Abstract
Context:
A single measure of knee laxity (ie, measurement of laxity in a single plane of motion) is probably inadequate to fully describe how knee joint laxity is associated with anterior cruciate ligament injury.
Objective:
To characterize interparticipant differences in the absolute and relative magnitudes of multiplanar knee laxity (ie, sagittal, frontal, and transverse planes) and examine physical characteristics that may contribute to these differences.
Design:
Descriptive laboratory study.
Setting:
University research laboratory.
Patients or Other Participants:
140 participants (90 women, 50 men).
Main Outcome Measure(s):
Using cluster analysis, we grouped participants into distinct multiplanar knee laxity profiles based on the absolute and relative magnitudes of their anterior knee laxity (AKL), genu recurvatum (GR), and varusvalgus (VV) and internal-external rotation (IER) knee laxity. Using multinomial logistic regression, we then examined associations between the different laxity profile clusters and physical characteristics of sex, age, activity level, general joint laxity, body mass index, thigh strength, and 8 measures of lower extremity anatomical alignment.
Results:
Six clusters were identified: low (LOW), moderate (MOD) and high (HIGH) laxity overall and disproportionally higher VV/IER (MODVV/IER), GR (HIGHGR), and AKL (HIGHAKL) laxity. Once all other physical characteristics were accounted for, the LOW cluster was more likely to be older, with longer femur length. Clusters with greater magnitudes of VV and IER laxity were more likely to be younger and to have lower body mass index, smaller Q-angle, and shorter femur length (MOD, HIGH, MODVV/IER) and less thigh strength (HIGH). The HIGHGR cluster was more likely to be female and to have a smaller tibiofemoral angle and longer femur length. The HIGHAKL cluster was more likely to have greater hip anteversion and navicular drop.
Conclusions:
The absolute and relative magnitudes of a person's multiplanar knee laxity are not always uniform across planes of motion and can be influenced by age, body composition, thigh strength, and structural alignment. Except in HIGHGR, sex was not a significant predictor of cluster membership once other physical characteristics were taken into account.
Keywords: hypermobility, anterior cruciate ligament injury risk factors, body composition, strength, lower extremity alignment, age, sex
Key Points.
We identified distinct clusters that differed in the absolute and relative magnitudes of their multiplanar knee laxity profiles.
A person's physical characteristics (ie, age, body composition, strength, lower extremity posture) in part predicted the probability of membership in a particular cluster.
The greater magnitudes of knee laxity often observed in females may be the result of innate sex differences in body composition and structure.
A growing body of literature reports an association between greater magnitudes of knee joint laxity (ie, anterior knee laxity [AKL]; genu recurvatum [GR]; general joint laxity [GJL], which encompasses GR; and internal rotation laxity) and a greater risk of anterior cruciate ligament (ACL) injury.1–9 Results from the few studies that have examined 2 or more laxity characteristics in combination suggest that the magnitude and direction of knee joint laxity may uniquely contribute to ACL injury risk.1,3,5,8 These unique contributions are supported by biomechanical studies demonstrating that high-risk knee joint biomechanics (eg, greater dynamic knee valgus) often occur in the same planes of motion as greater magnitudes of knee laxity (eg, frontal- and transverse-plane knee laxity).10–12 Moreover, the absolute and relative magnitudes of one's multiplanar knee laxity may be sex specific. That is, although females are reported to have greater sagittal plane laxity (ie, AKL, GR) than males,7,8,13–15 they have substantially greater varus-valgus (VV) and internal-external rotation (IER) laxities,16–18 even when matched with males on sagittal-plane knee laxity. Therefore, a single measure of knee laxity is probably inadequate to fully describe how laxity is associated with ACL injury. It is important to consider a more complete or multiplanar knee laxity profile (ie, one that considers both the absolute and relative magnitudes of knee laxity across the sagittal, transverse, and frontal planes) when examining a person's relative risk of injury. However, we are unaware of any authors to date who have attempted to characterize these multiplanar knee laxity profiles.
Also important to understand are the factors that may contribute to interindividual variations in multiplanar knee laxity profiles, so that we can better understand the underlying factors that contribute to high-risk knee joint laxity profiles in the future. Physical characteristics that contribute to the greater magnitudes of knee laxity in females than in males may include sex differences in body composition (eg, females have less thigh muscle mass and strength surrounding the knee)8 and hormone exposure19 (eg, females are exposed to large variations in sex hormone concentrations that may differentially affect intraarticular and extra-articular ligaments18,20). Other factors that may selectively load capsuloligamentous structures and promote greater knee laxity in a single plane of motion include condylar geometry,21,22 lower extremity alignment,23 types of habitual cutting and running activities,24 and height.25 For example, structural alignment at the hip (eg, greater pelvic angle and hip anteversion leading to greater femoral rotation and GR26–29), knee (eg, greater tibiofemoral angle and quadriceps angle leading to greater knee valgus and frontal-plane knee laxity30), and ankle (eg, greater navicular drop promoting greater GR and tibial rotation31,32) is thought to play a key role in the load distribution at the knee.33 Although many of these factors have been previously implicated in ACL injury,3,4,9,34,35 it is un-known whether these factors directly influence risk or whether they indirectly influence injury risk via more direct effects on other risk factors (eg, laxity). Previous authors23 have found associations between AKL and pelvic tilt, hip anteversion, GR, and navicular drop, but we are unaware of any researchers who have examined structural alignment associations with multiplanar knee laxity.
Therefore, our purpose was to use cluster analysis to group individuals based on the absolute and relative magnitudes of their AKL, GR, and VV and IER knee laxity values and then determine the physical characteristics that predicted membership in each of these multiplanar knee laxity clusters. Our hypothesis was that we would identify distinct clusters of multiplanar knee laxity profiles that differed in absolute and relative magnitudes across anatomical planes and that an individual's physical characteristics would, in part, predict the probability of membership in a particular multiplanar laxity cluster. Specifically, we expected that clusters with higher overall magnitudes of knee laxity would be more likely to be younger, less active, female, leaner (ie, have a lower body mass index [BMI]), and weaker (ie, have less thigh strength) and that clusters with disproportionally higher knee laxity in a given plane of motion would be more likely to have structural characteristics that selectively load capsuloligamentous structures in one or more planes (eg, greater or lesser tibiofemoral angle and knee valgus or varus laxity).
METHODS
Participants were 90 women (age = 21.2 ± 2.6 years, height = 163.9 ± 6.7 cm, mass = 61.3 ± 8.6 kg) and 50 men (age = 22.2 ± 2.7 years, height = 177.9 ± 9.3 cm, mass = 80.9 ± 13.3 kg) who were recreationally active (2.5–10 hours per week) for the past 3 months and nonsmokers who had a BMI ≤30, no history of ligament or cartilage injury to the knee, and no history of lower extremity injury in the past 6 months. Women had regular menstrual cycles and were nulligravida, as determined by self-report and a menstrual history questionnaire. All participants were enrolled in a larger study examining the effects of hormone-mediated knee laxity changes on weight-bearing knee joint biomechanics.12,36 At the time of initial enrollment, participants provided informed consent as approved by the University of North Carolina at Greensboro Institutional Review Board and were measured for height, mass, activity level, and 8 lower extremity anatomical characteristics (ie, structural factors that were not expected to change over time). After enrollment (1 week later for men, 2–3 months later for women), participants were measured for thigh strength and 5 joint laxity measures, with all women being measured during the first 6 days of their menstrual cycle to control for cyclic variations in knee laxity. The delay between initial enrollment and strength and laxity testing in women allowed us to track and document their knee laxity changes across the menstrual cycle18,37 and identify the days during menses when knee laxity values were expected to be at their baseline (nadir). Details of each measurement protocol follow.
Activity Level
To determine the exposure of the knee to different activity-related loads, we used the Activity Rating Scale by Marx et al.38 Participants rated their running, cutting, decelerating, and pivoting activities each as 0 (less than once per month), 1 (once per month), 2 (once per week), 3 (2–3 times per week) or 4 (4 or more times per week), resulting in a score from 0 to 16.
