to the absolute pHo value at the site of damage. However, the pronounced healing benefits resulting from the pharmacological inhibition of acid secretion are not due to simply raising pH at the gastric surface (or in the bulk lumen) at the site of damage. The mechanisms of gastric repair use cross-talk between parietal cell function and the restituting surface epithelium that is independent of these pH changes.
Significance of this study.
What is already known about this subject?
Gastric luminal acid is an aggressive factor that can limit tissue repair.
After damage to the gastric mucosa, luminal pH at the site of damage (surface pH) is physiologically elevated.
Inhibitors of gastric acid secretion speed ulcer healing.
What are the new findings?
Elevated pH values at the tissue surface are not sufficient to promote a more rapid repair of acute wounds.
Gastric repair can occur effectively whether or not surface pH values are predominantly the result of net acid secretion or net alkali secretion.
Inhibitors of acid secretion can accelerate gastric wound repair by mechanisms beyond luminal pH effects.
How might it impact on clinical practice in the foreseeable future?
Wound healing in the gastric mucosa may be accelerated by independently manipulating conditions beyond treatments designed to create a less acidic luminal pH environment.
Acknowledgments
Funding National Institutes of Health grant R01-DK54940.
Footnotes
Competing interests None.
Ethics approval This study was approved by the institutional animal care and use committee of the University of Cincinnati.
Provenance and peer review Not commissioned; externally peer reviewed.
Contributors ESD performed, analysed and interpreted the experiments, and drafted the manuscript. EA performed, analysed and interpreted the experiments, and edited the manuscript. SK performed and analysed the experiments. AV provided unique reagents and analysed the results. MHM contributed to the study concept and funding, and helped with the analysis and interpretation of results and manuscript editing.
References
- 1.Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41–60. doi: 10.1053/j.gastro.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 2.El-Omar EM. Mechanisms of increased acid secretion after eradication of Helicobacter pylori infection. Gut. 2006;55:144–6. doi: 10.1136/gut.2005.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holzer P, Wultsch T, Edelsbrunner M, et al. Increase in gastric acid-induced afferent input to the brainstem in mice with gastritis. Neuroscience. 2007;145:1108–19. doi: 10.1016/j.neuroscience.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen A, Flemstrom G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol. 2005;288:C1–19. doi: 10.1152/ajpcell.00102.2004. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner HK, Montrose MH. Regulated alkali secretion acts in tandem with unstirred layers to regulate mouse gastric surface pH. Gastroenterology. 2004;126:774–83. doi: 10.1053/j.gastro.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 6.Engel E, Guth PH, Nishizaki Y, et al. Barrier function of the gastric mucus gel. Am J Physiol. 1995;269:G994–9. doi: 10.1152/ajpgi.1995.269.6.G994. [DOI] [PubMed] [Google Scholar]
- 7.Chu S, Tanaka S, Kaunitz JD, et al. Dynamic regulation of gastric surface pH by luminal pH. J Clin Invest. 1999;103:605–12. doi: 10.1172/JCI5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillipson M, Atuma C, Henriksnas J, et al. The importance of mucus layers and bicarbonate transport in preservation of gastric juxtamucosal pH. Am J Physiol Gastrointest Liver Physiol. 2002;282:G211–19. doi: 10.1152/ajpgi.00223.2001. [DOI] [PubMed] [Google Scholar]
- 9.Ross IN, Turnberg LA. Studies of the ‘mucus–bicarbonate’ barrier on rat fundic mucosa: the effects of luminal pH and a stable prostaglandin analogue. Gut. 1983;24:1030–3. doi: 10.1136/gut.24.11.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schade C, Flemstrom G, Holm L. Hydrogen ion concentration in the mucus layer on top of acid-stimulated and -inhibited rat gastric mucosa. Gastroenterology. 1994;107:180–8. doi: 10.1016/0016-5085(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 11.Turnberg LA, Ross IN. Studies of the pH gradient across gastric mucus. Scand J Gastroenterol Suppl. 1984;92:48–50. [PubMed] [Google Scholar]