Lower Extremity Anatomical Characteristics
We evaluated pelvic angle, hip anteversion, quadriceps angle (Q-angle), tibiofemoral angle, tibial torsion, navicular drop, tibia length, and femur length. All measurement procedures and their validity and reliability have been previously described14,39,40 and illustrated14 in detail. Briefly, pelvic angle, Q-angle, tibiofemoral angle, navicular drop, and tibia and femur length were measured with the standing participant barefoot, with feet placed shoulder width apart, arms across the chest, and looking straight ahead.39 Pelvic angle was defined as the angle formed by a line from the anterior-superior iliac spine to the posterior iliac spine in the horizontal plane.39,41 The Q-angle was defined as the angle formed by the intersection of lines from the anterior-superior iliac spine to the patella center and from the patella center to the tibia tuberosity. Tibiofemoral (frontal-plane knee) angle was defined as the angle formed by the anatomical axis of the femur and the anatomical axis of the tibia.42 Navicular drop was defined as the change in navicular height (in millimeters) between standing subtalar joint neutral (with the medial and lateral talar heads equally palpable) and standing relaxed stances.43 Tibia and femur lengths (in centimeters) were measured as the distance from the knee joint line to the inferior aspect of the medial malleolus and the knee joint line to the most proximal aspect of the greater trochanter, respectively. Hip anteversion was defined as the torsion of the femur using the Craig test.44 Tibial torsion was measured as the angle between a line bisecting the medial and lateral epicondyles and a line bisecting the bimalleolar axis.39,45 A single investigator with excellent measurement reliability obtained all measures (intracorrelation coefficient [ICC] [2,3] > 0.87).14,39
Strength
Quadriceps and hamstring muscle torques (in newton-meters per kilogram) were obtained at 25° of knee flexion via maximal voluntary isometric contractions against a fixed dynamometer (Biodex System 3; Biodex Medical Systems, Inc, Shirley, NY). Participants were instructed to keep their arms over their chests and to extend the knee (quadriceps) or flex the knee (hamstrings) as hard as possible. Three 5-second maximal voluntary isometric contractions were obtained for each motion, and the mean peak torque across trials was recorded.
Laxity Measures
For each participant, 5 laxity characteristics were measured: AKL, GR, GJL, and VV and IER laxity. To control for the effects of exercise, all participants refrained from activity on the day that knee joint laxity values were obtained.46,47 Anterior knee laxity was defined as the anterior displacement (in millimeters) of the tibia relative to the femur when a 133-N anterior-directed load was applied to the posterior aspect of the tibia (KT-2000 Knee Arthrometer; MEDmetric Corporation, San Diego, CA). Genu recurvatum was defined as the amount of knee hyperextension (in degrees) when the participant maximally extended the knee with the distal thigh supported by a 4-in (10-cm) bolster.40 General joint laxity was measured with the Beighton and Horan Joint Mobility Index48 and scored from 0 to 9. Each characteristic was measured by the same tester, with excellent measurement reliability (ICC [2,3]; SEM = 0.96 [0.3 mm] for AKL, 0.97[0.5°] for GR, and 0.99 [0.3] for GJL).49
The VV and IER were measured with the Vermont Knee Laxity Device (University of Vermont, Burlington, VT) using the same procedures previously described.50 Participants were positioned with the knee flexed to 20°, the thigh securely fixed, the foot and ankle braced and restrained in the foot cradle, and counterweights applied to the thigh and shank to create an initial zero shear and compressive load across the tibiofemoral joint. Varus-valgus rotational laxity was defined as the total angular displacement (in degrees) of the tibia relative to the femur while 10 Nm of torque was applied to the lateral and medial aspects of the distal tibia via a handheld force transducer (model SM-50; Interface, Inc, Scottsdale, AZ) (Figure). Internal-external rotational laxity was defined as the total angular displacement (in degrees) about the long axis of the tibia when internal and external rotation torques of 5 Nm were applied using a T-handle connected to a force transducer (model MC3A; Advanced Medical Technology, Inc, Watertown, MA) affixed to the foot cradle (Figure). Electromagnetic sensors (Ascension Technology Corporation, Burlington, VT) and Motion Monitor software (Innovative Sports Training, Chicago, IL) measured the angular joint kinematics over 3 consecutive cycles of VV and IER loadings. Consistent laxity measurements (ICC range, 0.70–0.96, measurement error <2° VVLAX, and 3°–4° IERLAX) have been reported with these methods.50
Figure.
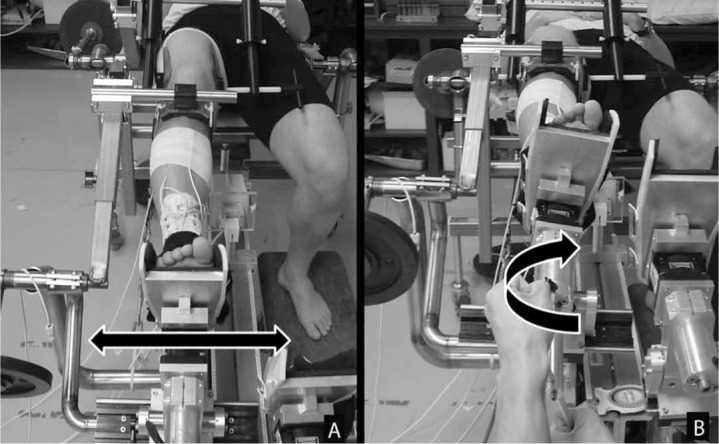
Measurement of rotational knee laxity using the Vermont Knee Laxity Device (University of Vermont, Burlington, VT). A, Varusvalgus. B, Internal-external.
Statistical Analysis
Data were analyzed in 2 steps. First, we conducted cluster analyses to group participants based on their measurements of AKL, GR, VV, and IER. Standardized scores for each laxity variable were used so that the magnitude of any one variable did not overwhelm the model.51 Following the Ward hierarchical cluster analysis,52 which estimated the number of clusters and the initial cluster centroids, k-means clustering further refined the cluster membership.53 Once cluster membership was determined, we performed separate 1-way analyses of variance and multiple comparisons with Bonferroni adjustments to compare the mean laxity values between the identified clusters. Based on the mean differences in laxity values between clusters, we characterized and named a multiplanar laxity profile for each cluster. Although GJL is also a measure of joint laxity, we did not include it in the cluster analysis because it is not knee specific. Rather, GJL was used as a predictor of the different laxity profiles (see next paragraph).
Once the multiplanar knee laxity profile was characterized for each cluster, a backward stepwise multinomial logistic regression analysis examined the extent to which the different physical characteristics predicted cluster membership.54,55 As before, standardized scores were used in the analysis for all predictors except the dichotomous variable of sex. For the initial regression analysis, the cluster that characterized the least amount of multiplanar knee laxity compared with all other clusters served as the reference group, and all other clusters were initially compared with this cluster. Then, we conducted post hoc multiple comparisons between the various multiplanar laxity clusters by changing the reference group (eg, using the cluster that characterized the greatest amount of multiplanar knee laxity as the reference group to which all other clusters were compared) in order to further distinguish characteristics of the different clusters. Because many of these variables differ by sex, we retained sex in the model to control for related confounding factors and to ensure that a given laxity profile was related to the actual physical characteristic, not an individual's sex. The criterion for retention of each predictor in the model (“P out”) was set at .20. Unless otherwise noted, predictors of the various laxity profiles were identified as those for which the odds ratio reached a significance level of P < .05; however, predictors that neared significance in each regression model (ie, odds ratios reaching P < .10 and P < .20) are also noted where appropriate. All analyses were performed using statistical software packages SAS (version 9.2; SAS Institute, Inc, Cary, NC) and PSAW (version 18; SPSS Inc, Chicago, IL).
RESULTS
Identifying Cluster Membership (Multiplanar Laxity Profiles)
The final cluster solution revealed 6 distinct clusters. Descriptive statistics for the laxity values within each cluster and results of the analysis of variance models comparing these values between clusters are shown in Table 1. Based on these comparisons, multiplanar laxity profiles for each cluster were characterized and named as follows (Table 2): clusters 1, 2, and 3 were named LOW, MOD, and HIGH, respectively, because people in these clusters were consistently low, moderate, or high on all laxity values. The laxity profile for cluster 4 was named MODVV/IER because people in this cluster were low on AKL and GR yet moderately high on VV and IER (ie, the VV and IER values were similar to those of the MOD cluster). The laxity profile for cluster 5 was named HIGHGR because these people were higher in GR than all other clusters, moderate in AKL, and low in VV and IER laxity. Finally, the laxity profile of cluster 6 was named HIGHAKL because these people had higher AKLs than those in other clusters while being low in all other laxity variables. These laxity profile names are used through the remainder of this article to more precisely describe each cluster.
Table 1.
Laxity Values Stratified by Cluster (N = 140) (Mean ± SD)
| Variable | Cluster (Women/Men/% of Participants in Cluster) |
|||||
| 1 (8/17/17.9) | 2 (19/9/20.0) | 3 (19/2/15.0) | 4 (27/6/23.6) | 5 (8/9/12.1) | 6 (9/7/11.4) | |
| Anterior knee laxity, mm | 5.3± 0.9a | 7.2 ± 1.1b | 8.7± 1.9c | 5.1± 0.9a | 7.2 ± 1.5b | 8.6 ± 1.5c |
| Genu recurvatum, ° | 0.6 ± 2.2d | 5.7± 1.7e | 7.6 ± 3.3f | 1.6 ± 1.9d | 8.8 ± 2.1f | 1.5 ± 1.6d |
| Varus-valgus rotation laxity, ° | 8.2 ± 1.6g | 13.2 ± 1.9h | 15.8 ± 2.8i | 12.7 ± 1.7h | 8.6 ± 1.7g | 10.0 ± 2.2g |
| Internal-external rotation laxity, ° | 16.9 ± 4.2g | 26.5 ± 4.3h | 36.7 ± 4.0i | 26.4 ± 4.1h | 19.7 ± 6.1g | 19.8± 5.5g |
Multiple comparisons performed with Bonferroni adjustment:
a Mean value different from the mean values of clusters 2, 3, 5, 6.
b Mean value different from the mean values of clusters 1, 3, 4, 6.
c Mean value different from the mean values of clusters 1, 2, 4, 5.
d Mean value different from the mean values of clusters 2, 3, 5.
e Mean value different from the mean values of clusters 1, 3, 4–6.
f Mean value different from the mean values of clusters 1, 2, 4, 6.
g Mean value different from the mean values of clusters 2–4.
h Mean value different from the mean values of clusters 1, 3, 5, 6.
i Mean value different from the mean values of clusters 1, 2, 4–6.
Table 2.
Characteristics of Identified Laxity Profiles
| Variable | Cluster Membership (Named Laxity Profile) |
|||||
| 1a (LOW) | 2 (MOD) | 3 (HIGH) | 4 (MODVV/IER) | 5 (HIGHGR) | 6 (HIGHAKL) | |
| Anterior knee laxity | LOW | MOD | HIGH | LOW | MOD | HIGH |
| Genu recurvatum | LOW | MOD | HIGH | LOW | HIGH | LOW |
| Internal-external rotation laxity | LOW | MOD | HIGH | MOD | LOW | LOW |
| Varus-valgus rotation laxity | LOW | MOD | HIGH | MOD | LOW | LOW |
Abbreviations: AKL, anterior knee laxity; GR, genu recurvatum; IER, internal-external rotation laxity; V V, varus-valgus rotation laxity.
a Initial reference group with which all other clusters were compared.
Physical Characteristics Distinguishing Laxity Profiles
Means and standard deviations for each predictor entered into the multinomial logistic regression model are presented in Table 3, stratified by clusters. Once all physical characteristics were taken into account, activity rating, pelvic angle, tibia length, hamstring peak torque, and GJL were not significant predictors in the overall model (all Ps > .367) and were removed from the analysis. The 10 predictors that remained in the model after stepwise removal and that predicted membership in 1 or more of the 6 multiplanar laxity clusters are listed in Table 4. The odds ratio (OR) for each predictor variable when each cluster was compared with LOW (ie, the initial reference group) is provided in Table 5. These ratios indicate higher (>1.0) or lower odds (<1.0) for membership in a given cluster relative to the LOW for each standard deviation increase in the predictor variable, with all other variables held constant. A summary of logistic regressions when each cluster was compared with all other clusters is provided in Table 6. The following sections summarize the primary distinguishing characteristics of each cluster, first as compared with those having the least amount of laxity (LOW) and then as compared with all other clusters.
Table 3.
Predictors Among the 6 Laxity Profiles (Mean ± SD)
| Variable | Laxity Profile |
|||||
| LOW | MOD | HIGH | MODVV/IER | HIGHGR | HIGHAKL | |
| Age, y | 23.7 ± 2.9 | 20.7 ± 2.3 | 21.3 ± 2.5 | 20.9 ± 2.0 | 21.5 ± 2.6 | 21.6 ± 3.2 |
| Body mass index, kg/cm2 | 26.3 ± 3.8 | 23.7 ± 3.2 | 21.3 ± 1.7 | 22.5 ± 2.2 | 24.4 ± 2.0 | 25.2 ± 3.0 |
| Activity rating total (range, 0 16) | 8.4±3.7 | 8.6 ± 4.5 | 8.7± 4.1 | 8.7 ± 4.6 | 9.2 ± 4.1 | 8.6 ± 4.4 |
| Pelvic angle, ° | 9.9±4.3 | 12.0 ± 3.6 | 12.5 ± 4.0 | 13.0± 4.8 | 11.8± 5.7 | 12.1 ± 4.4 |
| Hip anteversion, ° | 7.0 ± 6.4 | 13.8 ± 6.3 | 13.1± 5.0 | 11.8± 4.3 | 9.8 ± 5.6 | 13.5 ± 6.4 |
| Quadriceps angle, ° | 11.6 ± 4.7 | 11.9± 5.7 | 12.7 ± 3.9 | 14.5 ± 6.0 | 11.2 ± 4.3 | 14.4±4.7 |
| Tibiofemoral angle, ° | 11.1 ± 2.6 | 10.9 ± 2.7 | 11.4 ± 2.9 | 12.0 ± 2.5 | 9.4 ± 2.7 | 11.9 ± 2.4 |
| Tibial torsion, ° | 18.6 ± 5.2 | 15.6 ± 6.4 | 14.8± 7.3 | 19.9± 8.4 | 15.9± 5.7 | 19.1±8.7 |
| Navicular drop, mm | 5.2 ± 3.1 | 5.8 ± 3.2 | 7.1± 5.0 | 5.1± 3.4 | 5.3± 3.7 | 8.0 ± 4.6 |
| Tibia length, cm | 38.0 ± 2.9 | 36.1 ± 2.0 | 34.9 ± 2.3 | 35.2 ± 2.4 | 39.4± 3.1 | 36.6 ± 2.0 |
| Femur length, cm | 44.5 ± 2.5 | 42.0 ± 2.2 | 41.3 ± 2.6 | 41.6 ± 2.9 | 45.8± 3.4 | 42.8 ± 2.3 |
| General joint laxity (range, 0–9) | 1.0±1.3 | 2.0 ± 1.7 | 1.9 ± 2.1 | 1.6 ± 1.5 | 1.5± 1.7 | 1.3±1.3 |
| Quadriceps peak torque, Nm/kg | 2.5 ± 0.4 | 2.5 ± 0.5 | 2.3 ± 0.4 | 2.4 ± 0.5 | 2.6 ± 0.4 | 2.5 ± 0.5 |
| Hamstring peak torque, Nm/kg | 2.0 ± 0.3 | 1.8± 0.4 | 1.7± 0.3 | 1.8± 0.3 | 1.9± 0.3 | 1.9±0.4 |
Abbreviations: AKL, anterior knee laxity; GR, genu recurvatum; IER, internal-external knee rotation laxity; VV, varus-valgus knee rotation laxity.
Table 4.
Likelihood Ratio Test of Multinomial Logistic Regression for Distinguishing Cluster Membership a
| Effect | −2 Log Likelihood | χ2 | Significance |
| Intercept | 330.56 | 25.75 | .000 |
| Sex | 321.19 | 16.37 | .006 |
| Age | 317.23 | 12.42 | .029 |
| Body mass index | 353.74 | 48.93 | .000 |
| Hip anteversion | 331.45 | 26.64 | .000 |
| Quadriceps angle | 321.24 | 16.43 | .006 |
| Tibiofemoral angle | 318.47 | 13.66 | .018 |
| Tibial torsion | 320.88 | 16.07 | .007 |
| Navicular drop | 317.29 | 12.48 | .029 |
| Femur length | 346.46 | 41.65 | .000 |
| Quadriceps peak torque | 313.03 | 8.22 | .145 |
a Backward stepwise selection: P out = 0.2, df = 5. Results based on standardized scores for all independent variables except sex.