- 12.Baumgartner HK, Kirbiyik U, Coskun T, et al. Endogenous cyclo-oxygenase activity regulates mouse gastric surface pH. J Physiol. 2002;544:871–82. doi: 10.1113/jphysiol.2002.024620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starodub OT, Demitrack ES, Baumgartner HK, et al. Disruption of the Cox-1 gene slows repair of microscopic lesions in the mouse gastric epithelium. Am J Physiol Cell Physiol. 2008;294:C223–32. doi: 10.1152/ajpcell.00395.2006. [DOI] [PubMed] [Google Scholar]
- 14.Wallace JL, McKnight GW. The mucoid cap over superficial gastric damage in the rat. A high-pH microenvironment dissipated by nonsteroidal antiinflammatory drugs and endothelin. Gastroenterology. 1990;99:295–304. doi: 10.1016/0016-5085(90)91009-u. [DOI] [PubMed] [Google Scholar]
- 15.Wallace JL, Whittle BJ. The role of extracellular mucus as a protective cap over gastric mucosal damage. Scand J Gastroenterol Suppl. 1986;125:79–85. doi: 10.3109/00365528609093821. [DOI] [PubMed] [Google Scholar]
- 16.Demitrack ES, Soleimani M, Montrose MH. Damage to the gastric epithelium activates cellular bicarbonate secretion via SLC26A9 Cl(−)/HCO(3)(−) Am J Physiol Gastrointest Liver Physiol. 2011;299:G255–64. doi: 10.1152/ajpgi.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–32. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi K, Johnson LR. Effect of cell proliferation on healing of gastric and duodenal ulcers in rats. Digestion. 1986;33:92–100. doi: 10.1159/000199280. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi Y, Kitano S, Bandoh T, et al. Acceleration of gastric ulcer healing by omeprazole in portal hypertensive rats. Is its action mediated by gastrin release and the stimulation of epithelial proliferation? Eur Surg Res. 2003;35:75–80. doi: 10.1159/000069397. [DOI] [PubMed] [Google Scholar]
- 20.Baumgartner HK, Starodub OT, Joehl JS, et al. Cyclooxygenase 1 is required for pH control at the mouse gastric surface. Gut. 2004;53:1751–7. doi: 10.1136/gut.2004.040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dockray GJ, Hamer C, Evans D, et al. The secretory kinetics of the G cell in omeprazole-treated rats. Gastroenterology. 1991;100:1187–94. [PubMed] [Google Scholar]
- 22.Johnson LR, Guthrie PD. Mucosal DNA synthesis: a short term index of the trophic action of gastrin. Gastroenterology. 1974;67:453–9. [PubMed] [Google Scholar]
- 23.Carter KJ, Lee HH, Goddard PJ, et al. Cell survival in rabbit gastric glands: effect of extracellular pH, osmolarity, and anoxia. Am J Physiol. 1993;265:G379–87. doi: 10.1152/ajpgi.1993.265.2.G379. [DOI] [PubMed] [Google Scholar]
- 24.Svanes K, Takeuchi K, Ito S, et al. Effect of luminal pH and nutrient bicarbonate concentration on restitution after gastric surface cell injury. Surgery. 1983;94:494–500. [PubMed] [Google Scholar]
- 25.Svanes K, Ito S, Takeuchi K, et al. Restitution of the surface epithelium of the in vitro frog gastric mucosa after damage with hyperosmolar sodium chloride. Morphologic and physiologic characteristics. Gastroenterology. 1982;82:1409–26. [PubMed] [Google Scholar]
- 26.Matsueda K, Muraoka A, Umeda N, et al. Effect of the luminal hydrogen ion on alkali and mucus secretion in the rat stomach. Scand J Gastroenterol Suppl. 1989;162:35–8. doi: 10.3109/00365528909091119. [DOI] [PubMed] [Google Scholar]
- 27.Schmassmann A, Tarnawski A, Gerber HA, et al. Antacid provides better restoration of glandular structures within the gastric ulcer scar than omeprazole. Gut. 1994;35:896–904. doi: 10.1136/gut.35.7.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmassmann A, Tarnawski A, Peskar BM, et al. Influence of acid and angiogenesis on kinetics of gastric ulcer healing in rats: interaction with indomethacin. Am J Physiol. 1995;268:G276–85. doi: 10.1152/ajpgi.1995.268.2.G276. [DOI] [PubMed] [Google Scholar]