Table 5.
Odds Ratio a (95% Confidence Interval) for the Multinomial Logistic Regression When the Different Laxity Profiles Were Distinguished from LOW (Initial Reference Group)
| Variable | Cluster Comparison |
||||
| MOD Versus LOW | HIGH Versus LOW | MODVV/IER Versus LOW | HIGHGR Versus LOW | HIGHAKL Versus LOW | |
| Sex | 0.11 (0.01, 1.65)b | 0.78 (0.03, 17.55) | 0.22 (0.02, 2.77) | 9.38 (0.90, 97.15)c | 0.05 (0.00, 0.90)d |
| Age | 0.29 (0.13, 0.66)e | 0.38 (0.13, 1.06)c | 0.35 (0.16, 0.76)e | 0.46 (0.21, 1.02)c | 0.39 (0.17, 0.90)d |
| Body mass index | 0.26 (0.09, 0.80)d | 0.02 (0.00, 0.10)e | 0.13 (0.04, 0.40)e | 1.14 (0.42, 3.11) | 0.69 (0.24, 1.96) |
| Hip anteversion | 8.07 (2.45, 26.6)e | 3.04 (0.83, 11.2)c | 2.43 (0.75, 7.91)b | 1.36 (0.46, 3.98) | 10.10 (2.89, 35.4)e |
| Quadriceps angle | 0.22 (0.08, 0.65)e | 0.12 (0.03, 0.43)e | 0.40 (0.15, 1.06)c | 0.89 (0.30, 2.62) | 0.51 (0.17,1.52) |
| Tibiofemoral angle | 2.08 (0.70, 6.19)b | 1.87 (0.54, 6.47) | 2.46 (0.86, 6.99)c | 0.36 (0.12, 1.05)c | 2.69 (0.82, 8.86)b |
| Tibial torsion | 0.47 (0.19, 1.16)b | 0.21 (0.07, 0.62)e | 0.78 (0.34, 1.81) | 0.48 (0.18, 1.30)b | 0.75 (0.30, 1.89) |
| Navicular drop | 1.77 (0.77, 4.04)b | 2.77 (1.06, 7.29)d | 1.16 (0.54, 2.48) | 1.14 (0.48, 2.69) | 2.97 (1.20, 7.33)d |
| Femur length | 0.09 (0.02, 0.33)e | 0.06 (0.01, 0.28)e | 0.08 (0.02, 0.30)e | 1.73 (0.63, 4.75) | 0.15 (0.04, 0.57)e |
| Quadriceps peak torque | 0.86 (0.35, 2.11) | 0.29 (0.09, 0.99)d | 1.03 (0.43, 2.47) | 1.13 (0.47, 2.71) | 1.24 (0.49, 3.14) |
Abbreviations: AKL, anterior knee laxity; GR, genu recurvatum; IER, internal-external knee rotation laxity; VV, varus-valgus knee rotation laxity.
a Ratios indicate higher (>1.0) or lower odds (<1.0) for membership in a given laxity profile relative to the LOW laxity profile for each standard deviation increase in the predictor variable, with all other variables held constant. Results based on the standardized scores for all independent variables except sex.
b P < .2.
c P < .1.
d P < .05.
e P < .01.
Table 6.
Logistic Regression Summary a
| Reference Cluster | Cluster |
|||||
| LOW | MOD | HIGH | MODVV/IER | HIGHGR | HIGHAKL | |
| LOW | — | ↓Age | ↓BMI | ↓Age | W> M | M> W |
| ↓BMI | ↓QPT | ↓BMI | ↓Age | ↓Age | ||
| ↑HA | ↓QA | ↓FL | ↓TFA | ↑HA | ||
| ↓QA | ↓FL | ↓QA | TT | ↑ND | ||
| ↓FL | ↓TT | ↑TFA | ↓FL | |||
| TFA | ↑ND | HA | TFA | |||
| ND | ↑HA | |||||
| TT | ↓Age | |||||
| M> W | ||||||
| MOD | — | ↓BMI | ↓HA | W> M | ↑BMI | |
| ↓QPT | BMI | ↑BMI | ↑QA | |||
| ↓HA | QA | ↓HA | ||||
| ↓TT | TT | ↓TFA | ||||
| W>M | ↑QA | |||||
| ↑FL | ||||||
| HIGH | — | BMI | ↑BMI | ↑BMI | ||
| QPT | ↑QPT | ↑QPT | ||||
| QA | ↓TFA | ↑HA | ||||
| TT | ↑QA | ↑QA | ||||
| ND | ↑FL | ↑TT | ||||
| ↓ND | M>W | |||||
| TT | ||||||
| W > M | ||||||
| MODVV/IER | — | W> M | ↑BMI | |||
| ↑BMI | ↑HA | |||||
| ↓TFA | ↑ND | |||||
| ↑FL | ||||||
| QA | ||||||
| HIGHGR | — | M> W | ||||
| ↑HA | ||||||
| ↑TFA | ||||||
| ↓FL | ||||||
| ↑ND | ||||||
| HIGHAKL | — | |||||
Abbreviations: AKL, anterior knee laxity; BMI, body mass index; FL, femur length; GR, genu recurvatum; HA, hip anteversion; IER, internal-external knee rotation laxity; M, men; ND, navicular drop; QA, quadriceps angle; QPT, quadriceps peak torque; TFA, tibiofemoral angle; TT, tibial torsion; V V, varus-valgus knee rotation laxity; W, women.
a Arrows indicate that the cluster noted in the header row is more likely to have greater (↑) or lesser (↓) values than the reference cluster noted in the left column. Bold text: P < .05; normal text: P < .10; italic text: P < .20.
Predictors of Membership in the LOW Cluster.
Once sex and all other physical characteristics were accounted for, participants who were older and had longer femur lengths were more likely to be members in LOW (Table 1). The only exception was that femur length did not distinguish between memberships in the LOW and HIGHGR clusters.
Predictors of Membership in the MOD Cluster.
Participants who had more hip anteversion, were younger, and had a lower BMI, smaller Q-angle, and shorter femur length were more likely to be in MOD than in LOW (ORs = 8.06, 0.29, 0.26, 0.22, and 0.09, respectively; all Ps < .05). That is, for every 1-SD increase in hip anteversion, participants were 8.06 times more likely to be in MOD than in LOW. Similarly, for every 1-SD increase in age, BMI, Q-angle, and femur length, the odds were 0.29, 0.26, 0.22, and 0.09 lower for participants to be in MOD than in LOW. Participants in MOD were also more likely to have greater hip anteversion than those in MODVV/IER, HIGH, or HIGHGR (ORs = 3.3, 2.7, and 5.9, respectively; all Ps < .05) and smaller Q-angles than HIGHGR (OR = 0.25, P < .05) or HIGHAKL (OR = 0.44, P < .10).
Predictors of Membership in the HIGH Cluster.
Participants who had greater navicular drop and a tendency toward greater hip anteversion (P = .09); were somewhat younger (P = .07); and had lower values for BMI, Q-angle, tibial torsion, and peak quadriceps torques and shorter femur lengths were more likely to be in HIGH than in LOW (ORs = 3.04, 2.77, 0.28, 0.02, 0.11, and 0.21, respectively; all Ps < .05 unless otherwise stated). Participants with lower BMI and less quadriceps peak torque were also more likely to be in HIGH than in all other laxity clusters (ORs = 0.07, 0.14, 0.02, and 0.03 for MOD, MODVV/IER, HIGHGR, and HIGHAKL, respectively, for BMI; 0.34, 0.28, 0.26, and 0.24 for MOD, MODVV/IER, HIGHGR, and HIGHAKL, respectively, for quadriceps peak torque). Participants with smaller Q-angles were also more likely to be in HIGH than in MODVV/IER, HIGHGR, and HIGHAKL (ORs = 0.29, 0.13, and 0.23, respectively).
Predictors of Membership in the MODVV/IER Cluster.
Participants who were younger and had lower BMIs and shorter femur lengths were more likely to be in MODVV/IER than in LOW (ORs = 0.23, 0.35, and 0.08, respectively). Participants in MODVV/IER were also more likely to have smaller BMIs than those in HIGHGR (OR = 0.11) and HIGHAKL (OR = 0.18), less navicular drop than those in HIGH (OR = 0.42) or HIGHAKL (OR = 0.39), and greater Q-angles (OR = 3.50), quadriceps peak torque (OR = 3.52), and tibial torsion (OR = 3.69) than those in HIGH.
Predictors of Membership in the HIGHGR Cluster.
No significant predictors (P < .05 level) differentiated HIGHGR from LOW. However, participants in HIGHGR were more likely to have smaller tibiofemoral angles (ie, a more relative varus knee; ORs = 0.17, 0.19, 0.15, and 0.13, respectively) and longer femur lengths (ORs = 19.54, 29.1, 21.84, and 11.8, respectively) than were MOD, HIGH, MODVV/IER, and HIGHAKL participants, respectively. Participants in HIGHGR were also more likely to be women than those in MOD, MODVV/IER, and HIGHAKL (ORs = 0.012, 0.023, and 0.005, respectively) and more likely to have less hip anteversion than those in MOD and HIGHAKL (ORs = 0.17 and 0.13, respectively) and larger Q-angles than those in MOD and HIGH (ORs = 4.0 and 7.8, respectively).
Predictors of Membership in the HIGHAKL Cluster.
Participants who were younger men and who had greater hip ante-version and navicular drop and shorter femur lengths were more likely to be in the HIGHAKL than LOW cluster. Participants in HIGHAKL were also more likely to have greater hip anteversion than those in HIGH, MODVV/IER, and HIGHGR (ORs = 3.3, 4.2, and 7.4, respectively), greater navicular drop than those in MODVV/IER (OR = 2.6), and shorter femur lengths than those in HIGHGR (OR = 0.09).
DISCUSSION
Our goal was to cluster individuals by their multiplanar knee laxity profiles and determine some of the physical characteristics that predict membership in each cluster. In general, our hypotheses were supported: We were able to identify distinct clusters that differed in the absolute and relative magnitudes of their multiplanar knee laxity profiles, and an individual's physical characteristics in part predicted the probability of membership in a particular cluster. The following paragraphs address the characterization and implications of the different multiplanar knee laxity profiles defined for each cluster, followed by a discussion of the observed associations between physical characteristics and each multiplanar knee laxity cluster.
Multiplanar Knee Laxity Clusters
Six distinct multiplanar knee laxity profiles were identified based on the cluster analysis. The first 3 clusters (LOW, MOD, HIGH) represented participants with systematically low, moderate, or high overall multiplanar knee laxity, respectively. The last 3 clusters (MODVV/IER, HIGHGR, HIGHAKL) represented participants with disproportionately higher magnitudes of VV/IER, GR, and AKL laxity, respectively. The latter 3 laxity profiles suggest that the envelope of laxity about the knee is not uniform in all planes of motion in all people. Current evidence suggests that higher-risk knee joint biomechanics occur in the same planes of motion in which greater magnitudes of knee laxity are observed10–12 and that each laxity value may uniquely contribute to high-risk landing biomechanics11 and ACL injury risk.1,3,5,8 Based on these collective observations and the findings that correlations of knee laxity values across the different planes of motion with one another are low to moderate,18,36 the associations among joint laxity, ACL injury risk, and other knee conditions (eg, osteoarthritis) may be more complex than any single laxity measure. It will be important for future authors to account for multiplanar knee joint laxity in order to fully understand the implications of greater magnitudes of knee joint laxity on knee joint biomechanics and injury risk.
Associations Between Physical Characteristics and Cluster Membership
We then considered the primary physical characteristics that predicted membership in a particular cluster in an effort to elucidate the underlying factors that contribute to interparticipant differences in multiplanar knee laxity.
When LOW (the cluster with the least amount of laxity) was compared with all other clusters and after all other physical characteristics were accounted for, participants were more likely to be older than those in all other clusters and to have longer femur lengths than did all other clusters except for HIGHGR (ie, for 1-SD increases in age and femur length, they were 2.2 to 3.4 times more likely and 6.7 to 16.7 more likely, respectively, to be in LOW). Participants in this study population were young adults; the age range was 18 to 30 years. Although the mean ages across the different laxity profiles were not dramatically different, fewer than 20% of participants were 20 years of age or less in LOW, whereas 38% to 50% were 20 years or less in all other laxity profiles. Therefore, there is a greater likelihood that all participants in LOW had achieved full skeletal maturity as compared with those in other laxity profiles. Previous studies40,56,57 have demonstrated a reduction in joint laxity as males and females mature up to 19 years of age. Although we are not aware of any investigators who have continued to follow these maturational laxity trends into young adult years (ie, beyond the age of 19 years), it is possible that laxity continues to decrease as bone and muscle mass increase up to 30 years of age. Moreover, more men were represented in the LOW laxity cluster (68%) than in other clusters (9.5% to 53.0%), which may explain the greater likelihood of longer femur lengths in this cohort.
Distinguishing Characteristics of LOW, MED, and HIGH.
We hypothesized that people with greater overall magnitudes of knee laxity were more likely to be younger, less active, female, and weaker (having less thigh strength) and to have less mass (lower BMI). Distinguishing characteristics among LOW, MOD, and HIGH laxity clusters suggest that this hypothesis was only partially supported: Members in MOD were more likely to be younger and have lower BMIs than LOW, and members in HIGH were more likely to be younger and have lower BMIs and less quadriceps strength than those in both MOD and LOW. The MOD and HIGH clusters were also more likely to have shorter femur lengths than did the LOW. Physical activity was not a predictor of cluster membership, and it is interesting to note that although women represented an increasing proportion of participants assigned to the LOW (32%), MOD (68%), and HIGH (90.5%) laxity clusters, sex was not a strong predictor of cluster membership once other physical characteristics (many of them sex dependent) were taken into account.
Body mass index is often used as a surrogate method of estimating body composition.58 However, a higher BMI can represent greater lean mass in males and greater fat mass in females.59 When other predictors in the model (less quadriceps strength) were considered, every 1-SD increase in BMI and quadriceps strength decreased the odds of being in MOD (0.26 and 0.86 for BMI and strength, respectively) or HIGH (0.02, 0.29). Because lean mass and strength are reported to be positively correlated,60 these findings suggest that members of MOD and HIGH probably had lower overall mass, as well as lower relative lean mass. Many authors7,8,13–18 have reported higher average knee laxity values in females than in males, so sex differences in body composition and, in particular, lean body mass may explain these differences to some extent.
The associations between body composition and knee joint laxity may be particularly true for VV and IER laxity, as members in MODVV/IER were also more likely to have a smaller BMI than those in LOW, HIGHGR, and HIGHAKL (clusters characterized by low VV and IER) but greater BMI than those in HIGH (cluster characterized by greater VV and IER). Specifically, for every 1-SD increase in BMI, participants were less likely to be in MODVV/IER than in LOW (0.35), HIGHGR (0.11), or HIGHAKL (0.18) but more likely to be in MODVV/IER (14.3) than in HIGH. These distinguishing characteristics may in part explain why females, who carry less lean body mass relative to their body weight compared with males after puberty, tend to have disproportionately higher VV and IER laxity than males, even with similar sagittal-plane laxity.16–18 These potential underlying physical characteristics may be important (particularly because BMI and strength are modifiable), as women who have above-average VV and IER laxity demonstrate greater dynamic knee valgus motion when landing.10 These factors may also partially explain why females begin to demonstrate poorer hip and knee neuromuscular control than do males about the time that body composition changes begin to emerge during physical maturation.61–64 However, further work using a more accurate measure of lean body mass and stratifying analyses within sex (to control for other sex confounding factors) is needed to confirm whether these physical characteristics are the key underlying factors leading to the development of greater VV and IER laxity.
Another distinguishing factor of membership in the MOD and HIGH clusters (but not MODVV/IER) was a lower likelihood of having large Q-angles compared with other laxity clusters, especially when compared with participants in LOW and HIGHGR (for every 1-SD increase in Q-angle, participants were less likely to be in MOD than in LOW {0.22} or HIGHGR {0.25}, respectively, and less likely to be in HIGH than in the LOW {0.12} or HIGHGR {0.13} clusters). The Q-angle represents a composite measure of pelvic position, hip rotation, tibial rotation, patellar position, and foot position, such that smaller angles are associated with a more neutral pelvis (changing the orientation of the acetabulum and externally rotating the femur), less femoral anteversion and knee valgus (laterally displacing the patella relative to the anterior-superior iliac spine and tibial tuberosity), and greater internal tibial rotation (displacing the tibial tuberosity laterally).29 These multiple contributions make it difficult to fully interpret how Q-angle magnitude may affect biomechanical loading of the knee during weight bearing, particularly because these laxity profiles also had a trend toward a greater likelihood of hip anteversion compared with LOW (P < .10). Interesting to note is that the higher or lower Q-angles associated with the odds of being in a specific laxity profile (once other physical characteristics are accounted for) were somewhat inconsistent with the comparative mean values across the different laxity profiles (Table 3), which we did not observe with the other predictors. It may be that the predictive value of the Q-angle depends largely on its anatomical contributions, which were also entered in the model.
A final observation is that GJL was not a significant predictor of LOW or HIGH membership. This was surprising in that greater GJL has been associated with higher magnitudes of VV and IER,65 and GR is one of the criterion measures for GJL. Although the mean values were somewhat higher in clusters with higher magnitudes of VV, IER, or GR or a combination of these (Table 3), GJL was not a significant predictor of cluster membership once other physical characteristics were accounted for (P = .530). These findings suggest that GJL may represent a laxity phenomenon independent of knee joint laxity and thus a separate but important risk factor for ACL injury.8
Distinguishing Characteristics of Disproportionally Higher Laxity in One Plane of Motion.
Our hypothesis, that structural characteristics were more likely to predict membership in clusters with disproportionally higher knee laxity in a given plane of motion, was in large part supported. Individuals in clusters characterized by higher magnitudes of AKL had a 3 to 10 times greater likelihood of having more hip anteversion (MOD, HIGH, HIGHAKL), a 2.8 to 3.0 times greater likelihood of having more navicular drop (HIGH, HIGHAKL), and a 0.06 to 0.15 times lower likelihood of having longer femur length (MOD, HIGH, HIGHAKL) than did LOW (Table 1). One or more of these characteristics was also consistently found when any of these clusters was compared with MODVV/IER or HIGHGR, clusters that did not reflect proportionally higher AKL values (Table 6). An association between greater magnitude of navicular drop and AKL is consistent with the previous literature.15,32 When the foot pronates excessively during weight bearing, the obligatory internal rotation of the tibia on the foot is thought to lead to internal rotation of the tibia on the femur,35,66–68 which can increase ACL loads in the weight-bearing knee.69 It is also logical that greater magnitudes of hip anteversion may combine with greater magnitudes of navicular drop to promote greater rotary stress on the knee. Greater hip anteversion is commonly associated with an in-toeing gait,70,71 which can lead to compensations in other parts of the lower extremity, including excessive internal rotation of the tibia and overpronation of the subtalar joint during walking.70 Because shorter femur lengths were common to profiles with either moderate to high AKL or moderate to high VV and IER laxity, it is more likely that a longer femur length is a distinguishing characteristic of LOW (as previously noted) and HIGHGR (section to follow).
The primary distinguishing characteristics of HIGHGR were a lower likelihood (0.13 to 0.19) of having high tibiofemoral angles (or, conversely stated, a 5.3 to 7.7 times higher likelihood of having a more varus knee) and an 11.8 to 29.1 times higher likelihood of having a longer femur length than did all other laxity profiles except for LOW. Genu recurvatum can result from capsuloligamentous laxity, structural factors, or the combination of both.72 Because those in HIGHGR were more likely to have greater GR without greater VV and IER (or GJL), the cause of GR may be more structural, selectively stretching the posterolateral tissue constraints that control knee hyperextension.73,74 This possibility is supported by results from the logistic regression in that membership in HIGHGR was predicted primarily by structural factors. Tibiofemoral angle describes the angulation of the knee in the frontal plane, where reduced angulation is associated with a varus knee and increased medial contact forces. Greater relative varus alignment may lead to greater varus accelerations, which have been associated with posterolateral instability and excessive GR.74,75 The combination of smaller tibiofemoral angles with longer femur lengths may tend to increase the length of the moment arm, increasing the varus stress more than would be experienced with a short moment arm. As further support for this concept of a lengthened moment arm, these clusters tended to have longer tibia lengths (Table 3); however, tibia length was not a significant predictor in the model, probably because of its high correlation with femur length (r = 0.889).
Members in HIGHGR were also more likely to be female than those in MOD, MODVV/IER, and HIGHAKL (a trend toward same was noted when HIGHGR was compared with LOW and HIGH [P < .20]), once all other physical characteristics were taken into account. This was the only cluster in which sex was a consistent predictor of cluster membership. Although it is difficult to explain why females would be more likely to be in HIGHGR than in other clusters (especially because they are less likely to have smaller tibiofemoral angles and longer femur lengths than males, the other predictors of membership in HIGHGR), sex may be acting as a surrogate for other sex-dependent physical factors not accounted for in the model (eg, hormones, tibial geometry). More work is needed to understand the underlying cause of this sex-dependent association.
In summary, knee joint laxity is not uniform across different directions and planes of motion, and a person's multiplanar knee laxity may in part be explained by age, body composition and strength, and lower extremity posture. Specifically, participants who were younger and had a lower BMI and less thigh muscle strength were typically associated with clusters characterized by greater overall frontal- and transverse-plane laxity profiles (regardless of sagittal-plane laxity profile), whereas structural factors were more often associated with clusters characterized by disproportionately greater AKL (ie, greater likelihood of having more hip anteversion and navicular drop) or GR (ie, greater likelihood of having smaller tibiofemoral angles and longer femur lengths). Except for HIGHGR, these associations did not depend strongly on a person's sex, which suggests that the greater magnitudes of knee laxity more often observed in females may be largely explained by innate sex differences in body composition and structure. More work is needed to elucidate how these interparticipant differences in multiplanar knee laxity affect stability at the knee during weight-bearing activity and, ultimately, ACL injury risk.
Acknowledgments
This project was supported by award number R01AR053172 from the National Institutes of Health (NIH) National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) awarded to the University of North Carolina at Greensboro (S.J.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the NIAMS.
REFERENCES
- 1.Branch TP, Browne JE, Campbell JD. Rotational laxity greater in patients with contralateral anterior cruciate ligament injury than healthy volunteers. Knee Surg Sports Traumatol Arthrosc. 2010;18(10):1379–1384. doi: 10.1007/s00167-009-1010-y. et al. [DOI] [PubMed] [Google Scholar]
- 2.Hewett TE, Lynch TR, Myer GD, Ford KR, Gwin RC, Heidt RS., Jr. Multiple risk factors related to familial predisposition to anterior cruciate ligament injury: fraternal twin sisters with anterior cruciate ligament ruptures. Br J Sports Med. 2010;44(12):848–855. doi: 10.1136/bjsm.2008.055798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramer LC, Denegar CR, Buckley WE, Hertel J. Factors associated with anterior cruciate ligament injury: history in female athletes. J Sports Med Phys Fitness. 2007;47(4):446–454. [PubMed] [Google Scholar]
- 4.Loudon JK, Jenkins W, Loudon KL. The relationship between static posture and ACL injury in female athletes. J Orthop Sports Phys Ther. 1996;24(2):91–97. doi: 10.2519/jospt.1996.24.2.91. [DOI] [PubMed] [Google Scholar]
- 5.Myer GD, Ford KR, Paterno MV, Nick TG, Hewett TE. The effects of generalized joint laxity on risk of anterior cruciate ligament injury in young female athletes. Am J Sports Med. 2008;36(6):1073–1080. doi: 10.1177/0363546507313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramesh R, Von Arx O, Azzopardi T, Schranz PJ. The risk of anterior cruciate ligament rupture with generalised joint laxity. J Bone Joint Surg Br. 2005;87(6):800–803. doi: 10.1302/0301-620X.87B6.15833. [DOI] [PubMed] [Google Scholar]
- 7.Scerpella TA, Stayer TJ, Makhuli BZ. Ligamentous laxity and non-contact anterior cruciate ligament tears: a gender-based comparison. Orthopedics. 2005;28(7):656–660. doi: 10.3928/0147-7447-20050701-12. [DOI] [PubMed] [Google Scholar]
- 8.Uhorchak JM, Scoville CR, Williams GN, Arciero RA, St Pierre P, Taylor DC. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31(6):831–842. doi: 10.1177/03635465030310061801. [DOI] [PubMed] [Google Scholar]
- 9.Woodford-Rogers B, Cyphert L, Denegar CR. Risk factors for anterior cruciate ligament injury in high school and college athletes. J Athl Train. 1994;29(4):343–346. [PMC free article] [PubMed] [Google Scholar]
- 10.Shultz SJ, Schmitz RJ. Effects of transverse and frontal plane knee laxity on hip and knee neuromechanics during drop landings. Am J Sports Med. 2009;37(9):1821–1830. doi: 10.1177/0363546509334225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shultz SJ, Schmitz RJ, Nguyen AD, Levine BJ. Joint laxity is related to lower extremity energetics during a drop jump landing. Med Sci Sports Exerc. 2010;42(4):771–780. doi: 10.1249/MSS.0b013e3181bbeaa6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shultz SJ, Schmitz RJ, Nguyen A. Knee joint laxity and its cyclic variations influence tibiofemoral joint motion during weight acceptance. Med Sci Sports Exerc. 2011;43(2):287–295. doi: 10.1249/MSS.0b013e3181ed118d. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beynnon BD, Bernstein IM, Belisle A. The effect of estradiol and progesterone on knee and ankle joint laxity. Am J Sports Med. 2005;33(9):1298–1304. doi: 10.1177/0363546505275149. et al. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen AD, Shultz SJ. Sex differences in lower extremity alignment. J Orthop Sports Phys Ther. 2007;37(7):389–398. doi: 10.2519/jospt.2007.2487. [DOI] [PubMed] [Google Scholar]
- 15.Trimble MH, Bishop MD, Buckley BD, Fields LC, Rozea GD. The relationship between clinical measurements of lower extremity posture and tibial translation. Clin Biomech (Bristol, Avon) 2002;17(4):286–290. doi: 10.1016/s0268-0033(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 16.Hsu WH, Fisk JA, Yamamoto Y, Debski RE, Woo SL. Differences in torsional joint stiffness of the knee between genders: a human cadaveric study. Am J Sports Med. 2006;34(5):765–770. doi: 10.1177/0363546505282623. [DOI] [PubMed] [Google Scholar]
- 17.Sharma L, Lou C, Felson DT. Laxity in healthy and osteoarthritic knees. Arthritis Rheum. 1999;42(5):861–870. doi: 10.1002/1529-0131(199905)42:5<861::AID-ANR4>3.0.CO;2-N. et al. [DOI] [PubMed] [Google Scholar]
- 18.Shultz SJ, Schmitz RJ, Beynnon BD. Variations in varus/valgus and internal/external rotational knee laxity and stiffness across the menstrual cycle. J Orthop Res. 2011;29(3):318–325. doi: 10.1002/jor.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shultz SJ, Sander TC, Kirk SE, Perrin DH. Sex differences in knee laxity change across the female menstrual cycle. J Sports Med Phys Fitness. 2005;45(4):594–603. [PMC free article] [PubMed] [Google Scholar]
- 20.Hart DJ, Boykiw R, Sciore P, Reno C. Complex alterations in gene expression occur in knee ligaments of the skeletally mature multiparous rabbit during pregnancy. Biochimica Biophysica Acta. 1998;1397(3):331–341. doi: 10.1016/s0167-4781(98)00018-9. [DOI] [PubMed] [Google Scholar]
- 21.Hashemi J, Chandrashekar N, Gill B. The tibial plateau and its influence on the biomechanics of the tibiofemoral joint: a sex-based comparison in a blinded study [abstract] J Athl Train. 2008;43(5):556. et al. [Google Scholar]
- 22.McLean SG, Lucey SM, Rohrer S, Brandon C. Knee joint anatomy predicts high-risk in vivo dynamic landing knee biomechanics. Clin Biomech (Bristol, Avon) 2010;25(8):781–788. doi: 10.1016/j.clinbiomech.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Shultz SJ, Nguyen AD, Levine BJ. The relationship between lower extremity alignment characteristics and anterior knee joint laxity. Sports Health. 2009;1(1):54–60. doi: 10.1177/1941738108326702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24(4):427–436. doi: 10.1177/036354659602400405. [DOI] [PubMed] [Google Scholar]
- 25.Vauhnik R, Morrissey MC, Rutherford OM, Turk Z, Pilih IA, Perme MP. Correlates of knee anterior laxity in sportswomen. Knee. 2009;16(6):427–431. doi: 10.1016/j.knee.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Hruska R. Pelvic stability influences lower extremity kinematics. Biomechanics. 1998;5(6):23–29. [Google Scholar]
- 27.Khamis S, Yishar Z. Effect of feet hyperpronation on pelvic alignment in a standing position. Gait Posture. 2007;25(1):127–134. doi: 10.1016/j.gaitpost.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Kendall FP, McCreary EK, Provance PG. Muscles: Testing and Function. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1993. [Google Scholar]
- 29.Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33(11):639–646. doi: 10.2519/jospt.2003.33.11.639. [DOI] [PubMed] [Google Scholar]
- 30.Van der Esch M, Steultjens M, Wieringa H, Dinant H, Dekker J. Structural joint changes, malalignment, and laxity in osteoarthritis of the knee. Scand J Rheumatol. 2005;34(4):298–301. doi: 10.1080/03009740510018651. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen AD, Shultz SJ, Schmitz RJ, Luecht RM, Perrin DH. A preliminary multifactorial approach describing the relationships between lower extremity alignment, hip muscle activation and lower extremity joint excursion. J Athl Train. 2011;46(3):246–256. doi: 10.4085/1062-6050-46.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coplan JA. Rotational motion of the knee: a comparison of normal and pronating subjects. J Orthop Sports Phys Ther. 1989;10(9):366–369. doi: 10.2519/jospt.1989.10.9.366. [DOI] [PubMed] [Google Scholar]
- 33.Sharma L, Song JS, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286(2):188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 34.Beckett ME, Massie DL, Bowers KD, Stoll DA. Incidence of hyper-pronation in the ACL injured knee: a clinical perspective. J Athl Train. 1992;27(1):58–60. [PMC free article] [PubMed] [Google Scholar]
- 35.Hertel JN, Dorfman JH, Braham RA. Lower extremity malalignments and anterior cruciate ligament injury history. J Sports Sci Med. 2004;3(4):220–225. [PMC free article] [PubMed] [Google Scholar]
- 36.Shultz SJ, Schmitz RJ, Kong Y. Cyclic variations in multi-planar knee laxity profiles influence landing biomechanics. Med Sci Sports Exerc. doi: 10.1249/MSS.0b013e31823bfb25. et al. In press. [DOI] [PubMed] [Google Scholar]
- 37.Shultz SJ, Nguyen AD, Perrin DH. A Comparison of Cyclic Variations in Anterior Knee Laxity, Genu Recurvatum and General Joint Laxity Across the Female Menstrual Cycle. doi: 10.1002/jor.21145. Presented at: 54th Annual meeting of the Orthopaedic Research Society; San Francisco, CA; February 20–23, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213–218. doi: 10.1177/03635465010290021601. [DOI] [PubMed] [Google Scholar]
- 39.Shultz SJ, Nguyen AD, Windley TC, Kulas AS, Botic TL, Beynnon BD. Intratester and intertester reliability of clinical measures of lower extremity anatomical alignment: implications for multi-center studies. Clin J Sport Med. 2006;16(2):155–161. doi: 10.1097/00042752-200603000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Shultz SJ, Nguyen AD, Schmitz RJ. Differences in lower extremity anatomical and postural characteristics in males and females between maturation groups. J Orthop Sports Phys Ther. 2008;38(3):137–149. doi: 10.2519/jospt.2008.2645. [DOI] [PubMed] [Google Scholar]
- 41.Gilliam J, Brunt D, MacMillan M, Kinard RE, Montgomery WJ. Relationship of the pelvic angle to the sacral angle: measurement of clinical reliability and validity. J Orthop Sports Phys Ther. 1994;20(4):193–199. doi: 10.2519/jospt.1994.20.4.193. [DOI] [PubMed] [Google Scholar]
- 42.Moreland JR, Bassett LW, Hanker GJ. Radiographic analysis of the axial alignment of the lower extremity. J Bone Joint Surg Am. 1987;69(5):745–749. [PubMed] [Google Scholar]
- 43.Brody DM. Techniques in the evaluation and treatment of the injured runner. Orthop Clin North Am. 1982;13(3):541–558. [PubMed] [Google Scholar]
- 44.Magee DJ. Orthopedic Physical Assessment. 2nd ed. Philadelphia, PA: WB Saunders; 1992. [Google Scholar]
- 45.Stuberg W, Temme J, Kaplan P, Clarke A, Fuchs R. Measurement of tibial torsion and thigh-foot angle using goniometry and computed tomography. Clin Orthop Relat Res. 1991;272:208–212. [PubMed] [Google Scholar]
- 46.Nawata K, Teshima R, Morio Y, Hagino H, Enokida M, Yamamoto K. Anterior-posterior knee laxity increased by exercise: quantitative evaluation of physiologic changes. Acta Orthop Scand. 1999;70(3):261–264. doi: 10.3109/17453679908997803. [DOI] [PubMed] [Google Scholar]
- 47.Stoller DW, Markolf KL, Zager SA, Shoemaker SC. The effects of exercise, ice and ultrasonography on torsional laxity of the knee. Clin Orthop Relat Res. 1983;174:172–180. [PubMed] [Google Scholar]
- 48.Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Ann Rheum Dis. 1973;32(5):413–418. doi: 10.1136/ard.32.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shultz SJ, Levine BJ, Nguyen AD, Kim H, Montgomery MM, Perrin DH. A comparison of cyclic variations in anterior knee laxity, genu recurvatum and general joint laxity across the menstrual cycle. J Orthop Res. 2010;28(11):1411–1417. doi: 10.1002/jor.21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shultz SJ, Shimokochi Y, Nguyen AD, Schmitz RJ, Beynnon BD, Perrin DH. Measurement of varus-valgus and rotational knee laxity in-vivo, part I: assessment of measurement reliability and bilateral asymmetry. J Orthop Res. 2007;25(8):981–988. doi: 10.1002/jor.20397. [DOI] [PubMed] [Google Scholar]
- 51.Everitt B, Landau S. Cluster Analysis. New York, NY: Oxford University Press; 2001. [Google Scholar]
- 52.Ward JH., Jr. Hierarchical grouping to optimise an objective function. J Am Stat Assoc. 1963;58(301):236–244. [Google Scholar]
- 53.Rencher AC. Methods of Multivariate Analysis. Vol 1. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- 54.Agresti A. Categorical Data Analysis. New York, NY: John Wiley & Sons; 1990. [Google Scholar]
- 55.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- 56.Ahmad CS, Clark AM, Heilmann N, Schoeb JS, Gardner TR, Levine WN. Effect of gender and maturity on quadriceps-to-hamstring strength ratio and anterior cruciate ligament laxity. Am J Sports Med. 2006;34(3):370–374. doi: 10.1177/0363546505280426. [DOI] [PubMed] [Google Scholar]
- 57.Flynn JM, Mackenzie W, Kolstad K, Sandifer E, Jawad AF, Galinat B. Objective evaluation of knee laxity in children. J Pediatr Orthop. 2000;20(2):259–263. [PubMed] [Google Scholar]
- 58.Smalley KJ, Knerr AN, Kendrick ZV, Colliver JA, Owen OE. Reassessment of body mass indices. Am J Clin Nutr. 1990;52(3):405–408. doi: 10.1093/ajcn/52.3.405. [DOI] [PubMed] [Google Scholar]
- 59.Loomba-Albrecht LA, Styne DM. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes. 2009;16(1):10–15. doi: 10.1097/med.0b013e328320d54c. [DOI] [PubMed] [Google Scholar]
- 60.Maughan RJ, Watson JS, Weir J. Strength and cross-sectional area of human skeletal muscle. J Physiol. 1983;338(1):37–49. doi: 10.1113/jphysiol.1983.sp014658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ford KR, Shapiro R, Myer GD, Van Den Bogert AJ, Hewett TE. Longitudinal sex differences during landing in knee abduction in young athletes. Med Sci Sports Exerc. 2010;42(10):1923–1931. doi: 10.1249/MSS.0b013e3181dc99b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hewett TE, Myer GD, Ford KR. Decrease in neuromuscular control about the knee with maturation in female athletes. J Bone Joint Surg Am. 2004;86(8):1601–1608. doi: 10.2106/00004623-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 63.Quatman CE, Ford KR, Myer GD, Hewett TE. Maturation leads to gender differences in landing force and vertical jump performance: a longitudinal study. Am J Sports Med. 2006;34(5):806–813. doi: 10.1177/0363546505281916. [DOI] [PubMed] [Google Scholar]
- 64.Schmitz RJ, Shultz SJ, Nguyen AD. Dynamic valgus alignment and functional strength in males and females during maturation. J Athl Train. 2009;44(1):26–32. doi: 10.4085/1062-6050-44.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shultz SJ, Shimokochi Y, Nguyen AD, Schmitz RJ, Beynnon BD, Perrin DH. Measurement of varus-valgus and internal-external rotational knee laxities— in-vivo, part II: relationship with anterior-posterior and general joint laxity in males and females. J Orthop Res. 2007;25(8):989–996. doi: 10.1002/jor.20398. [DOI] [PubMed] [Google Scholar]
- 66.Bates BT, Osternig LR, Mason B, James LS. Foot orthotic devices to modify selected aspects of lower extremity mechanics. Am J Sports Med. 1979;7(6):338–342. doi: 10.1177/036354657900700606. [DOI] [PubMed] [Google Scholar]
- 67.Fleming BC, Renstrom PA, Beynnon BD. The effect of weightbearing and external loading on anterior cruciate ligament strain. J Biomech. 2001;34(2):163–170. doi: 10.1016/s0021-9290(00)00154-8. et al. [DOI] [PubMed] [Google Scholar]
- 68.Cornwall MW, McPoil TG. Footwear and foot orthotic effectiveness research: a new approach. J Orthop Sports Phys Ther. 1995;21(6):337–344. doi: 10.2519/jospt.1995.21.6.337. [DOI] [PubMed] [Google Scholar]
- 69.Cowan DN, Jones BH, Frykman PN. Lower limb morphology and risk of overuse injury among male infantry trainees. Med Sci Sports Exerc. 1996;28(8):945–952. doi: 10.1097/00005768-199608000-00002. et al. [DOI] [PubMed] [Google Scholar]
- 70.Staheli LT. Torsional deformity. Pediatr Clin North Am. 1977;24(4):799–811. doi: 10.1016/s0031-3955(16)33499-x. [DOI] [PubMed] [Google Scholar]
- 71.Gulan G, Matovinovic D, Nemec B, Rubinic D, Ravlic-Gulan J. Femoral neck anteversion: values, development, measurement, common problems. Coll Antropol. 2000;24(2):521–527. [PubMed] [Google Scholar]
- 72.Dejour D, Bonin N, Locatelli E. Tibial antirecurvatum osteotomies. Oper Tech Sports Med. 2000;8(1):67–70. [Google Scholar]
- 73.Morgan PM, LaPrade RF, Wentorf FA, Cook JW, Bianco A. The role of the oblique popliteal ligament and other structures in preventing knee hyperextension. Am J Sports Med. 2010;38(3):550–557. doi: 10.1177/0363546509348742. [DOI] [PubMed] [Google Scholar]
- 74.Noyes FR, Dunworth LA, Andriacchi TP, Andrews M, Hewett TE. Knee hyperextension gait abnormalities in unstable knees: recognition and pre-operative gait retraining. Am J Sports Med. 1996;24(1):35–45. doi: 10.1177/036354659602400107. [DOI] [PubMed] [Google Scholar]
- 75.Loudon JK, Goist HL, Loudon KL. Genu recurvatum syndrome. J Orthop Sports Phys Ther. 1998;27(5):361–367. doi: 10.2519/jospt.1998.27.5.361. [DOI] [PubMed] [Google Scholar]


